Abstract
The physiologic roles of angiopoietin-like proteins (Angptls) in the hematopoietic system remain unknown. Here we show that hematopoietic stem cells (HSCs) in Angptl3-null mice are decreased in number and quiescence. HSCs transplanted into Angptl3-null recipient mice exhibited impaired repopulation. Bone marrow sinusoidal endothelial cells express high levels of Angptl3 and are adjacent to HSCs. Importantly, bone marrow stromal cells or endothelium deficient in Angptl3 have a significantly decreased ability to support the expansion of repopulating HSCs. Angptl3 represses the expression of the transcription factor Ikaros, whose unregulated overexpression diminishes the repopulation activity of HSCs. Angptl3, as an extrinsic factor, thus supports the stemness of HSCs in the bone marrow niche.
Introduction
Hematopoietic stem cells (HSCs) are defined by their abilities to self-renew and differentiate into all blood cell types.1-3 The balance between different cell fates—self-renewal, differentiation, apoptosis, and migration—determines the number of HSCs. Extrinsic factors, including stem cell factor (SCF), thrombopoietin (TPO), FGF-1, Notch ligands, BMPs, Wnts, prostaglandin E2 (PGE2), transforming growth factor β (TGFβ), angiopoietin 1, IGF-2, angiopoietin-like proteins (Angptls), and IGFBP2, provide environmental cues to regulate HSC fates in vitro and in vivo (see reviews4,5 ). This extrinsic regulation is achieved by controlling signal transduction and, eventually, the activities of transcription factors, cell-cycle regulators, and chromatin modulators as intrinsic regulators. The study of the extrinsic cue–initiated signaling in HSCs—from ligands and receptors to signaling pathways and nuclear factors—is important for understanding the basic biology of stem cells and will provide critical insight into their therapeutic applications.
Angptls belong to a 7-member family of secreted glycoproteins that share sequence homology with angiopoietins, which are important modulators of angiogenesis.6,7 Each Angptl contains an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain. However, unlike angiopoietins, Angptls do not bind to tyrosine kinase receptor Tie-1 or Tie-2, and their receptors are unknown.6 This suggests that Angptls have functions different from those of angiopoietins. It is known that several members of the Angptl family play roles in regulating lipid metabolism and angiogenesis.6 In particular, Angptl3 inhibits lipoprotein lipase activity in vitro and in vivo, and mice deficient in Angptl3 display defects in lipid metabolism.8 In addition, it was shown that Angptl3 is capable of binding to the cell-surface integrin αVβ3, through which Angptl3 stimulates the adhesion and migration of endothelial cells and induces blood vessel formation.9 Angptl3 also activates protein kinase B and increases the permeability of glomerular endothelial cells.10 Recently, several studies suggested that Angptl3 plays a role in the regulation of HSC activity. We showed that cultures of mouse bone marrow (BM) side population (SP) CD45+Sca-1+ cells in serum-free medium containing SCF, TPO, IGF-2, and FGF-1 and supplemented with one of several Angptls (Angptl2, Angptl3, Angptl5, Angptl7, or Mfap4) stimulated a dramatic expansion of mouse long-term (LT) HSC numbers and activities.11 We also found that the inclusion of Angptl with other proteins dramatically stimulated expansion of human HSCs.12 The Zon laboratory demonstrated that Angptl1 and Angptl2 are required for HSC development during zebrafish embryogenesis.13 Chou and Lodish indicated that mouse fetal hepatic progenitors produce multiple growth factors, including Angptl3, and serve as the primary stromal cells for the expansion of fetal liver HSCs in vivo.14 However, most of the physiologic activities of the Angptls, including those of Angptl3, remain unknown. To elucidate the mechanism by which Angptl regulates HSC activity, we sought to investigate the in vivo role of Angptl3 in the mouse BM.
Methods
Mice
C57BL/6 CD45.2 and CD45.1 mice were purchased from the National Cancer Institute and the University of Texas Southwestern Medical Center animal breeding core facility. The original Angptl3-null mice were provided by Nuvelo and were backcrossed to C57BL/6 CD45.2 mice 10 times to obtain Angptl3-null and wild-type (WT) control littermates. Mice were maintained at the University of Texas Southwestern Medical Center animal facility. All animal experiments were performed with the approval of University of Texas Southwestern Committee on Animal Care. To genotype mice, DNA was extracted from tail tips using a DNAeasy kit according to the manufacturer's instructions (Sigma). The Angptl3 and/or LacZ-neomycin (neo) insert was amplified in a 3-primer PCR using the following primers: 5′TGTCTGTCAATTCTAGACCTTCCTGC3′ and 5′ATGATCCCAGATTTGCGTGAATAACC3′ for Angptl3, and 5′ATGATCCCAGATTTGCGTGAATAACC3′ and 5′GGGCCAGCTCATTCCTCCCACTCAT3′ for the lacZ-neomycin insert. The cycling conditions were 94°C for 2 minutes, followed by 35 cycles of 94°C for 45 seconds, 60°C for 45 seconds, and 72°C for 60 seconds, followed by a final extension of 72°C for 5 minutes.
Culture medium
StemSpan serum-free medium (StemCell Technologies) was used as the basal medium. The basal medium supplemented with 10 μg/mL heparin (Sigma), 10 ng/mL mouse SCF (R&D Systems), 20 ng/mL mouse TPO (R&D Systems), and 10 ng/mL human FGF-1 (Invitrogen) was used as STF (as SCF + TPO + FGF-1) medium. FBS was included in the STF medium in the coculture experiment described in Figure 4C and supplemental Figure 11 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article.)
Angptl3 binding assay
BM cells were isolated from 8- to 12-week-old C57BL/6 mice. Lin+ cells were depleted by AutoMACS with a biotinylated lineage cocktail (anti-CD3, anti-CD5, anti-B220, anti–Mac-1, anti–Gr-1, anti-Ter119; StemCell Technologies) followed by streptavidin-magnetic beads. Total BM, Lin+, or Lin− cells were incubated with 1500 ng/mL of Angptl3 protein in PBS/2% FBS buffer for 1 hour followed by goat anti-Angptl3 antibody for 15 minutes, and anti–goat-APC alone, or further combined with anti–Sca-1-PE/CY5.5, anti-Kit-FITC, and anti–CD150-PE (or anti–Flk-2-PE, and anti–CD34-PE) to label LT-HSCs. The staining was conducted at 4°C. The cells were then subjected to flow cytometric analysis.
Mouse HSC culture
Indicated numbers of BM Lin−Sca-1+Kit+Flk2−CD34− cells were isolated from 8- to 12-week-old C57BL/6 mice and were plated into wells of a U-bottom 96-well plate (3799; Corning) with the indicated medium as described. Cells were cultured at 37°C in 5% CO2 and the normal level of O2. For the purpose of competitive transplantation, cells from 12 culture wells were pooled and mixed with competitor/supportive cells before the indicated numbers of cells were transplanted into each mouse.
Flow cytometry
Donor BM cells were isolated from 8- to 12-week-old C57BL/6 CD45.2 mice. Lin−Sca-1+Kit+Flk2−CD34− cells were isolated by staining with a biotinylated lineage cocktail (anti-CD3, anti-CD5, anti-B220, anti–Mac-1, anti–Gr-1, anti-Ter119; StemCell Technologies) followed by streptavidin-PE/Cy5.5, anti–Sca-1-FITC, anti–Kit-APC, anti–Flk-2-PE, and anti–CD34-PE. For analyzing the repopulation of mouse HSCs, peripheral blood cells of recipient CD45.1 mice were collected by retro-orbital bleeding, followed by lysis of red blood cells and staining with anti–CD45.2-FITC, anti–CD45.1-PE, anti–Thy1.2-PE (for T-lymphoid lineage); anti–B220-PE (for B-lymphoid lineage); anti–Mac-1-PE; or anti–Gr-1-PE (cells costaining with anti–Mac-1 and anti–Gr-1 were deemed to be of the myeloid lineage) monoclonal antibodies (BD PharMingen). The “percent repopulation” shown in all figures was based on the staining results with anti–CD45.2-FITC and anti–CD45.1-PE. In all cases, FACS analysis of the above listed lineages was also performed to confirm multilineage reconstitution as previously described.15 For limiting dilution analysis, the HSC frequencies were calculated using the L-Calc 1.1 program from StemCell Technologies.16
Cell-cycle analysis with Hoechst and pyronin Y staining was performed as follows. The Lin−Sca-1+Kit+Flk2−CD34− cells were collected in Hanks buffered salt solution medium containing 10% FBS, 1 g/L glucose, and 20mM Hepes (pH 7.2). Cells were washed, Hoechst 33 342 (20 μg/mL, Invitrogen) was added, and cells were incubated at 37°C for 45 minutes, after which pyronin Y (1 μg/mL, Sigma) was added. Cells were incubated for another 15 minutes at 37°C, washed, and resuspended in cold PBS. Samples were immediately analyzed by flow cytometry (BD Biosciences, FACSAria). To examine 5-bromo-2′-deoxyuridine (BrdU) incorporation, mice were given 3 intraperitoneal injections of BrdU (Sigma; 3 mg/24 hours) in PBS and maintained on 0.2 mg/mL of BrdU in the drinking water for 72 hours. After 72 hours, the BM was harvested and stained with antibodies against lineage markers, c-Kit, and Sca-1. Cells were fixed, permeablized, and denatured, followed by antibody staining with anti–BrdU-PE (BD PharMingen) to examine BrdU incorporation.
Competitive reconstitution analysis
The indicated numbers of mouse CD45.2 donor cells were mixed with 1 × 105 freshly isolated CD45.1 competitor BM cells and the mixture was injected intravenously via the retro-orbital route into each of a group of 6- to 9-week-old CD45.1 mice that had been previously irradiated with a total dose of 10 Gy. One million BM cells collected from primary recipients were used for the secondary transplantation. To measure reconstitution of transplanted mice, peripheral blood was collected at the indicated times after transplantation and the presence of CD45.1+ and CD45.2+ cells in lymphoid and myeloid compartments were measured as described.16,17
Quantitative RT-PCR
Total RNA was isolated from FACS-collected BM Lin−Sca-1+Kit+Flk2−CD34− cells, differentiated lineage cells, or nonhematopoietic cells. First-strand cDNA was synthesized using SuperScript II RT (Invitrogen). Samples were analyzed in triplicate 25 μL reactions (300nM of primers, 12.5 μL of Master mix), which was adapted from the standard SYBR Green PCR Master Mix and RT-PCR protocols provided by Applied Biosystems. Primers were purchased from Qiagen or designed with software from Primer 3, and ordered from Sigma (supplemental Table 1). The default PCR protocol was performed on an Applied Biosystems Prism 7000 Sequence Detection System. The mRNA level in each population was normalized to the level of β-actin RNA transcripts present in the same sample, as described previously.11,16,18
Immunohistochemistry
To detect the HSCs in BM, immunofluorescence analysis was performed as described.19 Briefly, freshly dissected undecalcified femurs from 6- to 12-week-old WT C57BL/6 mice were embedded in OCT and chilled in dry ice for 1-2 hours. Sections were generated using Superfrost/Plus microscope slides (Fisher Scientific, Cat No: CM-5951WPLUS-001), Leica High Profile microtome blades (Model 818, Cat No: 63 062-01), and a Leica Cryostat with Blade Holder for a High Profile Blade at −20°C. The 8 μm sections were air-dried overnight at room temperature and subsequently fixed in −20°C methanol for 10 minutes. Slides were then blocked with 20% goat serum in 0.1M phosphate buffer (pH 7.4) for 60 minutes and then primary antibodies overnight. The primary antibodies used for the labeling of HSCs included PE-conjugated anti-CD150 (Biolegend), FITC-conjugated anti-CD48 (Biolegend), and FITC-conjugated anti-CD41 (eBioscience), as well as FITC-conjugated lineage markers including anti-B220 (B cells), Thy1.2 (T cells), CD4 (T cells), Gr-1 (myeloid cells), CD11b (myeloid cells), and Ter119 (erythroid cells) antibodies. To ensure the accuracy of antibody labeling of HSCs, control slides were separately stained as above without addition of CD150 primary antibody. All antibodies were purchased from BD Pharmingen unless otherwise noted. Pan-endothelial cell antigen or Angptl3 antigen was visualized by staining with biotinylated anti–MECA-32 (Biolegend) or unconjugated rabbit anti-Angptl3 (Santa Cruz) followed by APC-conjugated streptavidin (BD Pharmingen) or Cy5-conjugated anti-rabbit IgG (Jackson Laboratory). In some experiments, the CD45−SSEA4+, and CD45−SSEA4− cells were sorted for analysis on cytospin slides and fixed in cold methanol. Slides were further incubated with rabbit anti-Angptl3 followed by Cy2-conjugated secondary antibodies (Jackson Laboratory). The nuclear dye DAPI was included in all stains to evaluate nuclear morphology. Finally, slides were mounted without drying using IMMU-MOUNT (Thermo Scientific). Microscopy was performed using an Olympus BX50 fluorescence microscope or a Zeiss LSM510 confocal microscope.
5-FU challenge
5-fluorouracil (5-FU) was administered intraperitoneally to 10-14 female WT or null mice at a dose of 150 mg/kg weekly for 2-3 weeks. The survival rates of the 2 groups were analyzed using a log-rank test (n = 10-14) as described.20
Homing
BM cells were labeled with 5-(and −6) carboxyfluorescein succinimidyl ester (CFSE), and 1-2 × 107 cells were transplanted into indicated strains of lethally irradiated mice. After 16 hours, the total number of CFSE+ cells in the BM, spleen, or liver was determined by flow cytometry. When CFSE+ LSK (CFSE+Lin−Sca1+Kit+) cells were analyzed, the BM cells were stained with a biotinylated lineage cocktail followed by streptavidin-PE/Cy5.5, anti–Sca-1-PE, and anti–Kit-APC before analysis.
Retrovirus infection
MSCV-Ikaros-IRES-GFP was a gift from Dr Susan Winandy.21 The retroviral plasmids with PCL-ECO (2:1) were transfected using Lipofectamine 2000 (Invitrogen) into 293T cells. The resulting retroviral supernatant was collected 48-72 hours later and used for infection. E15 fetal liver Lin− cells were resuspended in viral supernatants (1 × 105 cells/mL) with 4 μg/mL polybrene and centrifuged at 2000 rpm for 120 minutes before culturing for 24 hours in StemSpan (StemCell Technologies) in the presence of 10‖μg/mL heparin, 10 ng/mL SCF, 20 ng/mL TPO, and 10 ng/mL FGF-1. Cells were then resuspended in viral supernatant for another round of infection. Cells were used directly or cultured for another 3 days before transplantation and analysis.
Colony assays
Angptl3-null or WT BM cells were diluted to the indicated concentration in Iscove modified Dulbecco meduim with 2% FBS, and were then seeded into methylcellulose medium M3334 (StemCell Technologies) for CFU-E colony formation, M3434 (StemCell Technologies) for CFU-GM and BFU-E colony formation, or M3630 (StemCell Technologies) for CFU–Pre-B colony formation assays, according to the manufacturer's protocols and what we described previously.20
Results
Angptl3 binds to HSCs
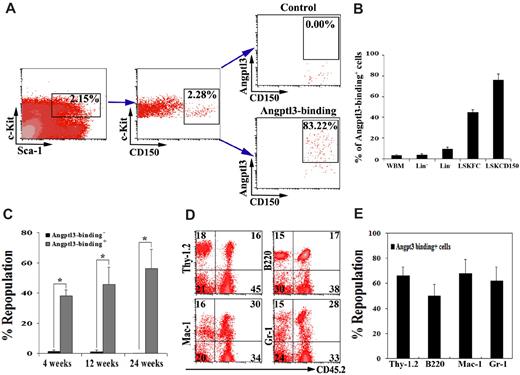
To determine whether HSCs are a direct cell target of Angptl3, we examined whether Angptl3 binds to HSCs. First we compared the abilities of total BM cells, differentiated hematopoietic Lin+ cells, enriched hematopoietic progenitor/precursor Lin− cells, and phenotypic LT-HSCs to bind Angptl3 (Figure 1A-B). While only 2.3% ± 0.9% freshly isolated mouse total BM cells and 3.7% ± 1.2% Lin+ cells bound to Angptl3, 9.3% ± 2.0% of the Lin− cells could do so. Importantly, most (83.2%, shown in the plot in Figure 1A and 76.0% ± 5.8% shown in Figure 1B) LT-HSCs as Lin−Sca-1+Kit+CD150+ cells bound to Angptl3. Similar Angptl3 binding assays were performed for LSKCD34−FLK-2− cells. As shown in Figure 1B, 44.43% ± 2.78% LSKCD34−FLK-2− cells bound Angptl3, and the binding of Angptl3 to HSCs was much higher than that to any other more differentiated BM populations. To further confirm that functional HSCs bind Angptl3, we used a previously described method22 to affinity-purify BM fractions based on their abilities to bind Angptl3, and used competitive reconstitution assays to measure which population contained repopulating HSCs (Figure 1C-E). As shown in Figure 1C, 30 000 BM cells that bound to Angptl3 resulted in 38.3%, 46.0%, and 56.2% of chimerism in the competitive reconstitution analysis at 4, 12, and 24 weeks after transplantation, when the short-term (ST)–HSC activity (4 weeks) and LT-HSC activity (12 and 24 weeks) were measured, respectively. In contrast, the same number of BM cells that did not bind to Angptl3 showed only 1.6%, 1.2%, and 0.2% repopulation, respectively. Therefore, Angptl3-binding cells had much greater repopulation activity than those that did not bind Angptl3. These Angptl3-binding cells were capable of repopulating both lymphoid and myeloid lineages (Figure 1D-E), attesting to their multilineage differentiation potential as HSCs. Limiting dilution analysis showed that the HSC frequency in Angptl3-binding BM cells was 1 per 8209, whereas the frequency in those cells that did not bind Angptl3 was 1 per 48 502 (Table 1). This represents a 6-fold enrichment of HSCs in those cells that bind Angptl3 and provides evidence that most BM HSCs bind to Angptl3. Angptl3 thus should have direct effects on HSCs. Similarly, we previously demonstrated that adult BM HSCs bind Angptl2.11
Most BM HSCs bind to Angptl3. (A-B) Freshly isolated adult BM Lin− cells isolated by AutoMACS were incubated with 1500 ng/mL Angptl3 and then stained with goat anti-Angptl3 antibody, followed by anti–goat-APC, anti–Sca-1-PE/CY5.5, anti–c-Kit-FITC, and anti–CD150-PE (or anti–Flk-2-PE, and anti–CD34-PE) antibodies. (A) Flow cytometry plots demonstrating that 83% Lin−Sca-1+Kit+CD150+ cells bound to Angptl3 compared with controls. (B) Summary of the percentages of total BM, Lin−, Lin+, and LSKCD34−FLK-2− cells (LSKFC) or LSKCD150+ cells (LSKCD150) that bound to Angptl3. (C-E) Freshly isolated adult CD45.2 BM cells were incubated with Angptl3 and then stained with goat anti-Angptl3 and anti–goat-Cy5 antibodies. Incubation without goat anti-Angptl3 and cells not treated with Angptl3 served as negative controls. (C) Thirty thousand positively stained cells and the same number of negatively stained cells were transplanted together with 1 × 105 CD45.1 competitor cells into lethally irradiated (10 Gy) CD45.1 mice (n = 8-9). Peripheral blood engraftments are shown at 4, 12, and 24 weeks after transplantation. (*statistically significant difference between 2 groups, P < .05). (D) Representative FACS plots of peripheral blood mononuclear cells, demonstrating the multilineage contributions of Angptl3-binding HSCs in 1 mouse at 24 weeks after transplantation. (E) Multilineage contribution of Angptl3-binding cells at 12 weeks after transplantation (n = 9).
Most BM HSCs bind to Angptl3. (A-B) Freshly isolated adult BM Lin− cells isolated by AutoMACS were incubated with 1500 ng/mL Angptl3 and then stained with goat anti-Angptl3 antibody, followed by anti–goat-APC, anti–Sca-1-PE/CY5.5, anti–c-Kit-FITC, and anti–CD150-PE (or anti–Flk-2-PE, and anti–CD34-PE) antibodies. (A) Flow cytometry plots demonstrating that 83% Lin−Sca-1+Kit+CD150+ cells bound to Angptl3 compared with controls. (B) Summary of the percentages of total BM, Lin−, Lin+, and LSKCD34−FLK-2− cells (LSKFC) or LSKCD150+ cells (LSKCD150) that bound to Angptl3. (C-E) Freshly isolated adult CD45.2 BM cells were incubated with Angptl3 and then stained with goat anti-Angptl3 and anti–goat-Cy5 antibodies. Incubation without goat anti-Angptl3 and cells not treated with Angptl3 served as negative controls. (C) Thirty thousand positively stained cells and the same number of negatively stained cells were transplanted together with 1 × 105 CD45.1 competitor cells into lethally irradiated (10 Gy) CD45.1 mice (n = 8-9). Peripheral blood engraftments are shown at 4, 12, and 24 weeks after transplantation. (*statistically significant difference between 2 groups, P < .05). (D) Representative FACS plots of peripheral blood mononuclear cells, demonstrating the multilineage contributions of Angptl3-binding HSCs in 1 mouse at 24 weeks after transplantation. (E) Multilineage contribution of Angptl3-binding cells at 12 weeks after transplantation (n = 9).
Comparison of the frequency of repopulating HSCs in Angptl3-positive and Angptl3-negative binding BM cells by limiting dilution analysis
| Group . | HSC frequency* . |
|---|---|
| Angptl3− binding cells | 1/48 502 (1/74 275-1/31 672) |
| Angptl3+ binding cells | 1/8209 (1/12 624-1/5338) |
| Group . | HSC frequency* . |
|---|---|
| Angptl3− binding cells | 1/48 502 (1/74 275-1/31 672) |
| Angptl3+ binding cells | 1/8209 (1/12 624-1/5338) |
L-Calc software was used to calculate the HSC frequency. n = 23; P < .05.
Angptl3 is required for maintaining the quiescence of HSCs
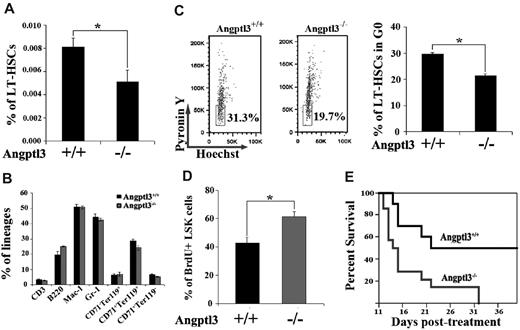
We asked whether Angptl3 had a functional role in HSCs by using Angptl3-null mice. The Angptl3-null and WT control mice used in our study were backcrossed onto the C57BL/6 background at least 10 times. RT-PCR analysis showed that the mutant mice were indeed deficient in Angptl3 expression (supplemental Figure 1). The Angptl3-null mice showed approximately 50% lower triglyceride, cholesterol, high-density lipoprotein, and low-density lipoprotein levels compared with WT mice (data not shown; consistent with published results8 ). We then analyzed the nature and number of hematopoietic cells from Angptl3-null and WT littermates. To examine the HSC compartment, we compared the frequencies of Angptl3-null and WT HSCs by flow cytometry. A significant decrease in phenotypic LT-HSCs (the BM Lin−Sca-1+Kit+Flk2−CD34− cells or LSKCD150+ cells) were found in adult Angptl3-null mice compared with WT mice (Figure 2A and supplemental Figure 2). While an average of 0.008%-0.009% LT-HSCs resided in the WT BM, an average of 0.005%-0.006% LT-HSCs were identified in the Angptl3-null BM (Figure 2A and supplemental Figure 2). The frequency of ST-HSCs in the null mice was not significant different from that of their WT counterparts (supplemental Figure 3). When BM cells were analyzed for their lineage distribution among T cells, B cells, myeloid cells, and erythroid cells, we found no significant differences in staining profiles between the WT and Angptl3-null animals (Figure 2B). The hematocrits, hemoglobin levels, and total red and white blood cell levels in peripheral blood were similar in the Angptl3-null and control mice (Table 2). We further assayed the levels of hematopoietic progenitors in Angptl3-null and WT BM. Angptl3-null BM cells had decreased numbers of granulocyte/monocyte progenitors (CFU-GM) and B lymphoid progenitors (CFU-Pre-B), but similar numbers of erythroid precursors (CFU-E) compared with WT BM (supplemental Figure 4). Thus, Angptl3-deficient BM had normal levels of terminally differentiated hematopoietic cells, but decreased myeloid and pre-B progenitors.
Angptl3 is required for the quiescence of HSCs in vivo. (A) Relative frequency of LT-HSCs as Lin−Sca-1+Kit+Flk2−CD34− cells in WT and Angptl3-null BM at 8-12 weeks (*P < .05, n = 16). (B) The major hematopoietic lineages were normal in Angptl3-null mice. The major lineages of hematopoietic cells in BM in WT and Angptl3-null mice were quantitated by flow cytometry analysis as T cells (CD3), B cells (B220), myeloid cells (Mac-1 and Gr-1), and erythroid cells (CD71−Ter119+, CD71+Ter119+, and CD71+Ter119−). (C) Angptl3-null BM HSCs are less quiescent than WT HSCs. In the left panel, LT-HSCs as Lin−Sca-1+Kit+Flk2−CD34− cells from a representative WT or Angptl3-null mouse, stained with Hoechst 33 342 and pyronin Y, were analyzed for cell cycle stage. In the right panel, the percentages of G0 cells for Angptl3-null and WT cells are shown (*P < .05, n = 9, right). (D) BrdU incorporation indicates decreased cycling in HSCs isolated from WT mice compared with those isolated from Angptl3-null mice (*P < .05, n = 5). (E) Angptl3-null mice were more sensitive than WT mice to myelotoxic injury. WT or null mice were treated with 150 mg/kg 5-FU intraperitoneally as described in Methods. Survival of the 2 groups was analyzed using a log-rank nonparametric test (P < .05, n = 10-14 in each group) and shown as a Kaplan-Meier survival plot.
Angptl3 is required for the quiescence of HSCs in vivo. (A) Relative frequency of LT-HSCs as Lin−Sca-1+Kit+Flk2−CD34− cells in WT and Angptl3-null BM at 8-12 weeks (*P < .05, n = 16). (B) The major hematopoietic lineages were normal in Angptl3-null mice. The major lineages of hematopoietic cells in BM in WT and Angptl3-null mice were quantitated by flow cytometry analysis as T cells (CD3), B cells (B220), myeloid cells (Mac-1 and Gr-1), and erythroid cells (CD71−Ter119+, CD71+Ter119+, and CD71+Ter119−). (C) Angptl3-null BM HSCs are less quiescent than WT HSCs. In the left panel, LT-HSCs as Lin−Sca-1+Kit+Flk2−CD34− cells from a representative WT or Angptl3-null mouse, stained with Hoechst 33 342 and pyronin Y, were analyzed for cell cycle stage. In the right panel, the percentages of G0 cells for Angptl3-null and WT cells are shown (*P < .05, n = 9, right). (D) BrdU incorporation indicates decreased cycling in HSCs isolated from WT mice compared with those isolated from Angptl3-null mice (*P < .05, n = 5). (E) Angptl3-null mice were more sensitive than WT mice to myelotoxic injury. WT or null mice were treated with 150 mg/kg 5-FU intraperitoneally as described in Methods. Survival of the 2 groups was analyzed using a log-rank nonparametric test (P < .05, n = 10-14 in each group) and shown as a Kaplan-Meier survival plot.
Results of complete blood count in WT and Angptl3-null mice
| . | WBC (103/μL) . | RBC (106/μL) . | Platelet (105/μL) . | HGB (g/DL) . | Neutrophils (%) . | Lymphocytes (%) . | Monocytes (%) . | Eosinophils (%) . | Basophils (%) . |
|---|---|---|---|---|---|---|---|---|---|
| WT | 9.2 ± 1.4 | 7.9 ± 0.4 | 10.8 ± 1.0 | 12.3 ± 0.2 | 17.0 ± 0.0 | 78.0 ± 1.7 | 2.0 ± 1.0 | 3.0 ± 1.00 | 0.0 ± 0.0 |
| KO | 8.5 ± 3.0 | 8.3 ± 0.2 | 11.8 ± 1.7 | 12.1 ± 0.6 | 16.3 ± 7.6 | 80.7 ± 5.1 | 2.0 ± 0.0 | 2.3 ± 0.6 | 0.0 ± 0.0 |
| . | WBC (103/μL) . | RBC (106/μL) . | Platelet (105/μL) . | HGB (g/DL) . | Neutrophils (%) . | Lymphocytes (%) . | Monocytes (%) . | Eosinophils (%) . | Basophils (%) . |
|---|---|---|---|---|---|---|---|---|---|
| WT | 9.2 ± 1.4 | 7.9 ± 0.4 | 10.8 ± 1.0 | 12.3 ± 0.2 | 17.0 ± 0.0 | 78.0 ± 1.7 | 2.0 ± 1.0 | 3.0 ± 1.00 | 0.0 ± 0.0 |
| KO | 8.5 ± 3.0 | 8.3 ± 0.2 | 11.8 ± 1.7 | 12.1 ± 0.6 | 16.3 ± 7.6 | 80.7 ± 5.1 | 2.0 ± 0.0 | 2.3 ± 0.6 | 0.0 ± 0.0 |
Three Angptl3-null mice and their WT littermates were analyzed for the complete blood count.
Next, we compared the cell-cycle/quiescence, apoptosis, and homing of HSCs in WT and Angptl3-null mice. The proportion of LT-HSCs (Lin−Sca-1+Kit+Flk2−CD34− cells or LSKCD150+ cells) in G0 was evaluated by Hoechst 33342 and pyronin Y staining.23 As shown in Figure 2C and supplemental Figure 5, approximately 30% of WT HSCs were in G0, which was significantly higher than that in Angptl3-null mice (21%-23%). This result was confirmed by BrdU incorporation analysis (Figure 2D). Therefore, HSCs in Angptl3-null mice are less quiescent than their WT counterparts. To confirm the role of Angptl3 in maintaining quiescence and to further evaluate its role in the stress response of HSCs, we treated mice with 5-FU, which is toxic to cycling cells and accelerates the entry of HSCs into the cell cycle.20 The survival of Angptl3-null mice was significantly lower than that of WT mice (Figure 2E). Therefore, consistent with a role in sustaining quiescence of HSCs, Angptl3 protects hematopoietic cells from exhaustion by toxic agents such as 5-FU. We conducted an apoptosis assay in LSK cells using Annexin V and 7-AAD staining. There was no difference in early (Annexin V+/7-AAD−) or late (Annexin V+/7-AAD+) apoptosis between WT and null LSK cells (supplemental Figure 6). Our analysis of homing suggested that HSCs in WT and null mice were not significantly different (supplemental Figure 7).
Angptl3 supports HSC repopulation
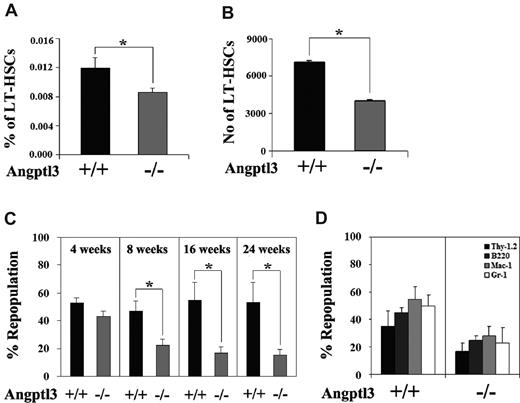
A hallmark of LT-HSCs is their ability to reconstitute the hematopoietic system of lethally irradiated mice. We compared the extent of repopulation of HSCs in Angptl3-null and in WT recipient mice as a measure of the effect of Angptl3 in the BM environment. Two types of experiments were performed. First, we transplanted WT donor BM cells into lethally irradiated Angptl3-null or WT recipients without competitors and measured the frequencies and numbers of donor Lin−Sca-1+Kit+Flk2−CD34− cells in the transplanted mice after 4 months (Figure 3A-B). The frequency and number of HSCs in the Angptl3-null recipients were approximately 71% and 57% of those in the WT recipients, respectively (Figure 3B). This result is concordant with the observation in homeostatic WT and null mice (Figure 2A), suggesting that Angptl3 supports the maintenance of the HSC pool. Second, after the same transplantation as described above, we conducted a secondary transplantation to measure the repopulation and self-renewal of HSCs. The BM from the primarily repopulated WT and Angptl3-null recipient mice was collected for secondary transplantation along with 105 competitor cells (Figure 3C). We found that the repopulating activity of cells originating from the primary Angptl3-null recipients was significantly decreased compared with those from the primary WT recipients. Over time, the secondary repopulation of original donor HSCs from the WT primary recipients was approximately 3-fold higher than that of the same donor cells from the null primary recipients (Figure 3C, see bars for 16 weeks and 24 weeks). Original donor HSCs repopulated both myeloid and lymphoid lineages (Figure 3D). Overall, these results indicate that Angptl3 in the BM environment supports in vivo expansion and self-renewal of HSCs. We also performed a competitive reconstitution analysis to compare the repopulation of HSCs isolated from null and WT mice. As shown in supplemental Figure 8, the null HSCs had a lower repopulation, suggesting that Angptl3 has a cell-intrinsic effect on HSCs, or the less quiescent null HSCs have decreased engraftment. We further tested whether Angptl3 had a cell-intrinsic effect on HSCs. Some HSCs take residence in extramedullary organs such as spleen.24 As shown in supplemental Figure 9, we isolated the same number of WT and null spleen cells for competitive reconstitution analysis. WT and null splenic HSCs had similar repopulation (supplemental Figure 9). Our results suggest that Angptl3 regulates the activity of BM HSCs mainly through an environmental effect.
Angptl3 supports HSC repopulation in vivo. (A, B) WT donor BM CD45.1 cells (5 × 105 cells) were transplanted into lethally irradiated CD45.2 Angptl3-null or WT recipient mice. The frequencies and numbers of donor Lin−Sca-1+Kit+Flk2−CD34− cells in the BM of the transplanted mice at 4 months after transplantation are shown in panel A and panel B, respectively (*P < .05, n = 5). (C) WT donor BM CD45.1 cells (5 × 105 cells) were transplanted into lethally irradiated CD45.2 Angptl3-null or WT recipient mice. These primary transplanted mice were killed at 2 months after transplantation and CD45.1 BM cells were collected and pooled. One million of pooled BM cells, along with 105 freshly isolated CD45.2 competitor cells, were injected into each of 5 lethally irradiated CD45.2 secondary recipient mice. The mice were then analyzed for the engraftment of original donor CD45.1 cells at 4-24 weeks after transplantation (*P < .05, n = 5). (D) Multilineage contribution of donor cells in secondary transplanted recipients at 24 weeks after transplantation (n = 5).
Angptl3 supports HSC repopulation in vivo. (A, B) WT donor BM CD45.1 cells (5 × 105 cells) were transplanted into lethally irradiated CD45.2 Angptl3-null or WT recipient mice. The frequencies and numbers of donor Lin−Sca-1+Kit+Flk2−CD34− cells in the BM of the transplanted mice at 4 months after transplantation are shown in panel A and panel B, respectively (*P < .05, n = 5). (C) WT donor BM CD45.1 cells (5 × 105 cells) were transplanted into lethally irradiated CD45.2 Angptl3-null or WT recipient mice. These primary transplanted mice were killed at 2 months after transplantation and CD45.1 BM cells were collected and pooled. One million of pooled BM cells, along with 105 freshly isolated CD45.2 competitor cells, were injected into each of 5 lethally irradiated CD45.2 secondary recipient mice. The mice were then analyzed for the engraftment of original donor CD45.1 cells at 4-24 weeks after transplantation (*P < .05, n = 5). (D) Multilineage contribution of donor cells in secondary transplanted recipients at 24 weeks after transplantation (n = 5).
Angptl3-producing endothelium and other stromal cells support HSC activity in the bone marrow
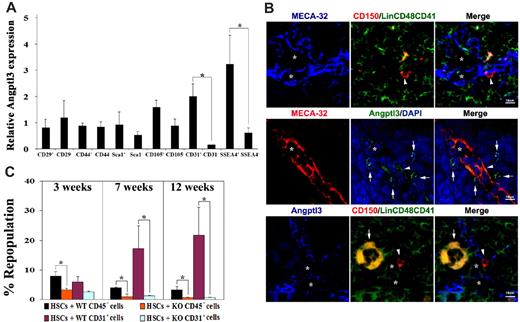
Because Angptl3 as an extrinsic factor that directly binds to HSCs, we investigated the expression pattern of Angptl3 in the stromal BM cells. We subfractionated BM stroma based on the markers for mesenchymal stem cells, including CD44, Sca1, CD105, CD29, and SSEA4, or endothelial cell marker CD31. We isolated CD45− BM cells from mice ranging in age from 8 to 12 weeks and measured Angptl3 expression using real-time RT-PCR. The Angptl3 expression level of the indicated population was normalized to that of the CD45− stromal cells (Figure 4A). We compared the Angptl3 expression of each positive population to that of the corresponding negative cells. As shown in Figure 4A, Angptl3 levels were 13-fold greater in BM CD45−CD31+ endothelial cells than in CD45−CD31− cells. Angptl3 was also expressed at 4-fold higher levels in CD45−SSEA4+ cells than in CD45−SSEA4− cells (Figure 4A). CD45−SSEA4+ cells represent 0.02%-0.1% of total BM nucleated cells. Whereas mouse CD45−SSEA4+ cells are enriched for mesenchymal stem cells upon culture,25 it is not clear whether they represent freshly isolated mesenchymal stem cells. The expression of Angptl3 protein by CD45−SSEA4+ cells was confirmed by immunofluorescence analysis (supplemental Figure 10). We found that 100% or CD45−SSEA4+ cells express Angptl3. By contrast, the level of Angptl3 in CD45−CD29+, CD45−CD44+, CD45−Sca-1+, or CD45−CD105+ cells was similar to that in their respective control counterparts (Figure 4A). Interestingly, in addition to Angptl3, we found that CD45−SSEA4+ cells expressed a significantly greater amount of other HSC cytokines compared with CD45−SSEA4− cells. These factors included SCF (9.71-fold), Notch ligands (more than 9-fold higher for both Delta-1 and Jagged-1), Wnt3a (40.39-fold), SDF-1 (123.29-fold), and IGFBP2 (8.96-fold), but not TPO or TGF-β (1.05-fold and 0.97-fold, respectively; supplemental Figure 11). Nestin expression was also significantly higher in this population (5.23-fold higher than CD45−SSEA4− cells; supplemental Figure 11). In summary, the significantly higher expression of Angptl3 in certain BM cells, especially in endothelial cells (as the BM vascular niche for HSCs19 ), and the enrichment of Angptl3 and other HSC-supportive factors in CD45−SSEA4+ cells suggests these Angptl3-producing cells share similar features with known HSC niche cells.
Angptl3-producing BM endothelial cells support HSC activity. (A) Angptl3 is highly expressed in BM endothelium and CD45−SSEA4+ cells. Indicated populations of BM cells were collected by flow cytometry and Angptl3 expression was measured by real-time RT-PCR. The cells sorted for analysis were gated from the CD45− fraction. (*significantly different between 2 groups, P < .05, n = 6). The levels of Angptl3 mRNA in each population were normalized to the level of β-actin transcripts present in the same sample (Mean ± s.e.m). (B) BM Angptl3+ endothelium is close to HSCs. Top, HSCs were associated with sinusoidal endothelial cells in mouse BM. BM was stained to reveal CD150+CD48−CD41−Lin− HSCs (red, with arrowhead); MECA-32+ sinusoidal endothelial cells (blue, with star); and CD41+, CD48+, or Lin+ differentiated hematopoietic cells (green or orange). Middle, most sinusoidal endothelial cells in mouse BM were Angptl3+, and some Angptl3+ cells were close to the sinusoidal endothelium. Most sinusoidal endothelial cells were also positive for Angptl3 (arrowhead); many Angptl3+ cells (green, with arrows) were also in contact with MECA-32+ sinusoidal endothelial cells (red). Nuclei were counterstained with DAPI (blue). Bottom, HSCs were associated with Angptl3+ cells in mouse BM; 31 of 51 (60.8%) HSCs are adjacent to Angptl3+ cells ( ≤ 2 cell distances). CD150+CD48−CD41−Lin− HSCs (red, with arrowhead), Angptl3+ cells (blue), and CD41+, CD48+, or Lin+ differentiated hematopoietic cells (green or orange). One megakaryocyte is marked by an arrow. Star markers indicate the possible vascular niche. Scale bar applies to all images. (C) Angptl3-deficient BM CD45− cells and CD45−CD31+ cells had decreased ability to support expansion of HSCs. One hundred and fifty BM CD45.1 Lin−Sca-1+Kit+Flk-2−CD34− cells were cocultured with 450 CD45− BM stromal cells isolated from CD45.2 WT mice (black bar), 450 CD45−CD31+ BM endothelial cells isolated from CD45.2 WT mice (purple bar), or the same number of CD45− or CD45−CD31+ cells from Angptl3-null mice (orange or green bars, respectively) in serum-containing StemSpan supplemented with SCF, TPO, and FGF-1. After 5 days, the cocultured cells were cotransplanted with 1 × 105 CD45.2 competitors into lethally irradiated CD45.2 recipient mice. The figure shows the engraftment at 3, 7, and 12 weeks after transplantation (*significantly different between 2 groups. P < .05, n = 7-8).
Angptl3-producing BM endothelial cells support HSC activity. (A) Angptl3 is highly expressed in BM endothelium and CD45−SSEA4+ cells. Indicated populations of BM cells were collected by flow cytometry and Angptl3 expression was measured by real-time RT-PCR. The cells sorted for analysis were gated from the CD45− fraction. (*significantly different between 2 groups, P < .05, n = 6). The levels of Angptl3 mRNA in each population were normalized to the level of β-actin transcripts present in the same sample (Mean ± s.e.m). (B) BM Angptl3+ endothelium is close to HSCs. Top, HSCs were associated with sinusoidal endothelial cells in mouse BM. BM was stained to reveal CD150+CD48−CD41−Lin− HSCs (red, with arrowhead); MECA-32+ sinusoidal endothelial cells (blue, with star); and CD41+, CD48+, or Lin+ differentiated hematopoietic cells (green or orange). Middle, most sinusoidal endothelial cells in mouse BM were Angptl3+, and some Angptl3+ cells were close to the sinusoidal endothelium. Most sinusoidal endothelial cells were also positive for Angptl3 (arrowhead); many Angptl3+ cells (green, with arrows) were also in contact with MECA-32+ sinusoidal endothelial cells (red). Nuclei were counterstained with DAPI (blue). Bottom, HSCs were associated with Angptl3+ cells in mouse BM; 31 of 51 (60.8%) HSCs are adjacent to Angptl3+ cells ( ≤ 2 cell distances). CD150+CD48−CD41−Lin− HSCs (red, with arrowhead), Angptl3+ cells (blue), and CD41+, CD48+, or Lin+ differentiated hematopoietic cells (green or orange). One megakaryocyte is marked by an arrow. Star markers indicate the possible vascular niche. Scale bar applies to all images. (C) Angptl3-deficient BM CD45− cells and CD45−CD31+ cells had decreased ability to support expansion of HSCs. One hundred and fifty BM CD45.1 Lin−Sca-1+Kit+Flk-2−CD34− cells were cocultured with 450 CD45− BM stromal cells isolated from CD45.2 WT mice (black bar), 450 CD45−CD31+ BM endothelial cells isolated from CD45.2 WT mice (purple bar), or the same number of CD45− or CD45−CD31+ cells from Angptl3-null mice (orange or green bars, respectively) in serum-containing StemSpan supplemented with SCF, TPO, and FGF-1. After 5 days, the cocultured cells were cotransplanted with 1 × 105 CD45.2 competitors into lethally irradiated CD45.2 recipient mice. The figure shows the engraftment at 3, 7, and 12 weeks after transplantation (*significantly different between 2 groups. P < .05, n = 7-8).
To study the spatial relationship of Angptl3-expressing cells and BM HSCs, we examined mouse BM using immunohistochemistry (Figure 4B). Markers validated in previous studies were used to confirm that HSCs were close to MECA-32+ sinusoidal endothelium in BM frozen sections (Figure 4B, top). We further found that most BM sinusoidal endothelial cells were Angptl3+, and some nonendothelial Angptl3+ cells were also close to MECA-32+ sinusoidal endothelium (Figure 4B, middle). Importantly, 60.8% of HSCs were adjacent to Angptl3-producing cells in the BM (Figure 4B, bottom). Because sinusoidal endothelium is an HSC niche19 and Angptl3 supports ex vivo and in vivo expansion of HSCs as shown here and in previous work,11 our results suggest that Angptl3-producing BM endothelial cells are a component of the HSC niche in vivo.
To directly test whether Angptl3-producing BM cells support HSC expansion, we cocultured HSCs and BM CD45− stromal cells or CD45−CD31+ endothelial cells, and used competitive reconstitution analysis to measure HSC activity. As shown in Figure 4C, we cocultured 150 CD45.2 Lin−Sca-1+Kit+Flk2−CD34− HSCs and 450 CD45− stromal cells or CD45−CD31+ endothelial cells isolated from WT mice. HSCs that were cocultured with CD45− cells or CD45−CD31+ cells from the Angptl3-null mice served as controls. After 5 days, the cocultured cells were used for competitive reconstitution to measure HSC activity. At 3, 7, and 12 weeks after transplantation, HSCs cocultured with WT CD45− stromal cells had a significantly higher repopulation relative to those cocultured with Angptl3-null CD45− cells. Importantly, HSCs cocultured with WT CD45−CD31+ cells had much higher repopulation relative to those cocultured with Angptl3-null CD45−CD31+ cells at 7 or 12 weeks after transplantation (17.1% ± 7.7% versus 1.2% ± 0.3% at 7 weeks, and 21.0% ± 9.4% versus 0.7% ± 0.1% at 12 weeks; Figure 4C). Similarly, Angptl3 expressed by BM CD45−SSEA4+ cells also supported expansion of HSCs (supplemental Figure 12). These experiments provide functional evidence that Angptl3 produced by BM CD45−CD31+ endothelial cells and other stromal cells supports HSC activity in the BM.
Angptl3 down-regulates Ikaros expression to enhance HSC stemness
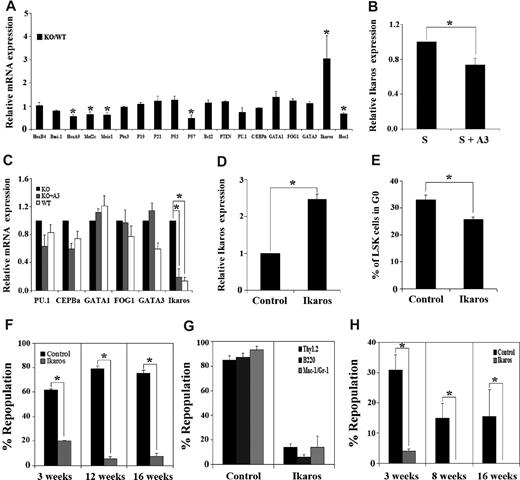
To identify intracellular targets of Angptl3 in HSCs, we compared the expression of several transcription factors and cell cycle regulators in HSCs isolated from Angptl3-null and WT mice using real-time RT-PCR. We were intrigued by the observation that Angptl3-null HSCs had 3-fold higher levels of Ikaros expression than their WT counterparts (Figure 5A). To determine whether extrinsic Angptl3 directly suppresses Ikaros expression, we treated HSCs with recombinant Angptl3 and found that the expression of Ikaros was decreased to 74% of untreated levels after 24 hours (Figure 5B). An 8-day culture of Angptl3-null HSCs in the presence of Angptl3 showed an 81% decrease in Ikaros expression compared with that in Angptl3-null HSCs without Angptl3 treatment (Figure 5C). Therefore, Angptl3 suppresses Ikaros expression in HSCs.
Angptl3 represses the expression of Ikaros to maintain stemness. (A) Angptl3-null HSCs had increased expression of Ikaros compared with WT HSCs. Lin−Sca-1+Kit+Flk2−CD34− cells were collected from WT or Angptl3-null BM. Gene expression analyzed by real-time RT-PCR is shown. The gene expression in WT samples was normalized to 1. (*significantly different from WT values, P < .05, n = 7). (B) The real-time RT-PCR analysis of bone marrow Lin−Sca-1+Kit+Flk2−CD34− cells cultured in SCF-containing serum-free medium, treated with or without 500 ng/mL Angptl3 for 24 hours. Gene expression in untreated samples was normalized to 1. (C) The real-time RT-PCR analysis of WT and Angptl3-null bone marrow Lin−Sca-1+Kit+Flk2−CD34− cells cultured in serum-free STF medium, treated with or without 500 ng/mL Angptl3 for 8 days. Gene expression in Angptl3-null samples was normalized to 1. Results of averages of all real-time RT-PCR reactions are shown. (*P < .05, n = 3). (D) Forced expression of Ikaros increased Ikaros expression 2.5-fold. Mouse E16 fetal liver CD45.1 Lin− cells were infected by retroviruses encoding GFP or Ikaros-IRES-GFP. The infection efficiency of Ikaros-IRES-GFP was approximately 20%. Three days later, 50 000 GFP+ cells were transplanted into lethally irradiated CD45.2 recipients. The expression of Ikaros in HSCs was quantitated by real-time RT-PCR 4 months after transplantation. (E) Overexpression of Ikaros decreased quiescence of HSCs. Cell cycle status of the donor GFP+Lin−Sca-1+Kit+ cells shown in panel D was analyzed by Hoechst/pyronin Y staining 4 months after transplantation (n = 6). (F) Mouse E16 fetal liver CD45.2 Lin− cells were infected by retroviruses encoding GFP or Ikaros, and 1.7 × 105 of these sorted GFP+ cells were cotransplanted with 105 CD45.1 bone marrow competitors into lethally irradiated CD45.1 recipients for competitive repopulation analysis. Donor engraftment at indicated time after transplantation is shown (*P < .05, n = 9). Data shown are representative of 4 different experiments that produced similar results. (G) Multilineage contribution of donor cells in recipients at 13 weeks after transplantation (n = 9). (H) Ikaros-overexpressing HSCs had diminished repopulation capacity in secondary transplantation. Fifteen hundred CD45.2 GFP+Lin−Sca-1+Kit+ cells isolated from primary transplanted mice as described in panel F were cotransplanted with 105 CD45.1 bone marrow competitors into lethally irradiated CD45.1 recipients for secondary competitive repopulation analysis. Donor engraftment at 3, 8, and 16 weeks after transplantation is shown (*P < .05, n = 5).
Angptl3 represses the expression of Ikaros to maintain stemness. (A) Angptl3-null HSCs had increased expression of Ikaros compared with WT HSCs. Lin−Sca-1+Kit+Flk2−CD34− cells were collected from WT or Angptl3-null BM. Gene expression analyzed by real-time RT-PCR is shown. The gene expression in WT samples was normalized to 1. (*significantly different from WT values, P < .05, n = 7). (B) The real-time RT-PCR analysis of bone marrow Lin−Sca-1+Kit+Flk2−CD34− cells cultured in SCF-containing serum-free medium, treated with or without 500 ng/mL Angptl3 for 24 hours. Gene expression in untreated samples was normalized to 1. (C) The real-time RT-PCR analysis of WT and Angptl3-null bone marrow Lin−Sca-1+Kit+Flk2−CD34− cells cultured in serum-free STF medium, treated with or without 500 ng/mL Angptl3 for 8 days. Gene expression in Angptl3-null samples was normalized to 1. Results of averages of all real-time RT-PCR reactions are shown. (*P < .05, n = 3). (D) Forced expression of Ikaros increased Ikaros expression 2.5-fold. Mouse E16 fetal liver CD45.1 Lin− cells were infected by retroviruses encoding GFP or Ikaros-IRES-GFP. The infection efficiency of Ikaros-IRES-GFP was approximately 20%. Three days later, 50 000 GFP+ cells were transplanted into lethally irradiated CD45.2 recipients. The expression of Ikaros in HSCs was quantitated by real-time RT-PCR 4 months after transplantation. (E) Overexpression of Ikaros decreased quiescence of HSCs. Cell cycle status of the donor GFP+Lin−Sca-1+Kit+ cells shown in panel D was analyzed by Hoechst/pyronin Y staining 4 months after transplantation (n = 6). (F) Mouse E16 fetal liver CD45.2 Lin− cells were infected by retroviruses encoding GFP or Ikaros, and 1.7 × 105 of these sorted GFP+ cells were cotransplanted with 105 CD45.1 bone marrow competitors into lethally irradiated CD45.1 recipients for competitive repopulation analysis. Donor engraftment at indicated time after transplantation is shown (*P < .05, n = 9). Data shown are representative of 4 different experiments that produced similar results. (G) Multilineage contribution of donor cells in recipients at 13 weeks after transplantation (n = 9). (H) Ikaros-overexpressing HSCs had diminished repopulation capacity in secondary transplantation. Fifteen hundred CD45.2 GFP+Lin−Sca-1+Kit+ cells isolated from primary transplanted mice as described in panel F were cotransplanted with 105 CD45.1 bone marrow competitors into lethally irradiated CD45.1 recipients for secondary competitive repopulation analysis. Donor engraftment at 3, 8, and 16 weeks after transplantation is shown (*P < .05, n = 5).
Ikaros is a zinc finger transcription factor important for differentiation of lymphoid, myeloid, and erythroid cells.26 While Ikaros is important for the development of fetal liver HSCs,27 the effect of Ikaros expression on the activity of adult BM HSCs is less clear. A recent study using the Ikaros-GFP reporter mouse showed that Ikaros expression is low in adult multipotent HSCs, but high in progenitors with lymphoid-myeloid potential.28 A dominant negative form of Ikaros can immortalize hematopoietic progenitors.29 Because Angptl3 down-regulates Ikaros in HSCs, we examined the effect of forced expression of Ikaros on HSC activities. Because the efficiency of retroviral infection for fetal liver HSCs/progenitors is significantly higher than that for adult BM cells, we infected fetal liver hematopoietic progenitor Lin− cells with an MSCV-Ikaros-IRES-GFP retrovirus and transplanted infected GFP+ cells into lethally irradiated recipient mice. MSCV-GFP virus-infected cells were transplanted as the control. At 4 months after transplantation, we isolated BM GFP+Lin−Sca-1+Kit+ cells and measured the expression of Ikaros. On average, there was 2.5-fold higher Ikaros expression in the Ikaros/GFP+ Lin−Sca-1+Kit+ cells compared with the control counterparts (Figure 5D). This forced expression of Ikaros reached similar levels as that of up-regulated Ikaros in Angptl3-null HSCs as shown in Figure 5A, which would allow for the study of the role of elevated Ikaros expression in Angptl3-null HSCs. To determine the effect of Ikaros overexpression on HSC quiescence, we measured the cell cycle status of Ikaros-overexpressed and control HSCs by Hoechst 33 342 and pyronin Y staining. While 33% of cells infected by GFP control virus were in G0, 26% of those infected by Ikaros virus were in G0 (Figure 5E). This result indicates that the overexpression of Ikaros in HSCs led to significantly decreased quiescence in the BM, which is consistent with the decreased quiescence of HSCs in Angptl3-null mice (Figure 2C-E).
We then determined the effect of forced expression of Ikaros on HSC repopulation. Figure 5F shows the result of a representative experiment. Here, we cotransplanted 1.7 × 105 CD45.1 GFP+ Ikaros-overexpressing cells or control GFP+ cells with 1 × 105 CD45.2 competitors into lethally irradiated CD45.2 recipients. At 3-16 weeks after transplantation, those recipients that received HSCs that overexpressed Ikaros had dramatically decreased repopulation compared with those that received control HSCs (Figure 5F-G). This result was representative of 4 independent experiments that produced similar results, suggesting that, on average, a 2.5-fold elevated Ikaros expression (similar to that in Angptl3-null HSCs) diminishes HSC repopulating activity. We then performed secondary transplantation to determine the self-renewal ability of HSCs with and without Ikaros overexpression. HSCs with overexpressed Ikaros lost their repopulation activity 8 weeks after transplantation (Figure 5H). Because the real-time RT-PCR only detected the Ikaros message expression at the population level, we do not exclude the possibility that a range of expression levels of Ikaros existed in our infected HSCs. Nevertheless, the consistent, dramatic decrease of the repopulation of Ikaros-overexpressing cells in the primary transplantation in 4 independent experiments, and the complete loss of engraftment of the Ikaros-overexpressed cells after 8 weeks in the secondary transplantation, indicate that unregulated overexpression of Ikaros is detrimental to HSC repopulating activity. This result is in agreement with the observations that HSCs in Angptl3-null mice (with 3-fold greater Ikaros expression) have decreased numbers and capacity for self-renewal than their WT counterparts (Figures 2 and 3).
To test whether Ikaros regulates the expression of other genes down-regulated in the null HSCs (Figure 5A), we overexpressed Ikaros and found that this resulted in a 74% decrease of Hes1 expression relative to cells transfected with control vector (supplemental Figure 13). Thus, down-regulation of Ikaros by Angptl3 may stimulate Notch signaling. Together, our results demonstrated that overexpression of Ikaros in HSCs decreases quiescence and impairs their repopulation, and Angptl3 is capable of supporting the stemness of HSCs through down-regulation of the expression of the transcription factor Ikaros.
Discussion
We previously showed that several Angptls, including Angptl3, support ex vivo expansion of HSCs,11,12,16 but the mechanisms responsible for this activity were unknown. Here, we studied the role of Angptl3 in the regulation of HSC activity in vivo by analyzing Angptl3-null and WT mice. For the first time, we demonstrated that HSCs in Angptl3-null mice had decreased numbers, quiescence, and repopulating activity. In addition, we showed that Angptl3 was expressed by BM endothelial and other stromal cells, and that these Angptl3-producing cells were adjacent to HSCs in the BM and support expansion of repopulating HSCs in coculture experiments. Furthermore, in our study of the underlying mechanism by which Angptl3 regulates HSC activity, we found that Angptl3 repressed the expression of the transcription factor Ikaros, whose overexpression diminished the repopulation activity of HSCs. This study thus provided the functional evidence that Angptl3 supports the repopulating activity of HSCs in the BM.
An important question in stem cell biology is how environmental cues regulate the potential of stem cells. Here we showed an example of an extrinsic factor that regulates HSC stemness by maintaining quiescence of HSCs in homeostatic mice while enhancing HSC repopulation in transplantation. First, we tried to determine whether Angptl3 directly binds to HSCs. It is known that Angptl3 binds to cell surface integrinαVβ3,9 and that integrin β3 is expressed on HSCs.30 Nevertheless, it is likely that there exists another signaling surface receptor for Angptl3. Using flow cytometry and reconstitution analysis (Figure 1), we showed that most HSCs bind to Angptl3. Therefore, Angptl3 is an extrinsic factor that directly acts on HSCs. How does a secreted factor that stimulates the ex vivo expansion of HSCs also support HSC quiescence in the BM? Theoretically, the maintenance of HSC quiescence could arise from a direct effect or an indirect effect. For example, cell cycle inhibitors such as p21 act directly to decelerate cycling to prevent the stem cell potential from exhaustion.23 In an indirect effect, HSC quiescence may occur in response to an increase in the total stem cell pool. If the stem cell pool is large enough, only a fraction of HSCs need to undergo asymmetric and symmetric divisions to maintain hematopoiesis; therefore, a decrease in the cell cycle is observed. This is the case in Lnk-null mice, which have increased HSC numbers and increased HSC quiescence.31 Moreover, if HSCs are engaged in their niche, they are maintained to be more quiescent than those HSCs that are losing interactions with their niche components. These scenarios possibly explain that, although SCF and TPO are positive factors that support HSC repopulation, HSCs isolated from the Kit (W41/W41) mice and TPO-null mice are less quiescent than their WT counterparts.32-34 The effect of Angptl3 on the quiescence of HSCs in the BM may represent a similar situation. This explanation is supported by our data demonstrating that Angptl3-null mice have significantly fewer HSCs in the BM, Angptl3 supports the ex vivo and in vivo expansion of HSCs, and Angptl3-producing endothelial cells are a component of the HSC niche.
Previously, we demonstrated that, in combination with other factors, Angptl3 stimulates ex vivo expansion of the number and activity of repopulating HSCs.11 Importantly, HSCs are different from many differentiated cells in that they have multiple cell fates. This may be why we needed multiple extrinsic factors to support HSC expansion; different factors may modulate distinct cell fates of HSCs. Here we showed that Angptl3 supported in vivo expansion of HSCs. Consistently, Angptl3 up-regulates Hoxa9 and Hes1, which play important roles in HSC self-renewal and differentiation (Figure 5A). Interestingly, we found that Angptl3 represses the expression of a transcription factor Ikaros, which is important for multilineage differentiation.26 While Ikaros plays an important role in the development of fetal liver HSCs,27 its dose effect on the activity of adult BM HSCs is unknown. Because there have been no reports about the effect of Ikaros on HSCs through a gain-of-function mechanism, we used retroviral infection to introduce Ikaros to HSCs and found that its forced expression reached levels similar to those seen when Ikaros is up-regulated upon Angptl3 deletion. We showed that this level of Ikaros overexpression in HSCs decreases the quiescence of HSCs and impairs their repopulation activity. Our data are consistent with the finding that a dominant negative form of Ikaros can immortalize hematopoietic progenitors,28 and that Ikaros expression is low in adult BM HSCs but high in certain progenitors.28 Our data thus suggest that maintenance of an appropriate level of Ikaros in HSCs is important for their normal function, and are further supported by our preliminary result showing that the forced expression of Ikaros impairs ex vivo expansion of HSCs (data not shown). Moreover, we found that forced expression of Ikaros negatively regulates the Notch pathway by decreasing Hes1 expression in HSCs (supplemental Figure 6), which could explain why Hes1 is down-regulated in Angptl3-null HSCs (Figure 5A). It is therefore possible that Ikaros provides a link between Angptl and the Notch pathway, which plays important roles in supporting self-renewal and inhibiting differentiation of HSCs. Based on these results, one mechanism by which Angptl3 may preserve the stemness of HSCs is to control the appropriate dose of Ikaros so that self-renewal and differentiation may be balanced.
Currently, we know of the existence of at least 2 BM HSC niches in vivo35 ; each is proposed to contain specific HSC-supportive cell types. The endosteal HSC niche contains osteoblasts as the main supportive cell type for maintenance of hematopoiesis.36,37 The vascular HSC niche is mainly composed of sinusoidal endothelial cells.19 Most recently, it was suggested that virtually every osteoblast is in close apposition to sinusoidal endothelial cells; therefore, these 2 types of cells may establish a compound niche.38,39 Additional cell types have also been isolated that represent components of HSC niche.35 The sympathetic nervous system40 and osteoclasts41 have been implicated in HSC localization and retention within the niche. CXCL12 (SDF-1)–abundant reticular (CAR) cells42 that surround the sinusoidal endothelial cells or are located near the endosteum are likely components of both vascular and endosteal niches. Similarly, CD146-expressing subendothelial stromal cells in human BM stroma that express high levels of CXCL12 (SDF-1) are capable of transferring the hematopoietic microenvironment to heterotopic sites upon transplantation.43 As shown in our study, Angptl3 produced by BM endothelial cells supports HSC activity, as determined in our functional reconstitution analysis. This is consistent with the findings that endothelial cells support dramatic ex vivo expansion of HSCs,44 and suggests that Angptl3 may play an important role in the vascular niche. In addition, we found that the CD45−SSEA4+ BM cells highly express Angptl3 and support HSC expansion. It was reported that cultured mouse SSEA4+ cells were enriched mesenchymal stem cells.25 Yet it is unclear whether the freshly isolated mouse CD45−SSEA4+ BM cells are also mesenchymal stem cells, and BM stromal cells isolated based on other mesenchymal stem cell markers such as CD44, Sca1, CD105, or CD29 do not highly express Angptl3. Nevertheless, BM CD45−SSEA4+ cells are enriched for the expression of Angptl3 and several HSC-supportive cytokines, including SCF, Notch ligands, Wnt 3a, IGFBP2, Nestin, and SDF-1. The significantly higher expression of Nestin and other factors in CD45−SSEA4+ cells suggests that these cells may have a certain level of overlap with mesenchymal stem cells.45 Further characterization of this population will clarify this issue. In sum, our data suggest that Angptl3-producing cells in the adult BM are composed of multiple cell types that share common features with other known BM niche cells. A similar situation may exist in the fetal liver. Most recently, it was reported that Angptl3-producing hepatocyte progenitors support in vivo expansion of fetal liver HSCs14 ; therefore, it is possible that Angptl3-producing cells are a part of the niche for fetal liver HSCs. Importantly, the results of our coculture of HSCs with several Angptl3-null and WT BM stromal cells followed by the “gold standard” of BM reconstitution analysis indicate that Angptl3 is likely a novel molecular component of the HSC niche.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nuvelo Inc for providing the Angptl3 null mice and Dr Susan Winandy from Northwestern University for providing the MSCV-Ikaros-IRES-GFP plasmid.
C.C.Z. is supported by National Institutes of Health grant K01CA120099, the Michael. L. Rosenberg Endowed Scholar Fund from University of Texas Southwestern Medical Center, Welch Foundation Grant I-1701, a Basil O'Connor Award from the March of Dimes Foundation, an American Heart Association Grant-in-Aid, and an American Society of Hematology Junior Faculty Award.
National Institutes of Health
Authorship
Contribution: J.Z. and C.C.Z. contributed to design, experimental performance, interpretation, and writing; and H.H., M.U., and R.S. contributed to experimental performance, interpretation, and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng Cheng Zhang, Departments of Physiology and Developmental Biology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390; e-mail: Alec.Zhang@UTSouthwestern.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal