Abstract
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-associated mortality in the United States and other countries. In most TRALI cases, human leukocyte antigen (HLA) class II antibodies are detected in implicated donors. However, the corresponding antigens are not present on the cellular key players in TRALI: neutrophils and endothelium. In this study, we identify monocytes as a primary target in HLA class II–induced TRALI. Monocytes become activated when incubated with matched HLA class II antibodies and are capable of activating neutrophils, which, in turn, can induce disturbance of an endothelial barrier. In an ex vivo rodent model, HLA class II antibody–dependent monocyte activation leads to severe pulmonary edema in a relevant period of time, whenever neutrophils are present and the endothelium is preactivated. Our data suggest that in most TRALI cases, monocytes are cellular key players, because HLA class II antibodies induce TRALI by a reaction cascade initiated by monocyte activation. Furthermore, our data support the previous assumption that TRALI pathogenesis follows a threshold model. Having identified the biologic mechanism of HLA class II antibody–induced TRALI, strategies to avoid plasma from immunized donors, such as women with a history of pregnancy, appear to be justified preventive measures.
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-associated death in the United States and other countries.1 It typically presents within 6 hours after transfusion as a clinical syndrome characterized by acute respiratory distress, hypoxemia, and a bilateral pulmonary edema on chest x-ray.2 The incidence of TRALI has been estimated as 1/5000 for all blood components, and current mortality rates are in the range of 5%-25%.3 All blood components have been implicated in TRALI, but those containing large amounts of plasma are mainly responsible.3-5 A recently published literature review states that in 80% of all TRALI cases, white blood cell antibodies can be identified in the implicated blood donor,6 and most implicated donors have been women with a history of pregnancy. Pregnancy results in alloimmunization against paternal white blood cell antigens in 21%-24% of women,7,8 with 3 main antibody specificities: human leukocyte antigen (HLA) class I, HLA class II, and human neutrophil antigens (HNAs).
The capability of antibodies recognizing either HNA9-11 or HLA class I12,13 to precipitate TRALI has been proved in different animal models. It is consensus opinion that neutrophil activation is the key mechanism by which antibodies to HNA and to HLA class I mediate TRALI,4,14 be it by direct binding to the cognate antigen on the surface of the neutrophil9-11,13 or, in the case of HLA class I antibodies, possibly by binding to HLA class I molecules on pulmonary endothelial cells, which leads to neutrophil trapping by the neutrophils' Fc receptors and subsequent neutrophil activation by receptor cross-link.12
Of note, it remains unclear how HLA class II antibodies can induce TRALI. HLA class II molecules are usually not expressed on neutrophils and endothelial cells. Epidemiologic studies, however, documented the clinical relevance of anti-HLA class II antibodies in TRALI. Kopko et al15 were the first to report an association between anti-HLA class II antibodies and TRALI in a small series of patients. A number of case reports were published subsequently,16-19 and plasma containing anti-HLA DR was shown to cause TRALI in a healthy male volunteer in an experimental setting.20 Currently available data from large series and published hemovigilance reports indicate that HLA class II antibodies matching the recipients' antigens are present in ∼ 50% of all TRALI cases and thus represent the most frequently detected matched antibodies in implicated donors.15,21-25
HLA class II molecules can be found on alveolar macrophages, which are unlikely to be reached by a transfused antibody before the breakdown of the blood-lung barrier and on some blood leukocytes, including monocytes. Kopko et al26 was the first to demonstrate that incubation of test monocytes with serum containing anti-HLA class II antibodies increases the percentage of cytokine-positive cells whenever the corresponding antigen was present. This finding was corroborated by a subsequent report on the release of leukotriene B4 (LTB4) from antigen-positive monocytes being incubated with a serum containing HLA class II antibodies,27 indicating that monocyte activation could indeed represent the initial step in HLA class II antibody–induced TRALI. We performed the following study to unravel the potential mechanism of HLA class II antibody–induced TRALI.
Methods
Plasma samples
Immunized female donors were identified by HLA class I and HLA class II screening with the use of commercially available enzyme immune assays (AbScreen; Biotest). Identification of the HLA class II antibody specificity was performed by enzyme immunoassay as well (AbIdent; Biotest). Plasma from 3 donors, 2 with anti-DR7 and 1 with anti-DR52, was used for further experiments. These plasma samples were investigated for the presence of HNA antibodies by 2 different laboratories; all samples were found to be free of HLA class I and HNA antibodies. Plasma was recovered from whole blood donations by standard blood banking procedures according to Good Manufacturing Practice guidelines and stored at −30°C. Before use, plasma was heat inactivated (56°C, 30 minutes). For immunoglobulin G (IgG) depletion, plasma samples were incubated in the presence of protein G–conjugated sepharose B (Pharmacia Biotech) at room temperature overnight and then centrifuged. Supernatant was used as IgG-depleted plasma. IgG levels were quantitated by turbidimetric standard methods (Siemens Healthcare Diagnostics). All study procedures involving humans were approved by the ethics committee of the Medical Faculty of the University of Giessen, Germany, and informed consent was obtained from all subjects who provided plasma and leukocytes in accordance with the Declaration of Helsinki.
Neutrophils and monocytes
Voluntary blood donors were genotyped for HLA class I and HLA class II antigens. Suitable (matched or unmatched) donors were selected for the preparation of neutrophils and monocytes. After dextran sedimentation, neutrophils were isolated from supernatant white blood cell–rich plasma by Ficoll-Hypaque gradient centrifugation followed by hypotonic red cell lysis. Monocytes were obtained from ethylenediaminetetraacetic acid–anticoagulated whole blood by Ficoll-Hypaque gradient centrifugation and subsequent automated magnetically activated cell sorting separation (Miltenyi Biotec), applying CD14 immunomagnetic beads according to the manufacturer's instructions. Purity of cell preparations was > 97% as determined by flow cytometry.
Monocyte cross-match by flow cytometry
Purified monocytes were fixed with 1% paraformaldehyde (pH 7.2) for 10 minutes and washed twice in phosphate-buffered saline; 1 × 107 cells were resuspended in 60 μL of phosphate-buffered saline buffer containing 0.5% bovine serum albumin (Serva) and 2mM ethylenediamine tetraacetate (Sigma-Aldrich). To block the monocytes' Fc γ receptors, 20 μL of FcR blocking reagent (Miltenyi Biotec) were added before 100 μL of monocyte suspensions (containing 1 × 106 cells) were incubated with 20 μL of human plasma for 30 minutes at 37°C. After extensive washing, 40 μL of fluorescein isothiocyanate (FITC)–conjugated rabbit anti–human IgG or IgM monoclonal antibody (Dako; 1:50 dilution) were added, and, after 30 minutes of incubation, cells were again washed extensively before they were analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Monocyte cultures and preparation of supernatants
Purified monocytes from HLA class II–genotyped donors were suspended in RPMI-1640 medium (PAA Laboratories) at a cell density of 1.5 × 106/mL, supplemented with human plasma (20%) either containing matched or nonmatched HLA class II antibodies. After incubation at 37°C for 6 hours at 5% CO2, cultures were centrifuged, and supernatant was stored at −70°C until use. Negative controls were incubated with plasma from a healthy male volunteer, and positive controls with 1 μg/mL lipopolysaccharide (LPS) dissolved in negative control plasma.
Detection of cytokines and LTB4
The concentration of interleukin-8 (IL-8), growth-related oncogene-α (GRO-α), tumor necrosis factor-α (TNF-α), and LTB4 in culture supernatant was determined by commercially available enzyme immunoassay (BD Biosciences) according to the manufacturer's instructions.
Production of reactive oxygen species in activated neutrophils
Purified neutrophils (5 × 105) were incubated with 30 μL of supernatant or control serum for 5 minutes in a total volume of 50 μL (neutrophil priming). N-formyl-MetLeuPhe (fMLP; final concentration 1μM; Sigma-Aldrich) was added; after 10 minutes of incubation, oxidative burst activity was measured by flow cytometry with the use of the BurstTest (Orpegen Pharma) according to the manufacturer's instructions. Monoclonal antibody 7D8 specific for CD177 served as positive control. Oxidative burst activity of isolated neutrophils was controlled with either phorbol myristate acetate, opsonized Escherichia coli, or no stimulus (data not shown). In some experiments, diphenyleneiodonium chloride (DPI; final concentration 50μM) was used to block nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. All experiments were performed in triplicate.
Permeability assays
The permeability of human pulmonary microvascular endothelial cells (Cambrex Bioscience) was assessed by the passage of FITC albumin (relative molecular mass, 56 000; Sigma-Aldrich) as previously described.28 Briefly, 3 × 105 human pulmonary microvascular endothelial cells were plated onto 48 Transwells fibronectin-coated 3-μm pore size polycarbonate membrane inserts (Costar) and left for 2 days to form confluent monolayers. Confluency of cells was monitored by microscopy. Aliquots of 200 μL of RPMI media (Gibco) containing FITC albumin (40 μg/mL) were applied in the upper chamber. Neutrophils (1.5 × 104), fMLP (final concentration, 10−8M), and supernatant from monocytes cultures (100 μL) were added. In some experiments, DPI (final concentration, 50μM) was used to block NADPH oxidase activity. At indicated time points, a sample from the bottom chamber was read in triplicate in a fluorescent microtiter plate reader (BioTek). After incubation with 200 μL of RPMI media containing 0.2 U/mL thrombin (Siemens Healthcare Diagnostics) at 37°C for 15 minutes, the passage of FITC albumin was measured as described. All data are from ≥ 3 independent experiments.
Ex vivo rat lung model
The rat lung model has been described elsewhere.29 In brief, adult male CD rats (∼ 350 g) purchased from Charles River, raised on a regular diet, were weighed and anesthetized with sodium pentobarbital (80 mg/kg intraperitoneally) and injected with heparin intraperitoneally (1000 IU/kg). Lungs were explanted while being perfused with Krebs Henseleit buffer (120mM NaCl, 4.3mM KCl, 1.1mM KH2PO4, 2.4mM CaCl2, 1.3mM MgCl2, and 13.32mM glucose as well as 5% [wt/vol] hydroxyethylamylopectin [molecular weight, 200 000], flow rate 4.0 mL/min, 6°C) by cannulas in the pulmonary artery and the left ventricle. After explantation of the lung the entire system was heated to 37°C in parallel with an increase of the flow rate to 13 mL/min. NaHCO3 was adjusted to result in a pH of 7.37-7.40. After flushing the lungs free of blood, the perfusion system was closed for recirculation (total system volume, 140 mL; left ventricular pressure, 2 cm H2O). In some experiments, rats were injected intraperitoneally with LPS (Sigma-Aldrich), 2 mg/kg body weight, 2 hours before lung explantation. Isolated rat lungs were ventilated with a gas mixture of 5.3% CO2, 21.0% O2, and 73.7% N2 with a tidal volume of 4 mL at a frequency of 60 breaths/min. A positive end-expiratory pressure of 1.5 cm H2O was chosen. The capillary filtration coefficient (Kfc) was quantified to assess changes in capillary permeability. Kfc was determined gravimetrically from the slope of the lung weight–gain curve induced by a venous pressure elevation of 10 cm H2O for 8 minutes as described previously.30 After an isogravimetric steady-state period of 30 minutes, a first Kfc measurement maneuver was performed (t = 0). In the respective experiments 15 mL of the buffer fluid was replaced by the supernatant from monocyte cultures or heat-inactivated (56°C, 30 minutes) human plasma after the first Kfc measurement. Neutrophils (1 × 108) and, in some experiments, monocytes (1 × 107) were added directly afterward into the pulmonary artery. Controls were treated similarly with application of the solvent. Subsequent repetitive Kfc measurements were performed every 35 minutes. Experiments were terminated after 140 minutes or earlier (if severe lung edema was induced). All experiments were performed in accordance with German animal protection legislation. Statistics were calculated by applying the Mann-Whitney U test with SPSS Version 9.0 (SPSS Software).
Depletion of rat neutrophils
Depletion of rat neutrophils was performed as described previously.13 In brief, rats were injected with 250 μL of rabbit–antirat PMN (Accurate Chemical & Scientific Corporation) intravenously and returned to their cages for 24 hours. Before these rats were used for the ex vivo protocol as described in “Ex vivo rat lung model,” a manual leukocyte differential was performed on a May-Grünwald-Giemsa (Merck) stained blood smear to ensure neutrophil depletion.
Lung histology
After the final venous pressure elevation, rat lungs were instilled with formaldehyde solution (4.5%, pH 7.2) through the trachea. Fixation was allowed to proceed overnight at room temperature. Subsequently, tissue samples were embedded in paraffin, and sections of 5 μm were stained with hematoxylin-eosin and antimyeloperoxidase. Pictures are representative for experiments and controls (antimyeloperoxidase; APAAP). A Zeiss Axioskop 40 (Carl Zeiss) with Plan-NEO Fluar 5×/0.5 NA objective lenses, in connection with a JVC KY-S75U digital camera (JVC) was used to acquire the micrographs. Images were processed with Diskus acquisition software, Version 4.50 (Hilgers).
Results
Human plasma containing HLA class II antibodies leads to specific monocyte activation
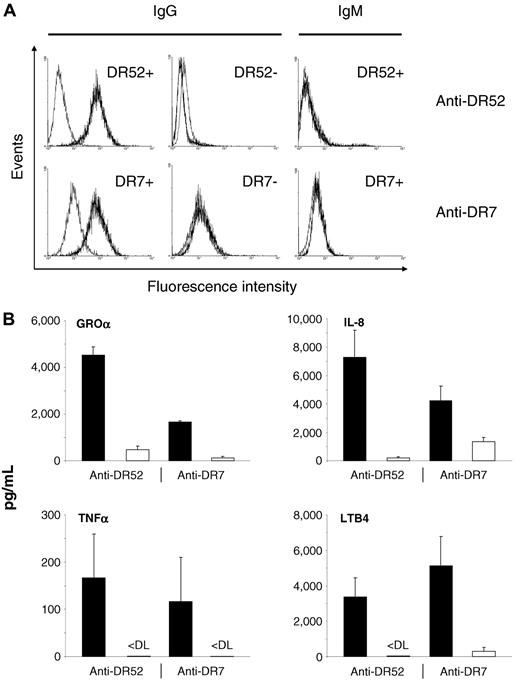
In a first set of experiments, we could demonstrate that HLA class II antibodies (anti-DR7 or anti-DR52) present in human plasma bind specifically to allogeneic human monocytes bearing cognate antigens (HLA-DR7 or HLA-DR52), whereas monocytes lacking these antigens are not recognized (Figure 1A). Subsequently, supernatants obtained after 6 hours of incubation were tested for the presence of different cytokines and LTB4. As shown in Figure 1B, GROα, IL-8, TNF-α, and LTB4 production by monocytes was significantly increased whenever the antibody specificity present in plasma matched the HLA class II antigens expressed on the cells compared with nonmatched controls. These findings indicate that HLA class II antibodies can bind to their cognate antigen(s) on the surface of monocytes and lead to monocyte activation.
HLA class II antibodies bind to and induce activation of matched monocytes. (A) Anti-DR52 (top) and anti-DR7 (bottom) were incubated with matched or unmatched monocytes as indicated. FITC-labeled anti-IgG or -IgM was used as a secondary antibody (black histograms). Gray histograms indicate results obtained with control plasma. (B) Matched (black bars) and unmatched (white bars) human monocytes were incubated with human anti-DR52 or anti-DR7 as indicated. Supernatants were investigated for the presence of cytokines and LTB4 after 6 hours, as indicated. P < .01 between matched and unmatched experiments for all groups. < DL indicates below detection limit. Concentrations are given as mean values + SDs.
HLA class II antibodies bind to and induce activation of matched monocytes. (A) Anti-DR52 (top) and anti-DR7 (bottom) were incubated with matched or unmatched monocytes as indicated. FITC-labeled anti-IgG or -IgM was used as a secondary antibody (black histograms). Gray histograms indicate results obtained with control plasma. (B) Matched (black bars) and unmatched (white bars) human monocytes were incubated with human anti-DR52 or anti-DR7 as indicated. Supernatants were investigated for the presence of cytokines and LTB4 after 6 hours, as indicated. P < .01 between matched and unmatched experiments for all groups. < DL indicates below detection limit. Concentrations are given as mean values + SDs.
Matched monocyte supernatants can activate neutrophils
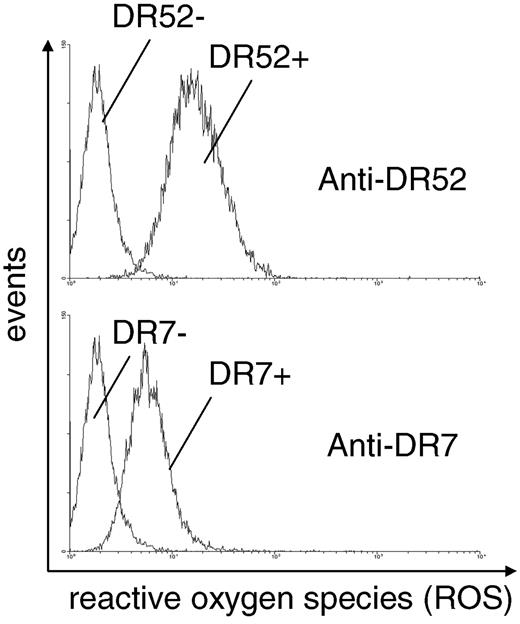
Supernatants from monocyte cultures were used to investigate whether soluble substances released by activated monocytes are able to stimulate neutrophils. Production of reactive oxygen species (ROS) was used to monitor neutrophil activation. Neutrophils were thus incubated with supernatants, and ROS production was measured after the addition of fMLP by a flow cytometric assay. In this assay, the amount of ROS produced is correlated with the production of rhodamine from dihydro rhodamine, which can be measured in flow cytometry (Figure 2). Incubation of neutrophils with supernatants obtained from matched monocytes induced a significantly higher production of ROS in neutrophils, as reflected in a higher mean fluorescence intensity than did incubation of neutrophils with supernatants obtained from nonmatched monocytes. Preincubation of neutrophils with DPI led to a 71%-98% reduction in ROS production, indicating that radicals may be produced by NAD(P)H-like oxidase systems of the neutrophil rather than by other oxidases (not shown).
Supernatant from matched, but not from unmatched, monocytes primes neutrophils for fMLP-induced oxidative burst. Human neutrophil suspensions were incubated with different supernatants from monocyte cultures preincubated with human HLA class II antibodies (matched or unmatched). After addition of fMLP, the oxidative burst activity of neutrophils was measured by flow cytometry. Pictures are representative for n = 7 separate experiments.
Supernatant from matched, but not from unmatched, monocytes primes neutrophils for fMLP-induced oxidative burst. Human neutrophil suspensions were incubated with different supernatants from monocyte cultures preincubated with human HLA class II antibodies (matched or unmatched). After addition of fMLP, the oxidative burst activity of neutrophils was measured by flow cytometry. Pictures are representative for n = 7 separate experiments.
Neutrophils activated by matched monocyte supernatant disturb endothelial permeability
It is known that ROSs are able to disturb the endothelial barrier by interfering with cell-cell contacts.31 We seek to investigate this phenomenon by studying the flux of FITC-labeled albumin through endothelial barriers in the presence of predefined stimuli. As shown in Figure 3, neutrophils and supernatant from matched monocytes changed the flux profile of FITC-albumin. After 30 and 60 minutes of time, a significantly larger amount of albumin has passed the endothelial barrier compared with the flux obtained in the presence of neutrophils treated with nonmatched monocyte supernatant. In the presence of the flavoprotein and thus NADPH oxidase inhibitor DPI, the amount of penetrated albumin through endothelial barrier at time points 30 and 60 minutes was no longer increased. The flux profile was comparable to the one obtained with nonmatched supernatants. No effects were observed when neutrophils were not added.
Neutrophils activated by monocyte supernatants disturb the barrier function of a human pulmonary microvascular endothelial cell monolayer. Human pulmonary microvascular endothelial cells were grown to confluence on a Transwell filter system. FITC-labeled albumin, neutrophils, and supernatant were added to the upper chamber. Presence of FITC-albumin in the bottom chamber was read at the indicated time points. Experiments were performed with monocyte supernatants obtained after incubation with matched or unmatched HLA class II antibodies as indicated (continuous lines). Thrombin was used as a positive control. Experiments were repeated in the presence of DPI to block NADPH oxidase activity (dashed lines). P < .01 for matched versus unmatched supernatant at 30 minutes and 60 minutes in the absence, but not in the presence, of DPI; n = 5 for each experiment. Results are given as mean values ± SDs.
Neutrophils activated by monocyte supernatants disturb the barrier function of a human pulmonary microvascular endothelial cell monolayer. Human pulmonary microvascular endothelial cells were grown to confluence on a Transwell filter system. FITC-labeled albumin, neutrophils, and supernatant were added to the upper chamber. Presence of FITC-albumin in the bottom chamber was read at the indicated time points. Experiments were performed with monocyte supernatants obtained after incubation with matched or unmatched HLA class II antibodies as indicated (continuous lines). Thrombin was used as a positive control. Experiments were repeated in the presence of DPI to block NADPH oxidase activity (dashed lines). P < .01 for matched versus unmatched supernatant at 30 minutes and 60 minutes in the absence, but not in the presence, of DPI; n = 5 for each experiment. Results are given as mean values ± SDs.
These data indicate that HLA class II antibodies may induce the production of stimuli in monocytes, which then cause neutrophil activation, ROS production, and, finally, disturbance of the endothelial barrier. This amplification cascade may explain the rapid development of lung edema in patients with TRALI. This hypothesis was then further investigated in an ex vivo rat lung model.
Matched monocyte supernatant causes TRALI in ex vivo perfused and ventilated rat lungs only after endothelial preactivation
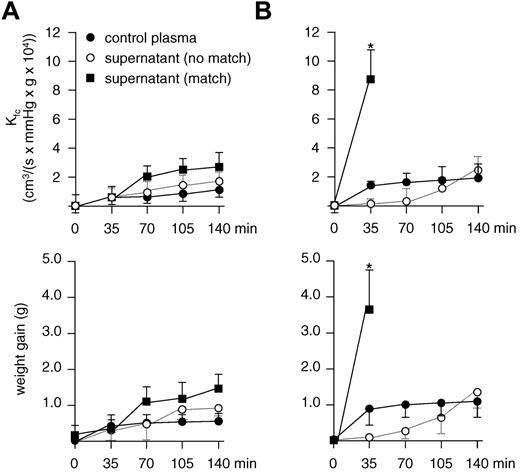
In a first set of experiments, isolated rat lungs were perfused with human neutrophils, and either supernatant from nonmatched monocytes or control plasma derived from a male donor was used as a control for further experiments (Figure 4A). Rat lungs were then perfused with human neutrophils and supernatant from matched monocytes. To our surprise, repetitive determination of the capillary filtration coefficient as well as continuous monitoring of the lung weight did not show a significant increase in endothelial permeability compared with baseline (Figure 4A open circles). Because neutrophil activation is a multistep process, we next investigated whether priming of neutrophils by the addition of fMLP would be sufficient to allow TRALI to precipitate in the presence of matched supernatant and human neutrophils. A tendency toward a slight weight gain and increase in the capillary filtration coefficient could be observed after 140 minutes, but this was not statistically significant (P > .05 for both parameters). In a second set of experiments, we decided to inject rats with LPS intraperitoneally 2 hours before the isolated lung perfusion to allow activation of the lung endothelium. When these lungs were perfused with human neutrophils and matched supernatant, a rapid increase in the capillary filtration coefficient and the lung weight occurred already within 35 minutes, leading to severe edema, precluding continuation of the experiment (Figure 4B). In the presence of human neutrophils and control plasma and in the presence of human neutrophils and nonmatched supernatant, no significant biophysical changes were observed. These data indicate that the occurrence of lung edema was specific for endothelial preactivation, human neutrophils, and matched supernatant.
HLA class II antibody induced TRALI in an ex vivo rat lung model. Isolated rat lungs are perfused with human neutrophils, and supernatant from human monocyte cultures were incubated with matched or unmatched human HLA class II antibodies or human control plasma as indicated. (A) No significant increase in the capillary filtration coefficient (Kfc) or lung weight occurred in ventilated and perfused rat lungs in the absence of LPS prestimulation (P > .05 for matched vs unmatched supernatant at t = 140 minutes for both parameters comparing matched and nonmatched supernatant). (B) After prestimulation of rats with LPS 2 hours before ex vivo lung perfusion, a significant increase in Kfc and lung weight is observed after 35 minutes in the presence of matched monocyte supernatant (P < .01); n = 7 for each experiment. Results are given as mean values ± SDs.
HLA class II antibody induced TRALI in an ex vivo rat lung model. Isolated rat lungs are perfused with human neutrophils, and supernatant from human monocyte cultures were incubated with matched or unmatched human HLA class II antibodies or human control plasma as indicated. (A) No significant increase in the capillary filtration coefficient (Kfc) or lung weight occurred in ventilated and perfused rat lungs in the absence of LPS prestimulation (P > .05 for matched vs unmatched supernatant at t = 140 minutes for both parameters comparing matched and nonmatched supernatant). (B) After prestimulation of rats with LPS 2 hours before ex vivo lung perfusion, a significant increase in Kfc and lung weight is observed after 35 minutes in the presence of matched monocyte supernatant (P < .01); n = 7 for each experiment. Results are given as mean values ± SDs.
HLA class II–dependent TRALI can be mimicked ex vivo
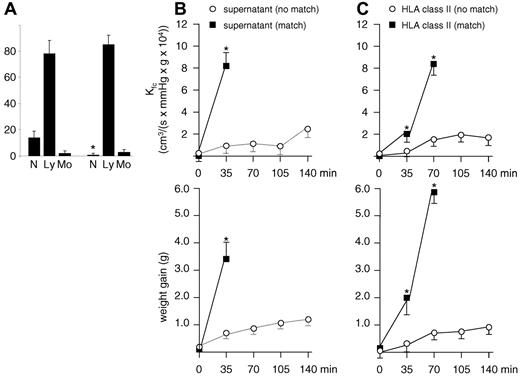
Next, we mimicked the physiologic setting as it occurs in patients, with the addition of human neutrophils, human monocytes, and donor plasma to the ex vivo model (Figure 5A). Rats were prestimulated with LPS as in the previous experiments. If the plasma contained HLA class II antibodies that did not match with the circulating monocytes, no significant increase in endothelial permeability and lung weight was observed (Figure 5A open circles) compared with control plasma (Figure 5A closed circles). In contrast, if there was a match between antibodies present in plasma and the antigen expressed on the monocytes, a significant increase in the capillary filtration coefficient was observed already after 35 minutes, leading to severe edema within 70 minutes (Figure 5A squares).
HLA class II antibodies induce TRALI in an ex vivo rat lung model in the presence of human neutrophils and matched human monocytes. (A) In the presence of neutrophils, HLA class II antibodies and monocytes that do not bear cognate antigen(s), a minor increase in the capillary filtration coefficient (Kfc) and lung weight is observed (○), but this increase is not different from results obtained with control plasma (●). In contrast, the presence of the same antibodies, neutrophils, but matched monocytes, leads to a significant increase in Kfc and lung weight after 35 minutes and severe edema after 70 minutes (■; P < .01). (B) The effect obtained with plasma, matched monocytes, and neutrophils can be abolished if human monocytes (▵) or human monocytes (▴) are not added to the circulation; n = 3 for each experiment. Results are given as mean values ± SD. (C) Clustering of leukocytes (red) in pulmonary capillaries after the addition of monocyte supernatant or plasma containing HLA class II antibodies to the perfusion circuit (hematoxylin and eosin and antimyeloperoxidase staining). If rat lungs were perfused with human neutrophils and supernatant from monocyte cultures incubated with plasma containing either unmatched (i) or matched (ii) HLA class II antibodies, leukocyte clustering is only observed after the addition of matched supernatant (ii). If rat lungs were perfused with human neutrophils, monocytes, and human plasma containing HLA class II antibodies that either were unmatched (iii) or matched (iv) to the circulating monocytes, clustering of leukocytes is only observed in the presence of matched HLA class II antibodies (iv).
HLA class II antibodies induce TRALI in an ex vivo rat lung model in the presence of human neutrophils and matched human monocytes. (A) In the presence of neutrophils, HLA class II antibodies and monocytes that do not bear cognate antigen(s), a minor increase in the capillary filtration coefficient (Kfc) and lung weight is observed (○), but this increase is not different from results obtained with control plasma (●). In contrast, the presence of the same antibodies, neutrophils, but matched monocytes, leads to a significant increase in Kfc and lung weight after 35 minutes and severe edema after 70 minutes (■; P < .01). (B) The effect obtained with plasma, matched monocytes, and neutrophils can be abolished if human monocytes (▵) or human monocytes (▴) are not added to the circulation; n = 3 for each experiment. Results are given as mean values ± SD. (C) Clustering of leukocytes (red) in pulmonary capillaries after the addition of monocyte supernatant or plasma containing HLA class II antibodies to the perfusion circuit (hematoxylin and eosin and antimyeloperoxidase staining). If rat lungs were perfused with human neutrophils and supernatant from monocyte cultures incubated with plasma containing either unmatched (i) or matched (ii) HLA class II antibodies, leukocyte clustering is only observed after the addition of matched supernatant (ii). If rat lungs were perfused with human neutrophils, monocytes, and human plasma containing HLA class II antibodies that either were unmatched (iii) or matched (iv) to the circulating monocytes, clustering of leukocytes is only observed in the presence of matched HLA class II antibodies (iv).
Interestingly, when we repeated this experiment with blood cells from the same donors, but after depletion of IgG antibodies by protein G–coupled sepharose from the plasma, TRALI could no longer be reproduced in our ex vivo model (data not shown). In the absence of either neutrophils or monocytes, no increase in the capillary filtration coefficient and the lung weight occurred (Figure 5B). Representative lung sections are given in Figure 5C.
HLA class II–induced TRALI in the ex vivo rat lung model is independent from the presence of rat neutrophils
LPS prestimulation supports adherence of rat neutrophils to the pulmonary vasculature which could possibly interfere with the biologic mechanism presented here. Rats were thus depleted from their neutrophils, and experiments were repeated as summarized in Figure 6. The neutrophil depletion protocol proved to be successful (Figure 6A). With the use of lungs obtained from these rats, TRALI could be mimicked in our model both in the presence of human neutrophils and supernatant obtained from matched monocytes (Figure 6B) and in the presence of human neutrophils, human monocytes, and plasma containing HLA class II antibodies (Figure 6C). Time course and magnitude of these reactions as well as those from negative controls were unaltered compared with those obtained with lungs from nondepleted rats (Figures 4–5).
Reproduction of TRALI in an ex vivo rat lung model after depletion of rat neutrophils. To exclude a possible contribution of rat neutrophils adherent to the pulmonary vasculature, rats were depleted from neutrophils 24 hours before LPS injection and subsequent lung explantation. (A) Differential blood counts obtained before (left) and after (right) neutrophil depletion; N indicates neutrophils; Ly, lymphocytes; Mo, monocytes. Results are given as mean values ± SDs. P < .01 for neutrophils before and after depletion (n = 6). (B) As in previous experiments (Figure 4B), lungs were perfused with human neutrophils and supernatant from human monocyte cultures incubated with matched or unmatched human HLA class II antibodies. A significant increase in endothelial permeability (Kfc) and lung weight is observed after 35 minutes in the presence of matched monocyte supernatant (P < .01; n = 3). (C) As in previous experiments (Figure 5A), lungs were perfused with human neutrophils, HLA class II antibodies, and monocytes that bear or do not bear the cognate HLA class II antigen. A significant increase in Kfc and lung weight after 35 minutes and severe edema after 70 minutes is observed in the presence of monocytes that bear the cognate antigen (P < .01; n = 3).
Reproduction of TRALI in an ex vivo rat lung model after depletion of rat neutrophils. To exclude a possible contribution of rat neutrophils adherent to the pulmonary vasculature, rats were depleted from neutrophils 24 hours before LPS injection and subsequent lung explantation. (A) Differential blood counts obtained before (left) and after (right) neutrophil depletion; N indicates neutrophils; Ly, lymphocytes; Mo, monocytes. Results are given as mean values ± SDs. P < .01 for neutrophils before and after depletion (n = 6). (B) As in previous experiments (Figure 4B), lungs were perfused with human neutrophils and supernatant from human monocyte cultures incubated with matched or unmatched human HLA class II antibodies. A significant increase in endothelial permeability (Kfc) and lung weight is observed after 35 minutes in the presence of matched monocyte supernatant (P < .01; n = 3). (C) As in previous experiments (Figure 5A), lungs were perfused with human neutrophils, HLA class II antibodies, and monocytes that bear or do not bear the cognate HLA class II antigen. A significant increase in Kfc and lung weight after 35 minutes and severe edema after 70 minutes is observed in the presence of monocytes that bear the cognate antigen (P < .01; n = 3).
Discussion
The main findings of our study are that (1) HLA class II antibodies can activate monocytes bearing cognate antigens; (2) effector substances released by these monocytes will result in neutrophil activation, which leads to increased lung vascular permeability in vitro and in an ex vivo rat lung model; and (3) the transfusion of HLA class II antibodies will cause severe lung injury in an ex vivo model in a clinically relevant time period if both monocytes and neutrophils are present.
HLA class I and HNA antibodies identified in TRALI can bind antigens present on the surface of neutrophils. It is generally thought, although unproven, that either receptor cross-linking or direct activation of the receptor may lead to signaling events that cause neutrophil activation and, subsequent, deterioration of the lung vascular integrity.10,13,32,33 This mechanism is not likely in the case of HLA class II antibodies, because HLA class II antigens are normally not present on neutrophils (see below). Here, we observed, however, that HLA class II antibodies can bind to monocytes that express the cognate antigen and thereby induces the release of potent cytokines and LTB4. This finding is in line with previous reports showing significant increase of intracellular cytokine expression and a release of LTB4, when monocytes were treated with HLA class II antibodies.26,27
We could further show that the effector substances released by monocytes after priming with HLA class II antibodies are able to prime neutrophils for fMLP-induced oxidative burst. Only in the presence of neutrophils, monocyte supernatants induced increased vascular permeability in vitro. This effect was abrogated in the presence of DPI, a flavoprotein, and thus NADPH oxidase inhibitor, which is capable of blocking ROS production. ROS production has been suspected to play a pivotal role in disturbing the endothelial barrier in TRALI,10 and there is evidence that oxidative stress increases vascular endothelial permeability in several experimental settings. In vitro, ROS increased transendothelial permeability in cultured endothelial cell monolayers and the transendothelial flux of tracer albumin; the duration and severity of the increase corresponded positively with increased ROS generation.31,34 The perfusion of ROS or xanthine/xanthine oxidase (which generates O2−) into isolated lungs of guinea pigs35 and rabbits36,37 resulted in pulmonary edema. Here, we demonstrate in an ex vivo model that monocyte supernatants can precipitate TRALI in the presence, but not in the absence, of neutrophils, indicating that effector substances released from antibody-stimulated monocytes are potent stimulators of neutrophils.
Strong candidates for these effects are IL-8 and GRO-α, because they have been shown to prime human neutrophils for ROS production38,39 and to induce the release of secondary granules from neutrophils.40 LTB4 is also a main product of activated monocytes and can both recruit and prime neutrophils efficiently.41 All 3 substances were identified in high concentrations in monocyte supernatants after incubation with matched HLA class II antibodies, and it is probable that these substances are responsible for the subsequent activation of neutrophils in HLA class II–dependent TRALI. Although TNF-α was also elevated, concentrations were substantially less than for the other 3 substances. We believe that because our experiments are performed in a platelet-free environment might be one reason for this observation, because P-selectin, which can be found on the surface of activated platelets, has been identified as a relevant inducer of TNF-α release from monocytes.42 A recent publication assigns platelets a relevant role in TRALI induced by monoclonal antibodies against MHC I in a murine model.43 It is conceivable that further monocyte activation by platelets might trigger TRALI under certain conditions in vivo.
Of note, when mimicking the TRALI reaction ex vivo by adding human plasma together with monocytes and neutrophils, we observed a lag phase that was not seen if supernatant was added to the model. It is possible that this lag phase is because TRALI does not occur until a critical concentration of monocyte-derived effectors required for neutrophil activation has accumulated. Activated neutrophils are indispensible for the TRALI reaction because they are responsible for disturbing the endothelial barrier function.
Our experiments do not exclude the possibility that TRALI induced by HLA class II antibodies may occur because of direct binding of HLA class II–positive neutrophils. There are partly contradictory reports to indicate that HLA class II expression on neutrophils can be induced in vitro by several agents (including interferon-γ, IL-3, and granulocyte-macrophage colony-stimulating factor [GM-CSF]). In addition, GM-CSF (but not G-CSF) or interferon-γ administration was also shown to induce HLA class II expression in patients,44,45 but this appears to be true for a small window of time only. Patients with Wegener granulomatosis may express HLA class II on their neutrophils,46 and the endothelium may also be activated to express HLA class II under certain conditions.47 Thus, in rare cases, some patients experiencing TRALI may have HLA class II expression on either cell type. To our knowledge, the presence of HLA class II antigens on neutrophils has not been studied systematically in patients with TRALI. There are, however, a few reported observations that are supporting a theory of indirect neutrophil activation by monocytes. First, in one autopsy case that was investigated for the presence of HLA-DR on the alveolar capillary endothelium and the neutrophils, no staining for HLA class II was seen.48 Second, in an in vivo experiment, HLA class II antibodies caused TRALI in a healthy male volunteer.20 Finally, it has been reported that monocytopenia occurs in patients with TRALI because of HLA class II antibodies.20,21
In contrast to previous observations with antineutrophil antibodies,9,10 where the antibody itself was capable of precipitating TRALI in an ex vivo rodent lung model without any additional stimuli, we were unable to induce TRALI with HLA class II antibodies without preactivation of the rat endothelium by LPS. It can be assumed that cellular costimuli from the endothelium, induced by endothelial preactivation, play a relevant role.
Because only 2 polyclonal anti-HLA class II antisera were used, it is unclear whether this represents a general phenomenon of HLA class II antibodies or whether the capability of inducing TRALI without additional stimuli depends on the binding to specific epitope(s) of the target antigen, which were probably not recognized by our antisera. The latter aspect cannot be excluded because a healthy volunteer developed TRALI after infusion of HLA class II antibodies.20 Plasma that has been used in our experiments were from females with a history of pregnancy, but none of these donors was involved in a TRALI case. It is thus not clear whether they are representative of TRALI. Another possible weakness of our experiments is the use of human cells in a rat lung model which might involve variables we could not control for. Although we believe that we could demonstrate convincingly that endothelial preactivation, human neutrophils, human monocytes, and plasma are required to precipitate TRALI in this model and that rat neutrophils do not participate in the observed reaction, results need to be interpreted with caution. The same, however, holds true for experiments were monoclonal antibodies are used instead of plasma.12,13,43
As we have recently suggested, the development of TRALI may follow a threshold model.4 Several factors define that threshold, including underlying individual (eg, genetic) and medical conditions (eg, infections). Depending on the heights of the threshold and the strength of the transfusion-related trigger (eg, antibody), TRALI will or will not occur. Although purely speculative, it is possible that the indirect activation of neutrophils by monocyte stimulation is a less strong transfusion-related trigger than direct neutrophil activation by antibody binding. If this holds true, the threshold for HLA class II antibody–induced TRALI needs to be lowered, which can be achieved by activation of the vascular endothelium.49
In conclusion, our study indicates that antibodies against HLA class II antigens are capable of inducing TRALI by monocyte activation and subsequent activation of neutrophils. Data obtained here support our previous assumption on a threshold model of TRALI and underline the importance of screening for HLA class II antibodies in suspected TRALI cases, supporting current strategies to avoid plasma from female blood donors with a history of pregnancy to reduce the number of TRALI cases.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part at the 52nd annual meeting of the American Society of Hematology, New Orleans, LA, December 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stephanie Gembardt, Christine Hofmann, Ingrid Wallon, and Silke Werth for their excellent assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Excellence Cluster–Cardiopulmonary System; U.J.H.S. and N.W.), by the University Medical Center Giessen and Marburg (UKGM, research grant 10/2009GI; S.S. and U.J.H.S.), and a fellowship from the Foundation for Hemotherapeutic Research, Bonn, Germany (U.J.H.S.).
This work contains parts of the doctoral thesis of W.W. (Faculty of Veterinary Medicine, Justus Liebig University, Giessen).
Authorship
Contribution: U.J.H.S. designed research, analyzed and interpreted data, made figures, and wrote the manuscript; W.W., B.B., K.H., and H.B. performed experimental work, analyzed data, and made figures; A.R. and J.B. contributed valuable reagents and provided input on the manuscript; R.M.B. performed histology, made figures, and contributed to the manuscript; W.W., G.B., and S.S. interpreted data and contributed to the manuscript; and N.W. designed animal experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulrich J.H. Sachs, Institute for Clinical Immunology and Transfusion Medicine, Justus Liebig University, Langhansstr 7, 35392 Giessen, Germany; e-mail: ulrich.sachs@med.uni-giessen.de.