Abstract
Natural hemozoin (nHZ), prepared after schizogony, consists of crystalline ferriprotoporphyrin-IX dimers from undigested heme bound to host and parasite proteins and lipids. Phagocytosed nHZ alters important functions of host phagocytes. Most alterations are long-term effects. We show that host fibrinogen (FG) was constantly present (at ∼ 1 FG per 25 000 HZ-heme molecules) and stably bound to nHZ from plasma-cultured parasites. FG was responsible for the rapid 100-fold stimulation of reactive oxygen species production and 50-fold increase of TNF and monocyte chemotactic protein 1 by human monocytes. Those effects, starting within minutes after nHZ cell contact, were because of interaction of FG with FG-receptors TLR4 and integrin CD11b/CD18. Receptor blockage by specific mAbs or removal of FG from nHZ abrogated the effects. nHZ-opsonizing IgGs contribute to the stimulatory response but are not essential for FG effects. Immediate increase in reactive oxygen species and TNF may switch on previously described long-term effects of nHZ, largely because of HZ-generated lipo-peroxidation products 15(S,R)-hydroxy-6,8,11,13-eicosatetraenoic acid and 4-hydroxynonenal. The FG/HZ effects mediated by TLR4/integrins represent a novel paradigm of nHZ activity and allow expansion of nHZ effects to nonphagocytic cells, such as endothelia and airway epithelia, and lead to a better understanding of organ pathology in malaria.
Introduction
Natural hemozoin (nHZ), as present in the food vacuole of Plasmodium falciparum and largely coincident with residual bodies (RBs) shed during schizogony, consists of a scaffold of crystalline ferriprotoporphyrin IX dimers (β-hematin, BH) from undigested host hemoglobin-heme1 bound to a vast array of host and parasite molecules.2-4 nHZ is phagocytosed in vivo and in vitro by host phagocytes and alters important functions in those cells. Most functional alterations were long-term postphagocytic effects.3,5-8 Some of those long-term effects were also reported in human and murine phagocytes fed with BH or variously manipulated HZ.9,10 By contrast, a powerful, short-term stimulation of oxidative burst by human monocytes was also shown to occur during phagocytosis of nHZ.8
Here, we show that host fibrinogen (FG) was constantly present and stably bound to nHZ and RBs prepared from parasites cultured in presence of plasma. FG in nHZ was responsible for a prephagocytic rapid and powerful stimulation of oxidative burst, accompanied by release of TNF and monocyte chemotactic protein 1 (MCP-1) by human monocytes. Those prephagocytic events were because of the interaction of FG with TLR4 and integrin CD11b/CD18 (CR3, Mac-1), known receptors for FG as an immune-active molecule.11-13 Present data are the first indication that nHZ-bound FG may act as a powerful self-signal molecule for early immune responses and a trigger for the persistent long-term effects that play multiple roles in malaria pathogenesis.
Methods
Reagents
Unless otherwise stated, reagents were obtained from Sigma-Aldrich. Monoclonal anti-TLR4 (mouse clone HTA125, isotype IgG2a), mouse IgG1, and IgG2a isotype control antibodies were from Ancell. Monoclonal anti-CD11b (mouse clone 2LPM19c, isotype IgG1) was from Santa Cruz Biotechnology, monoclonal anti-CD11b (mouse clone 44, isotype IgG1) was from Biosource (Invitrogen). Anti-TLR2 was a gift from Tiziana Musso, University of Torino. Mouse IgG1 isotype control Ab was from eBioscience. If not stated otherwise, cell culture media and additives were from Invitrogen.
Parasite culture
P falciparum (Palo Alto strain, mycoplasma-free) was kept in culture in medium supplemented with 10% (v/v) human plasma decomplemented at 56°C for 40 minutes as described.6-8 Alternatively, plasma was substituted by 0.5% (w/v) Albumax or 10% (v/v) human decomplemented serum.
Monocyte preparation
Peripheral blood mononuclear cells (PBMCs) were isolated as described6-8 and either kept in suspension at 1 × 108 cells/mL macrophage serum-free medium (M-SFM) or plated at 1 × 107 cells/mL RPMI 1640 medium/well in 24-well plates (Falcon; Becton Dickinson). The plates were incubated in a humidified CO2/air incubator at 37°C for 30 minutes, and nonadherent cells were removed by 3 washes. M-SFM was added, and cells were cultured overnight at 37°C with 200 U/mL IFNγ (R&D Systems). Immunopurified monocytes were prepared by depletion of B/T lymphocytes with Dynabeads Pan B and Pan T (Dynal Biotech). Monocyte enrichment was analyzed by FACS. Preparations with ≥ 80% monocytes were plated at 1 × 106 cells/mL M-SFM/well in 24-well plates. The use of human cells obtained after written informed consent as described for the present work was approved by the Bioethical Committee of the Torino University School of Medicine, the institutional review board for these studies.
Preparation and opsonization of HZ, RBs, BH, and RBCs
nHZ was isolated from parasite cultures supplemented with human plasma decomplemented as described.6-8 When indicated, HZ was isolated from plasma-free, serum-, or Albumax-supplemented cultures. The opsonized nHZ batches were tested for lipopolysaccharide (LPS) by the E-Toxate assay from Sigma (Limulus Amebocyte Lysate; gel solidification assay), sensitivity threshold at 0.05-0.1 EU/mL, and by the Limulus Amebocyte Lysate (kinetic chromogenic assay; Lonza, European Endotoxin Testing Service) with a sensitivity threshold at 0.005 EU/mL. In the nHZ batches used in this study, the LPS level was constantly below the sensitivity threshold and ≥ 500 times lower than the KD for human TLR4.14 Extensive washings with 10mM phosphate buffer (pH 8.0) were conducted after collecting nHZ from the 10%/40% interphase of a 6% mannitol-containing Percoll gradient to eliminate RBC membrane remnants. When indicated, nHZ (10% v/v, final) was resuspended in 50mM glycine buffer (pH 5.0), containing 10mM MgCl2 and 1mM CaCl2, treated for 1 hour at 37°C with 400 U/mL DNAse I, and washed extensively with PBS. RBs were isolated from culture supernatants after 6 hours from reinfection with ferromagnetic MACS LS columns (Miltenyi Biotec). FG-free nHZ was prepared by plasmin digestion of nHZ resuspended (20% w/v, final) in 50mM Tris HCl (pH 8.0) at 25°C for 14 hours, adding fresh plasmin (5 mU/mL) every 2 hours. Subsequently, nHZ washed extensively with PBS was resuspended in PBS. Heat-denatured HZ was prepared by incubating nHZ at 100°C for 5 minutes. In inhibition assays, nHZ was incubated with the F(ab′)2 fragment of anti–human IgG-Fc at 0.2 mg/mL for 15 minutes at 37°C and washed twice with PBS before starting phagocytosis. nHZ for phagocytosis was opsonized with 1 volume of fresh or decomplemented (see “Parasite culture”) human serum for 30 minutes at 37°C. BH was prepared according to Slater et al15 with minor modifications.16 When indicated, BH was incubated with fresh serum or plasma from the same donor at 37°C for 5 or 30 minutes, extensively washed with PBS, and resuspended in PBS. nHZ was added to cultures at 100nmol heme/well, corresponding to ∼ 50 RBC equivalents/monocyte. Control phagocytosis was performed with nonparasitized Rh+ RBCs obtained from healthy donors and treated with 0.75 U IgG anti-D/μL packed RBCs for 30 minutes at 37°C (Rhophylac; ZLB Behring).
Protein identification by Western blotting and sequencing
nHZ was solubilized in SDS-containing Laemmli buffer at 95°C for 5 minutes, and disulfide bonds were reduced with 65mM dithio-DL-threitol. Solubilized proteins were separated by 10% SDS-PAGE and stained with Coomassie R250 or silver. For FG detection by Western blotting, electrophoretically separated proteins were transferred to nitrocellulose and probed with monoclonal mouse anti–human FG-γ chain Ab (1:3000; Sigma; clone 85D4), or polyclonal sheep anti–human FG (1:5000; AbD Serotec; MorphoSys). For protein identification in nHZ, proteins were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon; Millipore). The Coomassie-stained bands were subjected to Edman degradation for N-terminal sequencing. Internal peptides of N-terminal–blocked proteins were generated by tryptic in situ digestion, and peptides were separated by reverse-phase HPLC with the use of a μRPC C2/C18 SC2.1/10 column (Pharmacia-LKB and Pharmacia-LKB SMART system). Peptides were microsequenced with an automated protein sequencer (477/120A or 494A/190A; Applied Biosystems), and the resultant sequences were screened in the SwissProt database for protein identification. For human IgG detection in nHZ, proteins were probed with goat anti–human IgG (whole molecule and Fab-specific, 1:80 000), both HRP conjugated. Complement C3 fragments were measured as C3c, making use of a rabbit anti–human complement C3c. Anti–mouse and anti–rabbit HRP-conjugated secondary antibodies were from Amersham Biosciences, anti–sheep HRP-conjugated secondary Ab was from Sigma. All Abs were used at 1:20 000.
Measurement of ROS release (oxidative burst)
ROS release (equivalent to oxidative burst) was quantified in suspended freshly prepared human monocytes (see “Monocyte preparation”) as indicated.8 Immediately after nHZ addition at 50 RBC equivalents/monocyte, cells were centrifuged (150g, 5 seconds, room temperature) to accelerate and synchronize nHZ contact with monocytes and to improve quantification of initial burst. Monocytes (1.5 × 105) were suspended in 1 mL of PBS supplemented with 5mM glucose and 20μM luminol (final), and luminescence was measured (Sirius Luminometer; Berthold). In parallel, control cells were either exposed to RBCs treated with anti-D IgG (0.75 U/μL packed RBCs) at 50 RBCs/monocyte or kept unfed and treated similarly. In inhibition assays, cells were preincubated with blocking anti-TLR4, anti-TLR2, and anti-CD11b Abs at 10 μg/106 monocytes for 15 minutes at 37°C before nHZ addition. Maximal burst was quantified by stimulation with phorbol myristate acetate (100nM, final).
FACS analysis
FACS analysis of monocytes was performed with a FACScan cytofluorimeter (BD Biosciences) and CellQuest software. Immunopurified human monocytes were judged by physical parameters and expression of the following surface antigens: CD14, MHC class I, and II. Abs used were anti-CD14 (mouse 3C10; ATCC), anti-MHC class I (mouse W6.32; ATCC), and anti-MHC class II (mouse BT-2.9). Saturating concentrations of mAbs recognizing TLR4 (mouse; e-Bioscience) and CD11b (mouse; Santa Cruz Biotechnology) were used to check FG receptor expression before functional studies. Bound Abs were revealed with FITC-conjugated F(ab′)2 goat anti–mouse IgG.
Measurement of TNF and MCP-1 release: phagocytosis assay
Adherent monocytes cultured at 1 × 106 cells/well in 24-well plates for 15 hours were exposed to nHZ (100nmol heme/well, corresponding to 50 RBC equivalents/monocyte). After 30 minutes the first HZ crystals were observed inside the monocyte, and phagocytosis was completed after 3 hours of incubation at 37°C. Three hours after HZ supplementation, cell-free/HZ-free supernatants were collected and frozen for later TNF and MCP-1 analyses. In parallel, control cells were either exposed to RBCs treated with anti-D IgG at 50 RBCs/monocyte or kept unfed and treated similarly. After removal of supernatants at 3 hours cells were washed 3 times with PBS, once with water, and finally with PBS to remove noningested HZ or RBCs. Phagocytosis was quantified as indicated.8 In inhibition assays, cells were preincubated with blocking anti-TLR4, anti-TLR2, and anti-CD11b antibodies at 10 μg/106 monocytes for 15 minutes at 37°C before nHZ addition. TNF and MCP-1 were quantified with enzyme immunoassay and the ELISA kits (Cayman Chemical and R&D Systems, respectively). Data on single experiments are averages of triplicate cultures, each assayed in duplicate. To exclude LPS contamination, nHZ and human monocytes were preincubated for 10 minutes with polymyxin B at 10 or 25 μg/mL, respectively. TNF release elicited by 1μM LPS was blocked by cell pretreatment with polymyxin B by 70%.
Statistical analysis
Statistical analysis was conducted with SPSS 14.0 (SPSS Inc). All data were assessed for normal distribution with the use of a Shapiro-Wilks test, and data were compared with the nonparametric Mann-Whitney U test. P values < .05 were considered to be statistically significant.
Results
Host FG is firmly bound to nHZ
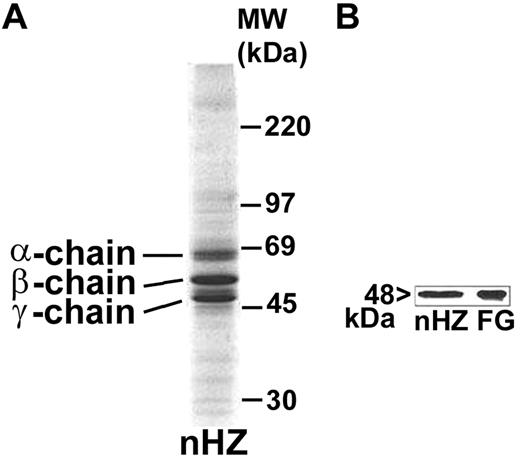
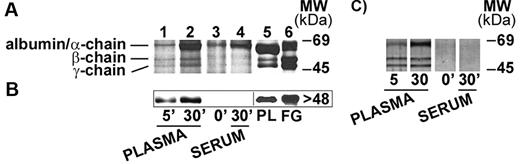
nHZ, isolated from culture supernatant after schizogony and extensively washed showed a characteristic, reproducible pattern in SDS-PAGE with 3 prominent protein bands appearing at 63.5, 56, and 48 kDa (Figure 1A), identified as α-, β-, and γ-chains of human FG by sequencing of the N-terminus of γ-chain and internal peptides of β-chain. In addition, FG chains were detected with specific antibodies in Western blot (γ-chain shown in Figure 1B). FG was by far the most abundant protein in nHZ from plasma-supplemented cultures, suggesting a high affinity of FG for HZ. RBs also have bound FG (not shown). Probably, FG binding to HZ occurs also in vivo after schizogony as concluded from in vitro binding studies of FG to FG-free HZ performed in whole plasma. FG incubated at physiologic concentrations with FG-free HZ isolated from FG-free cultures binds with high affinity to HZ (Figure 2A-B lanes 1 and 2). Already 5 minutes after plasma addition, the FG bands appear, and ∼ 25 000 HZ-heme molecules bound 1 FG molecule (Figure 2A). FG successfully competes with other plasma proteins for HZ binding. Albumin bound to FG-free HZ (Figure 2A lane 3) did not hinder FG binding when plasma was supplemented (Figure 2A lane 2). Protein-free synthetic BH also preferentially bound FG (Figure 2C).
Detection of FG bound to nHZ. (A) Coomassie stain of 20 μg of nHZ proteins separated by a 7%-17% gradient SDS-PAGE (corresponding to 50nmol heme/lane in terms of heme content). nHZ was isolated from P falciparum in vitro cultures in RPMI-1640 growth medium supplemented with 10% plasma. (B) Western blot of 10 μg of nHZ proteins separated by 10% SDS-PAGE transferred to nitrocellulose and probed with a mouse monoclonal anti-FG γ-chain and a peroxidase-linked secondary Ab. FG chains were detected by ECL. Solubilized FG (15 μg) was run in parallel as standard. One representative protein separation in SDS-PAGE and Western blot of 15 and 6, respectively, are shown.
Detection of FG bound to nHZ. (A) Coomassie stain of 20 μg of nHZ proteins separated by a 7%-17% gradient SDS-PAGE (corresponding to 50nmol heme/lane in terms of heme content). nHZ was isolated from P falciparum in vitro cultures in RPMI-1640 growth medium supplemented with 10% plasma. (B) Western blot of 10 μg of nHZ proteins separated by 10% SDS-PAGE transferred to nitrocellulose and probed with a mouse monoclonal anti-FG γ-chain and a peroxidase-linked secondary Ab. FG chains were detected by ECL. Solubilized FG (15 μg) was run in parallel as standard. One representative protein separation in SDS-PAGE and Western blot of 15 and 6, respectively, are shown.
FG binding to HZ under physiologic conditions. (A) FG binding to FG-free HZ. FG-free HZ, obtained from Albumax-supplemented parasite cultures (lane 3), was incubated in undiluted fresh plasma for 5 and 30 minutes (lane 1 and lane 2, respectively), or in undiluted fresh serum from the same healthy donor (lane 4). After extensive washings, proteins extracted from HZ (corresponding to 100nmol heme in terms of heme content) were separated by 10% SDS-PAGE and stained with Coomassie R250. Plasma-proteins (PL; 15 μg) or FG run as standard (lane 5 and lane 6, respectively). (B) Western blot of proteins extracted from the same samples of panel A (corresponding to 50nmol heme/lane) were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with a mouse monoclonal anti-FG γ-chain and a peroxidase-linked secondary Ab. FG γ-chain was detected by ECL. PL (15 μg) or solubilized FG was used as standard. (C) FG binding to BH. BH (corresponding to 100nmol heme in terms of heme content) was incubated and separated by SDS-PAGE as indicated for FG-free HZ and silver stained. Representative blots selected from 4 with similar results are shown.
FG binding to HZ under physiologic conditions. (A) FG binding to FG-free HZ. FG-free HZ, obtained from Albumax-supplemented parasite cultures (lane 3), was incubated in undiluted fresh plasma for 5 and 30 minutes (lane 1 and lane 2, respectively), or in undiluted fresh serum from the same healthy donor (lane 4). After extensive washings, proteins extracted from HZ (corresponding to 100nmol heme in terms of heme content) were separated by 10% SDS-PAGE and stained with Coomassie R250. Plasma-proteins (PL; 15 μg) or FG run as standard (lane 5 and lane 6, respectively). (B) Western blot of proteins extracted from the same samples of panel A (corresponding to 50nmol heme/lane) were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with a mouse monoclonal anti-FG γ-chain and a peroxidase-linked secondary Ab. FG γ-chain was detected by ECL. PL (15 μg) or solubilized FG was used as standard. (C) FG binding to BH. BH (corresponding to 100nmol heme in terms of heme content) was incubated and separated by SDS-PAGE as indicated for FG-free HZ and silver stained. Representative blots selected from 4 with similar results are shown.
Rapid induction of ROS release (oxidative burst) and release of TNF and MCP-1 by nHZ
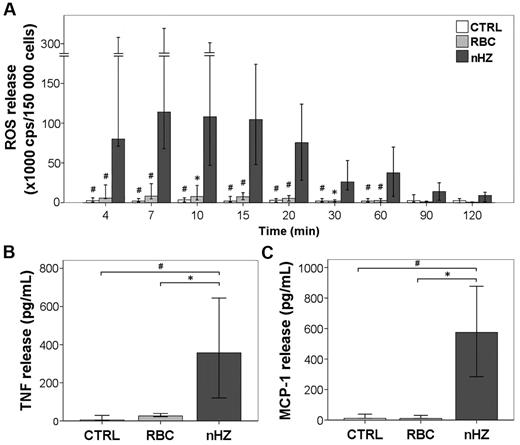
The addition of FG-containing HZ to adherent monocytes immediately induced a remarkably intense ROS release (equivalent to oxidative burst) (Figure 3A). After reaching a peak value 7 minutes after nHZ addition, burst remained high for 20 minutes, decreased slowly thereafter, attaining basal levels after 120 minutes. The nHZ-elicited burst exceeded ∼ 100-fold the short-term burst observed in monocytes interacting with IgG-opsonized RBCs. Three hours after the addition to monocytes, nHZ induced a 40- and 50-fold increase in the level of TNF and MCP-1, respectively (Figure 3B-C). The amount of TNF released after 1 hour from nHZ addition was 50% of peak level at 3 hours, suggesting a time dependency of stimulatory effect. However, only minor amounts (∼ 10%) of TNF were still released after 3 hours from monocytes grown for 24 hours in M-SFM, and those quantities were FG independent (not shown). TNF released during 3 hours by HZ-fed monocytes was one-half the amount produced after LPS stimulation (not shown). Soluble plasma FG from healthy donors did not stimulate ROS, TNF, and MCP-1 release by monocytes up to a concentration of 0.3 mg/mL (corresponding to 10% of its plasma concentration). This underlines the need for a conformational change in FG during its binding to HZ to become immune active and the absence of LPS. We excluded LPS contaminations in nHZ-FG further by negative Limulus test, heat resistance of nHZ-FG-effects, and unchanged nHZ-FG effects after preincubation of nHZ-FG with the LPS-chelator polymyxin B (not shown). Monocytes fed with opsonized RBCs ingested similar amounts of heme but produced distinctly less TNF and MCP-1 compared with nHZ-fed cells (Figure 3B-C). Similar amounts of ROS, TNF, and MCP-1 were also measured during 3 hours in monocytes fed with RBs (not shown). Because IFNγ is increased in patients with malaria, monocytes were exposed overnight to exogenous IFNγ. Not surprisingly, IFNγ-conditioned monocytes were more responsive to nHZ, releasing ∼ 10-fold TNF as unstimulated cells, suggesting that priming of monocytes was necessary for optimal TNF release. In contrast, MCP-1 release was not dependent on IFNγ pretreatment (not shown). Contaminating lymphocytes (average contamination ≤ 40%) did not interfere with the rapid FG effects, because nHZ-fed immunopurified monocytes (85%-90% CD14+ cells) elicited similar release of TNF and MCP-1 (not shown).
nHZ induces immunostimulatory effects in human monocytes. (A) ROS release (oxidative burst) from monocytes after the addition of nHZ. Suspended nonprimed monocytes were supplemented with nHZ at 50 RBC equivalents in terms of heme-content/monocyte or RBCs treated with anti-D IgG at 50 RBCs/monocyte immediately after isolation of PBMCs from healthy donors at time 0 or kept as unfed control (CTRL) and incubated at 37°C. Aliquots of 1.5 × 105 monocytes were taken at indicated times, and ROS was quantified by luminol-enhanced luminescence. Results from 12 independent experiments are shown. (B) TNF and (C) MCP-1 release from adherent monocytes after the addition of nHZ. Monocytes obtained from healthy donors were enriched by adherence of PBMCs. Approximately 106 monocytes per well were cultured in 1 mL of serum-free MSFM overnight in the presence of 200 U/mL IFNγ and then supplemented with either nHZ (100nmol/106 monocytes in terms of heme content) or nonparasitized RBCs opsonized with anti-D IgG (100 RBCs/monocyte) or kept as unfed control (CTRL). After 3 hours, HZ- and cell-free supernatants were collected and analyzed for TNF and MCP-1 by ELISA. Seven and 5 independent experiments were conducted for TNF and MCP-1 analysis, respectively. All results are expressed as medians with 95% CI. P values were obtained comparing each nHZ-fed sample with its relative nonfed (CTRL) and RBC-fed control. *P < .05, and #P < .01.
nHZ induces immunostimulatory effects in human monocytes. (A) ROS release (oxidative burst) from monocytes after the addition of nHZ. Suspended nonprimed monocytes were supplemented with nHZ at 50 RBC equivalents in terms of heme-content/monocyte or RBCs treated with anti-D IgG at 50 RBCs/monocyte immediately after isolation of PBMCs from healthy donors at time 0 or kept as unfed control (CTRL) and incubated at 37°C. Aliquots of 1.5 × 105 monocytes were taken at indicated times, and ROS was quantified by luminol-enhanced luminescence. Results from 12 independent experiments are shown. (B) TNF and (C) MCP-1 release from adherent monocytes after the addition of nHZ. Monocytes obtained from healthy donors were enriched by adherence of PBMCs. Approximately 106 monocytes per well were cultured in 1 mL of serum-free MSFM overnight in the presence of 200 U/mL IFNγ and then supplemented with either nHZ (100nmol/106 monocytes in terms of heme content) or nonparasitized RBCs opsonized with anti-D IgG (100 RBCs/monocyte) or kept as unfed control (CTRL). After 3 hours, HZ- and cell-free supernatants were collected and analyzed for TNF and MCP-1 by ELISA. Seven and 5 independent experiments were conducted for TNF and MCP-1 analysis, respectively. All results are expressed as medians with 95% CI. P values were obtained comparing each nHZ-fed sample with its relative nonfed (CTRL) and RBC-fed control. *P < .05, and #P < .01.
Removal of nHZ-bound FG abrogates monocyte responses to nHZ
To check the role of FG in nHZ, the effects of FG-free and heat-denatured nHZ were compared with nHZ. P falciparum was cultured in FG-free media and supplemented with serum or Albumax to avoid FG binding after schizogony. Alternatively, FG was either eliminated from nHZ by plasmin digestion or denatured by heat treatment. No FG chains were detectable by SDS-PAGE (silver stain) in serum-HZ, Albumax-HZ, or plasmin-treated nHZ (not shown). The absence of FG was confirmed by Western blot analysis (Figure 4A lanes 2, 5, and 6). Plasmin degraded nHZ-bound FG efficiently to the final degradation products detected in the discarded supernatant (Figure 4A lane 3).
nHZ-bound FG induces rapid ROS, TNF, and MCP-1 release. (A) FG content of different HZ types was analyzed by Western blot. HZ was obtained from supernatants of plasma- (nHZ, lane 1) or serum- (HZ SERUM, lane 5) or Albumax- (HZ ALBUMAX, lane 6) supplemented cultures of P falciparum. nHZ-bound FG was either degraded by plasmin treatment (see “Methods”), and both pellet (HZ PLASMIN, lane 2) and supernatants (SN PLASMIN, lane 3) were analyzed for undegraded FG and FG fragments or were denatured by heating at 100°C for 5 minutes (HZ 100°C, lane 4). Proteins extracted from 50nmoles HZ (in terms of heme content), and from the supernatant of the same amount of HZ, were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti-FG γ-chain. FG (15 μg) and plasmin-digested FG (FG PLASMIN) were separated and blotted as reference (lane 7 and lane 8, respectively). (B) Immediate ROS release by FG. Suspended monocytes were supplemented with differently treated HZ of equal heme content (100nmol/106 monocytes) immediately after isolation at time 0, briefly spun down, resuspended, and incubated at 37°C (n = 4). Aliquots of 1.5 × 105 monocytes were taken at indicated times, and ROS was quantified by luminol-enhanced luminescence. (C) Immediate TNF and MCP-1 release by FG. Human monocytes were enriched by Ficoll passage and adhesion, maintained in culture overnight with 200 U/mL IFNγ, and then fed with differently treated HZ of equal heme content (100nmol/106 monocytes) 15 hours after isolation. After 3 hours supernatants were collected and analyzed for TNF and MCP-1 by ELISA. TNF and MCP-1 release values of each HZ species are expressed as percentages of control nHZ values. Medians are shown of 8 (TNF) and 4 (MCP-1) independent experiments with a 95% confidence interval (CI). Absolute values of nHZ-induced TNF and MCP-1 release were, respectively 357 pg/mL (120-644 pg/mL) and 575 pg/mL (284-877 pg/mL). P < .05 up to 30 minutes for all modified HZ forms (B); *P < .05 and #P < .01 (C).
nHZ-bound FG induces rapid ROS, TNF, and MCP-1 release. (A) FG content of different HZ types was analyzed by Western blot. HZ was obtained from supernatants of plasma- (nHZ, lane 1) or serum- (HZ SERUM, lane 5) or Albumax- (HZ ALBUMAX, lane 6) supplemented cultures of P falciparum. nHZ-bound FG was either degraded by plasmin treatment (see “Methods”), and both pellet (HZ PLASMIN, lane 2) and supernatants (SN PLASMIN, lane 3) were analyzed for undegraded FG and FG fragments or were denatured by heating at 100°C for 5 minutes (HZ 100°C, lane 4). Proteins extracted from 50nmoles HZ (in terms of heme content), and from the supernatant of the same amount of HZ, were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti-FG γ-chain. FG (15 μg) and plasmin-digested FG (FG PLASMIN) were separated and blotted as reference (lane 7 and lane 8, respectively). (B) Immediate ROS release by FG. Suspended monocytes were supplemented with differently treated HZ of equal heme content (100nmol/106 monocytes) immediately after isolation at time 0, briefly spun down, resuspended, and incubated at 37°C (n = 4). Aliquots of 1.5 × 105 monocytes were taken at indicated times, and ROS was quantified by luminol-enhanced luminescence. (C) Immediate TNF and MCP-1 release by FG. Human monocytes were enriched by Ficoll passage and adhesion, maintained in culture overnight with 200 U/mL IFNγ, and then fed with differently treated HZ of equal heme content (100nmol/106 monocytes) 15 hours after isolation. After 3 hours supernatants were collected and analyzed for TNF and MCP-1 by ELISA. TNF and MCP-1 release values of each HZ species are expressed as percentages of control nHZ values. Medians are shown of 8 (TNF) and 4 (MCP-1) independent experiments with a 95% confidence interval (CI). Absolute values of nHZ-induced TNF and MCP-1 release were, respectively 357 pg/mL (120-644 pg/mL) and 575 pg/mL (284-877 pg/mL). P < .05 up to 30 minutes for all modified HZ forms (B); *P < .05 and #P < .01 (C).
The presence of FG in nHZ was apparently essential, because contact of FG-free HZ with phagocytes induced distinctly reduced monocyte responses. The rapid burst activation and ROS release observed 4 minutes after monocyte contact with nHZ was completely abrogated when FG-free HZ was used (Figure 4B). The TNF and MCP-1 release measured 3 hours after HZ addition was also significantly decreased by ≥ 50% in monocytes fed with plasmin-digested or heat-denatured nHZ and was almost reduced to nil with Albumax-HZ (Figure 4C). Interestingly, heat denaturation inhibited the stimulatory activity of nHZ as effectively as other methods of FG abrogation, suggesting that the native conformation of HZ-bound FG was essential for full effects (Figure 4B). FG depletion did not impair HZ uptake by monocytes, though. Phagocytosis of all HZ preparations was not significantly different from 12.75 RBC equivalents per monocyte (range, 10.37-21.25 RBC equivalents per monocyte; n = 30), as observed in monocytes fed with nHZ. Hence, FG appears to modify monocyte response in a phagocytosis-independent manner, suggesting the activation of surface receptors not essentially involved in phagocytosis.
FG receptors on monocytes
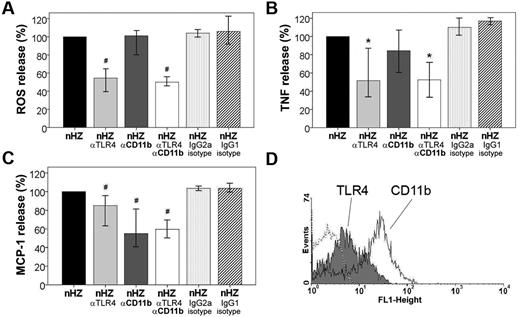
Because of the rapid, prephagocytic occurrence of the stimulatory nHZ-bound FG-mediated effects, involvement of receptor-mediated pathways was plausible. Two FG receptors, the TLR4 and the integrin CD11b/CD18, are expressed on the monocyte at low (400-3200) and high (20 000-60 000) copy numbers per cell, respectively (Figure 5D).13,17 Their role in the FG-signal transmission was evaluated, applying receptor-specific blocking Abs before the immune response to nHZ was elicited in functional assays. TLR4-blocking Abs significantly inhibited burst enhancement by ∼ 45% at 4 minutes after nHZ-bound FG to monocyte contact, whereas CD11b-blocking Abs had no effect. No inhibition was seen with isotype-matched control Abs (see Figure 5A). The TLR4-dependent inhibition coincided temporally with burst activation by nHZ, remained high up to burst peak at 15-20 minutes, but went down time dependently thereafter (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, initial burst enhancement was mainly elicited by TLR4, whereas CD11b/CD18 integrin did not play any significant role. TLR4-blocked monocytes maintained their ability to generate burst and were fully responsive to phorbol myristate acetate, the receptor-independent potent burst activator (not shown). Blocking anti-TLR4 and anti-CD11b/CD18 Abs significantly reduced TNF release from nHZ-treated monocytes by 50% and 15%, respectively (Figure 5B). To better define the role of CD11b, anti-TLR4 Abs were combined with anti–CD11b/CD18-blocking Abs (Figure 5B column 4). CD11b/CD18 binding of FG seemed to be permissive but not additive for signal transduction by TLR4, because no additional inhibition was found when both receptors were simultaneously blocked, confirming the primary role of TLR4 in FG-dependent TNF release. MCP-1 secretion was reduced by ∼ 55% when both FG receptors were blocked (Figure 5C). In contrast to TNF release, MCP-1 secretion was mostly CD11b/CD18 dependent. Here, the TLR4 played only a secondary role but was also responsive in non–IFNγ-treated cells (not shown). The same anti–TLR4-blocking Abs inhibited LPS binding by 50% (not shown). Both TNF and MCP-1 release was not inhibited by isotype-matched control Abs, confirming the receptor dependency of the nHZ-bound FG-elicited effects.
TLR4 and CD11b/CD18 mediate rapid ROS, TNF, and MCP-1 release induced by nHZ. (A) Inhibition of nHZ-elicited ROS release (oxidative burst) by TLR4- and CD11b/CD18-blocking antibodies from human monocytes. Suspended nonprimed monocytes were supplemented with nHZ immediately after isolation from healthy donors at time 0 and incubated at 37°C (nHZ, n = 8). For inhibition studies, cells were incubated for 15 minutes with blocking Abs, anti-TLR4 (nHZ αTLR4), and anti-CD11b (nHZ αCD11b), or nonspecific IgG2a (nHZ IgG2a isotype) and IgG1 (nHZ IgG1 isotype) Abs as isotype controls for anti-TLR4 and anti-CD11b, respectively, before nHZ addition. nHZ-elicited ROS release was quantified 4 minutes after nHZ addition by luminol-enhanced luminescence. (B) TNF and (C) MCP-1 release from monocytes pretreated or not with TLR4- and CD11b/CD18-blocking Abs and subsequent addition of nHZ. Monocytes obtained from healthy donors were enriched by adhesion, maintained in culture overnight with 200 U/mL IFNγ, and then supplemented with nHZ (100nmol/106 monocytes in terms of heme content). After 3 hours supernatants were collected and analyzed for TNF and MCP-1 by ELISA. For inhibition studies, cells were treated as indicated in panel A. ROS, TNF, and MCP-1 release values after Ab treatment are expressed as the percentage of control nHZ values. Four and 7 independent experiments were performed for TNF and MCP-1 analysis, respectively. Results are expressed as medians with 95% CI; *P < .05 and #P < .01. Absolute values of nHZ-induced TNF, MCP-1, and ROS release were, respectively, 357 pg/mL (120-644 pg/mL), 575 pg/mL (284-877 pg/mL), and 106 × 103 cps/150 000 cells (65-156 × 103 cps/150 000 cells). (D) Surface expression of TLR4 (gray full) and CD11b (open solid line) antigens in adherent human monocytes was analyzed by flow cytofluorimetry after overnight stimulation with 200 U/mL IFNγ. Background is shown as dashed line.
TLR4 and CD11b/CD18 mediate rapid ROS, TNF, and MCP-1 release induced by nHZ. (A) Inhibition of nHZ-elicited ROS release (oxidative burst) by TLR4- and CD11b/CD18-blocking antibodies from human monocytes. Suspended nonprimed monocytes were supplemented with nHZ immediately after isolation from healthy donors at time 0 and incubated at 37°C (nHZ, n = 8). For inhibition studies, cells were incubated for 15 minutes with blocking Abs, anti-TLR4 (nHZ αTLR4), and anti-CD11b (nHZ αCD11b), or nonspecific IgG2a (nHZ IgG2a isotype) and IgG1 (nHZ IgG1 isotype) Abs as isotype controls for anti-TLR4 and anti-CD11b, respectively, before nHZ addition. nHZ-elicited ROS release was quantified 4 minutes after nHZ addition by luminol-enhanced luminescence. (B) TNF and (C) MCP-1 release from monocytes pretreated or not with TLR4- and CD11b/CD18-blocking Abs and subsequent addition of nHZ. Monocytes obtained from healthy donors were enriched by adhesion, maintained in culture overnight with 200 U/mL IFNγ, and then supplemented with nHZ (100nmol/106 monocytes in terms of heme content). After 3 hours supernatants were collected and analyzed for TNF and MCP-1 by ELISA. For inhibition studies, cells were treated as indicated in panel A. ROS, TNF, and MCP-1 release values after Ab treatment are expressed as the percentage of control nHZ values. Four and 7 independent experiments were performed for TNF and MCP-1 analysis, respectively. Results are expressed as medians with 95% CI; *P < .05 and #P < .01. Absolute values of nHZ-induced TNF, MCP-1, and ROS release were, respectively, 357 pg/mL (120-644 pg/mL), 575 pg/mL (284-877 pg/mL), and 106 × 103 cps/150 000 cells (65-156 × 103 cps/150 000 cells). (D) Surface expression of TLR4 (gray full) and CD11b (open solid line) antigens in adherent human monocytes was analyzed by flow cytofluorimetry after overnight stimulation with 200 U/mL IFNγ. Background is shown as dashed line.
Mutually independent binding of FG and opsonins to nHZ
The role of opsonin binding on FG-dependent cell stimulation by nHZ was assessed. IgG and complement were quantified in various HZ preparations containing or not functionally intact FG and were correlated with FG binding. FG did not seem to interfere with the binding of complement and IgG to HZ (Figure 6A). Absence of FG in HZ prepared from Albumax and serum-supplemented cultures or the presence of denatured FG in heat-treated HZ did not influence binding of IgG and complement fragments to HZ. The release of ROS, TNF, and MCP-1 exclusively correlated with the presence of FG was independent of bound opsonins and was not surrogated by binding of IgG and C3 fragments to their receptors (Figure 6B).
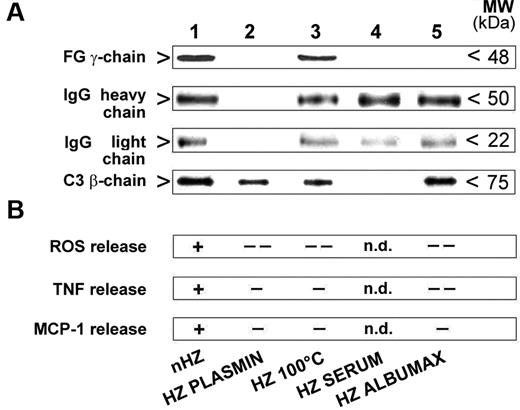
IgG and complement bind to nHZ in FG-independent manner but do not elicit the full ROS, TNF, and MCP-1 response in the absence of native FG. (A) IgG and complement binding to HZ during opsonization. HZ obtained from the supernatants of either plasma- (nHZ, lane 1), serum- (HZ SERUM, lane 4), or Albumax- (HZ ALBUMAX, lane 5) supplemented cultures of P falciparum or nHZ digested with plasmin (HZ PLASMIN, lane 2) or denatured by heating at 100°C for 5 minutes (HZ 100°C, lane 3) were opsonized with freshly drawn serum for 30 minutes at 37°C. One representative blot of 3 with similar results is shown. Binding of FG, the opsonins IgG (IgG heavy chain, IgG light chain), and complement (C3β-chain) to the different HZ types was analyzed by Western blotting of extracted and 10% SDS-PAGE separated proteins with specific anti-FG γ-chain, anti-IgG, and anti-C3c Abs. Proteins from 50nmoles, 10nmoles, and 10nmoles HZ (in terms of heme content) were analyzed for FG, both IgG chains, and complement, respectively. (B) FG is essential for full ROS, TNF, and MCP-1 release. IgG and complement alone are no substitutes for FG. Full responses as seen with opsonized FG-containing HZ are indicated with ‘+’ and significantly lower or no responses with ‘-’ or ‘- -’ (see Figure 4 for numeric data on which this qualitative summary is based); n.d. indicates not determined.
IgG and complement bind to nHZ in FG-independent manner but do not elicit the full ROS, TNF, and MCP-1 response in the absence of native FG. (A) IgG and complement binding to HZ during opsonization. HZ obtained from the supernatants of either plasma- (nHZ, lane 1), serum- (HZ SERUM, lane 4), or Albumax- (HZ ALBUMAX, lane 5) supplemented cultures of P falciparum or nHZ digested with plasmin (HZ PLASMIN, lane 2) or denatured by heating at 100°C for 5 minutes (HZ 100°C, lane 3) were opsonized with freshly drawn serum for 30 minutes at 37°C. One representative blot of 3 with similar results is shown. Binding of FG, the opsonins IgG (IgG heavy chain, IgG light chain), and complement (C3β-chain) to the different HZ types was analyzed by Western blotting of extracted and 10% SDS-PAGE separated proteins with specific anti-FG γ-chain, anti-IgG, and anti-C3c Abs. Proteins from 50nmoles, 10nmoles, and 10nmoles HZ (in terms of heme content) were analyzed for FG, both IgG chains, and complement, respectively. (B) FG is essential for full ROS, TNF, and MCP-1 release. IgG and complement alone are no substitutes for FG. Full responses as seen with opsonized FG-containing HZ are indicated with ‘+’ and significantly lower or no responses with ‘-’ or ‘- -’ (see Figure 4 for numeric data on which this qualitative summary is based); n.d. indicates not determined.
Role of opsonins in nHZ-mediated release of TNF, MCP-1, and oxidative burst
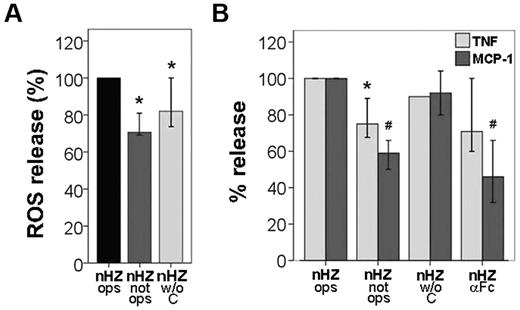
To assess the contribution of nHZ-bound opsonins IgG and complement, the monocyte responses to serum-opsonized, nonopsonized, and IgG-opsonized nHZ were compared. Burst was largely opsonin independent. Absence of IgG or complement abrogated burst by ∼ 20% or 30%, respectively (Figure 7A). In addition, opsonized but FG-free HZ did not enhance oxidative burst (Figure 4B). Maximal release of TNF and MCP-1 was observed with serum-opsonized nHZ, depending on IgG, to ∼ 25% and 50% for TNF and MCP-1, respectively, as concluded both from nonopsonized nHZ and anti–Fc-treated nHZ columns in Figure 7B. Complement-free but IgG-opsonized nHZ activated cells as much as fully opsonized nHZ, excluding a significant role of complement in TNF and MCP-1 release (Figure 7B). Thus, a considerable portion of cytokines and chemokines released by nHZ was opsonin independent, whereas the opsonin-dependent release was mainly because of nHZ-bound IgGs.
IgGs and complement bound to nHZ play a subsidiary role in the immediate nHZ-elicited release of ROS, TNF, and MCP-1 from monocytes. (A) Suspended monocytes from healthy donors were fed with serum-opsonized nHZ (nHZ ops), nonopsonized nHZ (nHZ not ops), or nHZ opsonized with decomplemented serum (nHZ w/o C). Cells were fed immediately after isolation at time 0, briefly spun down, resuspended, and incubated at 37°C. nHZ-elicited burst was quantified 4 minutes after nHZ addition by luminol-enhanced luminescence. (B) Monocytes from healthy donors were enriched by Ficoll passage and adhesion, maintained in culture overnight with 200 U/mL IFNγ, and then supplemented with differently treated nHZ at equal heme content (100nmol/106 monocytes) after 15 hours from isolation. Monocytes were supplemented with opsonized nHZ (nHZ ops), nonopsonized nHZ (nHZ not ops), nHZ opsonized with decomplemented serum (nHZ w/o C), or nHZ opsonized and treated with anti IgG-Fc Abs (nHZ αFc). After 3 hours, supernatants were collected and analyzed for TNF and MCP-1 by ELISA. Four, 5, and 7 independent experiments were performed for TNF, MCP-1, and ROS analysis, respectively. Results are expressed as medians with 95% CI; *P < .05 and #P < .01. Absolute values of nHZ-induced TNF, MCP-1, and ROS release were, respectively, 110 pg/mL (88-122 pg/mL), 430 pg/mL (200-877 pg/mL), and 146 × 103 cps/150 000 cells (74-369 × 103 cps/150 000 cells).
IgGs and complement bound to nHZ play a subsidiary role in the immediate nHZ-elicited release of ROS, TNF, and MCP-1 from monocytes. (A) Suspended monocytes from healthy donors were fed with serum-opsonized nHZ (nHZ ops), nonopsonized nHZ (nHZ not ops), or nHZ opsonized with decomplemented serum (nHZ w/o C). Cells were fed immediately after isolation at time 0, briefly spun down, resuspended, and incubated at 37°C. nHZ-elicited burst was quantified 4 minutes after nHZ addition by luminol-enhanced luminescence. (B) Monocytes from healthy donors were enriched by Ficoll passage and adhesion, maintained in culture overnight with 200 U/mL IFNγ, and then supplemented with differently treated nHZ at equal heme content (100nmol/106 monocytes) after 15 hours from isolation. Monocytes were supplemented with opsonized nHZ (nHZ ops), nonopsonized nHZ (nHZ not ops), nHZ opsonized with decomplemented serum (nHZ w/o C), or nHZ opsonized and treated with anti IgG-Fc Abs (nHZ αFc). After 3 hours, supernatants were collected and analyzed for TNF and MCP-1 by ELISA. Four, 5, and 7 independent experiments were performed for TNF, MCP-1, and ROS analysis, respectively. Results are expressed as medians with 95% CI; *P < .05 and #P < .01. Absolute values of nHZ-induced TNF, MCP-1, and ROS release were, respectively, 110 pg/mL (88-122 pg/mL), 430 pg/mL (200-877 pg/mL), and 146 × 103 cps/150 000 cells (74-369 × 103 cps/150 000 cells).
Discussion
A large number of HZ studies have used HZ stripped free of proteins or lipids or both or variously prepared BH, considered to be equivalent or comparable to nHZ. These materials are not the physiologic meals of phagocytes, though. Therefore, results obtained here with nHZ or RBs will be preferentially considered and discussed. nHZ has been shown to induce both stimulatory3,5,18 and inhibitory effects in HZ-laden human monocytes.6,8,19 Inhibitory effects, such as inhibition of erythropoiesis,7 dendritic cell maturation,19 antigen presentation,6 and abrogation of the ability to kill ingested bacteria3 and to repeat phagocytic cycles,8 are long-term effects that typically start 4-6 hours after completion of phagocytosis and may persist during several days.7,16,18 Short-term stimulatory effects5,8 elicited by yet undisclosed mechanisms were occasionally described but have received little attention so far.
In summary, this study shows, first, that nHZ bound large amounts of FG. Second, recognition of nHZ-bound FG or RB-bound FG by monocytes in the prephagocytic phase elicited the FG-dependent stimulation of oxidative burst and release of TNF and MCP-1. Third, those rapid effects were mediated by TLR4 and integrin CD11b/CD18 (CR3, Mac-1), both well-known FG-receptors on the monocyte.11-13 Finally, short-term FG effects appear to be the starting event leading to the persistent long-term effects described in previous studies and largely mediated by HZ-generated lipoperoxidation products such as 15(S,R)-hydroxy-6,8,11,13-eicosatetraenoic acid4,18,19 and 4-hydroxynonenal (4-HNE).7
FG is a large 340-kDa plasma glycoprotein formed by 2 identical sets of disulfide-bridged α-/β-/γ-chains.20 In addition to its role in hemostasis, FG has been proposed as a bridging molecule for intercellular adhesions and, more importantly, as a regulator of the innate immune response.11-13,20 This study shows that FG firmly and rapidly bound to both nHZ and RBs, successfully competing with high-concentration plasma proteins such as albumin. Assuming ∼ 2 × 108 heme molecules per HZ crystal,1 one HZ crystal binds 8 × 103 and one RB binds 8 × 104 FG molecules, respectively. FG binding to nHZ escaped attention until present probably because plasma is rarely used as a culture supplement.
A first short-term response elicited by FG bound to nHZ/RB was the rapid and remarkably strong oxidative burst starting immediately after cell contact and thus clearly a receptor-mediated event. Peak burst was enhanced > 100-fold compared with burst elicited by contact with opsonized RBCs.8 Several studies indicate that TLR4 and integrin CD11b/CD18 are FG receptors.11-13,17 TLRs, expressed in monocytes,17 are “guardian/alert receptors” and recognize numerous unique and well-conserved exogenous ligands, known as pathogen-associated molecular patterns.21 The best-known exogenous pathogen-associated molecular pattern ligand of TLR4 is LPS from Gram-negative bacteria.22 A number of host-derived endogenous “danger signal” molecules, such as FG, are also TLR4 ligands. TLR4 activation enhances oxidative burst and increases expression of proinflammatory cytokines, such as TNF, IL-1β, IL-8, IL-12, and IL-18, by activation of NF-κB and MAP kinases.11,12,23
The present study confirms involvement of TLR4 but not of CD11b/CD18 in burst enhancement. Burst was extensively or totally abrogated by pretreatment of cells with anti-TLR4 Abs or removal of FG from nHZ, respectively, but was not modified by anti-CD11b/CD18 Abs. Burst is produced by NADPH oxidase (NOX), which transfers electrons from NADPH to O2 generating ROS (equivalent to oxidative burst).24 Membrane assembly of subunits and activation of NOX depend on phosphorylation of the cytosolic component p47phox.24 A series of TLR4 downstream-activated kinases, such as PKC, IRAK-4 (directly or by p38 MAPK and Akt), and Src (directly or by PI3K), phosphorylate p47phox and stimulate burst.25 The long-term burst inhibition observed 6-14 hours after nHZ treatment8 was accompanied by defective phosphorylation of p47phox, compatible with the 4-HNE–mediated inhibition of PKC, a kinase responsible for above phosphorylation.3
A second short-term effect of FG bound to nHZ/RB was the fast release of TNF ∼1 hour and 3 hours after nHZ/RB contact. Burst enhancement immediately after contact with nHZ-bound FG was possibly instrumental for the fast release of TNF. In fact, NOX-generated ROSs rapidly activate TNF-α converting enzyme, a member of the metalloproteinase family that cleaves the membrane-bound 26-kDa nonglycosylated transmembrane TNF precursor,26 generating the soluble, circulating TNF.
A third short-term effect elicited by FG bound to nHZ/RB was the enhanced release of MCP-1 which mainly occurred by CD11b/CD18, a second FG receptor,13,20 as shown by the extensive abrogation of MCP-1 release by pretreatment of monocytes with anti-CD11b/CD18 Abs or removal of FG from nHZ. Interaction of FG with CD11b/CD18 in vitro induces activation of the NF-κB system, up-regulation of adhesion molecules, increase in cell migration, enhanced cytokine and chemokine expression, and degranulation of secretory vesicles.13,20 MCP-1, a 8.7-kDa chemokine, is a key molecule in both innate and adaptive immunity, known to induce Th1-type responses, destabilize the endothelial barrier, regulate recruitment of peripheral white blood cells (WBCs) to inflammatory sites,27-29 and induce migration of naive monocytes toward nHZ-fed cells (E.S., V.B., unpublished observations, October 2004). Therefore, FG bound and presented in the right conformation by nHZ may function as an “immune sensor” and a first priming signal for long-lasting responses by MCP-1–mediated recruitment of inflammatory cells.
The TLR4-mediated short-term responses to nHZ continues with a number of long-term nHZ effects apparently mediated by lipoperoxidation products interacting with receptors and downstream elements of signaling cascades,30 for example, the activation of the NF-κB system.31 Reports indicate that the cellular redox status regulates, and ROS production triggers, the nuclear translocation of NF-κB, whereas antioxidants abolish NF-κB activation.31-33 Activation of NF-κB is thus expected to play an essential role in the long-term nHZ effects, inducing the expression of proinflammatory cyto-chemokines, adhesion molecules and their receptors, a number of transcription factors, signaling molecules, and transporters and various enzymes, notably matrix metalloproteinase-9.31-33
The stable binding of FG to HZ and RBs and the FG effects mediated by TLR4 and integrins represent a novel paradigm of HZ activity. The presence of multiple FG receptors on a variety of cells would expand the number of specific cellular targets of HZ. FG may bind by TLR4, integrin, ICAM-1, or any combination of them, to a variety of cells, such as endothelia, airway epithelia, platelets and leukocytes, kidney podocytes, smooth muscle cells, and glia cells, and evoke a variety of responses according to the specifics of each cellular target.34-38
nHZ and RBs may play important roles in organ pathogenesis, because parasitized RBCs adhere to endothelia for longer periods of time, undergo schizogony, and liberate nHZ, that is expected to rapidly bind FG and be recognized and phagocytosed by adherent phagocytes. Similarly to the effects on phagocytes discussed before, interaction of nHZ-bound FG with endothelial TLR4 may activate the NF-κB system and enhance expression of matrix metalloproteinases and the cytokine-chemokine cascade, even in the absence of nHZ endo- or phagocytosis.35-38 Those activation processes in microvessels might explain the elevated levels of microparticles found in the plasma of patients with cerebral malaria39,40 and probably damage endothelium integrity, upset the structure of basal lamina, and abrogate the tight barrier function of endothelium in specific organs. The different forms of organ malaria are correlated with sequestration of parasitized RBCs and HZ-laden phagocytes in the brain,41,42 lungs (producing acute respiratory distress),43 placenta (poor birth outcome),37 and kidney (renal failure).44 It is plausible to assume that the pathogenic mechanism of organ malaria may be because of the combination of stimulatory effects of nHZ- or RB-bound FG induced in monocytes and endothelial cells as described earlier. Interestingly, preliminary studies (not shown) with IgGs isolated from an immune serum pool (kindly provided by Marcel Hommel, University of Liverpool) indicate that the TLR4/integrin-mediated and FG-dependent inflammatory responses might be attenuated by adaptive immune mechanisms, explaining the reduction of clinical symptoms with repetitive infections.
Specific interactions between nHZ and a highly specialized brain region may explain the mechanism of recurrent fever in malaria. Cyclic variation of TNF, a thermogenic cytokine, was directly linked to schizogony (and HZ release) in coincubation experiments with human monocytes. Interestingly, a delay of ∼ 6 hours between schizogony and peak release of TNF was observed.45 nHZ liberated during schizogony might directly adhere to endothelium in the vascular network of the thermoregulatory center in the anterior hypothalamus and start the chain of events that induce fever by TLR4 triggering.46 The duration of the febrile attacks (< 7 hours) is too short for ad hoc synthesis of cytokines but compatible with the timing of TLR4 effects elicited by nHZ-bound FG.
TLR involvement in malaria pathogenesis was the main focus of recent studies. In acute experimental human malaria, parasite infection was found to prime human TLR2 and TLR4 response with increased TNF production.35 Falciparum GPI was found to interact with TLR2 on release of merozoites, the main carriers of GPI.47 Present data do not support a role of TLR2 in mediating rapid TNF, MCP-1, and ROS release by nHZ, because those proinflammatory effects were unaffected by blocking GPI receptors on HZ-exposed monocytes (not shown). HZ was recently excluded to be a TLR9 ligand or to function as a carrier for malarial DNA to intracellular TLR9.48 We exclude any DNA/TLR9 involvement in the nHZ-elicited responses, which were unmodified with the use of DNase-treated nHZ completely devoid of parasitic DNA (not shown), while being considerably altered by blocking TLR4 with specific antibodies or removal of FG.
In conclusion, this study shows for the first time that host FG was constantly present and stably bound to nHZ isolated from plasma-cultured parasites. Although phagocytosed nHZ permanently alters important functions of host phagocytes, it is shown that FG contained in nHZ was responsible for the rapid 100-fold stimulation of ROS production and 50-fold increase in the level of TNF and MCP-1 by nHZ-supplemented monocytes. Those effects, starting within minutes after nHZ cell contact, were because of interaction of FG with FG -receptors TLR4 and integrin CD11b/CD18. Receptor blockage by specific mAbs or removal of FG from nHZ abrogated the effects. Immediate increase in ROS and TNF may switch on previously described long-term effects of nHZ, largely because of (and recapitulated by) HZ-generated liperoxidation products 15(S,R)-hydroxy-6,8,11,13-eicosatetraenoic acid and 4-HNE. The FG/HZ effects mediated by TLR4/integrin represent a novel paradigm of nHZ activity and may allow expansion of nHZ effects to nonphagocytic cells, such as endothelia, airway epithelia, smooth muscle cells, glia cells, and may lead to a better understanding of organ pathology in malaria.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Tatiana Ugarova (Arizona State University) for helpful suggestions on anti-CD11b antibodies and Dr Regine Kraft (Humboldt University Berlin, Germany) for FG identification.
This work was supported by Regione Piemonte, Progetto Ricerca Scientifica Applicata, and Progetti Ricerca Sanitaria Finalizzata (E.S. and P.A.); Compagnia di S. Paolo, Italian Malaria Network Project (P.A.); and EVIMALAR (European Virtual Institute dedicated to Malaria Research), Project No. 242095 (P.A.).
Authorship
Contribution: V.B., E.S., O.A.S., D.B., and G.G. conducted experiments; and V.B., E.S., and P.A. designed experiments, interpreted results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Evelin Schwarzer, Dipartimento di Genetica, Biologia e Biochimica, Università di Torino, Via Santena 5 bis, 10126 Torino, Italy; e-mail: evelin.schwarzer@unito.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal