Abstract
Delta-like ligand 4 (DLL4) is essential for the formation of mature vasculature. However, the role of DLL4-Notch signaling in pericyte/vascular smooth muscle cell (vSMC) development is poorly understood. We sought to determine whether DLL4-Notch signaling is involved in pericyte/vSMC formation in vitro and during vasculogenesis in vivo using 2 Ewing sarcoma mouse models. Inhibition of DLL4 with the antibody YW152F inhibited pericyte/vSMC marker expression by bone marrow (BM) cells in vitro. Conversely, transfection of 10T1/2 cells with the active domains of Notch receptors led to increased expression of pericyte/vSMC markers. Furthermore, the blood vessels of Ewing sarcoma tumors from mice treated with YW152F had reduced numbers of BM-derived pericytes/vSMCs, fewer open lumens, and were less functional than the vessels in tumors of control-treated mice. Tumor growth was also inhibited. These data demonstrate a specific role for DLL4 in the formation of BM-derived pericytes/vSMCs and indicate that DLL4 may be a novel therapeutic target for the inhibition of vasculogenesis.
Introduction
Mature blood vessels are composed of a single layer of endothelial cells, which form the inner tube of the vessel, surrounded by a supportive layer(s) of pericytes/vascular smooth muscle cells (vSMCs). Pericytes/vSMCs provide vessel structural support and contribute to the regulation of blood flow. In addition, pericytes/vSMCs provide growth and survival factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF2), and angiopoietin (Ang1) to endothelial cells, while preventing endothelial cell hyperproliferation. Thus, pericytes/vSMCs help to maintain a stable state of functional, nonproliferative, mature vasculature.1-3 Without pericytes/vSMCs, blood vessels are leaky, less stable, and more susceptible to antiangiogenic therapies and regression due to pathologic conditions such as hyperoxia.4
A functional vascular network is essential for the growth of solid tumors. Two of the processes by which tumors can form vessels are angiogenesis, the sprouting of preexisting blood vessels, and vasculogenesis, the recruitment of bone marrow (BM) cells to the tumor with subsequent formation of a de novo vessel network. During vasculogenesis, BM cells migrate to the tumor, adhere to sites of developing vasculature, and contribute to the endothelial and pericyte/vSMC populations within mature vasculature. We have previously shown that BM cells and the process of vasculogenesis are critical to the expansion of the tumor vascular network.5 We demonstrated that BM-cell migration to the tumor is regulated by the chemoattractant properties of VEGF165.6 Subsequently, BM cells differentiate into both endothelial cells and pericytes/vSMCs that help to form the new tumor vessels.7 However, little is known about the molecular mechanisms that direct BM-derived pericyte/vSMC formation once BM cells have reached the site of developing vasculature.
One molecular mechanism likely to contribute to this process is delta-like ligand 4 (DLL4)–Notch signaling. In mammals, the Notch family includes 5 ligands, DLL1, DLL3, DLL4, Jagged1, and Jagged2, and 4 receptors, Notch1-4. All ligands and receptors are membrane bound and signal by cell-to-cell contact. When a ligand binds to a receptor, 2 cleavage events occur to activate receptor signaling. The Notch intracellular domain (NICD) is released by the second cleavage event, which is mediated by the γ-secretase complex. NICD is then translocated to the nucleus, where it forms a transcriptional activating complex that includes recombination signal-binding protein-Jκ (RBP-Jκ) and mastermind-like protein (MAML). The NICD-RBP-Jκ-MAML complex induces transcription of Notch effectors such as the Hes and Hey family members, which are themselves transcription factors that go on to regulate the expression of downstream Notch targets.8,9
The Notch ligand DLL4 is essential for vascular formation.10,11 When DLL4 is inhibited in developing mouse retinas or in xenograft tumor models such as colon and lung carcinoma, excessive proliferation of nonfunctional vasculature occurs.12-14 Blood vessels become unorganized and display increased sprouting and microvessel density, but decreased perfusion and function. The etiology of this seemingly paradoxical situation of more vessels but less perfusion and the reason for the decreased functionality are poorly understood. The microvessels created by the hyperproliferation of endothelial cells in the absence of DLL4 are immature; they lack coverage by α-smooth muscle actin+ (α-SMA+) cells.14 A correlation between DLL4 expression and blood vessel maturation in bladder cancer has been demonstrated: 98.7% of DLL4+ tumor vessels were surrounded by α-SMA+ pericytes/vSMCs, while only 64.5% of DLL4− vessels had α-SMA+ cell coverage.15 Thesedata suggest that DLL4 may play a role in the formation of BM-cell–derived pericytes/vSMCs during vasculogenesis.
Recently, we demonstrated DLL4 expression by BM-derived pericytes/vSMCs in Ewing sarcoma patient samples and xenografts.16 Our previous studies showed that the majority of Ewing sarcoma tumor vessels were composed of a mosaic of locally derived and BM progenitor cell-derived endothelial cells and pericytes/vSMCs.17,18 This provided a model with which to investigate whether DLL4 contributes to the formation of BM-derived pericytes/vSMCs, and the correlation between DLL4, pericyte/vSMC coverage, and vessel perfusion.
To investigate the link between DLL4 and pericyte formation, we used YW152F DLL4-neutralizing antibodies to block DLL4 signaling in vitro and in vivo. Our in vitro data show for the first time that DLL4 plays a role in the regulation of pericyte/vSMC marker expression. Using 2 different Ewing sarcoma xenograft models, we show that treatment with the DLL4-neutralizing antibody YW152F results in decreased pericyte/vSMC coverage of the tumor vessels, impaired blood vessel functionality, and decreased tumor growth. These data confirm the importance of Notch signaling, in particular DLL4, in the formation of BM-derived pericytes/vSMCs in vivo. Our data also suggest that DLL4 may be a new therapeutic target to inhibit tumor vessel formation.
Methods
A4573, TC71, SC9-19, and 10T1/2 cell lines
All cells were cultured in completed Dulbecco modified Eagle medium as described in Reddy et al.7 TC71 and A4573 cells contain the t(11;22) translocation and the EWS-Fli1 fusion, which was confirmed by polymerase chain reaction (PCR). Both cells lines are mycoplasma free and were authenticated by short terminal repeat fingerprinting. A4573 cells were a gift from Dr V. Soldatenkov (Georgetown University Medical Center, Washington, DC), and TC71 cells were a gift from Dr T. Triche (University of Southern California, Los Angeles, CA). SC9-19 mouse embryonic stromal cells were a gift from Dr Akira Sugimoto (Hayashibara Biochemical Labs, Okayama, Japan). 10T1/2 mesenchymal cells were purchased from ATCC.
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center. For green fluorescent protein (GFP) BM-transplant experiments, donor mice of strain C57BL/6-Tg(UBC-GFP)30Scha/J were purchased from The Jackson Laboratory. The mice express enhanced GFP under control of the Rosa26 promoter. Recipients were athymic nu/nu nude mice. Nude mice were purchased at 6 weeks of age from the National Cancer Institute (strain 01B70).
GFP protein-positive BM-cell transplant
Whole BM was collected from GFP transgenic mice by flushing femurs with phosphate-buffered saline (PBS). Nude mice were given 900 rads of whole-body irradiation using a cesium irradiator (137Cs Mark 1 Irradiator; J. L. Shepherd & Associates) to eradicate endogenous BM. Irradiated mice were then rescued by intravenous injection of 1 × 106 GFP-positive (GFP+) BM cells. After transplant, 4 weeks were allowed for BM-cell engraftment before injection of tumor cells. At the end of each experiment, BM was collected from at least 3 representative mice to confirm GFP expression. More than 90% of BM cells in transplant-recipient mice were GFP+ according to the results of flow cytometry (FACSAria; BD Biosciences).
YW152F treatment in vivo
Experiments using YW152F DLL4-neutralizing antibodies were performed with either TC71 or A4573 xenograft models. For all experiments, a GFP BM transplant was performed and at least 4 weeks were allowed for BM cell engraftment before tumor inoculation. Mice were then injected subcutaneously with either 2 × 106 TC71 or 6 × 106 A4573 cells. Five days later, mice were randomly divided into either the treatment or the control group. Mice were treated twice weekly by intravenous injection of YW152F (1.3 mg of YW152F in 100 μL of PBS), immunoglobulin G (1 mg of human IgG in 100 μL of PBS; Southern Biotech), or 100 μL of PBS. TC71 cells were allowed to grow for a total of 21 days (5 treatments) and A4573 cells for 24 days (6 treatments). Tumor volumes were measured using calipers twice weekly and on the day of killing. Mice were killed by euthanasia or cervical dislocation and tumors were harvested and frozen for immunohistochemical analysis.
Experiments treating mice with TC71 tumors were done 3 times. In the first experiment, 9 mice were in the YW152F group and 8 mice were in the PBS group. In the second experiment, 9 mice were in the YW152F group, 9 mice were in the IgG group, and 7 mice were in the PBS group. In the third experiment, 6 mice were in the YW152F group and 7 mice were in the IgG group. Experiments treating mice with A4573 tumors were performed twice. Both times, 9 mice were in the YW152F group and 8 mice were in the IgG group.
YW152F treatment in vitro
A total of 1.33 × 105 TC71 or A4573 cells per well were plated in 6 well dishes in complete Dulbecco modified Eagle growth medium. One day after seeding, YW152F or IgG was added to the medium. Two doses, 2.8 and 5 μg/mL, were tested. The medium was changed and fresh YW152F or IgG was added daily. Total cell number and viability were counted at 48 and 72 hours by automated trypan blue assay using a Vi-Cell cell viability analyzer (Beckman Coulter). Experiments were done 2 times in triplicate each time.
