Abstract
We investigated possible cellular receptors for the human CXC chemokine platelet factor-4 variant/CXCL4L1, a potent inhibitor of angiogenesis. We found that CXCL4L1 has lower affinity for heparin and chondroitin sulfate-E than platelet factor-4 (CXCL4) and showed that CXCL10 and CXCL4L1 could displace each other on microvascular endothelial cells. Labeled CXCL4L1 also bound to CXCR3A- and CXCR3B-transfectants and was displaced by CXCL4L1, CXCL4, and CXCL10. The CXCL4L1 anti-angiogenic activity was blocked by anti-CXCR3 antibodies (Abs) in the Matrigel and cornea micropocket assays. CXCL4L1 application in CXCR3−/− or in wild-type mice treated with neutralizing anti-CXCR3 Abs, resulted in reduced inhibitory activity of CXCL4L1 on tumor growth and vascularization of Lewis lung carcinoma. Furthermore, CXCL4L1 and CXCL4 chemoattracted activated T cells, human natural killer cells, and human immature dendritic cells (DCs). Migration of DCs toward CXCL4 and CXCL4L1 was desensitized by preincubation with CXCL10 and CXCL11, inhibited by pertussis toxin, and neutralized by anti-CXCR3 Abs. Chemotaxis of T cells, natural killer cells, and DCs is likely to contribute to the antitumoral action. However, the in vivo data indicate that the angiostatic property of CXCL4L1 is equally important in retarding tumor growth. Thus, both CXCR3A and CXCR3B are implicated in the chemotactic and vascular effects of CXCL4L1.
Introduction
The chemokine family of chemotactic cytokines consists of rather small proteins (7-12 kDa) that signal via G protein-coupled receptors, designated CC chemokine receptor (CCR) or CXC chemokine receptor (CXCR), and regulate leukocyte recruitment to inflammatory sites, as well as leukocyte traffic between immunological compartments. Other target cells of chemokines include tumor cells and endothelial cells, and consequently, chemokines play a role in tumor development.1,2 For example, the CXCR3 ligands CXCL9, CXCL10, and CXCL11 are chemotactic for antitumoral lymphocytes and inhibit angiogenesis. However, some members of the chemokine family (eg, the CXCR2 ligands CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL8) favor tumor growth by attracting neutrophils, by stimulating the release of matrix metalloproteinases, by acting as growth factors, and by promoting angiogenesis. One of the first chemokines that was investigated as an anticancer therapeutic is CXCL4 or platelet factor-4.1-3
The human CXC chemokine platelet factor-4 (CXCL4) is encoded by 2 genes, located on chromosome 4 and probably arose through duplication.4,5 The 2 genes are indeed highly related and give rise to mature proteins that differ in only 3 amino acid residues in the carboxylic acid (COOH)–terminal part. Analysis of conditioned media from thrombin-treated platelets revealed that both CXCL4 genes are translated into proteins.6 Afterward, tumor cells and smooth muscle cells were identified as alternative cellular sources for CXCL4L1 but not for CXCL4, indicating that not every cell type that produces CXCL4 also releases CXCL4L1 and vice versa.7,8 The fact that tumor cells produce angiostatic CXCL4L1 provides a negative feedback on tumor growth, because CXCL4L1 is more potent than CXCL4 in angiostatic assays.6 In this manuscript, we confirm that the antitumoral activity of CXCL4L1 is exerted through inhibition of angiogenesis and demonstrate that this angiostatic activity is CXCR3-dependent in human and mouse models. Furthermore, we also show for the first time that CXCL4L1 attracts human and mouse activated T lymphocytes, human dendritic cells (DCs), and human natural killer (NK) cells and that CXCL4L1-induced chemotaxis of immature DCs is mediated by CXCR3, coupling to a Gαi protein. In addition to CXCR3A and CXCR3B interaction, binding to glycosaminoglycans (GAGs) was explored as well.
Methods
A supplemental Methods section is available on the Blood Web site (see the Supplemental Materials link at the top of the online article). All animal studies were approved by the institutional ethics committees of all participating universities.
Chemokines and antisera
Natural human CXCL4 was isolated from stimulated platelets as previously described.6 Recombinant human CXCL4L1 was produced in Escherichia coli and purified by a 4-step chromatographical procedure.9 Human CXCL11 and CXCL12 were chemically synthesized.10 Monoclonal anti–human CXCR3/CD183 (clone 1C6) and anti–human CXCR3/CD183 (clone 49 801) antibodies (Abs) and the corresponding isotype controls were purchased from BD Biosciences and R&D Systems, respectively. Other recombinant human and mouse chemokines were ordered from PeproTech. TAMRA [5(6)-carboxytetramethylrhodamine]-labeled CXCL10 was produced by Invitrogen. Polyclonal Abs were raised in goats or rabbits and were applied in several in vitro and in vivo assays.
Tumorigenesis models
Matrigel assay
Human dermal microvascular endothelial cells (HMVECs-d) were plated on Matrigel-coated 48-well plates (BD Matrigel matrix with high growth factor content; BD Biosciences). Reorganization of the endothelial cells into tubular structures was followed using an Axiovert 200 M inverted microscope. Results are expressed as percentage inhibition compared with the control cultures containing growth medium alone.
Rat cornea micropocket assay for angiogenesis
In vivo angiogenesis was assessed using the rat cornea micropocket assay as previously described.6 Chemokines were diluted in Hydron casting solution and phosphate-buffered saline plus 0.25% serum albumin to their final concentration with and without anti-murine CXCR3 or control antibody. Six days after implantation, corneas were harvested and photographed.
Chemotaxis
Human peripheral blood mononuclear cells were purified from buffy coats from healthy volunteers and cultured to generate human interleukin-2 (IL-2)–activated T lymphocytes and immature DCs.14,15 Boyden chamber chemotaxis assays with these cells were performed as previously described.14,15 Migration conditions for activated mouse T lymphocytes (generated from splenocytes by treatment with IL-2) were similar to conditions used for activated human T cells. Migration of human NK cells was evaluated using Transwell chambers.
Synthesis of fluorescently labeled CXCL4L1
Synthetic CXCL4L1, prepared by fluorenylmethoxycarbonyl solid phase peptide synthesis,10 was NH2-terminally labeled with TAMRA (Novabiochem) on the peptide synthesizer. Subsequently, unlabeled and TAMRA-labeled CXCL4L1 were deprotected, cleaved from the resin, and purified by reversed phase high-performance liquid chromatography as described.10 Proteins with the correct Mr were folded and repurified by reversed phase high-performance liquid chromatography. We demonstrated that chemical synthesis and addition of the TAMRA label to the NH2-terminus of CXCL4L1 did not affect its biological activity. Indeed, TAMRA-CXCL4L1 attracted immature DCs (data not shown).
Binding and signaling assays
CHO-K1 cells were transfected with CXCR3A, CXCR3B, or CXCR7 as described previously.16,17 These transfected cell lines were used in calcium and extracellular signal-regulated protein kinase signaling assays to identify the human CXCL4L1 receptor.16 Binding of TAMRA-CXCL4L1 to receptor-transfected cells was performed using confluent monolayers of CXCR3-transfected CHO cells seeded in 6-well plates. Cells were prechilled and incubated on ice for 2 hours with 300 ng/mL TAMRA-CXCL4L1 in the absence or presence of competing unlabeled chemokines. Cell lysates were cleared by centrifugation, and supernatants were collected. To visualize fluorescent chemokine in the lysates, samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and the gels were scanned afterward using the Cy3 filterset (excitation 540/25 and emission 595/25) of an Ettan DIGE scanner (GE Healthcare).
For endothelial cell binding tests with TAMRA-CXCL10 and TAMRA-CXCL4L1, HMVECs-L (human microsvascular endothelial cells isolated from lung tissue) were seeded in black 96-well plates. After incubation with the labeled and unlabeled chemokines (1 hour at 37°C), monolayers were washed and fixed with paraformaldehyde. Fluorescence (excitation 546 and emission 574) was quantified using a fluorescent plate reader (DYNEX Technologies).
To determine the binding affinity of CXCL4 and CXCL4L1 for GAGs, low molecular weight heparin (Sigma-Aldrich) or chondroitin sulfate-E (US Biologicals) was immobilized on a GAG-binding 96-well plate (BD Biosciences). After incubation with chemokines, biotinylated polyclonal rabbit anti–human CXCL4 or anti–human CXCL4L1 Ab was added.7 After removal of excess Ab, immune complexes were detected by streptavidin-coupled peroxidase.
Results
The antitumoral activity of human CXCL4L1 is mediated by inhibition of angiogenesis through CXCR3
In a previous study, we have demonstrated that natural CXCL4L1 is a more potent inhibitor of endothelial cell migration and angiogenesis than CXCL4.6 Here, this was complemented in both a physiologically representative in vitro assay and a clinically relevant animal model of human non-small cell lung cancer. Indeed, after subcutaneous injection in severe combined immunodeficiency mice, human A549 adenocarcinoma cell growth was inhibited by intratumoral treatment with pure natural CXCL4 or recombinant human CXCL4L1 (0.1 μg; 3 times per week) for 8 weeks, as previously shown.9 The implication of an angiostatic effect in this model was evidenced for both chemokines by the reduced number of blood vessels present in the resected tumors. Indeed, the number of Factor VIII-related antigen positive cells in the tumors was significantly higher in control mice than in CXCL4- or CXCL4L1-treated mice, the effect of the latter being more pronounced (supplemental Figure 1).
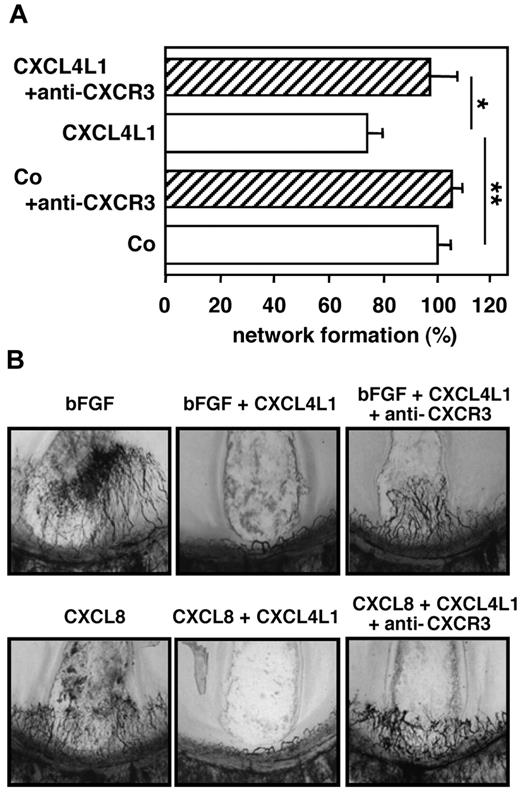
In the Matrigel assay with human microvascular endothelial cells, CXCL4L1 at 20-100 ng/mL significantly reduced tube formation, whereas CXCL4 at 1000 ng/mL did not (supplemental Figure 2). Because CXCR3 has been reported to act as a functional receptor for CXCL417 and to be involved in the inhibition of angiogenesis by CXCL9 and CXCL11,1,18 we investigated the role of this receptor in the angiostatic and antitumoral effect of CXCL4L1. Indeed, when we preincubated (30 minutes at 37°C) the microvascular endothelial cells with neutralizing monoclonal anti-CXCR3 Abs before addition to the Matrigel assay, the endothelial cell network in the presence of CXCL4L1 was similar to that of the cells incubated in assay medium without CXCL4L1 (Figure 1A). Thus, the anti-CXCR3 Ab significantly reduced the inhibitory effect of CXCL4L1 on endothelial cell migration and tube formation. After this in vitro observation, we applied anti-CXCR3 Abs in in vivo test systems. In the rat cornea micropocket assay, it was first confirmed that recombinant CXCL4L1 (80 ng/pellet) could inhibit the angiogenic activity of both CXCL8 (80 ng/pellet) and basic fibroblast growth factor (bFGF; 50 ng/pellet). Addition of Abs against mCXCR3 prevented the angiostatic activity of CXCL4L1 against CXCL8 and bFGF (Figure 1B).
The in vitro and in vivo angiostatic activity of human CXCL4L1 is CXCR3-dependent. HMVECs-d (A) were seeded in 48-well plates on Matrigel in the presence of 100 ng/mL CXCL4L1 and neutralizing monoclonal anti-CXCR3 Ab (2.5 μg/mL, clone 49 801; R&D Systems). After 12 hours, rearrangement of the endothelial cells into tubular structures was evaluated by microscopy. The total length of tubes in each well was determined, and results are expressed as the percentage inhibition of tube formation compared with control cultures stimulated with growth medium alone. Results (mean ± SEM of 5 independent experiments performed in duplicate or triplicate) were analyzed by the Mann Whitney test to detect differences between 2 conditions (*P < .05, **P < .005). Neovascularization (B) was evaluated after implantation of Hydron pellets containing 50 ng bFGF, 80 ng CXCL8, 80 ng CXCL4L1 plus 50 ng bFGF, or 80 ng CXCL4L1 plus 80 ng CXCL8, respectively, in rat corneas in the absence or presence of anti-CXCR3 Ab. Panels are at 40× magnification.
The in vitro and in vivo angiostatic activity of human CXCL4L1 is CXCR3-dependent. HMVECs-d (A) were seeded in 48-well plates on Matrigel in the presence of 100 ng/mL CXCL4L1 and neutralizing monoclonal anti-CXCR3 Ab (2.5 μg/mL, clone 49 801; R&D Systems). After 12 hours, rearrangement of the endothelial cells into tubular structures was evaluated by microscopy. The total length of tubes in each well was determined, and results are expressed as the percentage inhibition of tube formation compared with control cultures stimulated with growth medium alone. Results (mean ± SEM of 5 independent experiments performed in duplicate or triplicate) were analyzed by the Mann Whitney test to detect differences between 2 conditions (*P < .05, **P < .005). Neovascularization (B) was evaluated after implantation of Hydron pellets containing 50 ng bFGF, 80 ng CXCL8, 80 ng CXCL4L1 plus 50 ng bFGF, or 80 ng CXCL4L1 plus 80 ng CXCL8, respectively, in rat corneas in the absence or presence of anti-CXCR3 Ab. Panels are at 40× magnification.
Further, in a syngeneic model of subcutaneous LLC, intratumoral injection of CXCL4L1 (0.1 μg; 3× per week) reduced tumor growth (Figure 2A-C). Indeed, a significantly smaller tumor size was observed for the CXCL4L1-treated compared with control animals. However, when mice were co-injected with CXCL4L1 plus anti-CXCR3 Abs, the LLC tumor growth was not retarded (Figure 2A,C). There was no significant difference between control mice injected with either normal goat serum or anti-CXCR3 Abs in the absence of CXCL4L1. To obtain additional evidence that CXCR3 is implicated in the antitumoral effect of CXCL4L1, its antitumoral effect was evaluated in CXCR3−/− animals injected subcutaneously with LLC cells. It was noticed that CXCL4L1 was no longer able to reduce tumor growth and size in CXCR3−/− animals (Figure 2B-C). In addition, resected tumors were analyzed for changes in the number of microvascular cells. In the syngeneic model of LLC, intratumoral injection of CXCL4L1 in wt mice reduced (P < .05) the number of MECA32 positive cells (Figure 2D). However, injection of CXCL4L1 and anti-CXCR3 antibody together reverted this inhibition to levels observed in control mice receiving no CXCL4L1. Similarly, no difference in Factor VIII-related antigen (supplemental Figure 3A) or MECA32 (Figure 2D) positive cells was observed between CXCL4L1-injected or nontreated CXCR3−/− animals. Finally, when metastasis of tumor cells to the lungs was evaluated, absence or neutralization of CXCR3 increased the number of tumor cells in the lungs, compared with control or wt CXCL4L1-treated animals (supplemental Figure 3B-C). Taken together, these data indicate that the antitumoral and angiostatic activities of CXCL4L1 in mice are at least in part mediated through binding and activation of CXCR3.
Function of CXCR3 in tumor growth inhibition of LLC after treatment with the angiostatic chemokine CXCL4L1. LLC cells were subcutaneously implanted in C57Bl/6 mice in 2 separate experiments with different settings (first experiment 40 wt and 20 CXCR3−/− mice, ie, n = 10 per group; second experiment 90 wt and 30 CXCR3−/− mice; ie, n = 15 per group). In the first experiment, 6 treatment groups were included (control [Ctrl] versus CXCL4L1 normal goat serum-treated; Ctrl versus CXCL4L1 anti-CXCR3-treated; and Ctrl versus CXCL4L1 in CXCR3−/− mice). In the second experiment, 2 additional control groups of wt mice (WT Ctrl and WT CXCL4L1) were included that received no antibody treatment. Intratumoral chemokine treatment (vehicle control or 0.1 μg human CXCL4L1 per injection) started at the time of tumor inoculation and was repeated 3 times a week. Neutralization of CXCR3 was obtained by intraperitoneal injection of 0.5 mL polyclonal antiserum (4 mg immunoglobulin), 3× a week, starting at the time of tumor inoculation. Tumor dimensions were measured every week (A-C). (A-B) The mean tumor size (mm3) ± SEM per group from the second experiment. (C) The statistical analysis of the tumor sizes after 3 weeks when data from both experiments were combined. The WT Ctrl and WT CXCL4L1 group are not shown, because these treatments were only tested in the second experiment. To assess tumor vascularity, 5 tumors per group from both experiments were minced into single-cell suspensions for flow cytometric analysis using Abs against the endothelial cell marker MECA32 (panel D, n = 10/group). Statistically significant differences between the indicated groups were determined by the Mann Whitney test (*P < .05; **P < .01).
Function of CXCR3 in tumor growth inhibition of LLC after treatment with the angiostatic chemokine CXCL4L1. LLC cells were subcutaneously implanted in C57Bl/6 mice in 2 separate experiments with different settings (first experiment 40 wt and 20 CXCR3−/− mice, ie, n = 10 per group; second experiment 90 wt and 30 CXCR3−/− mice; ie, n = 15 per group). In the first experiment, 6 treatment groups were included (control [Ctrl] versus CXCL4L1 normal goat serum-treated; Ctrl versus CXCL4L1 anti-CXCR3-treated; and Ctrl versus CXCL4L1 in CXCR3−/− mice). In the second experiment, 2 additional control groups of wt mice (WT Ctrl and WT CXCL4L1) were included that received no antibody treatment. Intratumoral chemokine treatment (vehicle control or 0.1 μg human CXCL4L1 per injection) started at the time of tumor inoculation and was repeated 3 times a week. Neutralization of CXCR3 was obtained by intraperitoneal injection of 0.5 mL polyclonal antiserum (4 mg immunoglobulin), 3× a week, starting at the time of tumor inoculation. Tumor dimensions were measured every week (A-C). (A-B) The mean tumor size (mm3) ± SEM per group from the second experiment. (C) The statistical analysis of the tumor sizes after 3 weeks when data from both experiments were combined. The WT Ctrl and WT CXCL4L1 group are not shown, because these treatments were only tested in the second experiment. To assess tumor vascularity, 5 tumors per group from both experiments were minced into single-cell suspensions for flow cytometric analysis using Abs against the endothelial cell marker MECA32 (panel D, n = 10/group). Statistically significant differences between the indicated groups were determined by the Mann Whitney test (*P < .05; **P < .01).
Comparison of the chemotactic potency of CXCL4 and CXCL4L1 for activated lymphocytes and NK cells
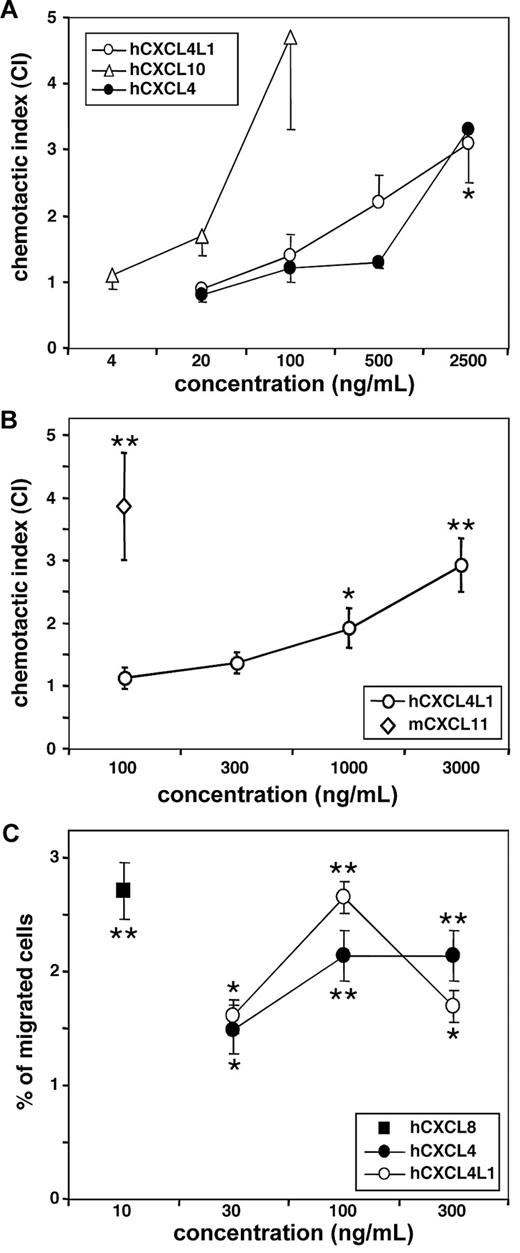
Despite early reports, showing chemotactic and/or activating effects of CXCL4 on various human leukocytic cell types, including monocytes and neutrophils, the purity of the chemokine preparations used and the micromolar concentration of CXCL4 required to observe these effects compared with nanomolar concentrations required for other chemokines, allowed to question the physiological relevance of some of these observations.19-21 Nevertheless, in view of its potent antitumoral activity, we investigated the in vitro chemotactic capacity of CXCL4L1 for antitumoral leukocytes (activated T lymphocytes and NK cells; Figure 3).
CXCL4 and CXCL4L1 attract activated T lymphocytes and NK cells. Chemotaxis experiments were performed to compare the chemotactic activity of CXCL4 and CXCL4L1 for (A) human (h) IL-2–activated T lymphocytes (n = 4 to 5), (B) murine (m) IL-2–activated T lymphocytes (n = 4 to 5), and (C) unstimulated NK cells (n = 5). Statistically significant migration toward chemokine is indicated (*P < .05; **P < .005; Mann Whitney test).
CXCL4 and CXCL4L1 attract activated T lymphocytes and NK cells. Chemotaxis experiments were performed to compare the chemotactic activity of CXCL4 and CXCL4L1 for (A) human (h) IL-2–activated T lymphocytes (n = 4 to 5), (B) murine (m) IL-2–activated T lymphocytes (n = 4 to 5), and (C) unstimulated NK cells (n = 5). Statistically significant migration toward chemokine is indicated (*P < .05; **P < .005; Mann Whitney test).
First, it was observed in the classical Boyden microchamber assay (Figure 3A) that in vitro activated human T lymphocytes revealed a weak, but significant chemotactic activity at a micromolar (2.5 μg/mL) concentration of pure recombinant CXCL4L1 (index of 3.1 ± 0.2; P < .05; n = 5). The minimal effective concentration of the CXCR3 ligand and known lymphocyte chemoattractant CXCL10 was 100 ng/mL (index of 4.7 ± 0.6; n = 4) in this test, confirming that CXCR3 was expressed and available for CXCL4L1 as well. Also activated mouse lymphocytes migrated in response to human CXCL4L1 at concentrations of 1000 ng/mL or more (Figure 3B). At the highest dose tested, 3000 ng/mL, human CXCL4L1 attracted activated mouse T lymphocytes as efficiently as 100 ng/mL murine CXCL11 (index of 2.9 ± 0.4 and 3.9 ± 0.8, respectively; n = 5).
Next, human NK cells were investigated for their capacity to chemotactically respond to the PF-4 variants (Figure 3C). A significant induction of NK cell migration was reached with an optimal concentration of 100 ng/mL for both CXCL4 (2.1% ± 0.5% migrated cells, n = 5) and CXCL4L1 (2.7% ± 0.2% migrated cells, n = 5), which is comparable to that obtained with CXCL8 at 10 ng/mL (2.7% ± 0.3%, n = 5). Thus, in contrast to the rather high CXCL4L1 concentrations required for the recruitment of effector lymphocytes, NK cells were attracted at a 10-fold lower CXCL4L1 concentration.
Interaction of PF-4 forms with GAGs
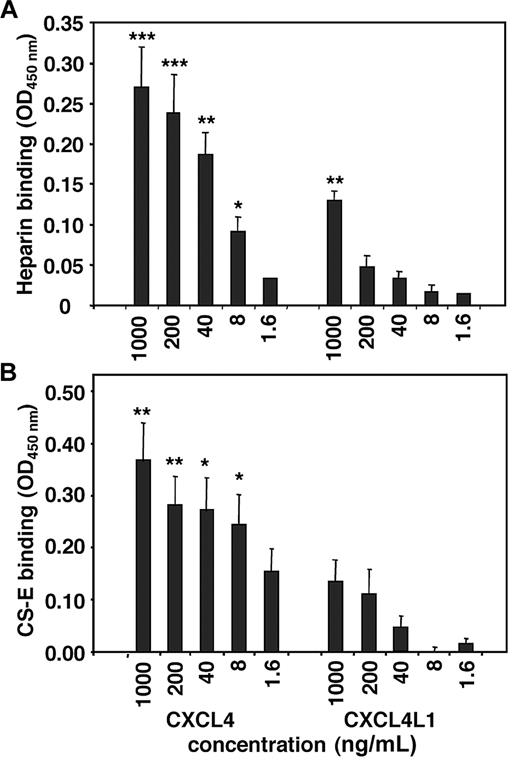
Because GAGs mediate some of the biological activities of CXCL4 (eg, activation of neutrophilic granulocytes inducing exocytosis),22 we investigated the affinity of both PF-4 forms for heparin and chondroitin sulfate-E (Figure 4). We observed strong interaction of authentic CXCL4 with both GAGs, because after addition of CXCL4 at concentrations as low as 8 ng/mL, immunoreactivity could still be detected. In contrast, high amounts (1000 ng/mL) of CXCL4L1 had to be added to heparin-coated plates to detect bound chemokine, whereas no interaction was observed between CXCL4L1 and chondroitin sulfate-E. The observed differential binding of CXCL4 and CXCL4L1 to GAGs corresponded well with the affinity of both chemokines for the heparin-Sepharose matrix used to purify the PF-4 forms. For elution of CXCL4 from the heparin-Sepharose column, higher concentrations of NaCl (1.6-1.8M) are required than needed for elution of CXCL4L1 (1-1.4M), reflecting its high affinity for heparin.6
Differential interaction of CXCL4 and CXCL4L1 with GAGs. Binding of CXCL4 and CXCL4L1 to immobilized low molecular weight heparin (A) or chondroitin sulfate-E (CS-E; B) was detected by biotinylated anti-CXCL4 or anti-CXCL4L1 Abs, respectively, as described in the Methods section. Results (mean ± SEM) shown are derived from 5 independent experiments. Statistical analysis testing for significant chemokine-GAG interaction is indicated (*P < .05; **P < .01; ***P < .001; Mann Whitney test).
Differential interaction of CXCL4 and CXCL4L1 with GAGs. Binding of CXCL4 and CXCL4L1 to immobilized low molecular weight heparin (A) or chondroitin sulfate-E (CS-E; B) was detected by biotinylated anti-CXCL4 or anti-CXCL4L1 Abs, respectively, as described in the Methods section. Results (mean ± SEM) shown are derived from 5 independent experiments. Statistical analysis testing for significant chemokine-GAG interaction is indicated (*P < .05; **P < .01; ***P < .001; Mann Whitney test).
Receptor binding of PF-4 forms
Although we provided evidence that CXCR3 is implicated in the angiostatic and antitumoral activity of human CXCL4L1 in mice (Figure 2), the question remained how the PF-4 variants function in the human system. Indeed, human CXCR3B, a variant of CXCR3, has been designated as a functional receptor for CXCL4.17 To our knowledge, mice have no splice variants of CXCR3. In contrast to the human CXCR3A receptor that facilitates lymphocyte migration, CXCR3B should rather mediate the endothelial cell growth inhibiting capacity of CXCL4, as well as that of the previously known CXCR3 ligands CXCL9, CXCL10, and CXCL11.17,23 We therefore investigated whether CXCL4 and CXCL4L1 might bind to human CXCR3A, CXCR3B, or CXCR7, a recently identified receptor for CXCL11 and CXCL12.24 It was found that CXCL4 and CXCL4L1 at 3 μg/mL did not compete for binding of 125I-CXCL11 to CHO-CXCR7 transfectants, whereas at 100 ng/mL cold CXCL11 and CXCL12 were able to displace 125I-CXCL11 for 67% and 97%, respectively (supplemental Figure 4). Interaction of CXCL4L1 with CXCR3A and CXCR3B was studied using fluorescently labeled TAMRA-CXCL4L1. TAMRA-CXCL4L1 bound to both CHO-CXCR3A and CHO-CXCR3B cells, because we observed the 8-kDa protein on fluorescence scanned SDS-PAGE gels loaded with the cell lysates (Figure 5A-C). TAMRA-CXCL4L1 could be displaced from CHO-CXCR3A cells by cold CXCL4L1, CXCL4, or CXCL10 (Figure 5A). The competition between labeled and cold CXCL4L1 on CXCR3A was dose-dependent, 3-fold excess of cold chemokine, but not equimolar concentrations, being able to displace 300 ng/mL of TAMRA-CXCL4L1 (Figure 5B). Similarly, CXCL4L1 could dose-dependently prevent TAMRA-CXCL4L1 binding to CXCR3B-transfectants (data not shown). Furthermore, CXCL4L1, CXCL4, and CXCL10 could displace TAMRA-CXCL4L1 from the CXCR3B receptor (Figure 5C). Figure 5D demonstrates that the interaction of TAMRA-CXCL4L1 with these CXCR3-transfected cells was receptor-specific. TAMRA-CXCL4L1 (300 ng/mL) was added to 3 × 106 mock-transfected, CXCR3A-, or CXCR3B-transfected CHO cells under conditions reducing GAG interaction (0.75M NaCl). The amount of fluorescence bound to CXCR3A- and CXCR3B-transfected cells was 2- and 3-fold higher, respectively, compared with the signal obtained with mock-transfected cells.
Receptor binding properties of CXCL4 and CXCL4L1 on endothelial cells and CXCR3 transfectants. TAMRA-CXCL4L1 [0 ng/mL (no label) or 300 ng/mL (all other lanes)] were added to CXCR3A- or CXCR3B-transfected CHO cells in the absence (Co) or presence of 3000 ng/mL cold CXCL4L1, CXCL4, or CXCL10 (A,C). Cell-bound 8-kDa TAMRA-CXCL4L1 in lysates was analyzed by SDS-PAGE scanned for fluorescence. The dose-dependency of competition for binding to CHO-CXCR3A cells is demonstrated in panel B. Competition for binding of TAMRA-CXCL4L1 to CHO-CXCR3B is shown in panel C. To detect aspecific interaction, binding of 300 ng/mL TAMRA-CXCL4L1 to mock-transfected cells (D) was compared with binding to equal numbers of CXCR3A- and CXCR3B-transfected cells. Results shown are representative of 2 (C), 3 (A-B), or 5 (D) experiments. Confluent monolayers of HMVECs (E-G) were incubated with TAMRA-CXCL4L1 or TAMRA-CXCL10 in the presence or absence of competing unlabeled chemokines (CXCL4L1 or CXCL10). Cell-bound fluorescence present in the fixed cell cultures was quantified by a fluorescence plate reader. Either the concentration of labeled chemokine (E-F) or cold chemokine (G) was kept constant. Results shown are the mean of 2 independent experiments performed in triplicate (E-G).
Receptor binding properties of CXCL4 and CXCL4L1 on endothelial cells and CXCR3 transfectants. TAMRA-CXCL4L1 [0 ng/mL (no label) or 300 ng/mL (all other lanes)] were added to CXCR3A- or CXCR3B-transfected CHO cells in the absence (Co) or presence of 3000 ng/mL cold CXCL4L1, CXCL4, or CXCL10 (A,C). Cell-bound 8-kDa TAMRA-CXCL4L1 in lysates was analyzed by SDS-PAGE scanned for fluorescence. The dose-dependency of competition for binding to CHO-CXCR3A cells is demonstrated in panel B. Competition for binding of TAMRA-CXCL4L1 to CHO-CXCR3B is shown in panel C. To detect aspecific interaction, binding of 300 ng/mL TAMRA-CXCL4L1 to mock-transfected cells (D) was compared with binding to equal numbers of CXCR3A- and CXCR3B-transfected cells. Results shown are representative of 2 (C), 3 (A-B), or 5 (D) experiments. Confluent monolayers of HMVECs (E-G) were incubated with TAMRA-CXCL4L1 or TAMRA-CXCL10 in the presence or absence of competing unlabeled chemokines (CXCL4L1 or CXCL10). Cell-bound fluorescence present in the fixed cell cultures was quantified by a fluorescence plate reader. Either the concentration of labeled chemokine (E-F) or cold chemokine (G) was kept constant. Results shown are the mean of 2 independent experiments performed in triplicate (E-G).
Finally, we performed binding studies using TAMRA-CXCL4L1 and TAMRA-CXCL10 on HMVECs. For both chemokines, we obtained a dose-dependent increase in binding of the labeled ligand to HMVECs, and mutual competition between CXCL4L1 and CXCL10 was observed (Figure 5E-G). These results suggest that CXCL4L1 shares its binding site on endothelial cells with CXCL10 and confirm the binding experiments with CXCR3B-transfected cells. Indeed, endothelial cells have been described to express exclusively CXCR3B.17 Based on the results, shown in Figure 5G, the Kd for interaction of CXCL4L1 with cellular receptors present on HMVECs was calculated to be equal to 0.28nM.
CXCL4 and CXCL4L1 are chemotactic for immature DCs via a pertussis toxin-sensitive receptor mechanism
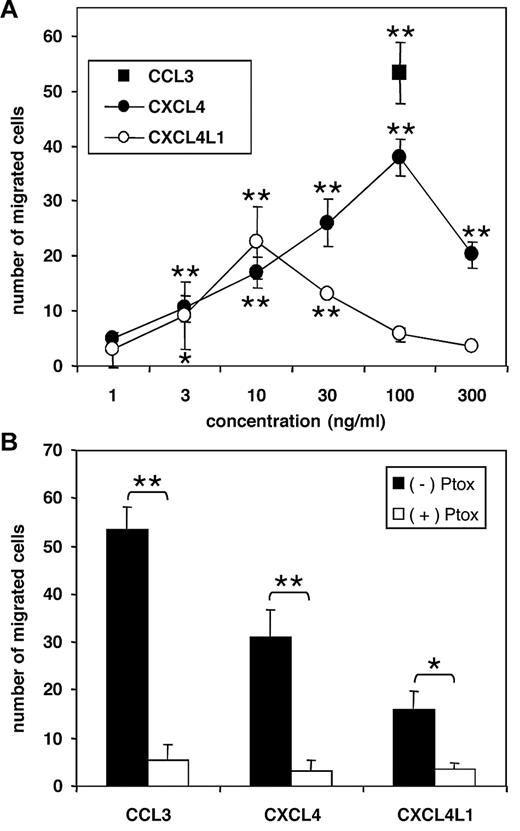
CXCL4, as well as CXCL4L1 provoked migration of immature DCs derived from human peripheral blood monocytes in a dose-dependent manner (Figure 6A). For CXCL4, the minimal effective concentration was 10 ng/mL (17 ± 3 migrated cells), whereas a maximal chemotactic response was reached at 100 ng/mL (38 ± 3 cells). The efficacy of CXCL4 on these cells was similar to that of CCL3 at 100 ng/mL (53 ± 6 cells). Furthermore, it was found that CXCL4L1 showed a different dose response curve on immature DCs by reaching maximal chemotactic activity already at 10 ng/mL (23 ± 6 cells). It can be deduced that both PF-4 variants exert a physiologically relevant chemotactic effect on immature DCs, which need to migrate to initiate immune responses. The participation of DCs in the antitumoral activity25 of both angiostatic PF-4 variants can therefore not be excluded.
Chemotactic activity of PF-4 variants for immature DCs. CXCL4L1 and CXCL4 were tested for their ability to induce chemotaxis of human immature DCs derived from peripheral blood in the Boyden microchamber (A). * (P < .05) indicates statistically significant migration toward chemokine (Mann Whitney test). The chemotactic response of CXCL4 (100 ng/mL), CXCL4L1 (100 ng/mL), and CCL3 (100 ng/mL) was reduced after treatment with 3 μg/mL pertussis toxin (Ptox; B) as determined by the Mann Whitney test (*P < .05; **P < .005). Results represent the mean ± SD of migrated cells of 4 (A) or 3 (B) independent experiments.
Chemotactic activity of PF-4 variants for immature DCs. CXCL4L1 and CXCL4 were tested for their ability to induce chemotaxis of human immature DCs derived from peripheral blood in the Boyden microchamber (A). * (P < .05) indicates statistically significant migration toward chemokine (Mann Whitney test). The chemotactic response of CXCL4 (100 ng/mL), CXCL4L1 (100 ng/mL), and CCL3 (100 ng/mL) was reduced after treatment with 3 μg/mL pertussis toxin (Ptox; B) as determined by the Mann Whitney test (*P < .05; **P < .005). Results represent the mean ± SD of migrated cells of 4 (A) or 3 (B) independent experiments.
Further, it was evidenced that the DC chemotactic activity of CXCL4 and CXCL4L1 is mediated by signaling through a pertussis toxin-sensitive G protein-coupled receptor mechanism (Figure 6B). Indeed, in parallel with CCL3, the migratory effect of both PF-4 variants (at 100 ng/mL) for immature DCs was completely abrogated in the presence of pertussis toxin. Finally, we wanted to confirm that CXCR3 is the tentative CXCL4 and CXCL4L1 receptor on human immature DCs and, therefore, we tested whether DC migration toward the PF-4 variants was affected by preincubation of the cells with CXCR3 ligands or anti-CXCR3 Abs. Figure 7A shows that preincubation of DCs with CXCL10 (50 ng/mL) or CXCL11 (20 ng/mL) almost completely or partially inhibited migration of DCs in response to CXCL4 (30 ng/mL) or CXCL4L1 (30 ng/mL), whereas the response to CCL3 (100 ng/mL) was not affected. Vice versa, CXCL4 (30 ng/mL) and CXCL4L1 (30 ng/mL) partially desensitized DCs to subsequent CXCL10- or CXCL11-induced migration. In addition, DC chemotaxis in response to CXCL4, CXCL4L1, CXCL10, or CXCL11 was reduced after preincubation of the cells with neutralizing anti–human CXCR3 Abs (Figure 7B). For CXCL4 and CXCL4L1, the neutralizing capacities of the monoclonal and polyclonal Ab preparations were similar. However, the monoclonal Ab fully abrogated the response to CXCL10 and CXCL11, which was only partially reduced by the polyclonal Abs. This suggests that compared with CXCL10 and CXCL11, CXCL4 and CXCL4L1 might have lower affinity for DC-expressed CXCR3 or distinct binding sites on this receptor. It has already been shown that compared with CXCL10, CXCL11 interacts differently with CXCR3.26,27 Taken together, we demonstrated that CXCL4 and CXCL4L1 share CXCR3 with CXCL10 and CXCL11 as a functional receptor on human DCs. Furthermore, we showed that both PF-4 variants can chemoattract immunocompetent cells at relatively low concentrations, suggesting a role in the immune response to infection and cancer.
The chemotactic activity of CXCL4L1 for human immature DCs is mediated via CXCR3. To demonstrate the involvement of CXCR3 in immature DC chemotaxis, cells were preincubated with CXCR3 ligands [(A) 30 ng/mL CXCL4, 30 ng/mL CXCL4L1, 50 ng/mL CXCL10, 20 ng/mL CXCL11, or dilution buffer (Ctrl)] or CXCR3-neutralizing Abs [(B) dilution buffer (Ctrl), 10 μg/mL monoclonal Ab (mAb), 28 μg/mL polyclonal Ab (pAb), or 10 μg/mL isotype control Ab] before addition to the Boyden microchamber. As chemoattractants, CCL3 (100 ng/mL), CXCL4 (30 ng/mL), CXCL4L1 (30 ng/mL), CXCL10 (50 ng/mL), or CXCL11 (20 ng/mL) were added to the lower wells. Results shown are the mean ± SD from 4 (A) or 3 (B) independent experiments. In panel A, statistically significant reduction of migration is indicated (*P < .05; Mann Whitney test).
The chemotactic activity of CXCL4L1 for human immature DCs is mediated via CXCR3. To demonstrate the involvement of CXCR3 in immature DC chemotaxis, cells were preincubated with CXCR3 ligands [(A) 30 ng/mL CXCL4, 30 ng/mL CXCL4L1, 50 ng/mL CXCL10, 20 ng/mL CXCL11, or dilution buffer (Ctrl)] or CXCR3-neutralizing Abs [(B) dilution buffer (Ctrl), 10 μg/mL monoclonal Ab (mAb), 28 μg/mL polyclonal Ab (pAb), or 10 μg/mL isotype control Ab] before addition to the Boyden microchamber. As chemoattractants, CCL3 (100 ng/mL), CXCL4 (30 ng/mL), CXCL4L1 (30 ng/mL), CXCL10 (50 ng/mL), or CXCL11 (20 ng/mL) were added to the lower wells. Results shown are the mean ± SD from 4 (A) or 3 (B) independent experiments. In panel A, statistically significant reduction of migration is indicated (*P < .05; Mann Whitney test).
Discussion
CXCL4 is the first chemokine structure identified, but its spectrum of biological activities is rather complex, because of the numerous molecules it is interacting with and because some of these partners are very different from the typical G protein-coupled chemokine receptors. One of the major physiological roles of CXCL4 is to influence blood coagulation, both procoagulant and anticoagulant properties being reported. CXCL4 on the one hand neutralizes heparin-like molecules on the endothelial surface of blood vessels, thereby inhibiting local antithrombin III activity and promoting coagulation. On the other hand, CXCL4 binds and induces conformational changes in protein C, thereby enhancing generation of activated protein C, which is a potent anticoagulant.28 In addition, by binding polysulfated GAGs, CXCL4 can indirectly interfere with activation of receptors by growth factors, such as vascular endothelial growth factor and FGF, improve adhesion of neutrophils to the vessel wall, and reduce proliferation of T cells.2,19,29 Furthermore, CXCL4 has been shown to directly interact with FGF-2, CXCL8, and CCL5.30-32 Finally, during the last decade, evidence accumulated that also CXCL4 activates a typical chemokine receptor,17 namely a variant of CXCR3 (vide infra).
Since the first description of CXCL4L1, no receptor has been identified for this rather new chemokine variant. In this manuscript, we identified CXCR3 to be a functional CXCL4L1 receptor both in the human and the mouse systems. In humans, several splice variants of CXCR3 have been described.17,33 For its angiostatic activity, CXCL4 is supposed to use CXCR3B, coupled to Gαs, whereas its chemotactic activity for activated T lymphocytes is mediated via CXCR3 coupled to a Gαi protein.17,20 However, the interaction with CXCR3 on activated T lymphocytes was of low affinity and Mueller et al20 could not dissect whether CXCL4 acted on this cell type via CXCR3A or CXCR3B or both CXCR3 variants. Furthermore, the data of Mueller et al20 are in agreement with the hypothesis of Fleischer et al19 that CXCR3 on activated T cells functions as a coreceptor that is activated after initial CXCL4 binding to proteoglycans. Also in our hands, assays with CXCL4- and CXCR-transfected cells were complicated because of the presence of GAGs. We must thus conclude that proteoglycans remain major cellular mediators of CXCL4 activity. In this context, CXCL4L1 has lower affinity for heparin6 and chondroitin sulfate-E than CXCL4. Here, we demonstrated that fluorescent TAMRA-CXCL4L1 binds to both CXCR3A- and CXCR3B-transfectants and that bound TAMRA-CXCL4L1 can be displaced by other CXCR3 ligands. In addition, chemotaxis of human immature DCs toward CXCL4L1 can be desensitized by the CXCR3 agonists CXCL10 and CXCL11 and can be neutralized by anti-CXCR3 Abs. The fact that pertussis toxin inhibited migration of human immature DCs toward CXCL4L1 suggests that CXCL4L1 attracts these cells via CXCR3, coupled to a Gαi protein. Thompson et al showed that CXCR3-mediated migration of murine activated T cells requires Gαi2.34 Because of its pertussis toxin sensitivity, probably the CXCR3A variant, and not CXCR3B, is activated by CXCL4L1 on human leukocytes. Indeed, earlier reports indicated the presence of CXCR3 on DCs,35,36 NK cells,37 and activated T cells.38 CXCL4L1 shares with CXCL4 these target cells and similarly to CXCL4L1, CXCL4 attracts human DCs via CXCR3. Only one other study described activity of CXCL4 on DCs.39 More specifically, the differentiation of monocyte-derived DCs in the presence of 10 μg/mL CXCL4 led to decreased expression of CD1a and lower secretion of tumor necrosis factor-α and IL-12. We observed chemotaxis of immature monocyte-derived DCs toward much lower concentrations of CXCL4L1 and CXCL4 (starting from 10 ng/mL). In addition, chemotaxis of NK cells occurred at relatively low levels (100 ng/mL) of these platelet chemokines. CXCL4 reportedly stimulates release of CXCL8 by NK cells from 10 ng/mL onwards,40 but has, until our study, never been shown to be chemotactic for these cells. We cannot exclude that the mode of action of CXCL4L1 is different on lymphocytes, NK cells, DCs, and endothelial cells, which might explain the different dose response curves. The finding that the chemotactic activity of CXCL4L1 for lymphocytes, DCs, and endothelial cells is mediated by CXCR3 shows that there is at least one common receptor, not excluding additional receptor binding (eg, GAGs) and signaling mechanisms or components present in one (eg, DC) but not another (eg, lymphocyte) cell type.
Finally, we demonstrated that CXCR3 is also the key receptor involved in the angiostatic activity of CXCL4L1. Indeed, neutralizing CXCR3 Abs blocked the inhibitory effect of CXCL4L1 on network formation in the Matrigel assay with HMVECs, and labeled CXCL4L1 and CXCL10 could displace each other on HMVECs. Further, the antitumoral activity for LLC of CXCL4L1 was completely abolished in the absence of CXCR3 or after neutralization of CXCR3. The lack of antitumoral activity in CXCR3−/− mice or antibody-treated mice concurred with enhanced tumor growth, a higher number of endothelial cells, and more metastasis to the lungs. It can be deduced that for its antitumoral activity, CXCL4L1 shares CXCR3 with CXCL4, CXCL9, CXCL10, and CXCL11. However, for CXCL4, several other mechanisms have been proposed to be involved in its inhibition of endothelial cell growth (eg, interaction with cell surface proteoglycans and direct complex formation with growth factors).30,31,41 We can at present only speculate whether heteromultimerization with growth factors occurs for CXCL4L1, but we demonstrated that the affinity of CXCL4L1 for heparin and chondroitin sulfate-E is lower compared with that of CXCL4. For this reason and because CXCL4L1 is angiostatic at much lower concentrations than CXCL4, we might speculate that the G protein-coupled CXCR3 is the major CXCL4L1 receptor. Indeed, chemokine-GAG interactions are mostly of lower affinity and often require chemokine dimerization or tetramerization (CXCL4).42,43 The 3 diverging amino acids of CXCL4 and CXCL4L1 are located in the COOH-terminal α helix, and because a proline residue (P58 in CXCL4) is involved, the 3-dimensional structure of CXCL4L1 might be different, as well as its tendency to form homomultimers. Taken together, our results provide evidence that both PF-4 forms can attract immature DCs in a CXCR3-dependent manner. Moreover, the angiostatic and antitumoral effects of CXCL4L1 in vivo are also mediated through CXCR3.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank N. Berghmans, K. Carrein, R. Conings, C. Dillen, K. Grauwen, J.-P. Lenaerts, I. Ronsse, and H. Verbeke for excellent technical assistance. The expertise of J.-Y. Springael is much appreciated.
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (Ministero dell'Istruzione dell'Università e della Ricerca; to S. Sozzani), the Center of Excellence (credit number EF/05/15) of the K.U. Leuven (to J.V.D.), the Concerted Research Actions (GOA) of the Regional Government of Flanders (to G.O. and J.V.D.), the Fund for Scientific Research of Flanders (FWO-Vlaanderen) contract number G064809 (to J.V.D.), the Interuniversity Attraction Poles (IAP) Program-Belgian Science Policy (to J.V.D. and M.P.), and the European Union 6FP EC contract INNOCHEM. (to J.V.D. and M.P.), as well as the National Institutes of Health (NIH) grant numbers CA87879, P50CA90388, and HL66027 (to R.M.S.). J.V. and M.G. hold a research assistant and a postdoctoral fellowship of the FWO-Vlaanderen, respectively.
National Institutes of Health
Authorship
Contribution: S. Struyf performed experiments and wrote the paper; L.S. performed experiments with DCs and NK cells; M.D.B. performed in vivo experiments and binding assays with endothelial cells; J.V. performed experiments with DCs and receptor-transfected cell lines and the GAG-binding assay; M.G. performed signaling experiments; S.N. performed Matrigel experiments; P.P. made synthetic TAMRA-CXCL4L1 and performed binding experiments; G.O. designed and supervised experiments; M.P. developed chemokine receptor-transfected cell lines; C.G. developed CXCR3−/− mice; S. Sozzani designed and supervised experiments; R.M.S. developed Abs and designed and supervised experiments; and J.V.D. designed and supervised experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jo Van Damme, Laboratory of Molecular Immunology, Rega Institute, K.U. Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium; e-mail: jo.vandamme@rega.kuleuven.be.
References
Author notes
S. Struyf, L.S., and M.D.B. contributed equally to this study.
S. Sozzani, R.M.S., and J.V.D. contributed equally to this study as senior authors.

![Figure 2. Function of CXCR3 in tumor growth inhibition of LLC after treatment with the angiostatic chemokine CXCL4L1. LLC cells were subcutaneously implanted in C57Bl/6 mice in 2 separate experiments with different settings (first experiment 40 wt and 20 CXCR3−/− mice, ie, n = 10 per group; second experiment 90 wt and 30 CXCR3−/− mice; ie, n = 15 per group). In the first experiment, 6 treatment groups were included (control [Ctrl] versus CXCL4L1 normal goat serum-treated; Ctrl versus CXCL4L1 anti-CXCR3-treated; and Ctrl versus CXCL4L1 in CXCR3−/− mice). In the second experiment, 2 additional control groups of wt mice (WT Ctrl and WT CXCL4L1) were included that received no antibody treatment. Intratumoral chemokine treatment (vehicle control or 0.1 μg human CXCL4L1 per injection) started at the time of tumor inoculation and was repeated 3 times a week. Neutralization of CXCR3 was obtained by intraperitoneal injection of 0.5 mL polyclonal antiserum (4 mg immunoglobulin), 3× a week, starting at the time of tumor inoculation. Tumor dimensions were measured every week (A-C). (A-B) The mean tumor size (mm3) ± SEM per group from the second experiment. (C) The statistical analysis of the tumor sizes after 3 weeks when data from both experiments were combined. The WT Ctrl and WT CXCL4L1 group are not shown, because these treatments were only tested in the second experiment. To assess tumor vascularity, 5 tumors per group from both experiments were minced into single-cell suspensions for flow cytometric analysis using Abs against the endothelial cell marker MECA32 (panel D, n = 10/group). Statistically significant differences between the indicated groups were determined by the Mann Whitney test (*P < .05; **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2009-11-253591/4/m_zh89991064380002.jpeg?Expires=1769112735&Signature=gDY-w19KMRlQt-w-UdgtX4~Eh7X0opz~vYmJfCkHqsMmfv~IGlX5Us6aoW2NinQM3IVqkwRXrHkrabTcmYo-aF5-X1hULAjlL-xo9xMCfeMSzKnyBi1TVLAgsZuJNcDfz4NWjCXmb4NaF51tN1SJmMaEC8GR7mJqE6cSTvEb7inDHFUZtO5~jmJoGGkme8da7RXT6QwW~s95C2aGc5dtjHtKMfgfAjkfnHQPvpO4indtP7EFAsenYogR1T~Bqoa-pC1MkOh1CUfrPszcVMAEVvPiB-lrD5RZRQ8qvG1XbN~gmkWKPFRNrBha-zzyEnTtYxtVkn5LzDK2rSLJxEowWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Receptor binding properties of CXCL4 and CXCL4L1 on endothelial cells and CXCR3 transfectants. TAMRA-CXCL4L1 [0 ng/mL (no label) or 300 ng/mL (all other lanes)] were added to CXCR3A- or CXCR3B-transfected CHO cells in the absence (Co) or presence of 3000 ng/mL cold CXCL4L1, CXCL4, or CXCL10 (A,C). Cell-bound 8-kDa TAMRA-CXCL4L1 in lysates was analyzed by SDS-PAGE scanned for fluorescence. The dose-dependency of competition for binding to CHO-CXCR3A cells is demonstrated in panel B. Competition for binding of TAMRA-CXCL4L1 to CHO-CXCR3B is shown in panel C. To detect aspecific interaction, binding of 300 ng/mL TAMRA-CXCL4L1 to mock-transfected cells (D) was compared with binding to equal numbers of CXCR3A- and CXCR3B-transfected cells. Results shown are representative of 2 (C), 3 (A-B), or 5 (D) experiments. Confluent monolayers of HMVECs (E-G) were incubated with TAMRA-CXCL4L1 or TAMRA-CXCL10 in the presence or absence of competing unlabeled chemokines (CXCL4L1 or CXCL10). Cell-bound fluorescence present in the fixed cell cultures was quantified by a fluorescence plate reader. Either the concentration of labeled chemokine (E-F) or cold chemokine (G) was kept constant. Results shown are the mean of 2 independent experiments performed in triplicate (E-G).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2009-11-253591/4/m_zh89991064380005.jpeg?Expires=1769112736&Signature=jgdNCnK5PZwTUikcQvyYMtIo9-1gyIohArTI-mek~V1t6zMI0PpKYrFxYmCyADZo~iRFV5ZthlPBYUXH4cB3BFUvanFesRoXWV7gx2SNGlteF-fJdOC1HQE5HMiAhmAqgF3RwCgS-JnwzfU-3yTmmuZZ4hWKjihMr2XLa41xpmzAkOwyDlFl2TLtRbWjbleFne2IbZsm1Pw5e-8bJnYmrXLq5S~2nniIC-BdbPcCYZCaBpI9C0qaDLaLhQG6Fuk6ONxpxqxrwCRQFoo~q2NF21O2DI~Cx1X55jANjnO4wkQuQFRD6NBCr-jbRdDpEX1D8G8vRDJBN9AI5KAQy1P-Ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. The chemotactic activity of CXCL4L1 for human immature DCs is mediated via CXCR3. To demonstrate the involvement of CXCR3 in immature DC chemotaxis, cells were preincubated with CXCR3 ligands [(A) 30 ng/mL CXCL4, 30 ng/mL CXCL4L1, 50 ng/mL CXCL10, 20 ng/mL CXCL11, or dilution buffer (Ctrl)] or CXCR3-neutralizing Abs [(B) dilution buffer (Ctrl), 10 μg/mL monoclonal Ab (mAb), 28 μg/mL polyclonal Ab (pAb), or 10 μg/mL isotype control Ab] before addition to the Boyden microchamber. As chemoattractants, CCL3 (100 ng/mL), CXCL4 (30 ng/mL), CXCL4L1 (30 ng/mL), CXCL10 (50 ng/mL), or CXCL11 (20 ng/mL) were added to the lower wells. Results shown are the mean ± SD from 4 (A) or 3 (B) independent experiments. In panel A, statistically significant reduction of migration is indicated (*P < .05; Mann Whitney test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2009-11-253591/4/m_zh89991064380007.jpeg?Expires=1769112736&Signature=awfKewwOBlwPSJY7AK9EyPDvcKSHZtzplRh8-Yz2y-1ANKotCxvmDdSh9kt8YN123qzLCn~g7dme-hCsowzDAFvqy1TLPsH22CoHa7JsBjfu9MbnRZQ7krE1vDTJGC9rKwRDiTIo6A~Hsf-yPJw585VrZqC9C~3tLmkNBsOtYdC6xzgCtYn97WK1e0Ep2pYrRHhcOLfsso-C4RVrhM57XayHnw7zADNX5HC1xuXmmTb8tF2BR93WTIpgDUFvubNT1oZQnJLk37PZYxlH3hATV2TGSu4Lxs2h0AVtGRnAnhQFT-bGWqW-Pk1WN-SuUPGIE-lrP~ikeQqQ~TdeJjkHiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal