Abstract
Acute chest syndrome describes new respiratory symptoms and findings, often severe and progressive, in a child with sickle cell disease and a new pulmonary infiltrate. It may be community-acquired or arise in children hospitalized for pain or other complications. Recognized etiologies include infection, most commonly with atypical bacteria, and pulmonary fat embolism (PFE); the cause is often obscure and may be multifactorial. Initiation of therapy should be based on clinical findings. Management includes macrolide antibiotics, supplemental oxygen, modest hydration and often simple transfusion. Partial exchange transfusion should be reserved for children with only mild anemia (Hb > 9 g/dL) but deteriorating respiratory status. Therapy with corticosteroids may be of value; safety, efficacy and optimal dosing strategy need prospective appraisal in a clinical trial. On recovery, treatment with hydroxyurea should be discussed to reduce the likelihood of recurrent episodes.
Introduction
Acute chest syndrome is (ACS) the second most common cause of hospitalization and challenges infection1 as the leading cause of sickle cell-related mortality2 in children. Still, management is largely determined by the experience of individual practitioners, and there are no conclusive randomized controlled clinical trials to guide therapy. What follows is a review of treatment options and related issues to assist in management; it will likely be especially useful to those who do not regularly encounter children with this potentially life-threatening complication.
What is in a name? That which we call a rose by any other name would smell as sweet. (William Shakespeare)
ACS is the term used to describe a new pulmonary infiltrate with respiratory findings in a person with sickle cell disease.3 The etiology of these episodes is often multifactorial and difficult to discern. Although children may be more likely to have infectious etiologies for their chest syndrome and adults more likely sickle cell-related causes,4,5 infection and inflammation may well induce sickling, and embolism/infarction may lead to superimposed infection. This is perhaps best illustrated by the fact that 2 common causes of ACS, Chlamydia pneumoniae and Mycoplasma pneumoniae, are associated with a severe clinical course in children with sickle cell disease6-9 rather than the “walking pneumonia” that typifies infection in others. It thus makes no sense to try to differentiate between “pneumonia” and ACS.
Chance is a word that does not make sense. Nothing happens without a cause. (Voltaire)
The investigators of a multicenter National Acute Chest Syndrome Study (NACSS)4 published in 2000 made aggressive attempts to identify etiology, including the performance of bronchoalveolar lavage (BAL) on consenting study enrollees; 72% of the 671 enrolled in the study had a lavage or sputum sample available. Despite these efforts, the authors only established potential causation in 38% overall and 70% of those with a complete assessment.
The most common identifiable cause of ACS in this study was pulmonary fat embolism (PFE).4 Originally described in trauma victims, PFE is a frequent complication of bone fracture and may progress to involve the lungs and CNS with multiorgan failure and death.10,11 The NACSS made the diagnosis by using BAL to identify fat-laden macrophages; less-invasive means of making the diagnosis are not well established, and even the specificity of fat-laden macrophages in BAL specimens has been questioned.12 Vichinsky et al,13 in the first systematic, prospective search for PFE in sickle cell disease, found that 44% of patients with moderate-to-severe ACS had PFE as a probable etiology. These patients had longer, more severe hospital courses and a greater decrease in hemoglobin concentration and platelet count than those without fat emboli. Fat from bone marrow, which is typically infarcted during acute pain episodes,14 embolizes to the lungs and, in its most extreme form, fat embolism syndrome may involve the brain, kidneys, and the liver15 with substantial mortality.16 It may be that, as with trauma,11 fat emboli occur commonly with acute bone infarction, and clinical severity is a reflection of site and volume of marrow infarction.
PFE is associated with activation of secretory phospholipase A2 (sPLA2), an enzyme that cleaves phospholipids to activate several potent inflammatory mediators,17 as well as free fatty acids, which are toxic to lung tissue.18 Serum levels of sPLA2 initially were shown to be uniformly elevated in a small group of patients with ACS, with more striking elevations in those with more severe episodes19 ; in a follow-up study elevation was detected 24-48 hours before clinical onset.20 In a multicenter trial,21 80% of subjects with ACS had sPLA2 elevation. The administration of packed red blood cell transfusion to patients hospitalized for pain and found to have elevation of sPLA2 appeared to prevent ACS22 ; a trial within the Sickle Cell Disease Clinical Research Network designed to confirm feasibility and efficacy of sPLA2 screening and preemptive transfusion (ClinicalTrials.gov ID NCT00951808) was closed before adequate subject accrual. Inhibitors of sPLA2 are under investigation for reduction of risk for atherosclerosis.23 One of them, varespladib, was studied similarly as a potential preemptive therapy of ACS (ClinicalTrials.gov ID NCT00434473); results have yet to be published.
C pneumoniae was the likely causative agent in 20% of patients when first described as an ACS pathogen in 1991.24 That C pneumonia is an obligate intracellular organism and may persist even in patients who mount an antibody response makes a firm establishment of causation difficult.25 Using PCR on BAL specimens and serology, Dean et al8 found that C pneumoniae was the most common identifiable infectious agent in the NACSS; it was present in 7.2% of all patients and in 30% of all patients tested at first episode and with a single agent isolated. C pneumoniae infection occurs in young children (< 5 years of age)26-28 and can be acquired nosocomially.29 Similarly, severe disease has been attributed to M pneumoniae,6,7 identified in the NACSS (serologically) in 6.6% of patients9 ; 12% of the 112 episodes of ACS that occurred in patients younger than 5 years were attributable to M pneumoniae. Macrolide therapy, or use of another antibiotic to which atypical bacteria are susceptible, is clearly indicated in patients diagnosed as having ACS, regardless of their age or location when diagnosed.
Bacterial infection commonly caused ACS in the 1960s, with Streptococcus pneumoniae the proven or suspected causative agent in 43% of an early series of cases.30 However, more recently S pneumoniae infection is an uncommon cause, occurring in only 1%-3%4,31,32 ; the incidence of S pneumoniae infection should only further decrease as more effective vaccines are available and widely used.33 In the NACSS, infection due to Staphylococcus aureus was found to be as common as S pneumoniae.
Parvovirus B19 infection, the common cause of aplastic crisis,34 is a notable viral cause of ACS, perhaps via its association with bone marrow infarction/necrosis35 and potentially PFE. Parvovirus B19 was identified by the use of PCR in 10 of the 216 episodes for which a pathogen was isolated in the NACSS; whether there was an association with the presence of fat emboli was not reported. We still manage (unfortunately) to surprise residents by asking for the reticulocyte count of a child admitted for ACS. Other viruses also were identified as pathogens during the NACSS. Respiratory syncytial virus was the most common, but influenza A was identified in 4 cases. Seasonal flu, and notably the H1N1 strain, may cause severe disease and is treatable36 ; influenza infection should be considered during times when prevalent and, if likely, antiviral treatment should be prescribed.
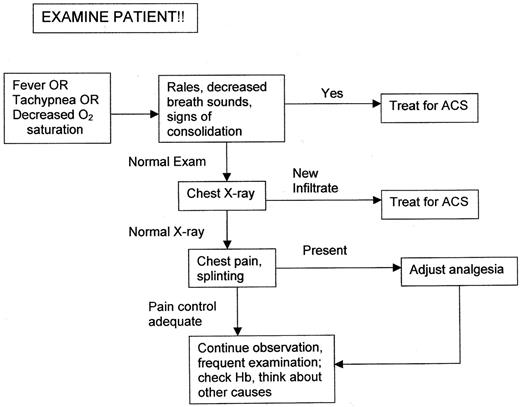
ACS was initially defined in patients with a new infiltrate on radiograph, and an abnormal x-ray is generally required to confirm the diagnosis. However, ACS is often a clinical diagnosis; a diagnostic approach is presented schematically in Figure 1. Careful assessment of clinical findings is essential. Children with tachypnea often appear deceptively comfortable. I generally count the respiratory rate of a patient at risk myself and find that tachypnea is sometimes overlooked and misreported by house staff and nurses. Respiratory rate varies with age and increases with fever, but in a resting, afebrile young child (ie, younger than 5 years of age) I am concerned with a rate in the upper twenties and in the mid-twenties or greater in an older child or adolescent.37 Asymmetry of breath sounds may be the only auscultatory finding, and persistent rales nearly always indicate ACS. Nasal flaring is often present even in older children as the syndrome progresses.
Radiographic changes may lag behind clinical findings, and therapy should commence once a clinical diagnosis is made. However, in the emergency department, 61% of patients with fever had unexpected infiltrates on the chest radiograph.38 It is important to note that nearly 50% of ACS occurs in patients hospitalized for other causes (notably pain)4 ; it is critical that all sickle cell inpatients be examined frequently and carefully, with specific attention to respiratory rate and signs of consolidation on physical examination, and be continuously monitored by pulse oximetry to detect any decrease in oxygenation.
Because of a right-shifted oxygen dissociation curve and the increased presence of hemolysis-related carboxyhemoglobin, pulse oximetry may indicate spurious oxygen saturation values39,40 and, particularly because some children may have baseline hypoxemia,41 treatment of ACS should not be initiated solely on the basis of low pulse oximetry readings. However, some authors have found good correlation in children with sickle cell anemia between oxygen saturation measured cutaneously and arterial oxygen saturation measured by cooximetry.42 We use pulse oximetry both in hospitalized patients at risk and during the course of ACS to detect changes in saturation; arterial blood gas determinations are reserved for the patient with persistent tachypnea who is suspected to have impending respiratory failure.
An idea is a feat of association. (Robert Frost)
Stroke has been associated with proximate (within 2 weeks)/concurrent ACS,43 perhaps reflecting the impact of inflammation on cerebral blood flow and impaired oxygenation in a population with a high prevalence of cerebral vasculopathy. This association makes urgent treatment of ACS with maintenance of adequate oxygenation all the more critical. A history of recurrent ACS increases stroke risk, which does not reconcile well with the observation that lower hemoglobin concentration is associated with stroke.43 whereas patients with greater hemoglobin level tend to get ACS.44
Children with asthma may be at increased risk for ACS, and a diagnosis, with therapy to control exacerbations in children with sickle cell disease, is recommended.45 Those with known asthma who develop acute pulmonary findings have a more difficult course46,47 and should be treated aggressively for wheezing if present; even in the absence of wheezing, we perform preventive bronchodilator therapy at least every 4 hours. The use of corticosteroids is also advisable in such patients, although there may be a risk for readmission for pain once ACS has resolved (see “All progress is precarious, and the solution of one problem brings us face to face with another problem. (Martin Luther King Jr)”). Although 20% of patients examined at the bedside for forced expiratory volume in 1 second had improvement after bronchodilator therapy,4 we do not routinely give bronchodilators to patients without asthma. We will give a therapeutic trial if wheezing is present; we do not, however, use a bronchodilator as adjunctive treatment of all ACS, as has been recommended on the basis of a favorable experience at a single institution.48 Children with asthma may be at increased risk for C pneumoniae infection and have a more severe clinical course than nonasthmatics,49,50 making macrolide therapy especially appropriate.
An ounce of prevention is worth a pound of cure. (Henry de Bracton)
Bellet et al51 demonstrated that incentive spirometry, given at a prescribed regimen of 10 puffs every 2 hours while the patient is awake, significantly and substantially reduced nosocomial ACS in patients hospitalized for chest or back pain. We prescribe and recommend this regimen of spirometry for all patients admitted for pain at any site who require opioid analgesia (virtually all patients hospitalized for pain), speculating that spirometry may reduce ACS risk by improving ventilation potentially compromised by the respiratory depressive effect of opioid analgesia. In a pilot study, intermittent positive airway pressure was well accepted and seemingly effective in reducing ACS risk52 ; the authors speculate this intervention may be better accepted than spirometry in children with chest pain.
When one administers opioids, it is tricky in patients with chest and back pain to achieve analgesia adequate to reduce respiratory splinting53 while avoiding respiratory depression. The nonsteroidal anti-inflammatory drug ketorolac is not a respiratory depressant and may provide analgesia comparable with opioids54 ; however, ketorolac is relatively expensive, can be used for only 5 days, and more than a single dose is not approved by the Food and Drug Administration for parenteral use in children younger than 16 years of age. Nonetheless, repetitive dosing is widely used for the treatment of postoperative pain in children, alone or with opioids.55 For sickle cell pain, in one small study ketorolac was found to be equianalgesic with meperidine56 and was adequate for pain control in 53% of children treated in the emergency department.57 We generally reserve its use for older children with chest and back pain, supplementing it with morphine or hydromorphone as needed.
We have not used nalbuphine hydrochloride, a mixed agonist antagonist opioid analgesic, which in a retrospective review of hospital records was associated with reduced incidence of nosocomial ACS.58 It appears to be equianalgesic with morphine with fewer side effects; combination therapy with morphine also was found to be superior to either medication alone in a recent randomized trial of treatment for postoperative pain.59 Optimizing pain control with decreased side effects, most critically ACS, should be a controlled trial priority; a prototype comparing 2 modes of administering patient-controlled analgesia was developed and piloted by the SCDRN (ClinicalTrials.gov ID NCT00999245).
Preemptive therapy with transfusion, either preceding diagnosis (as attempted using sPLA2 as a marker) or at diagnosis (to avoid a prolonged course and/or respiratory failure) needs further study. In the NACSS (48% age 20 years or older) radiographic evidence of extensive lobar involvement, a platelet count of < 200,000/cmm at diagnosis, and a history of cardiac disease were independent predictors of respiratory failure.4 In a small series of children, an arterial:alveolar oxygen gradient performed at diagnosis on room air > 30 was predictive of a severe course and the need for transfusion.60 In a retrospective review in which they examined choice of analgesia as a risk factor for ACS, Buchanan et al58 found that children who developed ACS had greater white blood cell counts (19.1 ± 4.8/cmm vs 13.2 ± 5.2/cmm, P < .0001) and lower admission hemoglobin levels (7.7 ± 1.2 g/dL vs 9.3 ± 2.0 g/dL) than those who did not. Identifying risk factors predictive enough to justify a randomized trial of preemptive therapy would be desirable; however, because ACS is often subtle at presentation and progresses rapidly, the examination of preemptive therapy, especially the use of transfusion, will be logistically difficult.
Children, at least those with homozygous sickle cell anemia, who recover from an episode of ACS should be offered therapy with hydroxyurea. In adults, hydroxyurea reduced the incidence of ACS by approximately 50% and the need for acute transfusion by 30% in the Multicenter Study of Hydroxyurea.61 It appears safe and similarly effective when given to children.62,63 Even infants, ages 9-17 months at entry, treated in the BABY HUG trial had a 73% reduction in ACS over the course of 2 years compared with a placebo group, with predictable myelosuppression the only toxicity noted.64 A single episode of ACS at any age should prompt discussion with the family regarding availability and potential use of hydroxyurea. Although the long-term impact of preventive intervention is unknown, recurrent ACS has been associated with “sickle cell lung disease” and early mortality,65 and repetitive episodes must be of concern.
Chronic transfusion therapy also may be offered as preventive therapy for children with recurrent ACS to reduce frequency of future episodes. At St Jude, patients who received a transfusion to maintain hemoglobin S < 30% had a reduced risk of ACS compared with their pretransfusion track record,66 and an analysis of non-neurologic events during the Stroke Prevention Trial demonstrated a dramatic reduction in incidence of ACS in children undergoing transfusion for primary stroke prevention compared with randomized control patients.67 We have rarely used transfusion specifically for recurrent ACS; certainly, it would be reserved now for the unusual child who did not respond or could/would not take hydroxyurea.
The rest is a mere matter of detail, to be settled with judgment, discretion, and caution. (John Griffin Carlisle)
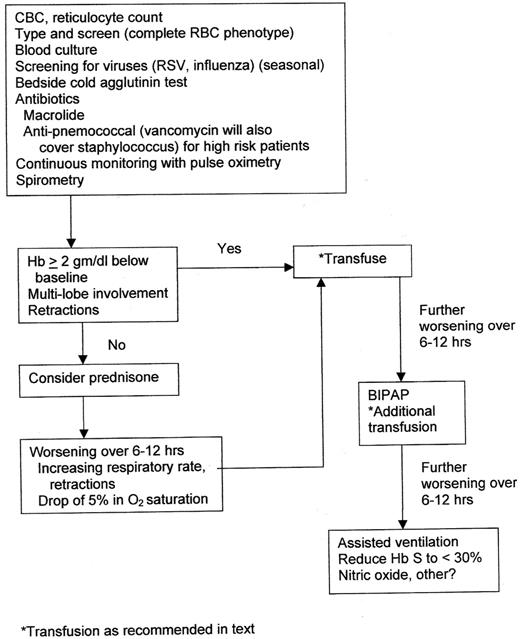
Figure 2 offers an overview of management. A blood culture should be performed and repeated if fever persists in the patient. Rapid screening tests for respiratory syncytial virus and influenza should be performed in season. Screening for M pneumoniae infection with the use of a bedside test for cold agglutinins offers good specificity if agglutination persists after rewarming and repeat cooling of the specimen.68
As discussed previously, treatment of ACS should always include a macrolide antibiotic. Children who have a high white blood cell count, high fever, or toxic appearance, especially those not immunized and/or not receiving recommended penicillin prophylaxis, should perhaps also be treated for pneumococcal infection.69 Although in most communities S pneumoniae remains sensitive to third-generation cephalosporins, vancomycin will additionally cover S aureus infection, a similarly uncommon but virulent pathogen. Since the H1N1 epidemic of 2010, we have often, when influenza is endemic, treated children with ACS with the antiviral medication oseltamivir, which ideally should be started within 48 hours of onset of symptoms; guidelines for diagnosis and treatment of influenza can be found at The Centers for Disease Control and Prevention.70
All children with ACS should be hospitalized and continuously monitored for oxygenation and clinical deterioration. Although oxygen therapy is not indicated for routine use in children hospitalized for pain,71 supplemental oxygen should be given to ACS patients with tachypnea and/or hypoxemia. Patients usually are febrile, feel well, and have near-baseline respiratory rate and oxygen saturation at discharge.
Hydration is generally given intravenously at approximately a maintenance rate. Patients with ACS may be at risk for pulmonary edema, especially if aggressive hypotonic fluid management is given72 ; in a patient who develops a clinical appearance suggestive of pulmonary edema within 6 hours of transfusion, a diagnosis of transfusion-associated acute lung injury should also be considered.73
Hyponatremia is known to be common in children admitted for pain episodes74 and likely occurs with ACS as well because of near-ubiquitous sodium-losing nephropathy.75 Induction of hyponatremia was not proven to be beneficial and was difficult to safely maintain in treating acute pain episodes76 ; it has not been studied in ACS. We generally give extra sodium (5% dextrose in water with 75 milliequivalents of sodium chloride per liter) with intent to reduce the incidence/degree of hyponatremia and its potential complications.77
Blood is thicker than water. (Proverb)
Blood transfusion therapy is extremely useful in the treatment of ACS. The earliest report of the benefit of transfusion was from Saudi Arabia several years ago. In a retrospective review, patients admitted with ACS and transfused within 24 hours had a prompt recovery relative to those who were not; many of the latter went on to require transfusion during a more prolonged clinical course.78 We have shown that transfusion therapy increases oxygenation beyond that attributable to the increase in hemoglobin alone.79 Transfused patients had dramatic improvements in arterial saturations and alveolar-arterial gradient compared with those who were not; similar improvements in oxygen saturation were observed in the NACSS.4
Transfusion most simply works by “diluting out” sickle cells. Abnormal up-regulation of adhesion molecules, specifically vascular cell adhesion molecule 1,80-83 may be attenuated by transfusion therapy84 (at least when given repetitively80 ). Provision of Duffy antigen by transfusion may attenuate the inflammatory response associated with ACS by binding chemokines, notably IL-8, via the Duffy antigen receptor for chemokines.85,86 Duffy antigen is both an inflammatory modulator and the entry portal to the red cell by malarial parasites.87 The latter role has made Duffy antigen positivity extremely rare in West Africa; approximately 70% of African Americans are Duffy antigen negative.86 White patients, who in most U.S. centers constitute the majority of blood donors, generally are positive for FYa and/or FYb,86 and it is speculated that transfusion of Duffy-positive blood may favorably attenuate the course of ACS by its impact on chemokines.85 Although no supportive clinical data are yet available, extended cross-matching to exclude Duffy incompatibility may be counterproductive, at least regarding treatment of ACS. Extended cross-matching to avoid incompatibility with antigens C, E, and Kell (in addition to D, A, and B) should be performed88 ; it reduces risk of allo-immunization and is not a major challenge for the blood bank, particularly because most white patients who are D-negative are also C- and E-negative.
A concern specific to patients with sickle cell disease who undergo transfusion is the delayed hemolytic transfusion reaction/hyperhemolysis syndrome.89 Within 6-10 days of transfusion, patients present with fever, hemoglobinuria, and often pain. Because of “bystander” hemolysis, the hemoglobin concentration is frequently decreased to less than pretransfusion levels, and additional transfusion may exacerbate the destruction of transfused and autologous red cells even if completely matched phenotypically. Although presumed to be allo- or auto-immune, new antibodies often are not detectable. Treatment with intravenous immunoglobulin, corticosteroids, and recombinant erythropoietin has been effective. Delayed hemolytic transfusion reaction/hyperhemolysis is relatively uncommon in children but important to recognize promptly so that further transfusion can (hopefully) be avoided.
In theory, there is no difference between theory and practice. But, in practice, there is. (Jan L. A. van de Snepscheut)
Urgent exchange transfusion has been recommended for treatment of ACS90 and is still used as front-line therapy at some centers.91 This preference is presumably determined by in vitro studies that show that increasing packed cell volume by adding even small increments of normal cells to sickle cells has such a substantial negative impact on blood viscosity that oxygen delivery to tissue would be reduced.92-94 There are also case reports that the high mortality from fat embolism syndrome, in which fat emboli disseminate and ultimately cause multiorgan failure, is reduced by repetitive or exchange transfusion.16
Despite these findings, clinical experience suggests simple transfusion is quite effective and adequate therapy for most children with ACS. Exchange transfusion as front-line therapy should be reserved for patients who are not sufficiently anemic to accommodate a simple transfusion. A second simple transfusion is sometimes needed if clinical improvement is less than satisfactory and is often possible because of frequent decrease in hemoglobin levels during the course of ACS (mean decrease, 0.7 g/dL)5 ; hemoglobin concentration and reticulocyte count should be monitored at least daily during treatment for ACS.
In the previously cited report from Saudi Arabia,78 patients who showed prompt clinical improvement presumably received simple transfusion because exchange is not discussed. The authors of the original paper describing PFE as a frequent finding in ACS,13 although again not specifically stated, presumably treated their transfused patients with simple transfusion; there were no deaths, although 6 of 27 (22%) had CNS findings, suggesting possible fat embolism syndrome. In our own report documenting improvements in tissue oxygenation,79 74% of those transfused received only simple transfusion. In our more recent retrospective look at the impact of prednisone therapy on ACS,95 all of 17 patients receiving blood had simple transfusion only; none required assisted ventilation, and all survived.
The NACSS initially was conceived to be a randomized trial of simple versus exchange transfusion for treatment of ACS. However, data regarding etiologies, risk factors for progression, and likely clinical impact of either simple or exchange transfusion were so lacking as to preclude a realistic study design; an observational study of etiology, management, and outcome was organized instead. Unfortunately, outcome data specifically regarding exchange versus simple transfusion were not reported in the NACSS. However, it is stated that patients who were given simple transfusion were no more likely to clinically worsen than those who received initial exchange; transfusion modality reflected preference of local investigators and was not necessarily on the basis of clinical findings.4 In the only randomized trial of exchange versus simple transfusion, also organized by Dr Vichinsky (a Brooklyn native and Downstate graduate), investigators examined preoperative preparation for elective surgery.96 In 604 surgeries, subjects were randomized to either simple (or, as the British say, “top-off”) transfusion to increase the hemoglobin concentration to 9-11 g/dL (mean posttransfusion hemoglobin, 10.6 g/dL; hemoglobin S, 59%), or exchange or repetitive simple transfusions to lower the hemoglobin S to < 30% (mean hemoglobin, 11 g/dL; mean hemoglobin S, 31%). There was no difference between the 2 transfusion groups in sickle cell-related or other surgical complications except that allo-immunization and other complications of transfusion were more common in the exchange group. The incidence of ACS, the most common sickle cell–related complication, was 10% in both groups.
The only circumstance in which we would use exchange transfusion as first-line therapy in ACS would be for a patient not sufficiently anemic to tolerate a simple transfusion; we do not administer transfusion volumes to sickle cell patients who are acutely ill that would increase their hemoglobin concentration to > 11 g/dL. If such patients require transfusion for the “dilute out sickle cells” effect, we generally use a partial exchange in which packed red blood cells are reconstituted with the use of either saline or plasma. Blood is removed from the patient and replaced with equal volume in 20- to 50-mL aliquots; the reconstituted packed cells ideally should have a hemoglobin concentration of 10-11 g/dL.
Alternatively, we have used donor bags for phlebotomy, as described by Lanzkowsky et al,97 for a “partial packed cell exchange,” but with concurrent infusion of reconstituted packed cells. We typically use an exchange volume of approximately 15-20 mL/kg of body weight (ie, 1 L of reconstituted blood for a 40- to 50-kg patient) to achieve a posttransfusion hemoglobin A percentage of 20%-40%; Piomelli et al98 actually performed the calculus to prepare tables indicating the volume of phlebotomized and transfused blood required, given a pretransfusion Hb level. Again, there is no evidence that further reduction in hemoglobin S concentration is indicated or beneficial. At institutions where experienced pediatric cytapheresis teams are readily available and adept at gaining peripheral venous access, similar results could be achieved by the use of a posttransfusion hemoglobin S target of 60%-70% rather than the usual 30% or less, with avoidance of any increase in total hemoglobin concentration; I am not certain how often this is performed.
All progress is precarious, and the solution of one problem brings us face to face with another problem. (Martin Luther King Jr)
Corticosteroid therapy (dexamethasone 0.3 mg/kg/dose every 12 hours for 4 doses) for ACS was examined in a blinded placebo-controlled trial several years ago in Dallas.99 All measures of severity (length of stay, days of oxygen therapy, febrile days, clinical deterioration) suggested efficacy of dexamethasone in attenuating the course of ACS. However there was a nonsignificant trend for an increase in readmission after corticosteroid therapy and resolution of ACS; generally those readmissions were within 72 hours and most commonly for pain. Investigators at Johns Hopkins subsequently did a retrospective review of their use of steroids in ACS.100 They showed that patients who received dexamethasone as adjunct therapy had as much as a 53% readmission rate (again mainly for pain) if they were not transfused during the episode. They also reported 2 steroid-treated patients who experienced intraventricular hemorrhage during or after the ACS episode. It is speculated that initial down-regulation of adhesion molecules to improve ACS might be followed by rebound up regulation and vasoocclusive pain, as demonstrated in sickle cell mice.101 Cautionary reports had previously been published regarding use of steroids in sickle cell disease,16,102 and in fact steroids have been suspected as a cause of fat embolism syndrome.16 A National Heart, Lung, and Blood Institute–sponsored trial designed to confirm the value of dexamethasone, as used in Dallas but with tapering doses over an additional 6 days (ClinicalTrials.gov ID NCT00530270), was stopped because of low accrual.
The use of corticosteroids preventively in trauma-induced fat embolism syndrome is controversial. A recent meta-analysis,103 however, suggested benefit in 7 trials that were analyzed, with a 78% reduction in fat embolism syndrome in subjects treated with 6-90 mg/kg over the course of 48 hours. Patients treated with lower doses of methylprednisolone among this group appeared to fare best; early therapy using low-dose corticosteroid therapy (1 mg/kg/d by continuous infusion for 14 days with tapering to day 28) has similarly been proposed for adult respiratory distress syndrome.104
We began using an asthma regimen of prednisone (2 mg/kg/d for 5-7 days; maximum dose 60- 80 mg/d) with the thought that this might provide the beneficial effects of dexamethasone without the readmission side effect95 ; prednisone actually reduces the relapse risk when used for treatment of status asthmaticus.105 Of 53 episodes of ACS treated with this regimen of prednisone, only 15.1% were readmitted for pain episodes after recovery from ACS, compared with 8.3% of 25 not receiving steroids. We also saw a suggestion of efficacy in that only 21.8% of the steroid-treated episodes underwent transfusion compared with 67.5% who underwent transfusion in our previously cited study79 and 72% of children in the NACSS.5
It is somewhat surprising that on the basis of review of discharges from institutions contributing data to the Pediatric Health Information System database, which contains administrative and billing data from more than 40 freestanding, tertiary care pediatric hospitals in the United States, that corticosteroids were used in 18%-92% (mean, 48%) of ACS patients with comorbid asthma but also in 2.3%-62% (mean, 23%) of patients without asthma; 75% (range, 45%-98%) of “severe” ACS patients (hemoglobin SS genotype, presence of comorbid asthma, requiring ventilator support, and stay in the intensive care unit) received corticosteroids.106 Steroid use was again associated with an increased readmission rate (odds ratio 2.3, 95% confidence interval 1.6-3.4) and also an increased length of stay (25% longer hospitalization, 95% confidence interval 14%-38%). Although a prospective trial is needed to clearly establish the role of corticosteroid therapy in treatment of ACS, we have continued to use asthma dosing of prednisone relatively early in the course of ACS and have not been impressed by a high readmission rate. I have, however, been impressed with the severity of diffuse pain in 2 of our patients who were readmitted after prednisone treatment of ACS.
Investigators at Hartford describe their experience treating “severe” ACS using both transfusion and dexamethasone.107 Although they believed the patient outcome was good and there was a low readmission rate, it is not clear whether or how much dexamethasone therapy may have added to the known beneficial effects of transfusion. It is noteworthy that transfusion therapy seems to reduce the risk of readmission if steroids are given99,100 ; however, it is not clear whether steroids are needed if transfusion is imminent or completed.
If at first you do not succeed, try, try again. Do not give up too easily; persistence pays off in the end. (Thomas H. Palmer)
Children who clinically worsen the despite management as outlined should be referred for intensive care. We have been impressed with prompt and dramatic clinical improvement with administration of bilevel positive airway pressure. Again, although its benefits were reported years ago and I suspect it is widely used, it is often not mentioned in reviews of management of ACS and has not been prospectively studied; it should not be forgotten as an important management tool.108,109
Although in the NACSS BAL was performed safely in the majority of enrolled research subjects, complications did occur in 13% and were more common in those patients with dyspnea and greater respiratory rates. In my experience pulmonologists have been uncomfortable doing the procedure, either because the patient is too well to justify BAL or too sick to perform it safely. Because treatment of ACS should be inclusive, covering the likely infectious pathogens as well as fat-embolism or other sickle cell-related causes, BAL results would uncommonly impact decisions regarding therapy.
Patients with sickle cell disease tend to be somewhat thrombophilic with elevated fibrinogen and factor VIII and increased platelet number and aggregability.110 Although in situ thrombosis has been suggested as a cause of otherwise undiagnosed ACS in the NACSS and adults do appear to be at risk for venous thromboembolism,111 we have not used prophylactic or therapeutic doses of anticoagulants in children for treatment of ACS.
Nitric oxide has been reported to improve survival in hypoxemic sickle cell (SAD) mice,112 may increase oxygen affinity in sickle erythrocytes,113 and has been effective in improving oxygenation with successful outcome in case reports of critically ill children with ACS114-116 and traumatic fat embolism117 ; however, a recent Cochrane Review failed to show any adequate clinical trials and could not conclude efficacy.118 Similarly, a Cochrane Review failed to show improved survival with NO treatment of acute respiratory distress syndrome, although transient improvement in oxygenation likely occurs.119 We have never used NO for treatment of ACS, but its use would be tempting in a case that is refractory to transfusion and not responding to maximal ventilatory support; a controlled trial is ongoing in France (ClinicalTrials.gov ID NCT00748423). It may be worth noting that the Cochrane Review also found no firm basis for the use of antibiotics120 or transfusion121 in ACS, and I would be loathe to forego the use of either, despite the unfortunate lack of data.
It ain't over 'til it's over. (Yogi Berra)
To summarize our management approach, which evolved over 30 years and care of hundreds of children, all children with ACS should be hospitalized. A chest x-ray should be performed on children with fever even if respiratory symptoms and signs are not perceived, but an x-ray does not obviate the need for clinical examination. Children hospitalized for fever or pain need frequent monitoring with careful attention to respiratory rate, oxygen saturation, and physical signs of pulmonary consolidation; we do not wait for a positive chest X-ray to make a diagnosis of ACS. Once diagnosed, antibiotic therapy must include a macrolide, and patients should be typed and screened for transfusion on diagnosis so extended cross matching can be done to include E, C, and Kell antigens should the need for transfusion arise; we would not extend the compatibility to include Duffy antigen. Patients who have worsening tachypnea, hypoxemia, and/or oxygen desaturation become candidates for transfusion; in such patients we often first start therapy with prednisone 2 mg/kg/d (maximum 60 mg/d) or its methylprednisolone equivalent, as used to treat asthmatic patients.
Patients and parents should be advised that the steroid therapy might increase the risk of a pain episode shortly after resolution of ACS, but in our experience that is uncommon. Patients who have worsening pulmonary findings/desaturation should then undergo transfusion. A simple transfusion of 7-13 mL/kg of packed red blood cells is the preferred initial treatment unless a patient's hemoglobin is > 9 gm/dL and a partial exchange using reconstituted packed cells is required. Assisted breathing with the use of bilevel positive airway pressure may be helpful. Patients who require mechanical ventilation should receive exchange transfusion or perhaps NO; hopefully, there will be few such patients. On recovery, the efficacy of hydroxyurea in reducing the risk of future ACS episodes, along with its other potential risks and benefits, should be conveyed to patient and family.
Acknowledgments
The “we” in this article refers to my friend and colleague of 30 years, Dr Sreedhar Rao, and me. I wish him the best in his “retirement.” Thanks to Dr Rao and Dr Stephanie J. Miller for their thoughtful comments and suggestions.
Authorship
Contribution: S.T.M. wrote the article.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Scott T. Miller, MD, SUNY-Downstate, Box 49, 450 Clarkson Ave, Brooklyn, NY 11203; e-mail: scott.miller@downstate.edu.