Hypoxyprobe
Hypoxyprobe-1 (pimonidazole HCl; HPI, Inc.) was reconstituted in PBS at a final concentration of 7 mg/mL. Mice received intravenous injections of 200 μL of Hypoxyprobe/PBS solution (total 1.4 mg of Hypoxyprobe). Two hours and 30 minutes later, mice were killed by cervical dislocation and tumors were harvested for immunohistochemical evaluation.
Immunohistochemical analysis
Frozen slides were fixed and permeabilized by submersion in acetone and blocked in 4% fish gelatin in PBS, as described in Schadler et al.16 For mouse anti–mouse primary antibodies, slides were then incubated overnight in mouse Fab fragment blocking solution (mouse Fab diluted 1:10 in 4% fish gel). Primary antibodies used were rabbit anti-DLL4, rabbit anti-desmin, and chicken anti-GFP (Abcam); anti–α-SMA-Cy3 (Sigma); rat anti-CD31 (BD Biosciences); rabbit anti-NG2 (Millipore); and mouse anti-Hypoxyprobe (HPI Inc.). Tissues were incubated with primary antibodies for 3 hours or overnight. Slides were then washed in PBS and incubated with 4% fish gel before the addition of secondary fluorescent antibodies. Cy3- and Cy5-labeled anti–rabbit and anti–mouse secondary antibodies (Jackson Immunoresearch Laboratories), Alexa 488–labeled anti–rat, Alexa 594–labeled anti–rabbit, and anti–chicken secondary antibodies (Molecular Probes) were used. Nuclei were labeled with Sytox Green (Jackson Immunoresearch Laboratories) or Hoescht 33342 (Molecular Probes).
Microscopy and immunohistochemistry quantification
Confocal microscopy images were captured using a laser confocal microscope (Carl Zeiss MicroImaging) and LSM software (Zeiss). Nonconfocal fluorescent images were captured using a Zeiss Axioplan fluorescence microscope equipped with narrow bandpass excitation filters, a 100-W Hg lamp, and a cooled charge-coupled device Hamamatsu C5810 camera (Hamamatsu Photonics).
For quantification, five 10× fields were captured from each slide. Total positive pixels of the color representing the marker of interest were normalized against total nuclei in each field using Simple PCI software (Hamamatsu). Ratios from each of the 5 fields for each slide were averaged to obtain 1 representative value for each tumor section. Values for tumor sections from each treatment group were pooled and the Mann-Whitney U test was used to determine statistical significance.
MigR1 plasmids
All MigR1 plasmids were a gift from P.Z.-M. The intracellular domains of each of the Notch receptors were cloned between the EcoRI and BamHI cloning sites under control of the MSCV LTR promoter. There is an internal ribosome entry site between the Notch intracellular domain and enhanced GFP, so that the 2 are not expressed as a fusion protein. For MigR1/DNMAM, a 61-amino acid portion of MAML-1 is directly fused to enhanced GFP.
Reverse transcription–PCR
RNA was collected using Trizol per the manufacturer's instructions (Invitrogen). cDNA was made using a reverse transcription (RT) kit (Promega). PCR primers are listed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Notch regulates pericyte/vSMC marker expression in vitro
The DLL4-neutralizing antibody YW152F was used to investigate the role of DLL4-Notch signaling in the regulation of BM-cell expression of pericyte/vSMC markers in vitro. GFP+ whole BM cells were isolated from the femurs of transgenic mice and seeded on top of a confluent layer of SC9-19 mouse stromal cells. Both SC9-19 cells and BM cells are DLL4+ (supplemental Table 1). After 1 week in coculture, the pericyte/vSMC markers desmin and RGS5 were induced in BM cells (Figure 1A). Daily treatment with YW152F for 1 week resulted in decreased expression of RGS5 (46%), desmin (40%), and α-SMA (45%) compared with treatment with IgG (Figure 1B). We next compared the effect of inhibiting all canonical Notch signaling using the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) to the specific inhibition of DLL4 by YW152F. BM cells were again cultured with SC9-19 stromal cells, and then treated with DAPT or dimethylsulfoxide as a control daily for 1 week. In addition to measuring the effect on pericyte/vSMC markers, we also examined the Notch effector Hes1. Expression of Hes1, like other Notch effectors, is induced by the active NICD-RBP-Jκ complex as an intermediate mediator of the signaling pathway. Hes1 expression is therefore a surrogate marker of Notch activity. In YW152F-treated BM cells, the Notch effector Hes1 was reduced by 24%, RGS5 by 46%, desmin by 40%, and α-SMA by 45% compared with the IgG control. Treatment with DAPT led to greater inhibition of Hes1 (82%), RGS5 (61%), desmin (41%), and α-SMA (63%) mRNA than treatment with YW152F compared with the respective controls (Figure 1C).
DLL4-Notch signaling is important for expression of pericyte markers in vitro. (A) Whole BM was isolated from the femurs of transgenic mice and grown in coculture with SC9-19 mouse embryonic stromal cells for 1 week. GFP+ BM cells were then isolated from the cocultures by FACS, and RNA was extracted. RT-PCR was performed for desmin and RGS5 on freshly isolated whole BM and on BM cells after 1 week in coculture. (B-C) GFP+ BM cells were isolated from the femurs of transgenic mice and grown in coculture with SC9-19 mouse embryonic stromal cells. Cells were treated daily with either IgG or YW152F (B) or dimethylsulfoxide or DAPT (C) for 1 week. GFP+ BM cells were then isolated from the cocultures by FACS and RNA was extracted. RT-PCR was performed to quantify RGS5, desmin, α-SMA, or Hes1 mRNA levels. Densitometry values are presented below each band. Experiments were repeated at least 2 additional times with similar results; data shown are representative. (D) 10T1/2 mesenchymal precursor cells were transfected with either MigR1 vector control or MigR1 containing the active domains of each of the 4 Notch receptors (NICD1-4). Forty-eight hours later, RNA was collected and mRNA levels were measured by RT-PCR for RGS5, α-SMA, and desmin. Experiments were repeated 3 times with similar results; results are representative. (E) TC71 cells were injected subcutaneously into nude mice that had previously received GFP+ BM transplants. When tumors reached 2 cm3, mice were killed and tumors were harvested and digested to form single-cell suspensions. GFP+ BM-derived cells were collected using FACS, and RNA was isolated from these tumor-derived GFP+ BM cells. RT-PCR was performed for DLL4 and Hes1.
DLL4-Notch signaling is important for expression of pericyte markers in vitro. (A) Whole BM was isolated from the femurs of transgenic mice and grown in coculture with SC9-19 mouse embryonic stromal cells for 1 week. GFP+ BM cells were then isolated from the cocultures by FACS, and RNA was extracted. RT-PCR was performed for desmin and RGS5 on freshly isolated whole BM and on BM cells after 1 week in coculture. (B-C) GFP+ BM cells were isolated from the femurs of transgenic mice and grown in coculture with SC9-19 mouse embryonic stromal cells. Cells were treated daily with either IgG or YW152F (B) or dimethylsulfoxide or DAPT (C) for 1 week. GFP+ BM cells were then isolated from the cocultures by FACS and RNA was extracted. RT-PCR was performed to quantify RGS5, desmin, α-SMA, or Hes1 mRNA levels. Densitometry values are presented below each band. Experiments were repeated at least 2 additional times with similar results; data shown are representative. (D) 10T1/2 mesenchymal precursor cells were transfected with either MigR1 vector control or MigR1 containing the active domains of each of the 4 Notch receptors (NICD1-4). Forty-eight hours later, RNA was collected and mRNA levels were measured by RT-PCR for RGS5, α-SMA, and desmin. Experiments were repeated 3 times with similar results; results are representative. (E) TC71 cells were injected subcutaneously into nude mice that had previously received GFP+ BM transplants. When tumors reached 2 cm3, mice were killed and tumors were harvested and digested to form single-cell suspensions. GFP+ BM-derived cells were collected using FACS, and RNA was isolated from these tumor-derived GFP+ BM cells. RT-PCR was performed for DLL4 and Hes1.
After demonstrating that inhibition of DLL4 reduces pericyte/vSMC marker mRNA expression by BM cells, we sought to determine whether active Notch signaling induces pericyte/vSMC marker expression. To this end, 10T1/2 mesenchymal precursor cells were transfected with MigR1 plasmids containing the intracellular domain of each of the 4 Notch receptors (NICD1-4) or the MigR1 control vector. 10T1/2 cells are multipotent and can become pericyte-like cells in culture when properly stimulated.19 Forty-eight hours after transfection, RGS5, α-SMA, and desmin mRNA levels were increased by all NICDs compared with the MigR1 empty vector control (Figure 1D).
Identification of DLL4+ BM-derived cells in Ewing sarcoma tumors
Having demonstrated that DLL4-Notch signaling induced the expression of pericyte/vSMC markers by BM cells and 10T1/2 mesenchymal progenitor cells in vitro, and that inhibiting DLL4 affected pericyte/vSMC formation by BM cells, we sought to confirm these findings in vivo using our Ewing sarcoma mouse model. We previously demonstrated the presence of BM-derived pericytes/vSMCs in Ewing tumor vessels. To confirm that these BM-derived pericytes express DLL4, nude mice were transplanted with GFP+ BM as described in Schadler et al.16 One month after transplant, engraftment was confirmed and mice were injected subcutaneously with TC71 Ewing sarcoma cells. Using this model, BM-derived cells in the tumor can be identified by GFP expression. When tumors reached 2 cm3, mice were killed and tumors were harvested. Tumors were digested to achieve a single-cell suspension, and GFP+ cells were collected using FACS. RT-PCR for DLL4 confirmed that BM-derived cells within Ewing sarcoma tumors express DLL4 (Figure 1E). We next demonstrated that the Notch signaling pathway was active in the BM-derived cells isolated from the tumor using RT-PCR for the Notch effector Hes1. As described above, the presence of Notch effectors is indicative of active Notch signaling within the cell. Hes1 mRNA was present in the GFP+ BM-derived cells (Figure 1E). Real-time PCR later confirmed the presence of the Notch effectors Hey1 and Hey2 as well, and demonstrated increased Notch 1 and Notch 4 mRNA in BM-derived cells isolated from the tumor compared with whole BM isolated from mice femurs (data not shown). The presence of DLL4 on both endothelial cells and BM cells and Notch effectors in BM cells from TC71 tumors, together with our in vitro data demonstrating a role for DLL4-Notch signaling in BM-cell expression of pericyte/vSMC markers, indicates that DLL4-Notch signaling may be important for the formation of BM-derived pericytes/vSMCs in vivo.
Effect of YW152F treatment on BM-derived pericytes/vSMCs, tumor vessel morphology, and vessel function
Having demonstrated that BM-derived cells in the tumors express DLL4 and that blocking DLL4 with YW152F inhibits pericyte marker expression in BM cells in vitro, we sought to determine whether YW152F affected BM-derived pericyte/vSMC formation and/or blood vessel structure and functionality in vivo. Nude mice were transplanted with GFP+ BM cells. One month later, BM engraftment was confirmed and mice were injected with subcutaneous TC71 or A4573 Ewing sarcoma cells. Beginning 5 days later, mice were treated twice weekly for 3 weeks with intravenous injections of YW152F, IgG control, or PBS. There was no difference in the total number of CD31+ cells between tumors from YW152F-treated mice and those from IgG control mice as assessed by immunohistochemistry; however, there was a difference in CD31+ vessel morphology. While CD31+ vessels in tumors from IgG-treated mice were elongated, with large, open lumens, CD31+ structures in tumors from YW152F-treated mice were small and punctate, with tiny lumens. The average number of large, open lumen vessels per 10× microscopic field was fewer in the YW152F-treated group than in the IgG control group (0.8 vs 2.1, P = .057, Figure 2A).
YW152F treatment affects tumor vessel morphology and the number of GFP+, α-SMA+, desmin+, and NG2+ cells. TC71 cells were subcutaneously injected in nude mice that had previously received GFP+ BM transplants. Mice were treated twice weekly with YW152F or IgG for a total of 5 treatments. Mice were then killed and tumors were analyzed by immunohistochemistry. (A) CD31+ endothelial cell structures. The number of open lumens per 10× field was manually counted. The average of 5 fields was determined for each tumor. (B) Anti-GFP was used to identify BM-derived cells; α-SMA, desmin, and NG2 were used to identify pericytes/vSMCs. Five 10× fields from each tumor were used to quantify cell numbers using Simple PCI software. The percentage reduction in the average positive pixel/nuclei ratio in YW152F-treated tumors compared with IgG-treated tumors was determined.
YW152F treatment affects tumor vessel morphology and the number of GFP+, α-SMA+, desmin+, and NG2+ cells. TC71 cells were subcutaneously injected in nude mice that had previously received GFP+ BM transplants. Mice were treated twice weekly with YW152F or IgG for a total of 5 treatments. Mice were then killed and tumors were analyzed by immunohistochemistry. (A) CD31+ endothelial cell structures. The number of open lumens per 10× field was manually counted. The average of 5 fields was determined for each tumor. (B) Anti-GFP was used to identify BM-derived cells; α-SMA, desmin, and NG2 were used to identify pericytes/vSMCs. Five 10× fields from each tumor were used to quantify cell numbers using Simple PCI software. The percentage reduction in the average positive pixel/nuclei ratio in YW152F-treated tumors compared with IgG-treated tumors was determined.
We next examined the BM-derived pericyte/vSMC population in tumors of YW152F-treated mice. Tumors in IgG control mice had thick layers of GFP+ BM-derived cells surrounding CD31+ endothelial cells, while the number of perivascular GFP+ cells was significantly reduced in tumors of YW152F-treated mice (85% reduction, P = .001, Figure 2B). Expression of all 3 pericyte/vSMC markers was also significantly reduced in tumors of YW152F-treated mice compared with IgG controls: α-SMA by 87% (P = .005), desmin by 70% (P = .002), and NG2 by 61% (P = .007, Figure 2B).
To determine whether the reduction in pericytes/vSMCs was due to a specific necessity for active Notch signaling in incoming BM cells, we used dominant-negative mastermind-like protein (DNMAM) to inhibit Notch signaling in BM cells. Whole BM transduced with either MigR1-GFP control or MigR1-GFP-DNMAM was transplanted into nude mice. One month later, TC71 cells were injected subcutaneously and tumors were allowed to form. Tumors from mice that had received MigR1-GFP-DNMAM BM were smaller and had fewer perivascular BM-derived cells than tumors from control transplanted mice (supplemental Figure 1).
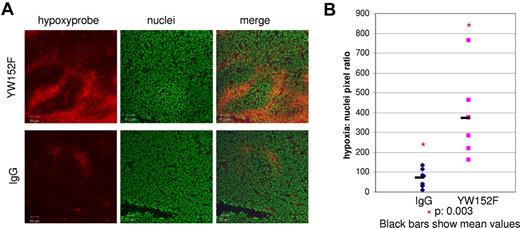
The reduced number of lumen-bearing vessels combined with the reduction in pericytes/vSMCs in tumors of YW152F-treated mice suggested that these vessels were less functional. As a surrogate marker of vessel functionality, we measured hypoxia within the tumors because increased hypoxia indicates reduced blood vessel functionality. TC71 tumors were treated with YW152F or IgG. Two-and-a-half hours before killing, mice were injected intravenously with Hypoxyprobe-1. Mice were then killed and tumors were harvested for immunohistochemical evaluation of hypoxia. Hypoxia was significantly increased in tumors from YW152F-treated mice compared with tumors from IgG control mice (5.3-fold increase, P = .003, Figure 3).
Hypoxia is increased in tumors treated with YW152F. TC71 cells were subcutaneously injected in nude mice. Mice were treated with YW152F or IgG as described in “YW152F treatment in vivo.” Before killing, mice were injected with Hypoxyprobe-1 to identify hypoxic cells. (A) Hypoxic regions (red) were identified by immunohistochemical analysis using anti–Hypoxyprobe-1. Nuclei were identified using Sytox Green (green). (B) The ratio of the total number of hypoxic cells to nuclei was determined using Simple PCI software for 5 fields per tumor, and the average values were calculated. Blue diamonds represent average hypoxia/nuclei ratios for IgG-treated tumors, and pink squares represent YW152F-treated tumors. Black bars show mean values.
Hypoxia is increased in tumors treated with YW152F. TC71 cells were subcutaneously injected in nude mice. Mice were treated with YW152F or IgG as described in “YW152F treatment in vivo.” Before killing, mice were injected with Hypoxyprobe-1 to identify hypoxic cells. (A) Hypoxic regions (red) were identified by immunohistochemical analysis using anti–Hypoxyprobe-1. Nuclei were identified using Sytox Green (green). (B) The ratio of the total number of hypoxic cells to nuclei was determined using Simple PCI software for 5 fields per tumor, and the average values were calculated. Blue diamonds represent average hypoxia/nuclei ratios for IgG-treated tumors, and pink squares represent YW152F-treated tumors. Black bars show mean values.
YW152F inhibits the growth of TC71 and A4573 Ewing sarcoma tumors in vivo
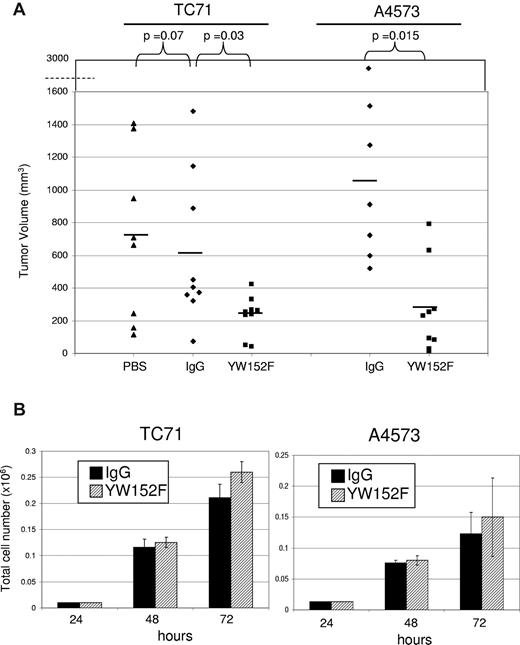
The reduced functionality of blood vessels in the tumors of mice treated with YW152F suggested that tumor growth may be affected. To determine whether inhibition of DLL4 using YW152F inhibited tumor growth, we again used the treatment schema described 4 paragraphs above. TC71 tumors from mice treated with YW152F were significantly smaller than those from IgG control mice (P = .03, Figure 4A). There was no difference between tumor size in IgG-treated and PBS-treated mice (P = .7). PBS control groups were therefore eliminated from future experiments. A4573 tumors were also significantly smaller in mice treated with YW152F than in IgG control mice (P = .015, Figure 4A).
YW152F inhibits the growth of Ewing sarcoma in vivo and does not effect tumor cell proliferation or viability in vitro. (A) TC71 or A4573 cells were subcutaneously injected in nude mice that had previously received GFP+ BM transplants. Mice were treated twice weekly with YW152F, IgG, or PBS and tumor growth was quantified. Black lines show mean volumes for each group. (B) TC71 or A4573 cells were seeded in triplicate in 6-well dishes. Cells were treated daily with either 5 μg/mL of YW152F or human IgG control. Cell viability and total cell number were assessed at 48 and 72 hours using the trypan blue assay and automated Vi-Cell cell counter. Black bars represent IgG-treated cells, and striped bars represent YW152F-treated cells. (P > .05 for all data.)
YW152F inhibits the growth of Ewing sarcoma in vivo and does not effect tumor cell proliferation or viability in vitro. (A) TC71 or A4573 cells were subcutaneously injected in nude mice that had previously received GFP+ BM transplants. Mice were treated twice weekly with YW152F, IgG, or PBS and tumor growth was quantified. Black lines show mean volumes for each group. (B) TC71 or A4573 cells were seeded in triplicate in 6-well dishes. Cells were treated daily with either 5 μg/mL of YW152F or human IgG control. Cell viability and total cell number were assessed at 48 and 72 hours using the trypan blue assay and automated Vi-Cell cell counter. Black bars represent IgG-treated cells, and striped bars represent YW152F-treated cells. (P > .05 for all data.)
Effect of YW152F on tumor-cell proliferation
To rule out the possibility that the inhibition of tumor growth by YW152F was secondary to a direct effect on Ewing sarcoma cell viability or proliferation, TC71 and A4573 cells were treated with YW152F or IgG in vitro. There was no significant difference in cell number or viability between the YW152F- and IgG-treated groups (Figure 4B). These data indicate that the tumor growth inhibition seen was not due to a direct effect of YW152F on tumor-cell proliferation.
Discussion
DLL4 has previously been demonstrated to play an essential role in vascular formation.11 DLL4-Notch signaling controls tip versus stalk cell fate assignment in endothelial cells, and is therefore a critical regulator of vascular sprouting during angiogenesis.20 In addition to its role in endothelial cell regulation, the importance of DLL4 in pericyte/vSMC formation during vasculogenesis is suggested by the correlation between DLL4 expression and blood vessel maturation, as measured by pericyte/vSMC coverage.14,21 However, the relationship between DLL4-Notch signaling and pericyte/vSMC formation during vessel development is poorly understood. Our studies demonstrate for the first time that DLL4 is critical for the formation of BM-derived pericytes/vSMCs, a key step in the process of vasculogenesis. When BM cells were cultured on an SC9-19 stromal cell feeder layer, the expression of desmin and RGS5 was induced, indicating a progression toward a pericyte/vSMC fate. DLL4 inhibition by YW152F reduced the mRNA levels of RGS, desmin, and α-SMA in BM cells. RGS5 and desmin mRNA levels were further reduced by the γ-secretase inhibitor DAPT, which inhibits Notch signaling. We used the Notch effector Hes1 as a surrogate marker for Notch signaling. Indeed, both YW152F and DAPT inhibited Hes1 expression. DAPT reduced mRNA levels of Hes1 to a greater extent than YW152F, indicating a more complete inhibition of the Notch pathway.
The greater reduction of Hes1, RGS5, and desmin mRNA levels by DAPT than by YW152F indicates that, in addition to DLL4, another Notch ligand may be involved in stimulation of the Notch pathway during pericyte/vSMC formation. Jagged1, for example, is important for pericyte/vSMC differentiation. An endothelial-cell–specific Jagged1 knockout is embryonically lethal in mice due to vascular defects, including a reduction in vSMC coverage.22 In addition, stimulation of human aortic SMCs with Jagged1 induces expression of smooth muscle actin in a Notch3-dependent manner.23 Jagged1 and DLL4 may play complementary roles in pericyte/vSMC formation. Contrary to the effects of inhibiting DLL4 or Notch, we demonstrated that transfection with NICD1-4, the active portion of Notch receptors, resulted in increased expression of RGS5, α-SMA, and desmin in 10T1/2 mesenchymal precursor cells. Each of the NICDs led to increased pericyte/vSMC marker expression over MigR1 controls. However, because of variance in transfection efficiency between NICD plasmids, the relative potency of one Notch receptor compared with another during pericyte/vSMC marker stimulation could not be evaluated. Our findings indicate a critical role for Notch signaling in pericyte/vSMC development, which is in keeping with data from other investigators, which include a role for Notch1 in SMC proliferation after vascular injury, a correlation between the expression of Notch3 and the expression of RGS5 in pericytes of developing teeth, and the presence of Notch3+ BM-derived cells in thickened neointima of mice after femoral artery injury.24,25 While the importance of functional Notch receptors for pericyte/vSMC development has been well documented, we are the first to demonstrate the critical role of DLL4 specifically in inducing expression of pericyte/vSMC markers. Our demonstration of a role for DLL4-Notch signaling in pericyte/vSMC marker regulation in vitro may be applicable to pericytes/vSMCs from non-BM origins. BM-derived pericytes/vSMCs are not the only cells that contribute to vessel formation in tumors.
After demonstrating the correlation between DLL4 and BM-cell expression of pericyte/vSMC markers in vitro, we confirmed the importance of DLL4-Notch signaling in pericyte/vSMC formation in vivo using our Ewing sarcoma mouse model. We previously demonstrated that BM cells contribute to the expansion of the tumor vascular network by migrating to the tumor and differentiating into both endothelial cells and pericytes/vSMCs.5,16,17,23-25 The tumor blood vessels were surrounded by thick layers of BM-derived pericytes/vSMCs. We also previously demonstrated that these BM-derived cells were essential for tumor growth using MEKK3-knockout BM cells, which cannot participate in vessel formation.5 Recently, we demonstrated that DLL4 is expressed by BM-derived pericytes/vSMCs in Ewing sarcoma xenografts and patient samples.16 The data presented here support our hypothesis that DLL4 regulates or stimulates the process of BM-cell–derived pericyte/vSMC formation. Furthermore, it suggests that DLL4 can be targeted for therapeutic purposes.
We used YW152F in vivo, which resulted in the inhibition of tumor growth. Tumors from control mice had elongated, well-organized, lumen-bearing CD31+ vessel networks, while CD31+ structures in tumors from mice treated with YW152F were small, punctate, and had few visible lumens. In addition, there was a significant reduction in the number of BM-derived pericytes/vSMCs in these tumor vessels, indicating inhibition of the vasculogenesis process. Several thick layers of GFP+ BM-derived, α-SMA+, desmin+, and NG2+ cells surrounded the majority of vessels in control tumors. GFP+ BM-derived cells and α-SMA+, desmin+, and NG2+ cells were reduced in tumors of YW152F-treated mice compared with controls. The importance of Notch signaling in BM cells specifically was demonstrated using DNMAM. Tumors from MigR1-GFP-DNMAM BM-transplanted mice were smaller and had fewer perivascular BM-derived cells, indicating that BM cells that are unable to receive a Notch signal have an impaired ability to form perivascular cells.
Although significantly reduced in tumors of mice treated with YW152F, a very thin layer of GFP+ cells surrounding CD31+ vessels remained. Thin layers of α-SMA+, desmin+, and NG2+ cells were also present in these tumors. We interpret this data to mean that a small number of BM-derived cells were able to become pericytes/vSMCs despite inhibition of DLL4. This may be due to incomplete inhibition of DLL4. Alternatively, other Notch ligands, such as Jagged1, may stimulate pericyte/vSMC differentiation in the absence of DLL4, or there may be a subpopulation of BM-derived pericytes/vSMCs that are not dependent on Notch signaling.
Correlating with the reduction in vessel lumens and pericytes/vSMCs, vessel functionality in YW152F-treated tumors was impaired as demonstrated using Hypoxyprobe-1. Tumor hypoxia was significantly increased by YW152F treatment, indicating decreased delivery of oxygenated blood to the tumor cells, most likely secondary to reduced blood vessel functionality.
These investigations are the first to demonstrate a direct correlation between DLL4 and expression of pericyte/vSMC markers in vitro and in vivo, and the efficacy of DLL4-neutralizing antibodies in inhibiting Ewing sarcoma tumor growth using 2 human xenograft models, TC71 and A4573. Both A4573 and TC71 tumors in YW152F-treated mice were significantly smaller than those in the controls. Tumor growth inhibition was not due to a direct effect on tumor cells. YW152F had no effect on TC71 or A4573 viability or proliferation in vitro. The inhibition of tumor growth can therefore be attributed, at least in part, to reduced pericyte/vSMC coverage, changes in vascular morphology, and the reduction in vessel functionality. In our in vivo model, YW152F is delivered systemically and therefore inhibits DLL4 expressed by both endothelial cells and incoming BM cells. It remains to be determined whether the DLL4 expressed by BM cells activates Notch receptors on neighboring BM cells, or whether the Notch receptors on incoming BM cells are activated solely by DLL4 expressed by the endothelial cells of the developing vasculature.
In summary, our data provide evidence that DLL4-Notch signaling is involved in the formation of BM-derived pericytes/vSMCs. Inhibiting DLL4 using YW152F or blocking Notch signaling with DAPT inhibited pericyte/vSMC marker expression by cultured BM cells. Conversely, stimulating Notch signaling in 10T1/2 cells triggered the expression of pericyte/vSMC markers. When DLL4-Notch signaling was blocked using YW152F in vivo, there was a reduction in BM-derived pericytes/vSMCs, a reduction in lumen-bearing vessels, a reduction in vessel functionality, and decreased tumor growth. These data establish DLL4 as a potential therapeutic target to block pericyte/vSMC formation, which will subsequently affect vessel functionality and vascular expansion. Our data also confirm the essential role of vasculogenesis in Ewing sarcoma tumor growth. Few clinically relevant molecular targets for the vasculogenesis process have been identified. Data presented here support the concept of using DLL4-neutralizing antibodies or other agents that target DLL4 for the treatment of Ewing sarcoma and possibly other tumors or disease processes in which vascular expansion needs to be inhibited.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Donna Reynolds for her help with tumor sectioning for immunohistochemical analysis.
This work was supported by the National Cancer Institute (R01 CA103986 to E.S.K. and core grant CA106672) and by the National Center for Research Resources (TL1RR024147 to K.S.S.).
National Institutes of Health
Authorship
Contribution: K.S.S. designed and performed experiments, interpreted data, and wrote the manuscript; Z.Z. performed experiments; P.Z.-M. interpreted data and contributed reagents; and E.S.K. conceived the original hypothesis, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eugenie S. Kleinerman, Division of Pediatrics, Unit 87, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ekleiner@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal