Abstract
Hepcidin is the master regulator of iron homeostasis. In the liver, iron-dependent hepcidin activation is regulated through Bmp6 and its membrane receptor hemojuvelin (Hjv), whereas, in response to iron deficiency, hepcidin repression seems to be controlled by a pathway involving the serine protease matriptase-2 (encoded by Tmprss6). To determine the relationship between Bmp6 and matriptase-2 pathways, Tmprss6−/− mice (characterized by increased hepcidin levels and anemia) and Bmp6−/− mice (exhibiting severe iron overload because of hepcidin deficiency) were intercrossed. We showed that loss of Bmp6 decreased hepcidin levels; increased hepatic iron; and, importantly, corrected hematologic abnormalities in Tmprss6−/− mice. This finding suggests that elevated hepcidin levels in patients with familial iron-refractory, iron-deficiency anemia are the result of excess signaling through the Bmp6/Hjv pathway.
Introduction
Iron supply in the body is provided both by iron recycling from senescent erythrocytes within the reticuloendothelial system and dietary iron absorption by duodenal enterocytes.1 The liver-iron regulatory hormone, hepcidin, controls these 2 iron-delivery pathways via its targeted degradation of the cell surface iron exporter, ferroportin. As a consequence, iron availability in the circulation is decreased, leading to hypoferremia.2,3 Neither the lack of hepcidin nor its excess can be compensated for by the body, the results of which ultimately manifest in either iron overload or iron-deficiency anemia, respectively.
Hepcidin gene expression is tightly regulated by body iron status and is dependent on bone morphogenetic protein 6 (Bmp6) and hemojuvelin (Hjv). Binding of the iron-regulated Bmp6 ligand4 to its receptors activates a signaling cascade leading to hepcidin transcription via phosphorylation of son of mother against decapentaplegic (Smad) 1/5/8 effectors.5 Hjv, a GPI-linked membrane protein synthesized by the hepatocytes, is a Bmp6 coreceptor.5 The critical role of the Bmp6/Hjv/Smad pathway in iron homeostasis is supported by the loss of hepcidin expression and massive parenchymal iron overload observed in Bmp6−/− and Hjv−/− mice as well as in mice with targeted liver deletion of Smad4.6-9
Recently, the serine protease matriptase-2 (also known as tmprss6) has been connected to this iron pathway10-12 because of its proteolysis of Hjv.13 Matriptase-2 is a type 2 serine protease that is predominately expressed in the liver (for review14 ). Matriptase-2–deficient mice10,12 have very high levels of hepcidin, which lead to the inhibition of dietary iron absorption and cause a severe iron-deficiency anemia phenotype. Matriptase-2 was thus characterized as a negative regulator of hepcidin gene expression. Accordingly, Du et al10 demonstrated that overexpression of normal matriptase-2 protein in hepatoma cells suppresses the activation of hepcidin expression. The anemic phenotype of matriptase-2–deficient mice is mirrored in patients with matriptase-2 mutations who present with iron-refractory, iron-deficiency anemia.11 Indeed, patients with iron-refractory, iron-deficiency anemia show inappropriately high hepcidin levels,11,14,15 which explain the lack of dietary iron absorption and partial response to parenteral iron treatment.16
The goal of this study was to characterize the in vivo relationship between matriptase-2 and the iron-regulated ligand of Hjv, Bmp6, by analyzing the role of Bmp6 in the setting of anemia in mice deficient for matriptase-2. Toward this purpose, we intercrossed matriptase-2 and Bmp6-deficient mice and compared the iron status of the double-mutant mice with that of wild-type controls or single-mutant mice.
Methods
Tmprss6tm1Otin mice on a mixed 129/Ola × C57BL/6 background12 were mated to Bmp6tm1Rob mice on an outbred CD1 background.6 F1 mice, heterozygous for both the Tmprss6tm1Otin (hereafter referred to as Tmprss6+/−) and the Bmp6tm1Rob alleles (referred to as Bmp6+/−) were then intercrossed and the F2 progeny genotyped as previously described.6,12 As expected, the 9 possible genotypic combinations were observed among the F2 mice.
Mice were cared for in accordance with the European convention for the protection of laboratory animals. Animals were given free access to tap water and standard laboratory mouse chow diet (AO3, iron content 280 mg/kg). Mice used in this study were 8- to 13-week-old females and had a mixed 129/Ola × C57BL/6 × CD1 background.
Hematologic parameters as well as plasma and liver iron were obtained as previously described.17
RNA extraction and real-time quantification of the hepcidin and β-actin transcripts were performed as reported by Chung et al.18 Standardized genetic nomenclature for mouse hepcidin is Hamp and Atcb for β-actin.
Student t tests were used to compare quantitative traits between mouse groups. P values less than .05 were considered as statistically significant.
Results and discussion
To investigate the role of Bmp6 in the pathogenesis of iron-refractory, iron-deficiency anemia, we intercrossed matriptase-2 and Bmp6-deficient mice and analyzed iron metabolism in their F2 progeny.
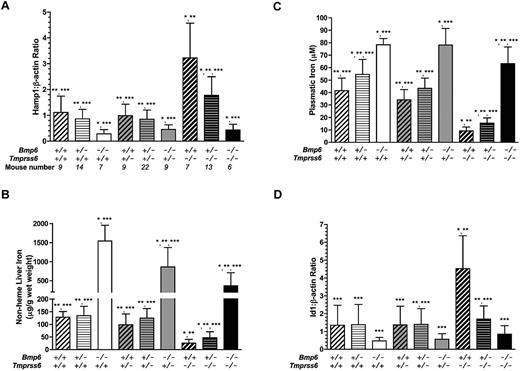
As recently published,6,7 liver hepcidin expression was repressed in Bmp6−/− mice compared with Bmp6+/+ controls (Figure 1A), leading to increased liver and plasma iron levels (Figure 1B-C). Id1, a marker of activation of the Bmp/Smad signaling pathway, had an expression pattern similar to that of hepcidin in these Bmp6−/− mice (Figure 1D). However, their expression of Tmprss6 was not significantly different from the wild-type controls (data not shown). Furthermore, as shown in Table 1, hematologic parameters of Bmp6−/− mice were similar to those of Bmp6+/+ controls.
Phenotypic analysis of female mice according to their Bmp6/Tmprss6 genotypes. The phenotypes analyzed include hepcidin mRNA expression relative to β-actin (A), nonheme iron concentration of liver (B), plasmatic iron (C), and Id1 mRNA expression relative to β-actin (D). Data are presented as mean ± SD. *P < .05 compared with Bmp6+/+Tmprss6+/+controls; **P < .05 compared with Bmp6−/−Tmprss6+/+ mice; ***P < .05 compared with Bmp6+/+Tmprss6−/− mice.
Phenotypic analysis of female mice according to their Bmp6/Tmprss6 genotypes. The phenotypes analyzed include hepcidin mRNA expression relative to β-actin (A), nonheme iron concentration of liver (B), plasmatic iron (C), and Id1 mRNA expression relative to β-actin (D). Data are presented as mean ± SD. *P < .05 compared with Bmp6+/+Tmprss6+/+controls; **P < .05 compared with Bmp6−/−Tmprss6+/+ mice; ***P < .05 compared with Bmp6+/+Tmprss6−/− mice.
Hematologic parameters of female mice according to their Bmp6/Tmprss6 genotype
| Genotype . | RBC (1012/L) . | Hb (g/dL) . | Hct (%) . | MCV (fL) . |
|---|---|---|---|---|
| Bmp6+/+Tmprss6+/+ | 9.77 ± 0.35‡ | 16.22 ± 0.63‡ | 48.12 ± 2.36‡ | 49.28 ± 1.86‡ |
| Bmp6+/−Tmprss6+/+ | 9.59 ± 0.49‡ | 16.01 ± 0.62‡ | 47.96 ± 2.46‡ | 50.04 ± 1.62‡ |
| Bmp6−/−Tmprss6+/+ | 9.52 ± 0.48‡ | 16.40 ± 0.66‡ | 48.61 ± 2.01‡ | 51.13 ± 2.12‡ |
| Bmp6+/+Tmprss6+/− | 9.54 ± 0.37‡ | 15.60 ± 0.45*†‡ | 46.27 ± 1.44†‡ | 48.52 ± 1.86†‡ |
| Bmp6+/−Tmprss6+/− | 9.53 ± 0.35‡ | 16.08 ± 0.59‡ | 47.67 ± 2.69‡ | 50.10 ± 2.26‡ |
| Bmp6−/−Tmprss6+/− | 9.45 ± 0.45‡ | 16.51 ± 0.72‡ | 48.98 ± 2.57‡ | 51.82 ± 1.62*‡ |
| Bmp6+/+Tmprss6−/− | 6.99 ± 1.1*† | 11.97 ± 1.44*† | 32.06 ± 4.86*† | 45.94 ± 1.02*† |
| Bmp6+/−Tmprss6−/− | 8.45 ± 0.63*†‡ | 13.48 ± 0.85*†‡ | 38.40 ± 2.54*†‡ | 45.47 ± 0.63*† |
| Bmp6−/−Tmprss6−/− | 9.28 ± 0.35*‡ | 15.92 ± 1.13‡ | 46.88 ± 4.08‡ | 50.42 ± 2.61‡ |
| Genotype . | RBC (1012/L) . | Hb (g/dL) . | Hct (%) . | MCV (fL) . |
|---|---|---|---|---|
| Bmp6+/+Tmprss6+/+ | 9.77 ± 0.35‡ | 16.22 ± 0.63‡ | 48.12 ± 2.36‡ | 49.28 ± 1.86‡ |
| Bmp6+/−Tmprss6+/+ | 9.59 ± 0.49‡ | 16.01 ± 0.62‡ | 47.96 ± 2.46‡ | 50.04 ± 1.62‡ |
| Bmp6−/−Tmprss6+/+ | 9.52 ± 0.48‡ | 16.40 ± 0.66‡ | 48.61 ± 2.01‡ | 51.13 ± 2.12‡ |
| Bmp6+/+Tmprss6+/− | 9.54 ± 0.37‡ | 15.60 ± 0.45*†‡ | 46.27 ± 1.44†‡ | 48.52 ± 1.86†‡ |
| Bmp6+/−Tmprss6+/− | 9.53 ± 0.35‡ | 16.08 ± 0.59‡ | 47.67 ± 2.69‡ | 50.10 ± 2.26‡ |
| Bmp6−/−Tmprss6+/− | 9.45 ± 0.45‡ | 16.51 ± 0.72‡ | 48.98 ± 2.57‡ | 51.82 ± 1.62*‡ |
| Bmp6+/+Tmprss6−/− | 6.99 ± 1.1*† | 11.97 ± 1.44*† | 32.06 ± 4.86*† | 45.94 ± 1.02*† |
| Bmp6+/−Tmprss6−/− | 8.45 ± 0.63*†‡ | 13.48 ± 0.85*†‡ | 38.40 ± 2.54*†‡ | 45.47 ± 0.63*† |
| Bmp6−/−Tmprss6−/− | 9.28 ± 0.35*‡ | 15.92 ± 1.13‡ | 46.88 ± 4.08‡ | 50.42 ± 2.61‡ |
Data are presented as mean ± SD.
RBC indicates red cell count; Hb, hemoglobin; Hct, hematocrit; and MCV, mean corpuscular volume.
P < .05 compared with Bmp6+/+Tmprss6+/+controls.
P < .05 compared with Bmp6−/−Tmprss6+/+ mice.
P < .05 compared with Bmp6+/+Tmprss6−/−mice.
Conversely, hepcidin and Id1 gene expressions were significantly up-regulated in Tmprss6−/− mice (Figure 1A-D), and as expected,12 these mice had reduced hepatic and plasma iron indices, compared with Tmprss6+/+ controls (Figure 1B-C). In addition, Tmprss6−/− mice were anemic and presented with significantly decreased hemoglobin levels, as well as red blood cell count, hematocrit, and mean corpuscular volume (Table 1). In addition, as previously reported,19 they exhibited lower Bmp6 gene expression than wild-type controls (data not shown).
It is interesting to note that, in double-mutant Bmp6−/−Tmprss6−/− mice, hepcidin expression was repressed to the same extent as in Bmp6−/− mice (Figure 1A). A similar pattern of expression was observed for Id1, although the comparison did not reach statistical significance because of a slightly higher variability of gene expression levels between mice for Id1 than for hepcidin (Figure 1D). In addition, phosphorylation of Smad1/5/8 appeared similarly decreased in Bmp6−/−Tmprss6+/+ mice and in Bmp6−/−Tmprss6−/− mice, compared with wild-type controls (data not shown), which is concordant with the similar reduction we observed in their levels of hepcidin expression. However, although liver and plasmatic iron levels were higher in Bmp6−/−Tmprss6−/− mice than in Bmp6+/+ mice, these levels remained significantly lower than in Bmp6−/− mice (Figure 1B-C). Lastly, and most importantly, iron-deficiency anemia observed in the Tmprss6−/− mice was completely rescued by Bmp6 deficiency. As shown in Table 1, values of hemoglobin, hematocrit, and mean corpuscular volume observed in Bmp6−/−Tmprss6−/− mice were comparable to control values. Furthermore, heterozygous loss of Bmp6 in Tmprss6−/− mice was able to partially correct systemic iron homeostasis by decreasing hepcidin gene expression and increasing plasma and liver iron levels (Figure 1).
Although hematologic parameters were found to be normal in mice deficient for both Hjv and matriptase-2,19,20 supporting the role of matriptase-2 as a regulator of Hjv expression at the hepatocyte membrane, the role of Bmp6 in this process was not clearly defined. The data obtained in this study indicate that hepcidin overexpression, which results from matriptase-2 inactivation, requires the presence of Bmp6. Indeed, neither the activation of the Hjv/Smad signaling pathway nor the establishment of the anemic phenotype was observed in double knockout mice.
However, in contrast to Hjv−/−Tmprss6−/− mice,19,20 which had a phenotype very similar to Hjv−/− mice, we found that loss of matriptase-2 in Bmp6−/−Tmprss6−/− mice attenuates the effects of Bmp6 deficiency on hepatic and plasma iron accumulation. It could be speculated that, because of the lack of matriptase-2, Hjv is stabilized at the hepatocyte plasma membrane and can serve as a coreceptor for ligands other than Bmp6. However, neither hepcidin nor Id1 gene expression were found to be up-regulated in the double-mutant female mice compared with Bmp6−/− mice. Alternatively, matriptase-2 could, in absence of Bmp6, regulate other iron-related proteins or initiate a signaling pathway involved in maintaining hepatic iron balance and/or systemic iron regulation, independently of hepcidin.
In conclusion, the present data further support that Bmp6 is the physiologic ligand of Hjv and demonstrate that the regulation of Hjv membrane expression by matriptase-2 serves to tightly control the signaling pathway induced by Bmp6.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of the hepcidin team in Cochin Institute and Hélène Coppin in U563 for fruitful discussions and critical reading of the manuscript.
This study was supported by funding from AFEF and EU contract (LSHM-CT-2006-037296).
Authorship
Contribution: A.L. and J.-C.D. designed and performed research and analyzed data; L.K. provided the Bmp6-deficient mice and performed research; A.J.R. generated the Tmprss6-deficient mice; M.-P.R. provided the Bmp6-deficient mice and contributed to the writing of the paper; C.L.-O. provided the Tmprss6-deficient mice and contributed to the writing of the paper; and S.V. and G.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gaël Nicolas or Sophie Vaulont, Département d'Endocrinologie, Metabolisme et Cancer, Institut Cochin, Faculté de Médecine Cochin-Port Royal, 24, rue du Fg St Jacques, 75014 Paris, France; e-mail: gael.nicolas@inserm.fr or sophie.vaulont@inserm.fr.
References
Author notes
A.L. and J.-C.D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal