Abstract
Mutations in the essential telomerase components hTERT and hTR cause dyskeratosis congenita, a bone marrow failure syndrome characterized by mucocutaneous features. Some (∼ 3%) sporadic aplastic anemia (AA) and idiopathic pulmonary fibrosis cases also carry mutations in hTERT and hTR. Even though it can affect clinical outcome, because the mutation frequency is rare, genetic testing is not standard. We examined whether the cooccurrence of bone marrow failure and pulmonary fibrosis in the same individual or family enriches for the presence of a telomerase mutation. Ten consecutive individuals with a total of 36 family members who fulfilled these criteria carried a germline mutant telomerase gene (100%). The mean age of onset for individuals with AA was significantly younger than that for those with pulmonary fibrosis (14 vs 51; P < .0001). Families displayed autosomal dominant inheritance and there was an evolving pattern of genetic anticipation, with the older generation primarily affected by pulmonary fibrosis and successive generations by bone marrow failure. The cooccurrence of AA and pulmonary fibrosis in a single patient or family is highly predictive for the presence of a germline telomerase defect. This diagnosis affects the choice of bone marrow transplantation preparative regimen and can prevent morbidity.
Introduction
Telomerase function is essential for the maintenance of stem cell compartments.1,2 The clinical manifestations of mutant telomerase genes were first studied in the setting of dyskeratosis congenita (DC), a rare bone marrow failure syndrome classically defined by the presence of mucocutaneous features: skin hyperpigmentation, oral leukoplakia, and nail dystrophy.3 The primary cause of mortality in these patients is aplastic anemia (AA).3 Pulmonary and liver fibrosis have been known to occur in a subset of DC patients, and are a common cause of morbidity after bone marrow transplantation.4 Mutations in the essential components of the enzyme telomerase—hTERT, the telomerase reverse transcriptase, and hTR, the RNA component—cause haploinsufficiency and lead to autosomal dominant inheritance of DC.5,6 In these families, the clinical phenotype is heterogeneous in part because of genetic anticipation: an earlier and more severe onset of disease across each generation due to progressive telomere shortening.5,7 Therefore, telomere length, and not just telomerase mutations, predicts the severity of organ failure.2,5
In the absence of the defining features of DC, a subset of patients with bone marrow failure also carries mutations in the essential telomerase genes. Germline mutations in hTERT and hTR are detectable in ∼ 3% of apparently sporadic AA cases.8,9 More commonly, mutations in telomerase cause pulmonary fibrosis, with germline defects accounting for inheritance in 8%-15% of familial and 1%-3% of sporadic cases.10-14 The genetic diagnosis of telomere-mediated bone marrow failure has implications for treatment, because affected patients generally do not respond to immunosuppression and, like DC patients, may be at increased risk for fatal complications after conventional bone marrow transplantation.4,9,15 Despite this, the rare incidence of mutations remains an impediment to routine genetic testing, and in the absence of the mucocutaneous features of DC, telomerase mutation carriers cannot be readily identified. We examined whether a personal and family history of both AA and pulmonary fibrosis can enrich for the presence of a telomerase mutation. We show that the cooccurrence of AA and pulmonary fibrosis is highly predictive for the presence of an inherited mutation in one of the essential telomerase genes.
Methods
We retrospectively studied 38 consecutive patients referred to Johns Hopkins Hospital from 2005-2009 for genetic evaluation of the etiology of bone marrow failure or pulmonary fibrosis. Patients were included in this study if they had AA or idiopathic interstitial lung disease and a personal or family history of a second feature: bone marrow failure or idiopathic interstitial lung disease. Exclusion criteria were clinical signs or family history of any of the typical mucocutaneous features of DC: skin hyperpigmentation, oral leukoplakia, and nail dystrophy. The study was approved by the Johns Hopkins institutional review board, and subjects gave written informed consent in accordance with the Declaration of Helsinki. The subjects were examined directly, and primary clinical records of family members were reviewed. hTERT and hTR were sequenced from genomic DNA.11 We measured telomerase activity directly5 and telomere length using flow-fluorescence in situ hybridization.11
Results
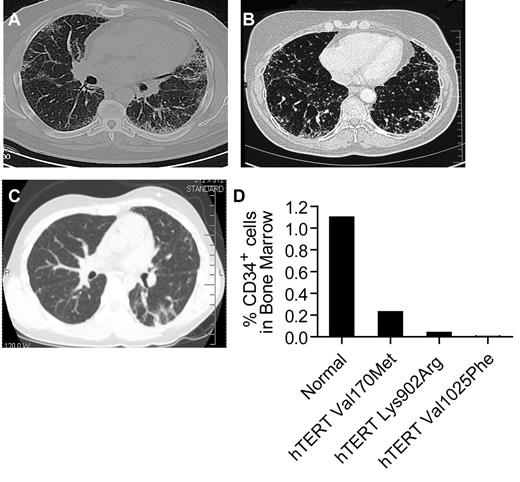
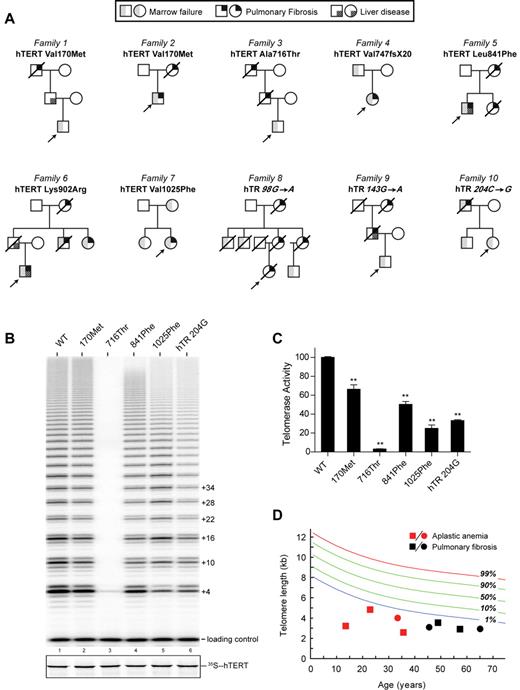
Ten consecutive individuals fulfilled the study criteria; 6 cases first came to clinical attention for symptoms related to AA, and 4 with symptoms related to idiopathic interstitial lung disease (Table 1). Six of the 10 individuals were subsequently diagnosed with a second feature (Table 1). Representative computed axial tomography (CAT) scan images and bone marrow studies are shown (Figure 1A-D). The mean age at first diagnosis for patients who presented with AA was significantly younger than those with pulmonary fibrosis: 14 years (range 9-21) vs 51 years (range 44-61; P < .001 by Student t test). For all 10 cases, there was at least one other first-degree relative who had bone marrow failure or pulmonary disease (Figure 2A). All of the pedigrees showed an inheritance pattern consistent with autosomal dominant transmission. In 8 of 10 families, we observed heterogeneity of phenotypes across generations. Specifically, within each of these 8 families, older generations first manifested with pulmonary fibrosis, and subsequent generations with bone marrow failure (Figure 2A). In 2 families, there was a case of cryptogenic liver cirrhosis that led to premature mortality. Two of the 10 probands were children with AA who subsequently developed progressive pulmonary disease within 3 years after myeloablative bone marrow transplantation. In both cases, reevaluation of the family history revealed cases of AA and pulmonary fibrosis in relatives. Two patients with pulmonary fibrosis, although they came to medical attention with respiratory symptoms, were later noted to have significant cytopenias, and one patient required prolonged transfusion support while on myelosuppressive drugs. None of the probands we examined had any of the classic features of DC or the less frequent features such as epiphora, but the majority reported a history of premature hair graying before age 25 (7 of 10, mean 17 years, range 6-23).
Age of onset and clinical presentation of telomerase mutation carriers
| Sex . | Age, y* . | First presentation . | Second feature(s) . | Family history† . | Mutation . |
|---|---|---|---|---|---|
| M | 9 | AA | Pulmonary fibrosis, liver fibrosis | Pulmonary fibrosis, liver cirrhosis | hTERT Lys902Arg |
| M | 10 | AA | Pulmonary disease | Pulmonary fibrosis, liver disease | hTERT Val170Met |
| F | 13 | AA | Pulmonary fibrosis | AA | hTERT Val1025Phe |
| M | 14 | AA | Pulmonary fibrosis, liver cirrhosis | hTR 143 G→A | |
| M | 18 | AA | Pulmonary fibrosis | hTERT Ala716Thr | |
| F | 21 | AA | Pulmonary fibrosis, MDS | hTR 204 C→G | |
| M | 44 | Pulmonary fibrosis‡ | Hypoplastic marrow | Pulmonary fibrosis | hTERT Val170Met |
| F | 44 | Pulmonary fibrosis§ | Pancytopenia, macrocytosis | Pulmonary fibrosis, pancytopenia | hTERT Val747AlafsX20 |
| M | 57 | Pulmonary fibrosis‡ | Hypoplastic marrow, liver disease | Pulmonary fibrosis | hTERT Leu841Phe |
| F | 61 | Pulmonary fibrosis‡ | AA, pulmonary fibrosis | hTR 98 G→A |
| Sex . | Age, y* . | First presentation . | Second feature(s) . | Family history† . | Mutation . |
|---|---|---|---|---|---|
| M | 9 | AA | Pulmonary fibrosis, liver fibrosis | Pulmonary fibrosis, liver cirrhosis | hTERT Lys902Arg |
| M | 10 | AA | Pulmonary disease | Pulmonary fibrosis, liver disease | hTERT Val170Met |
| F | 13 | AA | Pulmonary fibrosis | AA | hTERT Val1025Phe |
| M | 14 | AA | Pulmonary fibrosis, liver cirrhosis | hTR 143 G→A | |
| M | 18 | AA | Pulmonary fibrosis | hTERT Ala716Thr | |
| F | 21 | AA | Pulmonary fibrosis, MDS | hTR 204 C→G | |
| M | 44 | Pulmonary fibrosis‡ | Hypoplastic marrow | Pulmonary fibrosis | hTERT Val170Met |
| F | 44 | Pulmonary fibrosis§ | Pancytopenia, macrocytosis | Pulmonary fibrosis, pancytopenia | hTERT Val747AlafsX20 |
| M | 57 | Pulmonary fibrosis‡ | Hypoplastic marrow, liver disease | Pulmonary fibrosis | hTERT Leu841Phe |
| F | 61 | Pulmonary fibrosis‡ | AA, pulmonary fibrosis | hTR 98 G→A |
AA indicates Aplastic anemia; and MDS indicates myelodysplastic syndrome.
Age at first presentation.
Family history shown in Figure 2A.
Usual interstitial pneumonia/idiopathic pulmonary fibrosis.
Idiopathic interstitial lung disease, nonclassifiable.
Evidence of both pulmonary fibrosis and bone marrow failure in individuals with mutant telomerase genes. (A-B) Representative CAT scan images showing typical peripheral honeycombing pattern in 2 individuals with idiopathic pulmonary fibrosis and pancytopenia. Both individuals had biopsy confirmation of the usual interstitial pneumonia. The scans are from individuals in families 2 and 6. (C) CAT scan image of the proband of family 7, who developed pulmonary fibrosis after bone marrow transplantation for AA. Biopsy confirmed nonspecific interstitial pneumonia. (D) For the 3 individuals with images shown, the percentage of bone marrow CD34+ cells by flow cytometry was decreased in the setting of pancytopenia, macrocytosis, and hypocellular/aplastic marrow. The 95% confidence interval for CD34+ percentage is 0.4%-1.8%.
Evidence of both pulmonary fibrosis and bone marrow failure in individuals with mutant telomerase genes. (A-B) Representative CAT scan images showing typical peripheral honeycombing pattern in 2 individuals with idiopathic pulmonary fibrosis and pancytopenia. Both individuals had biopsy confirmation of the usual interstitial pneumonia. The scans are from individuals in families 2 and 6. (C) CAT scan image of the proband of family 7, who developed pulmonary fibrosis after bone marrow transplantation for AA. Biopsy confirmed nonspecific interstitial pneumonia. (D) For the 3 individuals with images shown, the percentage of bone marrow CD34+ cells by flow cytometry was decreased in the setting of pancytopenia, macrocytosis, and hypocellular/aplastic marrow. The 95% confidence interval for CD34+ percentage is 0.4%-1.8%.
Pedigrees of 10 probands with personal or family history of telomere-mediated disease. (A) Pedigrees showing males symbolized by squares, females by circles, and deceased individuals with a slash through them. The mutation is listed above each pedigree, and the shaded symbols indicate the diagnoses described in the key above the pedigrees. (B) Telomerase activity assay showing that mutations in hTERT or hTR decrease enzyme activity, as evident by the decreased intensity of the ladder repeat pattern compared with wild-type telomerase (WT). hTERT mutations are referred to by the residue number and the mutant residue. (C) Quantitation of telomerase activity based on at least 3 independent experiments. **P < .01 (2-sided Student t test). (D) Lymphocyte telomere length measured by flow-fluorescence in situ hybridization showing mutation carriers relative to healthy controls. The normal distribution is based on data from 400 controls. Both AA and pulmonary fibrosis probands had telomere length less than the first percentile compared with age-matched controls.
Pedigrees of 10 probands with personal or family history of telomere-mediated disease. (A) Pedigrees showing males symbolized by squares, females by circles, and deceased individuals with a slash through them. The mutation is listed above each pedigree, and the shaded symbols indicate the diagnoses described in the key above the pedigrees. (B) Telomerase activity assay showing that mutations in hTERT or hTR decrease enzyme activity, as evident by the decreased intensity of the ladder repeat pattern compared with wild-type telomerase (WT). hTERT mutations are referred to by the residue number and the mutant residue. (C) Quantitation of telomerase activity based on at least 3 independent experiments. **P < .01 (2-sided Student t test). (D) Lymphocyte telomere length measured by flow-fluorescence in situ hybridization showing mutation carriers relative to healthy controls. The normal distribution is based on data from 400 controls. Both AA and pulmonary fibrosis probands had telomere length less than the first percentile compared with age-matched controls.
Because mutations in hTERT and hTR cause a subset of pulmonary fibrosis and AA cases, we sequenced these genes. In all 10 cases (100%), we identified a germline mutation in either hTERT (n = 7) or hTR (n = 3). None of the 9 distinct mutations had been reported previously in large cohorts of controls,8,9 and mutations affected highly conserved residues within hTERT and hTR (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In 5 of the 10 families, we confirmed that the mutation segregated with the phenotype in at least 2 other family members. We tested the functional consequences of the 4 novel hTERT mutations and hTR 204C→G reported previously but not studied,16 and found that they all showed compromised telomerase activity (Figure 2B-C). The 4 remaining mutations were described previously and had been shown to decrease enzyme activity.5,7,11,13 The mutant telomerase gene was associated with very short lymphocyte telomere length in all of the subjects who had not undergone transplantation (8 of 8, less than the first percentile compared with age-matched controls; P < .001 by paired t test; Figure 2D). Importantly, there were no individuals in our cohort who fulfilled the study criteria who did not carry a mutation in hTERT or hTR (Table 2). In contrast, only 2 of the 28 patients (7%) who did not fulfill our study criteria carried mutations in telomerase (Table 2).
Clinical features of individuals with hTERT and hTR mutations
| . | Fulfilled study criteria (n = 10) . | Did not fulfill study criteria (n = 28) . |
|---|---|---|
| First diagnosis in index case | ||
| BMF | 6 (60%) | 10 (36%) |
| PF | 4 (40%) | 18 (64%) |
| Second feature (BMF or PF) in index case | 6 (60%) | 3 (11%) |
| Onset of premature graying < 25 y | 7 (70%) | 3 (11%) |
| Family history | ||
| BMF in index case and PF in family | 6 of 6 (100%) | 0 of 28 (0%) |
| PF in index case and BMF in family | 4 of 4 (100%) | 0 of 28 (0%) |
| hTERT or hTR mutation | 10 (100%) | 2 (7%) |
| . | Fulfilled study criteria (n = 10) . | Did not fulfill study criteria (n = 28) . |
|---|---|---|
| First diagnosis in index case | ||
| BMF | 6 (60%) | 10 (36%) |
| PF | 4 (40%) | 18 (64%) |
| Second feature (BMF or PF) in index case | 6 (60%) | 3 (11%) |
| Onset of premature graying < 25 y | 7 (70%) | 3 (11%) |
| Family history | ||
| BMF in index case and PF in family | 6 of 6 (100%) | 0 of 28 (0%) |
| PF in index case and BMF in family | 4 of 4 (100%) | 0 of 28 (0%) |
| hTERT or hTR mutation | 10 (100%) | 2 (7%) |
BMF indicates bone marrow failure; and PF, pulmonary fibrosis defined using criteria for idiopathic interstitial lung disease.
Discussion
Because our findings indicate that the complex of AA and pulmonary fibrosis is highly specific for the presence of a germline telomerase defect, affected patients should be counseled regarding genetic testing options. Telomere length less than the first percentile can distinguish telomere-mediated bone marrow failure from other congenital causes, and is highly specific for the presence of a mutant telomere gene.10,11,17 The findings of this study suggest that, in the presence of a relevant clinical history, genetic testing along with short telomere length will confirm the diagnosis of telomere-mediated disease.
The recognition of the telomere syndrome complex has several important clinical implications. First, relatives who may have subclinical disease can be identified by genetic testing and excluded from the bone marrow transplantation donor pool. Second, because patients with telomere-mediated bone marrow failure who undergo bone marrow transplantation using conventional preparative regimens are particularly prone to developing fatal complications, alternative transplantation protocols can be chosen. A tailored, nonmyeloablative choice of conditioning regimens has recently shown promise in improving short-term outcome.18 In patients with pulmonary fibrosis, the identification of a mutant telomerase gene may also be significant, because individuals with limited marrow reserves require vigilance during treatment with myelosuppressive drugs in the setting of cancer treatment or after lung transplantation. The diagnosis of a telomere syndrome can also influence the care of relatives of mutation carriers, who have also been shown to have short telomeres,5,10,11,19 and heighten the index of suspicion for potential life-threatening complications. For example, DC is a cancer-prone syndrome. DC patients may have as much as a 200-fold increased incidence of myelodysplastic syndrome and a significantly increased risk of squamous cell cancers of the skin, head, neck, and anogenital tract.20 It is possible that patients with germline defects in telomerase, even in the absence of DC features, may also be at increased risk. Dermatologic findings in patients with telomere syndromes may also be mistaken for a diagnosis of graft-versus-host disease after bone marrow transplantation, and this may lead to unnecessary treatment. Therefore, the genetic diagnosis of telomere-mediated disease can affect clinical care in multiple settings.
Pulmonary fibrosis and AA are known complications of DC. In the present study, we demonstrate that their cooccurrence in the absence of the classic DC features is highly specific for a germline mutation in hTERT or hTR. In our study, the clinical phenotype alone did not distinguish hTERT and hTR mutation carriers. However, we observed striking heterogeneity of phenotypes within families with a generation effect on disease type and onset. Within a single family, older generations were more likely to manifest with adult-onset pulmonary fibrosis, whereas in subsequent generations, bone marrow failure was the first presentation at a younger age. This observation suggests that genetic anticipation due to telomere shortening is not only associated with an earlier onset of phenotypes across generations, but also with a changing pattern of disease from pulmonary fibrosis to bone marrow failure. Telomere length that shortens across generations is therefore a determinant of both disease severity and disease type. Based on these patterns, we predict that in subsequent generations or with age, the classic features of DC may eventually appear. The extent of genetic anticipation and phenotype evolution are likely correlated with the degree of telomerase loss of function, with hypomorphic mutations causing more subtle genetic anticipation.19 In our study, we identified mutations in hTERT or hTR in all 10 index cases who fulfilled the study criteria. Mutations in TINF2, the gene for the telomere-binding protein TIN2, account for DC cases that usually present with bone marrow failure before the age of 10, although mutations have been reported in at least one multigenerational DC family.21,22 It is therefore possible that TINF2 mutations might account for inheritance in young bone marrow failure patients, who, similar to the patients we studied, lack mutations in hTERT or hTR at screening. Future studies may also identify similar families who have short telomeres, but who may carry mutations in yet-to-be-identified disease genes important in telomere biology. Independently of the discrete mutation, the cooccurrence of pulmonary fibrosis and AA in a single patient and family should prompt consideration of a telomere-mediated syndrome.
In summary, although mutant hTERT and hTR genes account for the inheritance of a relatively small subset of pulmonary fibrosis and AA cases independently, their cooccurrence within a single family is highly specific for the presence of a germline mutation in a telomerase gene. A thorough personal and family history, along with subsequent confirmation with genetic testing, can prevent life-threatening complications and have significant implications for the care of affected children and adults and their families.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the individuals who participated in this study and to all of their referring physicians.
This work was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation to M.A. E.M.P. received support from an National Heart, Lung and Blood Institute special supplement to T32GM007309, and J.K.A. received support from the Maryland Stem Cell and Parker B. Francis Foundations.
National Institutes of Health
Authorship
Contribution: E.M.P. and J.K.A. performed and analyzed the sequence data and alignment; X.Q. and J.J.-L.C. designed and X.Q. performed the telomerase activity assays; and M.A. designed the study, evaluated the clinical data with E.M.P., and wrote the manuscript. All authors had full access to the data in the study and take responsibility for both the integrity of the data and the accuracy of the data analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Armanios, MD, Department of Oncology, 1650 Orleans St, CRB I Rm 186, Baltimore, MD 21287; e-mail: marmani1@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal