Abstract
Most HIV+ individuals require lifelong highly active antiretroviral therapy (HAART) to suppress HIV replication, but fail to eliminate the virus in part because of residual replication in gut-associated lymphoid tissues (GALT). Naturally elicited HIV-specific CD8+ T cells generated in the acute and chronic infectious phases exhibit antiviral activity, but decrease in number after HAART. Therapeutic vaccines represent a potential strategy to expand cellular responses, although previous efforts have been largely unsuccessful, conceivably because of a lack of responding HIV-specific central-memory CD8+ T cells (Tcm). To determine whether patients receiving HAART possess CD8+ T cells with Tcm qualities that are amenable to augmentation, HIV-specific CD8+ T-cell clones were derived from HIV-reactive CD28+CD8+ T-cell lines isolated from 7 HIV+ HAART-treated patients, expanded ex vivo, and reinfused into their autologous host. Tracking of the cells in vivo revealed that clones could persist for ≥ 84 days, maintain expression and/or re-express CD28, up-regulate CD62L, secrete IL-2, proliferate on cognate Ag encounter and localize to the rectal mucosa. These results suggest some infused cells exhibited phenotypic and functional characteristics shared with Tcm in vivo, and imply that more effective therapeutic vaccination strategies targeting CD8+ Tcm in patients on HAART might provide hosts with expanded, long-lasting immune responses not only systemically but also in GALT. This study is registered at www.clinicaltrials.gov as NCT00110578.
Introduction
Highly-active antiretroviral therapy (HAART) can dramatically reduce the HIV burden but fails to clear all reservoirs and cannot eradicate the virus.1 GALT potentially remains a major reservoir in patients receiving HAART,2 in part because this large lymphoid compartment contains CCR5+CD4+ T cells chronically activated by dietary Ags which may render them more susceptible to HIV-1 entry and replication.3,4 Although multiply spliced HIV RNA suggestive of actively replicating virus are often undetectable in GALT after 6 months of HAART,5 CD4+ T cells producing virus have been observed in GALT after years of uninterrupted HAART.6,7 Whether detected virus represents ongoing cycles of viral replication as supported by studies demonstrating the evolution of HIV-envelope sequences,8 or steady release of virions from resting CD4+ T cells without viral evolution,9 remains controversial. However, the HIV present in residual reservoirs appears poised to more briskly replicate, as revealed by the rapid peak in viral replication when HAART is interrupted.10
CD8+ T cells are directly responsible for initial control of viremia in primary HIV infection, reducing peak levels to a lower set point, but ultimately the naturally elicited response fails as disease progresses.11-13 HAART is disappointingly associated with a decrease in the numbers of circulating HIV-specific CD8+ T cells, presumably by limiting effective Ag stimulation,14 and mitigates the contributions of host CD8+ T-cell responses to viral elimination. The use of therapeutic vaccines in this population represents a strategy to reinforce cellular immune responses, predominantly by augmenting preexisting HIV-specific CD8+ T cells.15 Such antiviral responses induced under more optimal conditions than persistent infection may establish T-cell responses that can be maintained or further boosted over time, proliferate on Ag re-encounter, and localize to sites of persistent viral replication such as the GALT to provide an ongoing effector response. Previous efforts aimed at augmenting T-cell responses in HAART treated patients with structured treatment interruptions (STI) or therapeutic vaccines have not been as successful as hoped, and may have been undermined in part by the quality of responses elicited.15-17
Reinfusion of ex vivo–expanded HIV-specific CD8+ T cells can reproduce the primary goal of therapeutic vaccination by increasing frequencies of HIV-reactive T cells, in a setting in which the fate of the elicited response can be tracked.18 Previous studies from our group showed that most HIV-specific CD8+ T-cell clones derived from and reinfused into HIV patients with high viral burdens have limited persistence after transfer, likely because of the isolation of clones from effector memory CD8+ T cells (Tem), which represent HIV-reactive CD8+ T cells most abundant in chronic infection, and which typically become terminally differentiated during the process of in vitro expansion.19 Reinfusion of Tcm cells or cells derived from Tcm, which possess the ability to self-renew and maintain robust responses over time, would presumably reconstitute a better response.20-23 The precise phenotype of Tcm cells remains controversial, but it is generally agreed that Ag-experienced CD8+ T-cell populations persisting after resolution of an acute infection that continue to express CD28 contain Tcm.24 Chronic HIV infection is characterized by the presence of CD28− HIV-specific CD8+ T cells.25 Although HAART has the potential to preserve/permit establishment of a pool of HIV-specific CD8+ Tcm with CD28 expression,26 as the frequency of HIV-specific CD8+ T cells detectable declines to very low levels in patients controlled with HAART, it has been difficult to determine how effectively CD8+ Tcm are preserved/rescued.
To determine whether patients on HAART have CD8+ T cells with Tcm qualities amenable to augmentation and capable of restoring in vivo immune responses, we derived HIV-specific CD8+ T-cell clones from T-cell lines with elevated fractions of HIV-specific CD8+CD28+ T cells that had been generated from patients with well-controlled HIV infection. Our findings indicate that HIV-specific CD8+ T cells in these patients can be expanded ex vivo and subsequently exhibit a phenotype and functional characteristics shared with Tcm in vivo after transfer. The infused HIV-specific CD8+ T-cell clones were capable of: (1) persisting after reinfusion; (2) maintaining CD28 expression throughout ex vivo expansion and infusion and/or re-expressing (CD28 after infusion if CD28 was no longer detectable after the ex vivo expansion process; (3) up-regulating CD62L in vivo; (4) secreting IL-2 and proliferating on encountering cognate Ag; and (5) localizing to mucosal sites independent of the presence of detectable local viral replication.
Methods
Clinical protocol and patient characteristics
Protocol 2077 was approved by the Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Board, the Food and Drug Administration, and the National Institute for Allergy and Infectious Diseases. All patients provided informed consent in accordance with the Declaration of Helsinki, were HIV seropositive by ELISA and Western blot (Bio-Rad), CMV seropositive by ELISA (Wampole), and had no prior history of opportunistic infections and CD4+ T cells > 200 cells/mm3 (ie, patients with CDC classification “C” or “3” were excluded). All patients were receiving HAART according to the current clinical practice guidelines with HIV plasma viremias below 30 copies/mL as determined by ultra-sensitive RT-PCR (Roche). Patients were enrolled and received infusions from June 2006 to August 2007 (Table 1).
Characteristics of HIV-seropositive study patients at the time of T-cell infusions
| Patient . | CD4 cells/μL . | % CD4 . | CD8 cells/μL . | % CD8 . | Viral load, copies/mL plasma . | Years of HIV infection . | Years of HAART . | Total CD4 at time of HAART initiation . | Route of infection . |
|---|---|---|---|---|---|---|---|---|---|
| 7701 | 577 | 19 | 1938 | 64 | < 30 | 8 | 5 | 211 (15%) | MSM |
| 7702 | 898 | 40 | 714 | 32 | < 30 | 20 | 9 | UNK | MSM |
| 7703 | 642 | 25 | 1368 | 54 | < 30 | 21 | 7 | UNK | MSM |
| 7704 | 575 | 34 | 779 | 46 | < 30 | 20 | 9 | 490 | MSM/IVDU |
| 7705 | 539 | 45 | 473 | 40 | < 30 | 6 | 5 | UNK | MSM |
| 7707 | 439 | 22 | 754 | 38 | < 30 | 2 | 1 | 219 | MSM |
| 7709 | 850 | 43 | 682 | 35 | < 30 | 17 | 9 | 320 | MSM |
| Mean | 646 | 33 | 958 | 44 | < 30 | 13.4 | 6.4 |
| Patient . | CD4 cells/μL . | % CD4 . | CD8 cells/μL . | % CD8 . | Viral load, copies/mL plasma . | Years of HIV infection . | Years of HAART . | Total CD4 at time of HAART initiation . | Route of infection . |
|---|---|---|---|---|---|---|---|---|---|
| 7701 | 577 | 19 | 1938 | 64 | < 30 | 8 | 5 | 211 (15%) | MSM |
| 7702 | 898 | 40 | 714 | 32 | < 30 | 20 | 9 | UNK | MSM |
| 7703 | 642 | 25 | 1368 | 54 | < 30 | 21 | 7 | UNK | MSM |
| 7704 | 575 | 34 | 779 | 46 | < 30 | 20 | 9 | 490 | MSM/IVDU |
| 7705 | 539 | 45 | 473 | 40 | < 30 | 6 | 5 | UNK | MSM |
| 7707 | 439 | 22 | 754 | 38 | < 30 | 2 | 1 | 219 | MSM |
| 7709 | 850 | 43 | 682 | 35 | < 30 | 17 | 9 | 320 | MSM |
| Mean | 646 | 33 | 958 | 44 | < 30 | 13.4 | 6.4 |
HAART indicates highly active antiretroviral therapy; UNK, unknown; MSM, men who have sex with men; and IVDU, intravenous drug use.
Epitope mapping
Identification of HIV and CMV epitopes for each patient was performed by testing the reactivity of bead-selected CD8+ T cells (Miltenyi Biotec) against pools of overlapping 15mer peptides spanning the HIV-1 clade B consensus sequence (NIH AIDS Research and Reference Reagent Program) and CMV pp65 protein (kindly provided by Dr T. Manley, FHCRC) grouped into pools. Smaller subpools aligned in a grid were used to stimulate selected CD8+ T cells for detection of IFNγ secretion in an ELISPOT assay (R&D Systems) to identify individually recognized peptides as described.27 Reactive 15-mer sequences were compared with previously identified peptide sequences restricted to HLA alleles expressed by the patient.28 MHC alleles that could be synthesized into a multimer to allow tracking the T cells after transfer were preferentially selected. For some patients, CD8+ T cells were expanded by weekly stimulations with peptide-pulsed autologous adherent cells to characterize IFNγ-reactivity if direct ex vivo reactivity was undetectable, reflecting a low endogenous frequency of HIV-specific CD8+ T cells.
Isolation and expansion of HIV- and CMV-specific CD8+ T-cell clones
All ex vivo manipulations were performed a GMP-facility as previously described.18,29 CD8+ T cells were stimulated twice in 7-day cycles with 9-11mer peptide (Anaspec)–pulsed adherent cells to obtain several polyclonal CD8+ T-cell lines. For each peptide specificity, lines that both expressed > 2% specific CD8+ T cells (an average of 3 lines per peptide) and contained the highest fraction of CD28+pentamer+ (ProImmune)–specific T cells were selected for cloning by limiting dilution. CD8+ T-cell clones that demonstrated the highest cytolytic activity were further expanded and infused after 42 days in culture. CD8+ T-cell clones were tested for monoclonality by analysis of TCR-Vβ usage30 (Table 2).
Characteristics of infused HIV-specific and CMV-specific CD8+ T cells
| Patient . | Clone specificity . | Epitope . | HLA restriction . | Corresponding multimer . | Vβ usage . |
|---|---|---|---|---|---|
| HIV-specific CD8+ T cells | |||||
| 7701 | HIV-GAG | GEIYKRWII | HLA B*0801 | B*0801/GEIYKRWII- HIV-GAG | Vβ13 |
| 7702 | HIV-GAG | GEIYKRWII | HLA B*0801 | B*0801/GEIYKRWII- HIV-GAG | Vβ13 |
| 7703 | HIV-GAG | GEIYKRWII | HLA B*0801 | B*0801/GEIYKRWII- HIV-GAG | Vβ2 |
| 7704 | HIV-RT-POL | ILKEPVHGV | HLA A*0201 | A*0201/ILKEPVHGV-HIV-RT-POL | Vβ3 |
| 7705 | HIV-ENV | IPRRIRQGL | HLA B*0702 | B*0702/IPRRIRQGL-HIV-ENV | Vβ14 |
| 7707 | HIV-NEF | QVPLRPMTYK | HLA A*0301 | A*0301/QVPLRPMTYK-HIV-NEF | Vβ6 |
| 7709 | HIV-GAG | KRWIILGLNK | HLA B*2705 | B*2705/KRWIILGLNK-HIV-GAG | Vβ13 |
| CMV-specific CD8+ T cells | |||||
| 7701 | CMV-pp65 | NLVPMVATV | HLA A*0201 | A*0201/NLVPMVATV-CMV-pp65 | Vβ8 |
| 7702 | CMV-pp65 | NLVPMVATV | HLA A*0201 | A*0201/NLVPMVATV-CMV-pp65 | Vβ3 |
| 7703 | CMV-pp65 | NLVPMVATV | HLA A*0201 | A*0201/NLVPMVATV-CMV-pp65 | V8 |
| 7704 | CMV-pp65 | IPSINVHHY | HLA B*3501 | B*3501/IPSINVHHY-CMV-pp65 | Vβ3/13 |
| 7705 | CMV-pp65 | RPHERNGFTVL | HLA B*0701 | B*0701/RPHERNGFTVL-CMV-pp65 | Vβ12 |
| 7707 | CMV-pp65 | IPSINVHHY | HLA B*3501 | B*3501/IPSINVHHY-CMV-pp65 | Vβ1 |
| 7709 | CMV-pp65 | YSEHPTFTSQY | HLA A*0101 | A*0101/YSEHPTFTSQY-CMV-pp65 | Vβ13 |
| Patient . | Clone specificity . | Epitope . | HLA restriction . | Corresponding multimer . | Vβ usage . |
|---|---|---|---|---|---|
| HIV-specific CD8+ T cells | |||||
| 7701 | HIV-GAG | GEIYKRWII | HLA B*0801 | B*0801/GEIYKRWII- HIV-GAG | Vβ13 |
| 7702 | HIV-GAG | GEIYKRWII | HLA B*0801 | B*0801/GEIYKRWII- HIV-GAG | Vβ13 |
| 7703 | HIV-GAG | GEIYKRWII | HLA B*0801 | B*0801/GEIYKRWII- HIV-GAG | Vβ2 |
| 7704 | HIV-RT-POL | ILKEPVHGV | HLA A*0201 | A*0201/ILKEPVHGV-HIV-RT-POL | Vβ3 |
| 7705 | HIV-ENV | IPRRIRQGL | HLA B*0702 | B*0702/IPRRIRQGL-HIV-ENV | Vβ14 |
| 7707 | HIV-NEF | QVPLRPMTYK | HLA A*0301 | A*0301/QVPLRPMTYK-HIV-NEF | Vβ6 |
| 7709 | HIV-GAG | KRWIILGLNK | HLA B*2705 | B*2705/KRWIILGLNK-HIV-GAG | Vβ13 |
| CMV-specific CD8+ T cells | |||||
| 7701 | CMV-pp65 | NLVPMVATV | HLA A*0201 | A*0201/NLVPMVATV-CMV-pp65 | Vβ8 |
| 7702 | CMV-pp65 | NLVPMVATV | HLA A*0201 | A*0201/NLVPMVATV-CMV-pp65 | Vβ3 |
| 7703 | CMV-pp65 | NLVPMVATV | HLA A*0201 | A*0201/NLVPMVATV-CMV-pp65 | V8 |
| 7704 | CMV-pp65 | IPSINVHHY | HLA B*3501 | B*3501/IPSINVHHY-CMV-pp65 | Vβ3/13 |
| 7705 | CMV-pp65 | RPHERNGFTVL | HLA B*0701 | B*0701/RPHERNGFTVL-CMV-pp65 | Vβ12 |
| 7707 | CMV-pp65 | IPSINVHHY | HLA B*3501 | B*3501/IPSINVHHY-CMV-pp65 | Vβ1 |
| 7709 | CMV-pp65 | YSEHPTFTSQY | HLA A*0101 | A*0101/YSEHPTFTSQY-CMV-pp65 | Vβ13 |
Treatment plan
Patients received 3.3 × 109 HIV-specific and control CMV pp65-specific CD8+ T-cell clones/m2, 28 days apart. The second infusion was followed by 14 days of low-dose subcutaneous IL-2 (2.5 × 105 IU/m2 every 12 hours) as a means to increase in vivo persistence without toxicity.29 Patients were monitored with serial blood-draws on days 1, 3, 7, 14, 21, 28, and then monthly after infusions for a total of 6 months after the second infusion. Throughout the follow-up period, patients remained on HAART. In all patients, except for 7709 who had a small burst of viral replication 84 days after the last infusion, plasma HIV viremia remained below 30 copies/mL (data not shown).
Rectal specimens
Rectal mucosal biopsies were obtained through an anoscope 3-4 cm above the squamo-columnar junction before, 14 days after infusions 1 and 2, and at 100+ days after infusion 2. At each time point, 3 samples of mucosa were obtained at 12, 4, and 8 p.m. and placed in RPMI (Invitrogen). In addition,3 snostrips (Chauvin Pharmaceuticals) for CMV DNA quantification were saturated with rectal mucosal secretions as described.31
Flow cytometry
Infused autologous T-cell clones in PBMCs obtained after transfer were identified by binding to APC-conjugated specific pentamer constructs (Table 2), and analyzed by flow cytometry after staining with fluorochrome-conjugated mAbs to CD3, CD4, CD16, CD19, CD8, CD28, CD27, CD62L, CCR7, CD45RA, CD45RO, CD137 (4-1BB), CD132 (IL-2Rγ), CD127 (IL7Rα), CD57, and PD-1 (BD PharMingen). Intracellular cytokine expression of IFNγ, TNFα, and IL-2 by pentamer+CD8+ T cells pulsed for 4-5 hours with relevant peptide were performed as described.32 Cells were analyzed on an LSRII (BD Biosciences) using FACSDiva software.
Cytotoxicity assays
Cytotoxic responses of HIV Ag-specific T cells were examined as described.18
Synthesis of Qdot multimers
Peptide–MHC multimeric complexes (pMHCs) were formed in vitro and conjugated to Qdots as described.33,34 Constructs used in this study included B*0801/GEIYKRWII(HIV-GAG) and A*0201/NLVPMVATV(CMV-pp65) for patients 7701, 7702, and 7703; B*0702/IPRRIRQGL(HIV-ENV) and B*0701/RPHERNGFTVL(CMV-pp65) for patient 7705; A*0301/QVPLRPMTYK(HIV-NEF) and B*3501/IPSINVHHY(CMV-pp65) for patient 7707; B*2705/KRWIILGLNK(HIV-GAG) and A*0101/YSEHPTFTSOY(CMV-pp65) for patient 7709.
In situ immunofluorescence staining
Detection and quantification of HIV- and CMV-specific CD8+ T cells in tissue biopsies
A confocal microscope (LSM 510; Carl Zeiss MicroImaging) or fluorescence microscopy (Nikon Eclipse TE 2000-S; Nikon Instruments Inc) was used. Qdot655+CD8+ double-positive T cells were visualized in fresh tissue biopsies 460 × 460 μm in size and 30 μm deep at room temperature. At least 2 sections were prepared for each cryopreserved biopsy and 5 fields were analyzed for each tissue section. Cells were enumerated in tissue fields of 460 × 460 μm in size and 7-μm deep by using a particle counting algorithm (ImageJ). Excitation lasers, detection bands, and the beam splitter parameters for the confocal microscopy were as described.34 CD8+ T cells/mm2, Qdot655-multimer+cells/mm2 and the percentage of Qdot655-multimer+ cells in the CD8+ T-cell population were calculated. Images were acquired with a Photometrix, CoolSNAP HQ camera (Photometrix).
PCR
Primers flanking the CDR3 region of infused HIV- and CMV-specific CD8+ T-cell clones were designed for patients 7701 (constant region C3 [ATCATAAATTCGGGTAGGATCC] and Vβ13.2 [AGGGTACCACTGACAAAGGAGA] for the HIV clone, Vβ8.1 [ACTTTAACAACAAGCTTCCG] for the CMV clone), 7704 (C3 and Vβ3 [GTCTCTAGAGAGAAGAAGGAGGCG] for both HIV and CMV clones) and 7707 (C3 and Vβ6.2 [AGGCCTAAGGGATCTTTCTC] for the HIV clone and Vβ1 [GCACAACAGTTCCCTGACTT] for the CMV clone), and used in TaqMan assays as an additional strategy to detect infused T cells in PBMCs and rectal biopsies collected after transfer. A TaqMan assay designed for the CD8β chain was used concurrently on the same cDNA (0.3-1 μg) isolated from samples (CD8βF: ATCTACTGGCTGAGACAGCGC; CD8βR: GCCAGGAACTCGTGGTGACT). Based on the assumption that each T cell makes proportionally the same relative number of copies of CD8β and TCR, this method was used to interpret the number of TCR copies as a percent of CD8+ T cells.
Results
HIV-specific CD8+ T-cell lines and clones that express CD28 can be generated from patients receiving HAART
Lines that contained the highest fraction of virus-specific CD28+CD8+ T cells were selected for cloning by limiting dilution (Figure 1A and supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Clones selected for infusions demonstrated specific lytic activity for the stimulating viral epitope in a 51Cr-release assay and IFNγ secretion in response to peptide-pulsed autologous B-lymphoblastoid cell lines (B-LCL), as well as binding to a designed HLA-pentamer (Figure 1B and supplemental Figure 1B). HIV peptide-specific clonal populations contained variable levels of CD28+ cells, ranging from 0.8% to 98.1% (Figure 1C). The mean frequency of CD28+ cells in CMV-specific T-cell lines, which were generated as specificity controls for analysis of the localization of HIV-specific CD8+ T cells to the GALT, was similar to that of HIV-specific CD8+ T-cell lines (80.25% vs 80.52%; supplemental Figure 1A). All clones, irrespective of specificity expressed CD3, CD8, and CD45RO but not CD4, CD16, CD19 (not shown), and CD45RA, consistent with an Ag-experienced but not terminally differentiated phenotype. Clones were also negative for CD62L, CCR7, and CD127 (Figure 1D) as well as CD137, CD132 (IL-2Rγ), CD57, and PD-1 (not shown). All clones lacked expression of CD103 and CCR9, molecules associated with migration to the GALT. However, clones exhibited a narrow coordinate expression of the individual α4 and β7 integrin chains compared with PBMCs (Abs to the human α4β7 dimer were unavailable) and also expressed CD11a, b, and c (Figure 1E and supplemental Figure 1E). Thus, in contrast to studies with transfer of in vitro–generated CD8+ T-cell clones in macaques,21,36,37 we found that some HIV- and CMV-CD8+ T clones isolated from HIV+ patients analyzed immediately before infusion expressed CD28 on at least a fraction of the clonal population. This may reflect species differences and/or the somewhat different procedure methods used to derive and expand the human clones compared with macaque clones.21
Phenotypic and functional characterization of HIV-specific CD8+ T-cell lines and clones isolated and expanded for infusion. (A) Percentages of HIV-specific CD8+ T cells in each cell line (left column) from which each infused clone was ultimately derived. Specific pentamer binding (y-axis) and CD8 (x-axis) are shown. Expression of CD28 (right column) on gated HIV-specific pentamer+CD8+ T cells (bold line) compared with isotype control (gray line). Inset values represent percentages of CD28+CD8+ T cells. (B) Data for patient 7705 is shown (HLA B*0702/IPRRIRQGL-HIV-ENV) and is representative of HIV-specific CD8+ T-cell clones used for adoptive transfer. Lytic activity (top panel) of the HIV-specific CD8+ T-cell clone to autologous B-LCL pulsed with decreasing concentrations of peptide, IFNγ secretion (bottom left panel), and binding to the corresponding MHC-peptide pentamer (bottom right panel) is shown. (C) Expression of CD28 on clonal HIV-specific CD8+ T cells infused (bold line) for each patient compared with isotype control (gray line). Inset values represent percentages of CD28+CD8+ T cells. (D) Expression of CD45RA, CD45RO, CD62L, CCR7, and CD127 (bold line) compared with isotype control (gray line) on a representative CD8+ T-cell clone used for infusion (7705). (E) Expression of CD103, CCR9, CD11a, CD11b, CD11c, integrin α4 and integrin β7 (bold line) compared with isotype control (gray line). The bottom right panel shows coexpression of integrin α4 and integrin β7 on the clone (black dots) compared with PBMC (gray dots).
Phenotypic and functional characterization of HIV-specific CD8+ T-cell lines and clones isolated and expanded for infusion. (A) Percentages of HIV-specific CD8+ T cells in each cell line (left column) from which each infused clone was ultimately derived. Specific pentamer binding (y-axis) and CD8 (x-axis) are shown. Expression of CD28 (right column) on gated HIV-specific pentamer+CD8+ T cells (bold line) compared with isotype control (gray line). Inset values represent percentages of CD28+CD8+ T cells. (B) Data for patient 7705 is shown (HLA B*0702/IPRRIRQGL-HIV-ENV) and is representative of HIV-specific CD8+ T-cell clones used for adoptive transfer. Lytic activity (top panel) of the HIV-specific CD8+ T-cell clone to autologous B-LCL pulsed with decreasing concentrations of peptide, IFNγ secretion (bottom left panel), and binding to the corresponding MHC-peptide pentamer (bottom right panel) is shown. (C) Expression of CD28 on clonal HIV-specific CD8+ T cells infused (bold line) for each patient compared with isotype control (gray line). Inset values represent percentages of CD28+CD8+ T cells. (D) Expression of CD45RA, CD45RO, CD62L, CCR7, and CD127 (bold line) compared with isotype control (gray line) on a representative CD8+ T-cell clone used for infusion (7705). (E) Expression of CD103, CCR9, CD11a, CD11b, CD11c, integrin α4 and integrin β7 (bold line) compared with isotype control (gray line). The bottom right panel shows coexpression of integrin α4 and integrin β7 on the clone (black dots) compared with PBMC (gray dots).
HIV-specific CD8+ T-cell clones isolated from T-cell lines containing CD28+ cells can persist in vivo after transfer
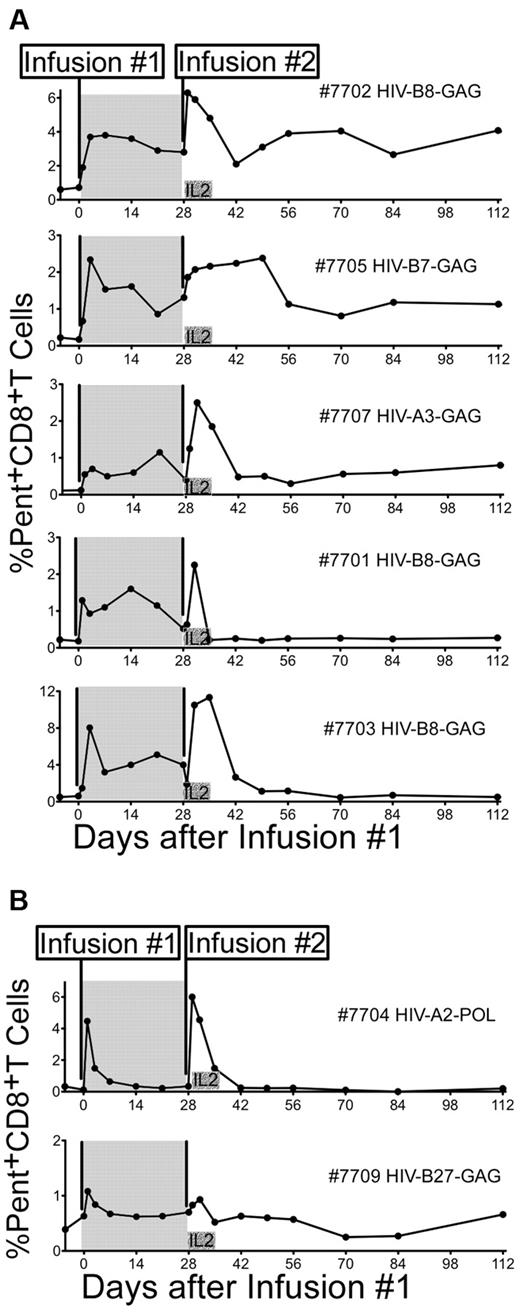
Seven patients receiving HAART were infused twice, 28 days apart, with 3.3 × 109 cells of the same monoclonal HIV-specific (Table 2 and Figure 1) and CMV-specific CD8+ T-cell clone (Table 2 and supplemental Figure 1). The sensitivity of pentamer staining was fixed at 0.1% of total CD8+ T cells, below which the capacity to distinguish between transferred pentamer+ cells and background was diminished. Persistence was calculated as the last time point at which pentamer+ T cells were 2 times background levels or > 0.1%. Patients had varying levels of preexisting HIV-specific pentamer+ T cells ranging from < 0.1% to 0.66% of total CD8+ T cells (mean 0.41%). Frequencies of endogenous CMV-specific T cells binding to pentamer were generally higher before infusions (range 1.3%-4.48%, mean 2.03%), and did not reach 2 times background levels after infusions. Because pentamer binding could not distinguish infused CMV-specific T cells from endogenous CMV-specific T cells, persistence of infused CMV-specific T cells in the peripheral blood could not be accurately monitored. In 5 of 7 patients, infused HIV-specific CD8+ T-cell clones without subcutaneous exogenous IL-2 persisted for at least 21 days, based on MHC class I/peptide pentamer staining (Figure 2A shaded area). Persistence beyond 28 days could not be directly assessed (second T-cell infusion).
In vivo persistence of HIV-specific CD8+ T-cell clones isolated from CD28-expressing CD8+ T-cell lines. (A shaded area) The percentage of pentamer+CD8+ T cells (x-axis) detected in PBMCs collected −7 days (+/−2 days), immediately before and after infusions are shown for 5 (7701, 7702, 7703, 7705, and 7707) of 7 infused HIV-specific CD8+ T-cell clones that showed persistence for ≥ 21 days after the first infusion. Persistence was calculated as the last time point at which pentamer+CD8+ T cells were 2 times background levels or > 0.1%. (A nonshaded area) The percentage of pentamer+CD8+ T cells after each infusion and up to 112 days after the first infusion is shown for the same patients. (B) Two patients (7704 and 7709) that showed a rapid disappearance of the infused clones within 3 days of both infusions are shown.
In vivo persistence of HIV-specific CD8+ T-cell clones isolated from CD28-expressing CD8+ T-cell lines. (A shaded area) The percentage of pentamer+CD8+ T cells (x-axis) detected in PBMCs collected −7 days (+/−2 days), immediately before and after infusions are shown for 5 (7701, 7702, 7703, 7705, and 7707) of 7 infused HIV-specific CD8+ T-cell clones that showed persistence for ≥ 21 days after the first infusion. Persistence was calculated as the last time point at which pentamer+CD8+ T cells were 2 times background levels or > 0.1%. (A nonshaded area) The percentage of pentamer+CD8+ T cells after each infusion and up to 112 days after the first infusion is shown for the same patients. (B) Two patients (7704 and 7709) that showed a rapid disappearance of the infused clones within 3 days of both infusions are shown.
Coadministration of IL-2 augments peak frequencies of adoptively transferred HIV-specific CD8+ T cells in vivo but has no effect on long-term persistence
The peak frequencies of CD8+ T cells achieved in peripheral blood 24-72 hours after infusions followed by low-dose subcutaneous IL-2 were higher (P = .05) in comparison to infusions of the T-cell clones alone (supplemental Figure 2). However, and in contrast to previous observations by our group with infusions of effector clones,29 the frequency of persisting transferred HIV-specific CD8+ T cells at day 14 and at day 21 after infusion 2 (4 of 7 compared with 5 of 7 patients and 3 of 7 compared with 5 of 7 patients after infusion 1, respectively) was not significantly different from the frequency of persisting transferred HIV-specific CD8+ T cells without IL-2 administration. Of the 5 infused HIV-specific T-cell clones that persisted at least 21 days after infusion 1, 3 remained detectable in the peripheral blood for at least 84 days after infusion 2, and 2, despite measurable persistence after infusion 1, persisted only for a short period of time after infusion 2 (3 and 7 days, respectively; Figure 2A). The results obtained with MHC pentamers were confirmed by RT-PCR for clone-specific TCR Vβ CDR3 regions (Table 2) in 2 patients (supplemental Figure 3). The 2 remaining patients showed persistence for at most 3 days after the first and second infusion (Figure 2B). Phenotyping of the clones before infusion and in vivo early after transfer could not distinguish the clones that ultimately persisted from the clones that exhibited a short in vivo survival.
HIV-specific CD8+ T cells show phenotypic characteristics associated with CD8+ Tcm over time after transfer in vivo
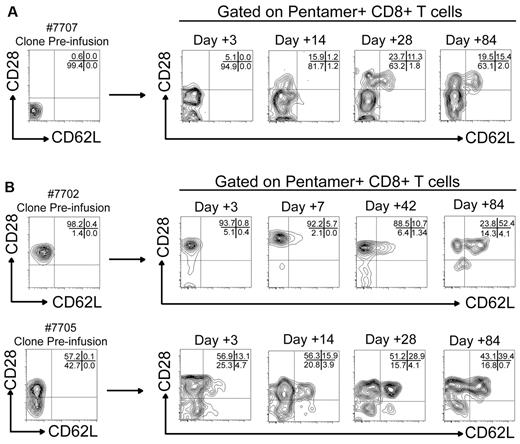
Of the 3 HIV-specific CD8+ T-cell clones that exhibited prolonged in vivo survival in the peripheral blood (7702, 7705, and 7707), one HIV-specific CD8+ T-cell clone (7707) expressed no CD28 or CD62L at the time of transfer (Figure 3A). However, gating on pentamer+CD8+ T cells from PBMC on days 3, 14, 28, and 84 after infusion 2 revealed an increasing fraction of the cells persisting from the infused clone expressed CD28 (15.9% at day 14, 35% at day 28, and 34.9% at day 84). In addition, a subset of the clonal subpopulation that now expressed CD28 also exhibited CD62L expression after 28 and 84 days (32.2% and 44.1% of pentamer+CD28+CD8+ T cells, respectively). CD28 was expressed before infusion on 98.2% and 57.2% of the clonal populations of patients 7702 and 7705, respectively. On all cells isolated after transfer from subject 7702, CD28 was detectable throughout the monitoring period, and on increasing numbers of cells from 7705, such that > 82% of cells were CD28+ at 84 days after transfer (Figure 3B). Expression of CD62L also progressively increased over time on the fraction of the infused cells that were CD8+CD28+ reaching 52% and 39%, respectively, at 84 days. Although CD8+ T-cell clones transferred into patients in this study were not specifically isolated from populations pre-sorted for a Tcm phenotype as has been reported with macaque studies,21 the clones were derived from specific T-cell lines which contained a high frequency of CD28, and the results suggest a fraction of the clones may have been derived from CD8+ Tcm.
Adoptively transferred HIV-specific CD8+ T cells show phenotypic characteristics associated with CD8+ Tcm over time after transfer in vivo. (A) Expression of CD28 (x-axis) and CD62L (y-axis) on pentamer+ cells from patient 7707 before and after transfer. The phenotype of pentamer+ T cells (A*0301/QVPLRPMTYK-HIV-NEF) analyzed by flow cytometry 3, 14, 28, and 84 days after the second infusion are shown. Percentages of pentamer+CD8+ T cells that express CD28 and CD62L in each gated quadrant are shown in the top right aspect of each plot. (B top panel) Expression of CD28 (x-axis) and CD62L (y-axis) on pentamer+CD8+ T cells (B*0801/GEIYKRWII-HIV-GAG) from patient 7702 obtained 3, 7, 42, and 84 days after the second infusion. (B bottom panel) Pentamer+CD8+ T cells (HLA B*0702/IPRRIRQGL-HIV-ENV) from patient 7705 obtained 3, 14, 28, and 84 days after the second infusion.
Adoptively transferred HIV-specific CD8+ T cells show phenotypic characteristics associated with CD8+ Tcm over time after transfer in vivo. (A) Expression of CD28 (x-axis) and CD62L (y-axis) on pentamer+ cells from patient 7707 before and after transfer. The phenotype of pentamer+ T cells (A*0301/QVPLRPMTYK-HIV-NEF) analyzed by flow cytometry 3, 14, 28, and 84 days after the second infusion are shown. Percentages of pentamer+CD8+ T cells that express CD28 and CD62L in each gated quadrant are shown in the top right aspect of each plot. (B top panel) Expression of CD28 (x-axis) and CD62L (y-axis) on pentamer+CD8+ T cells (B*0801/GEIYKRWII-HIV-GAG) from patient 7702 obtained 3, 7, 42, and 84 days after the second infusion. (B bottom panel) Pentamer+CD8+ T cells (HLA B*0702/IPRRIRQGL-HIV-ENV) from patient 7705 obtained 3, 14, 28, and 84 days after the second infusion.
CD28 expression by adoptively transferred HIV-specific CD8+ T-cell clones is associated with IL-2 secretion after specific antigenic exposure
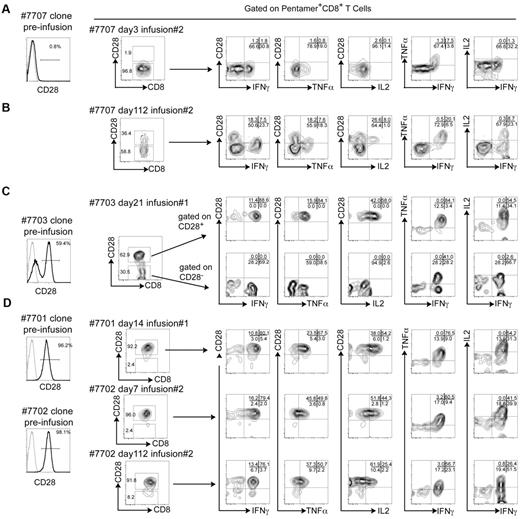
Infused HIV-specific CD8+ T-cell clones secreted IFNγ and TNFα in the presence of their cognate peptide as analyzed ex vivo after transfer, regardless of CD28 expression. However, only the CD28+CD8+ subset was additionally capable of secreting IL-2 (Figure 4). Patient 7707 was infused with a HIV-specific CD8+ T-cell clone uniformly lacking CD28 expression. When analyzed ex vivo early after transfer (3 days), the cells derived from the clone remained almost entirely CD28− (96.8%), and after stimulation with the cognate peptide (Table 2) produced TNFα and IFNγ but not IL-2 (Figure 4A). The same analysis performed on cells acquired 112 days after infusion 2 showed that a portion of cells now expressed CD28, and only cells from this fraction secreted IL-2 after stimulation with the cognate peptide (Figure 4B). Patient 7703 received a HIV-specific CD8+ T-cell clone that contained both CD28+ and CD28− fractions. When analyzed ex vivo 21 days after infusion 1, both positive and negative fractions remained detectable, and after stimulation with cognate peptide again only the subset of cells that expressed CD28 produced IL-2 (Figure 4C). Similar results were obtained for patient 7705, who also received a CD8+ T-cell clone containing CD28− and CD28+ fractions (data not shown). Patients 7701 and 7702 received HIV-specific CD8+ T-cell clones that were nearly uniformly CD28+ (96.2% and 98.1%, respectively). Ex vivo stimulation of cells derived from these clones with cognate peptide 14 days after infusion 1 (7701), 7 and 112 days after infusion 2 (7702), induced production of IFNγ, TNFα, and IL-2 (Figure 4D). IL-2 production was only detected in the fraction of HIV-specific CD8+ T cells that expressed CD28.
CD28 expression by adoptively transferred HIV-specific CD8+ T-cell clones is associated with IL-2 secretion after stimulation by specific Ag. (A) Expression of CD28 on the HIV-specific CD8+ T-cell clone before infusion (left histogram) for patient 7707 (A*0301/QVPLRPMTYK-HIV-NEF). Whole PBMCs were obtained at day 3 after the 2nd infusion and stained with specific pentamer (A*0301/QVPLRPMTYK-HIV-NEF). The expression of CD28 ex vivo on pentamer+ T cells is shown (plot to the right of the histogram). Cells were stimulated with specific peptide (QVPLRPMTYK) and pentamer+CD8+ T cells analyzed for the surface expression of CD28 and intracellular production of IFNγ, TNFα, and IL-2 (rightmost plots). (B) The same analysis was performed on whole PBMCs obtained at day 112 after transfer of the HIV-specific clone into patient 7707. (C) Data on PBMCs that were obtained on day 21 after the first infusion from patient 7703 (B*0801/GEIYKRWII-HIV-GAG) are shown. Analysis for pentamer+CD28+ and CD28− subpopulations are shown individually. (D) Data for patients 7701 and 7702 (B*0801/GEIYKRWII-HIV-GAG) are shown on PBMC that were obtained 14 days after the first infusion (7701), 7 and 112 days after the second infusion (7702), after stimulation with the cognate peptide (GEIYKRWII). Pentamer−CD8+ T cells in the same assays did not produce IFNγ, TNFα, or IL-2 (not shown).
CD28 expression by adoptively transferred HIV-specific CD8+ T-cell clones is associated with IL-2 secretion after stimulation by specific Ag. (A) Expression of CD28 on the HIV-specific CD8+ T-cell clone before infusion (left histogram) for patient 7707 (A*0301/QVPLRPMTYK-HIV-NEF). Whole PBMCs were obtained at day 3 after the 2nd infusion and stained with specific pentamer (A*0301/QVPLRPMTYK-HIV-NEF). The expression of CD28 ex vivo on pentamer+ T cells is shown (plot to the right of the histogram). Cells were stimulated with specific peptide (QVPLRPMTYK) and pentamer+CD8+ T cells analyzed for the surface expression of CD28 and intracellular production of IFNγ, TNFα, and IL-2 (rightmost plots). (B) The same analysis was performed on whole PBMCs obtained at day 112 after transfer of the HIV-specific clone into patient 7707. (C) Data on PBMCs that were obtained on day 21 after the first infusion from patient 7703 (B*0801/GEIYKRWII-HIV-GAG) are shown. Analysis for pentamer+CD28+ and CD28− subpopulations are shown individually. (D) Data for patients 7701 and 7702 (B*0801/GEIYKRWII-HIV-GAG) are shown on PBMC that were obtained 14 days after the first infusion (7701), 7 and 112 days after the second infusion (7702), after stimulation with the cognate peptide (GEIYKRWII). Pentamer−CD8+ T cells in the same assays did not produce IFNγ, TNFα, or IL-2 (not shown).
Adoptively transferred HIV-specific CD8+ T cells that express CD28 are capable of proliferation after specific Ag exposure in the absence of added exogenous cytokines
CD8+ Tcm cells have the ability to promote autocrine proliferative responses and expand on re-encounter with Ag. We therefore asked whether the expression of CD28 in vivo on infused HIV-specific CD8+ T-cell clones was associated with the ability of clones to proliferate ex vivo after exposure to cognate Ag in the absence of added exogenous cytokines. Whole PBMCs were collected after T-cell transfer, stained with CSFE and incubated in the presence of cognate peptide. Proliferation was assessed ex vivo by analyzing dilution of CFSE on infused HIV-specific pentamer+CD8+ T-cell clones. Patients 7701 and 7702 received HIV-specific CD8+ T-cell clones that were nearly uniformly CD28+ before and after transfer. Ex vivo, the infused clones within PBMC collected 14 days and 3 days, respectively, after infusion 1, proliferated extensively based on CFSE dilution at 5 and 7 days in the absence of supplemental cytokines (Figure 5A). In addition, the pentamer+CD28+ T cells remained CD28+ throughout the divisions (Figure 5A middle panels). We next evaluated cells derived from patients (7703 and 7705) that had received HIV-specific CD8+ T-cell clones containing both a CD28+ and CD28− fraction before and after transfer. After stimulation with cognate peptide, only the T-cell fractions that expressed CD28 diluted CFSE ex vivo in the absence of supplemental cytokines, and by the time of analysis of the proliferative response the CD28− fraction could no longer be detected (Figure 5B). The same analysis was performed on PBMCs collected from patient 7707 3 days after transfer of a CD28− clone. After 7 days ex vivo, pentamer-binding HIV-specific CD28− T cells could not be detected after stimulation without supplemental IL-2 (Figure 5C), but providing exogenous IL-2 supported the proliferation of the CD28−CD8+ T cells. CD28 was detected in vivo on a subpopulation of this infused clone (Figure 5D), and again only the CD28+ fraction had the ability to proliferate in response to cognate Ag without the addition of exogenous IL-2 (Figure 5D left panel). The addition of IL-2 to ex vivo cultures promoted the survival and proliferation of both CD28+ and CD28− fractions (5D right panel). These ex vivo results suggest that expression of CD28 on HIV-specific CD8+ T-cell clones would provide the cells with the ability to proliferate in vivo if Ag is encountered without requiring another cell such as a CD4+ Thelper cell to provide exogenous IL-2.
Adoptively transferred HIV-specific CD8+ T cells that express CD28 are capable of proliferation after specific Ag exposure in the absence of supplemental exogenous cytokines. All plots are gated on pentamer+ T cells or as otherwise indicated. (A-B) PBMCs from patients 7701 and 7702 collected, respectively, 14 and 3 days after the first infusion and PBMCs from patients 7703 and 7705 collected, respectively, 21 and 3 days after the first infusion were stained with CFSE and incubated in the presence of cognate peptide (B*0801/GEIYKRWII-HIV-GAG for 7701-7703 and B*0702/IPRRIRQGL-HIV-ENV for 7705). Expression of CD28 on pentamer+CD8+ T cells (plots in the top row) is shown. Plots in the second and third rows show CFSE dilution after 5 and 7 days ex vivo (x-axis) relative to CD28 expression (y-axis). Dilution of CFSE after 7 days ex vivo is shown (histogram, bottom row). (C) Pentamer+CD8+ T cells in PBMCs from patient 7707 collected 3 days after the second infusion and after 7 days of ex vivo stimulation with cognate peptide (A*0301/QVPLRPMTYK-HIV-NEF; left panel). The same experiment performed with the addition of exogenous IL-2 50 IU/mL (right panel). (D) CFSE dilution of detected pentamer+CD8+ T cells PBMC from patient 7707 collected 84 days after the second infusion and after 7 days of ex vivo stimulation with cognate peptide with (right panel) or without IL-2 (left panel).
Adoptively transferred HIV-specific CD8+ T cells that express CD28 are capable of proliferation after specific Ag exposure in the absence of supplemental exogenous cytokines. All plots are gated on pentamer+ T cells or as otherwise indicated. (A-B) PBMCs from patients 7701 and 7702 collected, respectively, 14 and 3 days after the first infusion and PBMCs from patients 7703 and 7705 collected, respectively, 21 and 3 days after the first infusion were stained with CFSE and incubated in the presence of cognate peptide (B*0801/GEIYKRWII-HIV-GAG for 7701-7703 and B*0702/IPRRIRQGL-HIV-ENV for 7705). Expression of CD28 on pentamer+CD8+ T cells (plots in the top row) is shown. Plots in the second and third rows show CFSE dilution after 5 and 7 days ex vivo (x-axis) relative to CD28 expression (y-axis). Dilution of CFSE after 7 days ex vivo is shown (histogram, bottom row). (C) Pentamer+CD8+ T cells in PBMCs from patient 7707 collected 3 days after the second infusion and after 7 days of ex vivo stimulation with cognate peptide (A*0301/QVPLRPMTYK-HIV-NEF; left panel). The same experiment performed with the addition of exogenous IL-2 50 IU/mL (right panel). (D) CFSE dilution of detected pentamer+CD8+ T cells PBMC from patient 7707 collected 84 days after the second infusion and after 7 days of ex vivo stimulation with cognate peptide with (right panel) or without IL-2 (left panel).
Infused HIV-specific CD8+ T-cell clones survey the rectal mucosa
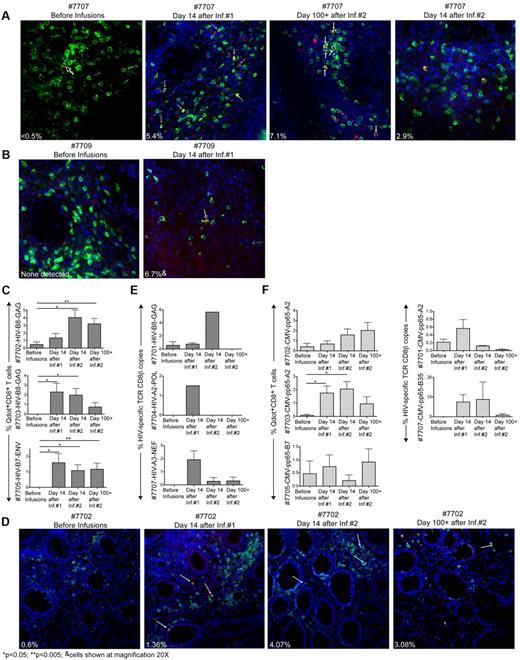
Patients consented to 4 sets of rectal biopsies performed before, 14 days after each infusion, and at a late time point 100+ days after the second infusion. Concurrent with the infusion of HIV-specific CD8+ T-cell clones, each patient was also infused with an autologous pp65-CMV–specific CD8+ T-cell clone as a comparative control for the ability to enter and accumulate in the rectal mucosa. Three different methods were used to determine the accumulation of HIV and CMV-specific clones in the rectal mucosa depending on the availability of specific reagents and the total amount of tissue obtained: staining with Qdot-labeled multimers on fresh tissue as a first choice (Qdot reagents were available for patients 7707 and 7709 when fresh tissue was collected), Qdot analysis on cryopreserved tissue as a second choice,35 or quantitative RT-PCR for the TCR genes (in settings in which Qdots were not available or in a selected individual for confirmation of Qdot data; Table 3). For patient 7707, clusters of HIV-NEF–specific Qdot655+CD8+ T cells not detectable before infusions were identified in the submucosa of rectal biopsies taken 14 days after the first, second, and 100+ days after infusion 2 (Figure 6A). These results were also confirmed by RT-PCR for the specific TCR gene (discussed in this paragraph). In patient 7709, HIV-GAG–specific Qdot655+CD8+ T cells not present before infusions were identified in the submucosa of rectal biopsies taken at 14 days after infusion 1 (∼6.7% of total CD8+ T cells), but were not found in subsequent biopsies (Figure 6B). Analysis of Qdot staining performed on cryopreserved biopsies of patients 7702, 7703, and 7705 demonstrated an increase in the frequencies of HIV-specific clones in rectal biopsies after the infusions, and for all 3 patients cells remained detectable at day 100+ after infusion 2 (Figure 6C-D). In the 3 patients analyzed by RT-PCR (7701, 7704, and 7707), 2 rectal samples were evaluated at each time point with the average of 2 amplifications computed (Figure 6E). Thus, an increase from baseline levels suggesting accumulation of transferred HIV-specific T cells in rectal mucosal tissue was detected in 7 of 7 patients after at least one of the 2 infusions evaluated either by Qdot staining or TCR-specific RT-PCR and in all rectal biopsies of patient 7707 obtained after infusions. HIV-specific T cells were detected in rectal biopsies obtained at 100+ days in 3 of 3 patients whose T cells were also detected in the peripheral blood for ≥ 84 days (patients 7702, 7705, and 7707) and 1 of 4 patients whose T cells had disappeared from the peripheral blood within 14 days after infusion 2 (patient 7703; Figure 2).
Methods used to determine the accumulation of transferred HIV- and CMV-specific T cells in the rectal mucosa
| Patient no. . | Clone specificity . | Epitope . | HLA restriction . | Vβ usage . | Method used to detect clones in rectal biopsies . | ||
|---|---|---|---|---|---|---|---|
| Quantitative TCR-PCR . | Qdot on fresh tissue . | Qdot on cryopreserved tissue . | |||||
| 7701 | HIV-GAG | GEIYKRWII | HLA B*0801 | Vβ13 | X | Not available† | |
| 7701 | CMV-pp65 | NLVPMVATV | HLA A*0201 | Vβ8 | X | Not available† | |
| 7702 | HIV-GAG | GEIYKRWII | HLA B*0801 | Vβ13 | Not available† | X | |
| 7702 | CMV-pp65 | NLVPMVATV | HLA A*0201 | Vβ3 | Not available† | X | |
| 7703 | HIV-GAG | GEIYKRWII | HLA B*0801 | Vβ2 | Not available† | X | |
| 7703 | CMV-pp65 | NLVPMVATV | HLA A*0201 | Vβ8 | Not available† | X | |
| 7704 | HIV-RT-POL | ILKEPVHGV | HLA A*0201 | Vβ3 | X | Not available† | |
| 7704 | CMV-pp65 | IPSINVHHY | HLA B*3501 | Vβ3/13 | Not done‡ | Not available† | |
| 7705 | HIV-ENV | IPRRIRQGL | HLA B*0702 | Vβ14 | Not available† | X | |
| 7705 | CMV-pp65 | RPHERNGFTVL | HLA B*0701 | Vβ12 | Not available† | X | |
| 7707 | HIV-NEF | QVPLRPMTYK | HLA A*0301 | Vβ6 | X | X | |
| 7707 | CMV-pp65 | IPSINVHHY | HLA B*3501 | Vβ1 | X | Not available† | |
| 7709 | HIV-GAG | KRWIILGLNK | HLA B*2705 | Vβ13 | Not done‡ | X | |
| 7709 | CMV-pp65 | YSEHPTFTSQY | HLA A*0101 | Vβ13 | Not done‡ | Not available† | |
| Patient no. . | Clone specificity . | Epitope . | HLA restriction . | Vβ usage . | Method used to detect clones in rectal biopsies . | ||
|---|---|---|---|---|---|---|---|
| Quantitative TCR-PCR . | Qdot on fresh tissue . | Qdot on cryopreserved tissue . | |||||
| 7701 | HIV-GAG | GEIYKRWII | HLA B*0801 | Vβ13 | X | Not available† | |
| 7701 | CMV-pp65 | NLVPMVATV | HLA A*0201 | Vβ8 | X | Not available† | |
| 7702 | HIV-GAG | GEIYKRWII | HLA B*0801 | Vβ13 | Not available† | X | |
| 7702 | CMV-pp65 | NLVPMVATV | HLA A*0201 | Vβ3 | Not available† | X | |
| 7703 | HIV-GAG | GEIYKRWII | HLA B*0801 | Vβ2 | Not available† | X | |
| 7703 | CMV-pp65 | NLVPMVATV | HLA A*0201 | Vβ8 | Not available† | X | |
| 7704 | HIV-RT-POL | ILKEPVHGV | HLA A*0201 | Vβ3 | X | Not available† | |
| 7704 | CMV-pp65 | IPSINVHHY | HLA B*3501 | Vβ3/13 | Not done‡ | Not available† | |
| 7705 | HIV-ENV | IPRRIRQGL | HLA B*0702 | Vβ14 | Not available† | X | |
| 7705 | CMV-pp65 | RPHERNGFTVL | HLA B*0701 | Vβ12 | Not available† | X | |
| 7707 | HIV-NEF | QVPLRPMTYK | HLA A*0301 | Vβ6 | X | X | |
| 7707 | CMV-pp65 | IPSINVHHY | HLA B*3501 | Vβ1 | X | Not available† | |
| 7709 | HIV-GAG | KRWIILGLNK | HLA B*2705 | Vβ13 | Not done‡ | X | |
| 7709 | CMV-pp65 | YSEHPTFTSQY | HLA A*0101 | Vβ13 | Not done‡ | Not available† | |
Qdot reagents not available at the time fresh biopsies were obtained.
Specific TaqMan probes could not be designed (short and/or G-rich regions within the rearranged TCR genes).
Infused HIV-specific CD8+ T-cell clones survey the rectal mucosa. (A-B) Fluorescence images of fresh rectal tissue sections (30 μm) from patient 7707 (A) and 7709 (B) stained with DAPI in blue, CD8 in green and Qdot655 multimer A*0301/QVPLRPMTYK-HIV-NEF for patient 7707 and Qdot655 multimer B*2705/KRWIILGLNK-HIV-GAG for patient 7709 in red. Arrows indicate Qdot655+CD8+cells (yellow). Inset percentage values reflect Qdot655+CD8+ T cells as a percent of total CD8+ T cells. (C) Cryopreserved rectal tissue sections stained concurrently with CD8 and the corresponding HIV-specific Qdot655 multimer B*0801/GEIYKRWII-HIV-GAG for 7702 and 7703; B*0702/IPRRIRQGL-HIV-ENV for 7705. Ten fields of 1 mm2 spanning 2 rectal biopsies per time point were enumerated for CD8+Qdot655+ T cells and results expressed as a percentage of total CD8+ T cells. (D) Fluorescence images of cryopreserved rectal tissue sections (7 μm) from patient 7702 stained with DAPI in blue, CD8 in green, and Qdot 655 multimer. B*0801/GEIYKRWII-HIV-GAG in red. Inset values represent the average of 10 counted fields. (E) Frequencies of HIV-specific T cells in rectal biopsies for patients 7701, 7704, and 7707 expressed as a percent HIV-specific Vβ copies relative to CD8β copies. Results are an average of 2 independent rectal samples taken at each time point. (F) CMV-specific CD8+ T cell clones quantified in rectal biopsies from patients 7701 and 7707 (TCR-specific RT-PCR) and CMV-specific CD8+ T-cell clones in rectal biopsies from patients 7702, 7703, and 7707 (specific Qdot655 multimers). One-tailed, paired Student t tests were used for statistical analysis.
Infused HIV-specific CD8+ T-cell clones survey the rectal mucosa. (A-B) Fluorescence images of fresh rectal tissue sections (30 μm) from patient 7707 (A) and 7709 (B) stained with DAPI in blue, CD8 in green and Qdot655 multimer A*0301/QVPLRPMTYK-HIV-NEF for patient 7707 and Qdot655 multimer B*2705/KRWIILGLNK-HIV-GAG for patient 7709 in red. Arrows indicate Qdot655+CD8+cells (yellow). Inset percentage values reflect Qdot655+CD8+ T cells as a percent of total CD8+ T cells. (C) Cryopreserved rectal tissue sections stained concurrently with CD8 and the corresponding HIV-specific Qdot655 multimer B*0801/GEIYKRWII-HIV-GAG for 7702 and 7703; B*0702/IPRRIRQGL-HIV-ENV for 7705. Ten fields of 1 mm2 spanning 2 rectal biopsies per time point were enumerated for CD8+Qdot655+ T cells and results expressed as a percentage of total CD8+ T cells. (D) Fluorescence images of cryopreserved rectal tissue sections (7 μm) from patient 7702 stained with DAPI in blue, CD8 in green, and Qdot 655 multimer. B*0801/GEIYKRWII-HIV-GAG in red. Inset values represent the average of 10 counted fields. (E) Frequencies of HIV-specific T cells in rectal biopsies for patients 7701, 7704, and 7707 expressed as a percent HIV-specific Vβ copies relative to CD8β copies. Results are an average of 2 independent rectal samples taken at each time point. (F) CMV-specific CD8+ T cell clones quantified in rectal biopsies from patients 7701 and 7707 (TCR-specific RT-PCR) and CMV-specific CD8+ T-cell clones in rectal biopsies from patients 7702, 7703, and 7707 (specific Qdot655 multimers). One-tailed, paired Student t tests were used for statistical analysis.
The accumulation of CMV-specific clones in rectal tissues was also assessed, using when possible the same method used to assess the presence of HIV-specific clones (quantitative TCR RT-PCR for patients 7701 and 7707, or Qdot staining of cryopreserved tissue for patients 7702, 7703, and 7705). All patients evaluated exhibited in situ and/or TCR RT-PCR evidence of increased CMV-specific T cells in rectal mucosa (Figure 6F). The transferred HIV- and CMV-specific CD8+ T-cell clones identified in the biopsies were found embedded in lymphocyte-rich tissue or in lymphoid aggregates located along colon crypts (Figure 6A,B,D). The HIV- and CMV-specific CD8+ T cells detected in the rectal biopsies by Qdot stains likely reflected infiltrating cells and not sampling of intravascular HIV- and CMV-specific CD8+ T cells, as H&E stains of the same rectal biopsies detected only sparse capillaries scattered throughout the connective tissue (supplemental Figure 4).
No evidence of HIV-RNA was detected in the rectal biopsy tissue before or after infusions. However, as 1 only biopsy sample/time point could be dedicated to determining HIV-RNA a sampling bias from limited tissue cannot be excluded. No evidence of CMV-DNA was found in sequential rectal swabs taken at biopsy time points suggestive of CMV viral replication (data not shown). These results demonstrate that infused CD8+ T cells have the capacity to extravasate from the intravascular compartment and may have the potential to survey the rectal mucosa for virally infected cells.
Discussion
Our findings show that HIV+ patients treated with HAART with no prior history of opportunistic infections and with CD4+ T cells > 200 cells/mm3 before the start of therapy possess circulating HIV-specific CD8+ T cells, albeit at very low levels, that can be isolated, cloned, and expanded ex vivo. Afterin vitro expansion and reinfusion back into the autologous host, some transferred CD8+ T cells localized to mucosal sites and some persisted and demonstrated Tcm characteristics. Although a concurrent expansion of endogenous CD8+ T cells contributing to the increase in pentamer+ T cells after infusions cannot formally be excluded as infused cells were not genetically tagged, the temporal relationship of the increase in pentamer+ T cells, as well as the documented in vivo expansion in some patients of T cells expressing the identical HIV-specific TCR as the infused cells, render this alternative unlikely. Overall, these observations suggest therapeutic vaccines capable of stimulating residual HIV-specific CD8+ T cells with Tcm qualities in patients receiving HAART may be able to provide hosts with long-lasting enhanced systemic immune responses and accumulation of specific CD8+T cells in the GALT.
CD8+Tcm cells specific for HIV could potentially provide a renewable source of effector cells for individuals infected with HIV. With murine LCMV infection, CD8+ T-cell adoptive transfer studies revealed enhanced protective immunity provided by Tcm, apparently as a result of more robust proliferation of Tcm than Tem after in vivo challenge, which likely reflects in part the distinct ability of Tcm to secrete IL-2 in response to Ag and use it as an autocrine growth factor.22 In humans, CD8+ T cells capable of secreting IL-2 in response to cognate Ag correlate with protection from acute viral infections, are detected in naturally contained chronic viral infections such as CMV or EBV25,38 and in HIV long-term nonprogressors.39 Although the ontogeny and plasticity of CD8+ Tcm and CD8+Tem remains controversial,40,41 a critical characteristic of Tcm cells is expression of the costimulatory molecule CD28, which is associated with promoting survival and the IL-2 production in response to Ag.42,43 In the context of chronic HIV infection, most HIV-specific CD8+ T cells are CD28− and exhibit characteristics of Tem. However, in patients treated with HAART, studies have demonstrated the presence, albeit in very low numbers, of HIV-specific CD28+CD8+ T cells suggesting that the absence of Ag may allow for the generation of, or conversion of existing HIV-specific CD8+ Tem to CD8+ Tcm.26 Patients in this study had insufficient frequencies of detectable HIV-specific CD8+ T cells before infusions to allow an accurate determination of the specificity and phenotype of the HIV-specific CD8+ T cells. However, after in vitro generation, most HIV-specific CD8+ T-cell lines contained a significant frequency of HIV-specific CD8+ T cells that expressed CD28 and that yielded progeny in some individuals that retained qualities of CD8+Tcm after transfer in vivo as reflected by the ability of some infused clones to persist, secrete high levels of IL-2 and proliferate extensively on Ag re-encounter. These functional responses are distinct from those generally found in HIV-specific CD8+ T cells in chronically infected HIV patients,25,44,45 which likely reflects the fact that most of the responding cells have Tem characteristics. Accumulation over time of persisting cells that express CD28 and CD62L is consistent with the fate of LCMV-specific murine CD8+ T cells found late after clearance of Ag22 and in some respects similar to the accumulation of CD27+ T cells in persisting cells after adoptive transfer studies in humans,46,47 as CD27 can deliver an alternative costimulatory signal to CD28 that facilitates cell survival and function. Tracking of the infused CD8+ T cells could not distinguish whether transferred cells progressively reacquired Tcm qualities or if a fraction of the transferred cells with retained Tcm characteristics survived/expanded better after transfer. However, such cells would be predicted more efficient than transferred CD8+ Tem with limited replication potential at controlling residual HIV viral replication in the recipient, although antiviral activity could not be directly assessed in our study as no HIV replication was detected in these HAART-treated patients.
Localization of HIV-specific cytotoxic CD8+ T cells to the GALT is essential to control residual HIV replication in patients receiving HAART. Viral RNA, and CD4+ T cells producing virus continue to be detected in the GALT of patients with undetectable blood HIV RNA.5-7,48 In this study, HIV-specific CD8+ T cells isolated from the peripheral blood, expanded, and then reinfused into the vascular compartment, readily trafficked to the rectal mucosa and remained detectable for 100+ days in 4 of 7 patients compared with 3 of 7 in the peripheral blood. However, trafficking to the rectal mucosa was not a unique characteristic of infused HIV-specific CD8+ T-cell clones, as CMV-specific CD8+ T-cell clones also trafficked to this tissue. The precise phenotype (Tem or Tcm) or lineage relationship of cells detected in the gut was not determined, as it was not feasible to obtain sufficient biopsy material for more extensive analysis. Taken together, these results suggest that an immunogenic HIV vaccine delivered systemically to patients controlled with HAART should be able to elicit CD8+ T cells with the ability to traffic to the GALT, and to persist and be available at this site if viral replication occurs.
Therapeutic immunization of HIV-infected individuals receiving HAART that could result in the expansion of HIV-specific CD8+ Tcm cells would be more readily applied to large populations than adoptive transfer of in vitro–expanded cells. Many HIV immunogens have been developed and administered to HIV-infected individuals in whom HAART was initiated early in the course of disease, with the intent of subsequently discontinuing HAART.15,17,49 Disappointingly, no correlation was found between the number and breadth of CD8+ T-cell responses after vaccination and the control of HIV viral replication after the interruption of HAART. There are many potential explanations for this outcome, but ultimately the results suggest the vaccine stimulus was inadequate.50 Our study did not follow T-cell transfer with a STI, as the infused T cells were expanded to target a single epitope for tracking purposes, making the risk of virus escape high. However, cells with Tcm characteristics that were specific for different viral proteins were isolated from the study individuals, and the data implies that vaccines engaging a broad repertoire of CD8+ Tcm might be able to provide hosts on HAART with both long-lasting enhanced systemic immune responses and trafficking of HIV-specific CD8+ T cells to the GALT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health/National Institute for Allergy and Infectious Diseases (NIH/NIAID) grant U01 AI054334. A.G.C. was supported in part by the Swiss National Science Foundation, Junior Investigator Award.
National Institutes of Health
Authorship
Contribution: A.G.C. analyzed and interpreted patient data, designed and performed experiments, and drafted the manuscript; C.C. and S.K. collected all patient data; J.Z. and A.T. performed Qdot stains; K.D. performed all PCRs; C.J.T. contributed to, and M.L.C. performed, flow cytometry experiments; R.V. performed the clinical trial support; S.R. and L.C. analyzed and interpreted data; P.D.G. designed research, analyzed, and interpreted data and revised the manuscript; and all authors approved and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip Greenberg, MD, Member and Head, Program in Immunology, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D3-100, Seattle, WA 98109; e-mail: pgreen@u.washington.edu.