Abstract
Protein tyrosine kinase 7 (PTK7) is a pseudokinase whose precise function in regulating angiogenesis remains unknown. The purpose of this study was to define the mechanisms by which PTK7 promotes vascular endothelial growth factor-A (VEGF-A)–induced angiogenesis in vivo and in vitro. Immunoblotting was used to measure PTK7 expression in several types of vascular endothelial cells. Using both immunoprecipitation and immunoblotting, PTK7 was found to join a receptor complex with Flt-1 (VEGFR1), but not with KDR/Flk-1 (VEGFR2) or with Flt-4 (VEGFR3). By surface plasmon resonance analysis, the interaction between Flt-1 and PTK7 was confirmed and found to be intensified by VEGF-A. Flt-1 phosphorylation and downstream signals of Akt, and focal adhesion kinase (FAK) thus induced were down-regulated by inhibition of PTK7 expression using siRNA. Moreover, PTK7 overexpression in endothelial cells resulted in enhanced angiogenesis in vitro. In contrast, neovascularization induced in vivo by VEGF-A pellets was significantly decreased by injection of siRNA targeting PTK7. These data suggest that PTK7 serves an essential role in Flt-1–mediated angiogenesis.
Introduction
Protein kinases regulate the functions of many proteins by catalyzing covalent attachment of phosphate to serine, threonine, and tyrosine residues in their targets. Genome-wide analyses have revealed that 2%-3% of all eukaryotic genes encode proteins containing a kinase domain, used to regulate cellular homeostasis.1-3 Unexpectedly, some protein kinases (< 10%) lack one or more of the conserved amino acids required for kinetic activity, and are thus predicted to be inactive. These kinases are termed pseudokinases or defective kinases. However, unlike tyrosine kinases, such pseudokinases have not been well studied and their physiologic roles remain obscure.
One such pseudokinase, protein tyrosine kinase 7 (PTK7, also termed colon carcinoma kinase 4 [CCK4]) was originally identified as a protein overexpressed in several cancer cells, including melanoma4 and colon carcinoma.5 Structurally, PTK7 consists of a single peptide, with 7 immunoglobulin-like extracellular domains, a transmembrane region, and a C-terminal domain with homology to tyrosine kinases. Although no specific ligand for PTK7 has yet been identified, mouse embryos expressing a truncated form of PTK7 die perinatally, with profound defects in neural tube closure and orientation of inner ear stereociliary bundles, indicating that PTK7 is involved in polarity regulation within cell planes.6 Interestingly, PTK7 is evolutionarily highly conserved among organisms as diverse as hydra (which occupy a near-basal position among metazoa) and humans.7 These studies indicate that, despite absence of both kinetic activity and known function, PTK7 may play an essential role in survival and embryogenesis.
Angiogenesis is controlled by various positive and negative regulators, including growth factors, cytokines, lipid metabolites, and cryptic hemostatic protein fragments. Of the various angiogenic pathways, the role of vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) in vascularization have been better characterized than other systems.8,9 VEGF-A binds with high affinity to Flt-1 (VEGFR1) and Flk-1/KDR (VEGFR2), an interaction that is essential for angiogenesis. There is now consensus that Flk-1/KDR is the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF-A in many pathologic states.10 However, the role of Flt-1 in angiogenesis remains controversial. Some reports have claimed that Flt-1 positively affects angiogenesis and vascular endothelial cell migration.11,12 In contrast, soluble Flt-1 is considered to function as a negative regulator and a decoy factor in angiogenesis. Contrary to these reports, other studies, using knockdown or knockout mouse models13-15 have found that Flt-1 increases vascular endothelial cell growth and proliferation, suggesting that Flt-1 is critical in facilitating angiogenesis. Shin and colleagues have reported that PTK7 is present in vascular endothelial cells (VECs) and plays an important role in human umbilical vein endothelial cell (HUVEC) migration.16 These authors have found that HUVEC migration was inhibited in vitro and in vivo when PTK7 expression was reduced. However, the exact mechanisms by which PTK7 regulates cell migration and angiogenesis were not explored.
The purpose of the present study was to define the mechanisms by which PTK7 promotes VEGF-induced angiogenesis, using both in vivo and in vitro models.
Methods
Reagents
Mouse monoclonal antibody against human PTK7 (clone 1A8) was purchased from GeneTex. Human recombinant VEGF-A165, mouse recombinant VEGF-A164, and goat polyclonal antibody against PTK7 were purchased from R&D Systems. Anti–human VEGFR1 (Flt-1), anti–human VEGFR2 (KDR), anti–phospho-Tyr (P99), and anti–human VEGFR-3 (Flt-4) were from Santa Cruz Biochemicals. Anti–phospho-tyrosine kinase, anti–phospho-Flt-1 (Tyr 1213), and anti–phospho-KDR (Tyr1054) were from Millipore. Mouse anti-FAK was purchased from Calbiochem, and both anti–phospho-FAK (Tyr 567/577) and anti–phospho-Akt (Ser/Thr) were from Cell Signaling Technology. Anti–mouse Akt was from Cell Sciences Inc. Murine PlGF was purchased from Sigma-Aldrich.
Cell culture
HUVECs (Clonetics) were cultured in EGM2-MV medium (Lonza). Bovine arterial endothelial cell (BAE) and the mouse endothelial cell line, MS1 (a kind gift from Dr Patricia D'Amore, Boston MA), derived from primary murine cells by immortalization with temperature-sensitive SV40 large T antigen,17 were cultured in Dulbecco modified Eagle medium (DMEM)/F12, with 10% (vol/vol) fetal bovine serum (Invitrogen), respectively. HUVECs were used between passages 5 and 10. As a negative control, HEK293 cells and NIH 3T3 fibroblasts (ATCC) were maintained in DMEM with 10% (vol/vol) FBS. Cells were grown under 5% (vol/vol) CO2 at 37°C and media were changed every 2-3 days.
Immunoprecipitation
Cell lysates for immunoprecipitation were prepared in mammalian cell lysis buffer supplemented with a protease inhibitor cocktail (Roche). Supernatants were collected after centrifugation at 15 000g for 20 minutes at 4°C. Between 350-500 μg of total protein was incubated with 1∼3 μg of appropriate antibody for at least 3 hours and 50 μL of protein A/G-conjugated agarose beads for an additional hour at 4°C. Beads were washed 3 times with lysis buffer and immunoprecipitates resuspended in 2× SDS sample buffer.
Western blot analysis
Total protein concentrations of supernatant fractions were determined using the BCA protein assay (BioRad Laboratories). Equal amounts of protein aliquots were boiled in equal volumes of 2× SDS Laemmli sample buffer, and resolved on 8% (wt/vol) or 10% (wt/vol) SDS-PAGE. Proteins were next transferred to polyvinylidene difluoride (PVDF) membranes and probed overnight with primary antibodies. Immunoreactive bands were detected with horseradish peroxidase–conjugated secondary antibodies and visualized by the enhanced chemiluminescence technique.
Plasmids
cDNAs encoding a fragment of human PTK7 (PTK715-59) and full-length PTK7 were amplified by PCR from a HeLa cell cDNA library, using Pfu polymerase (Stratagene). Each PCR fragment was inserted into the pcDNA3.1 expression vector (Invitrogen) and plasmids were transformed into Escherichia coli JM109 cells. Transformed JM109 cells were grown in 1 L of Luria broth (LB) and recombinant plasmids were purified using the QIAfilter approach (QIAGEN). Both PTK7 cDNA clones were fully sequenced.
Purification of proteins
To construct PTK7-GST and fragment PTK715-59-GST expression vectors, the cDNAs amplified in the previous section were introduced into the GST expression vector pGEX-5X (GE Healthcare). These vectors were introduced into E coli L21/DE3. Transformed cells were grown in LB medium with ampicillin, and after isopropyl-L-thio-β-D-galactopyranoside (IPTG) induction, were spun down, washed in PBS, and sonicated in the presence of 35mM octyl-glucopyranoside in PBS (pH 7.2). Cell debris was removed by centrifugation, and the GST-PTK7 or GST-fragment-PTK7 proteins purified on a GST affinity column (Amersham Biosciences). To construct the Flt-1 and Flk-1/KDR-GST plasmid, cDNA encoding Flt-1 and Flk-1 was amplified and inserted into the vector described. The plasmid was transformed into CHO cells maintained in Ham-F12 medium (GIBCO-BRL) supplemented with 10% (vol/vol) FCS in p60 plates at 5 × 105 cells per well. Transductions were carried out 1 day after initial plating using 10 μg of the pCl-Neo constructs and 24 μL of lipofectamine in a total volume of 1 mL medium/well. Transfected cells were selected using G418 sulfate (Geneticin; GIBCO-BRL) at 500 μg/mL and cloned by limiting dilution, and clones producing the highest levels of PTK7 proteins as assessed by ELISA were expanded for protein production. Flt-1-GST and Flk-1/KDR-GST were purified from tissue culture supernatants by affinity chromatography on an anti-Bb column. The eluate was concentrated and the protein subjected to final purification by gel filtration using Biacore buffer on Superdex 200. To cleave GST-tag from proteins, the eluted fractions were dialyzed overnight at 4°C against 5mM CaCl2, 100 mM NaCl, and 50mM Tris (pH 8.0), and incubated 16 hours at room temperature with 1.2 μg of bovine factor Xa (Promega) per 100 μg of fusion protein. Digestion products were resolved by on 3%-12% Tris-Bis Native Gel (Invitrogen) or SDS-PAGE. After electrophoresis, the gels were stained with Coomassie Blue R-250 and then destained.
Surface plasmon resonance analysis
Analyses were performed using a BIAcore 3000 instrument (GE Healthcare). Flt-1 or Flk-1/KDR was immobilized onto CM5 sensor chip (GE Healthcare) surfaces, using an amine coupling kit (GE Healthcare) according to the manufacturer's instructions. Protein binding was measured at a flow rate of 10 μL/min in 145mM NaCl/10mM HEPES (pH 7.4) containing varied concentrations of analyte proteins, unless otherwise indicated. As a control, each analyte protein was injected over a surface with no immobilized protein, to yield a blank sensorgram for subtraction of bulk refractive index background. Surface regeneration was achieved by injection of 20 μL of 10mM glycine-HCl (pH 2.0). Data were analyzed using BIAcore software (GE Healthcare).
Transient transduction of pPTK7 or siRNA directed against PTK7
To temporally knock down or overexpress PTK7, cells were transfected with siRNA directed against PTK7 mRNA (siPTK7), or plasmid (pPTK7), as previously described.18 In brief, 20 pmol of siRNA was mixed with 1 μL of Lipofectamine 2000 (Invitrogen) to form a transduction complex that was then added to a culture medium of low serum concentration. After 4 hours of transduction, the medium was changed to normal medium. Overexpression, and siRNA transduction effectiveness, were confirmed by real time RT-PCR and Western immunoblotting.
Real-time RT-PCR assay for PTK7
RNA was isolated with RNeasy Micro Kit (QIAGEN) and reverse transcribed using Superscript III Kit (Invitrogen). Real-time PCR was performed using TaqMan Universal PCR Mastermix and preformulated primers for PTK7 (assay ID Mm00613365_ml) and GAPDH (assay ID Mm99999915_gl; Applied Biosystems). The results were analyzed by the comparative threshold cycle method and normalized by GAPDH as an internal control.
Flt-1 and KDR gene silencing
To generate shVEGFR, retrovirus expression vector pSIREN-RetroQ (Clontech) was used to achieve the expression of short hairpin interference RNA (shRNA) in MS1 cells. The following inserts were used: KDR, shKDR-a (5′-ggatgaacattgtgaacga-3′) and shKDR-b (5′-gctcctgaagatctgtata-3′); Flt-1, shFlt-1-a (5′-gagcaaacgtgacttattt-3′), and shFlt-1-b(5′-gcagcacgctgtttattga-3′). Each sequence was separated by a 9-nucleotide noncomplementary spacer from the reverse complement of the same 19-nucleotide sequence. SMART vector 2.0 nontargeting negative control particle (Thermo Scientific) was used for nonsilencing control (shNeg).
Retroviral stock solutions were generated by transient cotransfection with the lentiviral packing vectors psPAX2, pMD2.G and sh-Flt-1, or sh-KDR into HEK 293T cell by calcium phosphate method. Supernatants containing lentiviruses were used to infect MS1 cells overnight. Cells were selected with 1 μg/mL puromycin (Invitrogen) for at least 1 week, and down-regulation of target genes were examined by RT-PCR and Western blot.
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Schepens Eye Research Institute and all mice were treated according to the Association for Research in Vision and Ophthalmology Statement for use of animals in Ophthalmic and Vision Research. Male Balb/c and C57BL/6 mice (6-8 weeks of age) were purchased from Taconics.
Corneal micropocket angiogenesis assay
This assay was performed as described previously.19 In brief, hydron-sucralfate pellets (1 μg) containing 160 ng/pellet VEGF-A164 were implanted into pockets surgically created in the avascular corneal midstroma of anesthetized male Balb/C mice. After pellet insertion, 20 pmol PTK7 siRNA or nonspecific random control siRNA was subconjunctivally injected, and injections repeated every 2 days. Slit-lamp photography for neovascularization extent evaluation was performed 7 and 14 days postoperatively. At each observation time, 3 mice from each group were killed, eyeballs were enucleated, and whole-mount corneal flaps prepared for CD31 staining of the vascular area. The details are described in “Immunohistochemical and immunocytochemical staining.”
Wound break assay
Vascular endothelial cell migration was determined by monolayer wound-healing assays as described previously.20 During these experiments, we omitted both medium serum and growth factors for 12 hours to mitigate the effects of migration-enhancing cytokines. All experiments were performed 3 times, at least in triplicate, and a minimum of 3 different areas were studied in each sample.
Cell proliferation assay
Transfected cells were seeded at a density of 2 × 105 cells per 100-mm dish and cultured with VEGF-A. Cell number was counted at each time point until 72 hours. Serum free DMEM medium was changed once at 48 hours.
Immunohistochemical and immunocytochemical staining
Cells were fixed for 5 minutes in 10% (vol/vol) formaldehyde. Single-label or double-label immunofluorescence was performed. As controls, samples were exposed to isotype primary antibodies or irrelevant IgG to measure nonspecific binding of secondary antibodies. Samples were incubated with anti-PTK7, -CD31, or -VEGF receptors for more than 2 hours at room temperature, followed by incubation for 1 hour with TRITC-conjugated or FITC-conjugated secondary antibodies. After rinsing with PBS, samples were observed under a fluorescence microscope (a Nikon Eclipse TE200 instrument equipped with a Nikon digital camera, model DXM 1200) using filters appropriate for fluorescein visualization.
Matrigel assay
HUVECs, MS1, and NIH 3T3 cells were trypsinized, counted, and resuspended (5 × 104/mL) in DMEM medium without serum. Some cells were transfected with siRNA directed against PTK7 before suspension. Matrigel basement membrane (Chemicon) was suspended in dilution buffer, without growth factors or heparin, and 150-μL aliquots placed into each well of flat-bottomed 48-well tissue culture plates and allowed to gel for 1-2 hours at 37°C before cell seeding. Next, cell suspensions were plated (100 μL/ well) onto Matrigel surfaces and incubated at 37°C. Tube formation and cell migration were observed over the next 36 hours under an inverted microscope.
Statistics
All data are expressed as mean ± SD. Differences between groups were examined by multivariate analyses using Newman-Keuls test or ANOVA followed by the Bonferroni procedure for comparison of means using SPSS 11.0. Values of P < .05 were considered statistically significant.
Results
PTK7 expression and induction in vascular endothelial cells
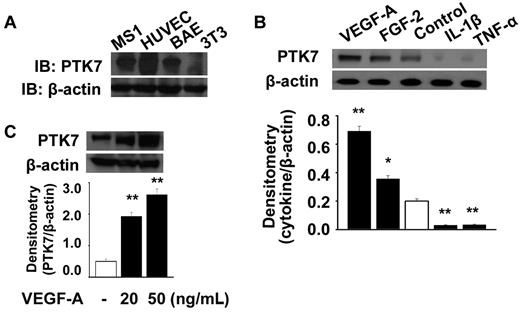
We determined PTK7 expression levels in various types of VECs, and confirmed using immunoblotting that PTK7 was highly expressed in such cells, including HUVECs, bovine arterial endothelial cells (BAEC cells), and MS1 cells (Figure 1A). However, NIH-3T3 dermal fibroblasts did not express PTK7. To investigate whether vasculogenic and/or proinflammatory cytokines induced PTK7 expression, HUVECs were treated with 20 ng/mL VEGF-A or FGF-2, or 30 ng/mL IL-1β or TNF-α, for 24 hours and PTK7 expression determined by immunoblotting. Both VEGF-A and FGF-2 enhanced PTK7 expression in HUVECs, with VEGF-A induction of PTK7 expression occurring (Figure 1B). In contrast, IL-1β and TNF-α suppressed PTK7 synthesis compared with controls. The VEGF-A induced PTK7 expression was increased in a dose-dependent manner (Figure 1C).
PTK7 expression and induction in vascular endothelial cells. (A) PTK7 expression was determined in HUVECs, a mouse endothelial cell line (MS1), and bovine arterial endothelial (BAE) cells by Western immunoblotting. (B) PTK7 expression was determined in the presence of 20 ng/mL VEGF-A or FGF-2, or 30 ng/mL IL-1β or TNF-α, added to cells in 6-well plates after 24 hours of serum starvation. Twenty-four hours later, MS1 cells were collected and immunoblotting performed (*P < .05, **P < .01, compared with control). (C) Induction of PTK7 was investigated 24 hours after treatment with VEGF-A (20 and 50 ng/mL) in MS1 cells using Western immunoblot.
PTK7 expression and induction in vascular endothelial cells. (A) PTK7 expression was determined in HUVECs, a mouse endothelial cell line (MS1), and bovine arterial endothelial (BAE) cells by Western immunoblotting. (B) PTK7 expression was determined in the presence of 20 ng/mL VEGF-A or FGF-2, or 30 ng/mL IL-1β or TNF-α, added to cells in 6-well plates after 24 hours of serum starvation. Twenty-four hours later, MS1 cells were collected and immunoblotting performed (*P < .05, **P < .01, compared with control). (C) Induction of PTK7 was investigated 24 hours after treatment with VEGF-A (20 and 50 ng/mL) in MS1 cells using Western immunoblot.
PTK7 receptor complex formation with Flt-1, Flk-1/KDR, and Flt-4
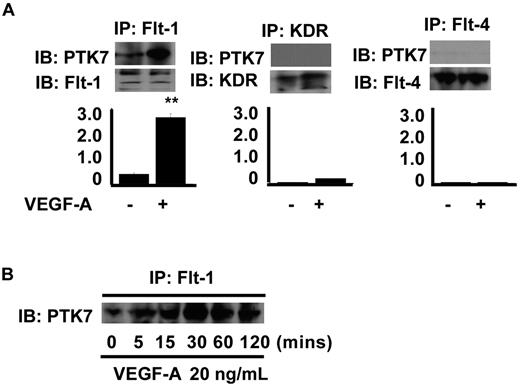
Although PTK7 was cloned nearly 2 decades ago, ligand or adaptor molecules that interact with PTK7 remain unknown. To clarify the mechanisms by which PTK7 mediates VEGF-A–induced angiogenesis, immunoprecipitation and immunoblot for 3 types of VEGF receptors and PTK7 were performed. We found that Flt-1 and PTK7 form a complex in VEC, and interaction between Flt-1 and PTK7 was enhanced by VEGF-A (Figure 2A). However, neither Flk-1/KDR nor Flt-4 formed a complex with PTK7. In addition, the complex formation between PTK7 and Flt-1 mediated by VEGF-A was found to be increased in a time-dependent fashion (Figure 2B). After 5 minutes of coincubation with VEGF-A, Flt-1-PTK7 complex formation commenced and continued to rise over the next 2 hours. KDR-PTK7 complex formation was not found at any tested levels of VEGF-A (data not shown).
Immunoprecipitation and immunoblot for VEGF receptors and PTK7. (A) Confluent MS1 cells were collected and lysed 2 hours after treatment with 20 ng/mL VEGF-A (control cells were not so treated). Immunoprecipitation was performed using 3 μg of antibodies specific for Flt-1, Flk-1/KDR, and Flt-4, and with 350-μg amounts of protein samples. Precipitates were collected and immunoblotting performed using antibodies against either PTK7 or VEGF receptors (**P < .01, compared with control). (B) Receptor complex formation between PTK7 and Flt-1 was assessed over time by immunoprecipitation and immunoblotting. MS1 cells, starved of serum and cytokines for 24 hours, were treated with 20 ng/mL VEGF-A and collected at various time points (5, 15, 30, 60, and 120 minutes). Immunoprecipitation and immunoblotting were performed as for panel A.
Immunoprecipitation and immunoblot for VEGF receptors and PTK7. (A) Confluent MS1 cells were collected and lysed 2 hours after treatment with 20 ng/mL VEGF-A (control cells were not so treated). Immunoprecipitation was performed using 3 μg of antibodies specific for Flt-1, Flk-1/KDR, and Flt-4, and with 350-μg amounts of protein samples. Precipitates were collected and immunoblotting performed using antibodies against either PTK7 or VEGF receptors (**P < .01, compared with control). (B) Receptor complex formation between PTK7 and Flt-1 was assessed over time by immunoprecipitation and immunoblotting. MS1 cells, starved of serum and cytokines for 24 hours, were treated with 20 ng/mL VEGF-A and collected at various time points (5, 15, 30, 60, and 120 minutes). Immunoprecipitation and immunoblotting were performed as for panel A.
Surface plasmon resonance analysis of receptor complex formation between Flt-1 and PTK7
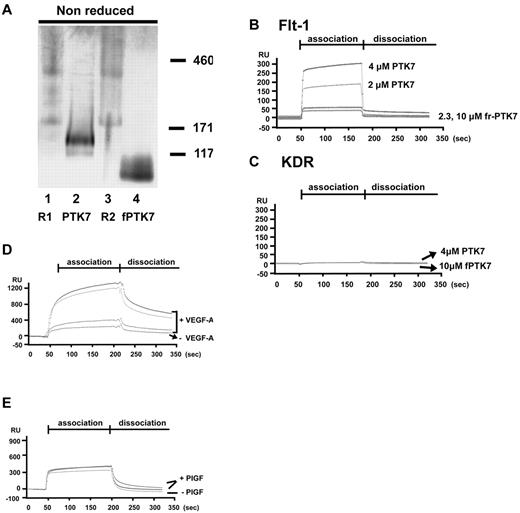
To further delineate the nature of the Flt-1/PTK7 interaction, we used surface plasmon resonance (SPR; BIAcore) analysis. Before performing the SPR experiment, the purity of each protein was confirmed by electrophoresis and gel staining (Figure 3A). Flt-1 (R1) and KDR (R2) were found to exist as monomers as well as dimers in vitro. Moreover, dimer formation was significantly increased by adding VEGF-A (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Surface plasmon resonance analysis of receptor complex formation between Flt-1 and PTK7. (A) A total of 0.5 or 1.0 μg of each purified protein was resolved in 3%-12% Tris-Bis Native Gel, and stained with Coomassie Blue R250 and then destained (R1, Flt-1, R2, Flk-1/KDR). (B) Purified full-length PTK7 (2μM or 4μM) or fragmented PTK715-59 (2.3μM or 10μM) was allowed to flow over chips bearing immobilized Flt-1 (B) or KDR (C) at concentrations of 500nM. All sensorgrams were corrected for unbound-chip background. (D) Purified Flt-1 was immobilized at a concentration of 500nM. Full-length PTK7 at different concentrations (0.2, 2 or 4μM) was allowed to flow over the chip surface in the presence or absence of 20 ng/mL VEGF-A. (E) Sensorgram was run with or without PlGF (50 ng/mL) in the same immobilized and analyte conditions described for panel D. All sensorgrams were corrected for unbound-chip background.
Surface plasmon resonance analysis of receptor complex formation between Flt-1 and PTK7. (A) A total of 0.5 or 1.0 μg of each purified protein was resolved in 3%-12% Tris-Bis Native Gel, and stained with Coomassie Blue R250 and then destained (R1, Flt-1, R2, Flk-1/KDR). (B) Purified full-length PTK7 (2μM or 4μM) or fragmented PTK715-59 (2.3μM or 10μM) was allowed to flow over chips bearing immobilized Flt-1 (B) or KDR (C) at concentrations of 500nM. All sensorgrams were corrected for unbound-chip background. (D) Purified Flt-1 was immobilized at a concentration of 500nM. Full-length PTK7 at different concentrations (0.2, 2 or 4μM) was allowed to flow over the chip surface in the presence or absence of 20 ng/mL VEGF-A. (E) Sensorgram was run with or without PlGF (50 ng/mL) in the same immobilized and analyte conditions described for panel D. All sensorgrams were corrected for unbound-chip background.
Either full-length mouse Flt-1 or Flk-1/KDR was immobilized on a sensor chip surface. Next, full PTK7 or fragment-PTK715-59 was allowed to flow over VEGF receptors immobilized on chip surfaces. Full-length PTK7 interacted with Flt-1, in a stable and dose-dependent manner (Figure 3B). On the other hand, fragment-PTK715-59 showed only a slight SPR increase from baseline, and was easily removed during washout. However, PTK7 did not interact with Flk-1/KDR (Figure 3B-C).
To examine whether the Flt-1 interaction with PTK7 was affected by VEGF-A, we added 50 ng/mL VEGF-A to the PTK7 analyte. This resulted in higher concentration-dependent SPR (Figure 3D). In the presence of VEGF-A, the maximum interaction capacity between PTK7 and Flt-1 was increased, and binding velocity also rose with declined dissociation between PTK7 and Flt-1. The maximum binding capacity increased up to 4-fold compared with the binding seen without VEGF-A. In addition, the intermolecular binding strength was significantly increased in the presence of VEGF-A, with slower complex breakdown during the dissociation period. However, another Flt-1 binding ligand, PIGF, did not affect the interaction between Flt-1 and PTK7 (Figure 3E).
Phosphorylation of VEGFRs and induction of downstream signal transduction by PTK7
To investigate the specific VEGFR activation cascades regulated by PTK7, the phosphorylation of VEGFRs and downstream signals were investigated using siRNA for PTK7 (siPTK7). The knockdown effect of PTK7 siRNA for PTK7 expression in VEC was confirmed (Figure 4A). Then, Flt-1 and Flk-1/KDR activations were compared between control and siPTK7-transfected VECs as the first step in studying PTK7-VEGFR signaling. After inhibition of PTK7, VEGF-induced Flt-1 phosphorylation (but not Flk-1/KDR phosphorylation) significantly decreased (Figure 4B). Because phosphorylation of focal adhesion kinase (FAK), and Akt are important downstream signals after VEGFR phosphorylation, the activation of the above regulators was determined by siPTK7. The phosphorylation of FAK was significantly decreased in PTK7-silenced cells after VEGF-A stimulation (Figure 4C). Similarly, phosphorylation of Akt by VEGF-A was also inhibited in the absence of PTK7. Akt phosphorylation began to increase 5 minutes after VEGF-A treatment of control cells. After silencing of PTK7 expression, however, Akt phosphorylation was significantly delayed to 30 minutes, and was suppressed compared with the control (Figure 4D).
Phosphorylation of VEGF receptors and its downstream signals by PTK7. (A) Expression of PTK7 24 hours after transduction of siRNA silencing PTK7 (siPTK7), nonspecific random siRNA (siNeg), or lipofectamine only (control; **P < .01). (B) Phosphorylation of Flt-1 and KDR subsequent to PTK7-silencing siRNA transduction was determined after treatment with 20 ng/mL VEGF-A. When cells were > 90% confluent, transduction with PTK7-silencing or control siRNA was performed. Twenty-four hours later, cells were detached, resuspended, and reattached to wells of a 6-well plate. Twenty-four hours after stabilization, cells were treated with 20 ng/mL VEGF-A and collected for immunoblotting. Immunoblotting was performed using 10% (wt/vol) SDS-PAGE gels, followed by incubation with primary antibodies to each of phosphorylated Flt-1 (Y1213; 1:1000) and phospho-KDR (Y1054; 1:800). Bands were detected by chemiluminescence (**P < .01). The time courses of FAK (C) and Akt (D) phosphorylation after PTK7 silencing and VEGF-A treatment were determined by immunoblotting.
Phosphorylation of VEGF receptors and its downstream signals by PTK7. (A) Expression of PTK7 24 hours after transduction of siRNA silencing PTK7 (siPTK7), nonspecific random siRNA (siNeg), or lipofectamine only (control; **P < .01). (B) Phosphorylation of Flt-1 and KDR subsequent to PTK7-silencing siRNA transduction was determined after treatment with 20 ng/mL VEGF-A. When cells were > 90% confluent, transduction with PTK7-silencing or control siRNA was performed. Twenty-four hours later, cells were detached, resuspended, and reattached to wells of a 6-well plate. Twenty-four hours after stabilization, cells were treated with 20 ng/mL VEGF-A and collected for immunoblotting. Immunoblotting was performed using 10% (wt/vol) SDS-PAGE gels, followed by incubation with primary antibodies to each of phosphorylated Flt-1 (Y1213; 1:1000) and phospho-KDR (Y1054; 1:800). Bands were detected by chemiluminescence (**P < .01). The time courses of FAK (C) and Akt (D) phosphorylation after PTK7 silencing and VEGF-A treatment were determined by immunoblotting.
The role of PTK7 in vascular endothelial cell migration and in vitro angiogenesis
To investigate the specific biologic role of Flt-1 with PTK7 association in angiogenesis, shRNA for Flt-1 and KDR were designed and transduced into MS1 cell, respectively. After puromycin selection and maintaining several passages, the knock-down effect of either shFlt-1 or KDR was confirmed by real time RT-PCR (Figure 5A) and immunoblot (supplemental Figure 2).
PTK7 expression and its role in vascular endothelial cell migration. (A) Expressions of Flt-1 and KDR mRNA were determined in short hairpin RNA for Flt-1(shFlt-1) or KDR (shKDR) transduction conditions by real-time RT-PCR. (B) shRNA-transduced cells were plated at a density of 2 × 104/well in a 6-well plate and cultured with VEGF-A (20 ng/mL). Then cell numbers were counted on each time point (**P < .01 vs shNeg). (C) Cell migration after wound breakage was determined by cell restitution assay. Some shFlt-1–transduced cells were transfected with PTK7-silencing siRNA (○). VEGF-A (20 ng/mL) was added to serum-free medium and cell migration was observed over the next 36 hours. Migratory cells were counted using inverted phase-contrast microscopy (×100; *P < .05, **P < .01). (D) PTK7 expression changes in healing wound breaks were measured by immunoblotting. After wound breaks were created by pipette tips in confluent cell layers, cell samples were serially collected over the next 36 hours and immunoblotting was performed as described. Band densities were measured and statistically analyzed (*P < .05, **P < .01). (E) Cells were stained using anti–mouse PTK7 monoclonal antibody during the wound break assay. MS1 cells were stained 6 hours (left and middle panels) and 18 hours (right panel) after wounding. Enhanced localized PTK7 expression was observed in the wound margin (white arrow; ×200, except bottom right [×400]; green: PTK7; blue: DAPI). Arrowhead indicates lamellipodia and filopodia staining with PTK7 antibody.
PTK7 expression and its role in vascular endothelial cell migration. (A) Expressions of Flt-1 and KDR mRNA were determined in short hairpin RNA for Flt-1(shFlt-1) or KDR (shKDR) transduction conditions by real-time RT-PCR. (B) shRNA-transduced cells were plated at a density of 2 × 104/well in a 6-well plate and cultured with VEGF-A (20 ng/mL). Then cell numbers were counted on each time point (**P < .01 vs shNeg). (C) Cell migration after wound breakage was determined by cell restitution assay. Some shFlt-1–transduced cells were transfected with PTK7-silencing siRNA (○). VEGF-A (20 ng/mL) was added to serum-free medium and cell migration was observed over the next 36 hours. Migratory cells were counted using inverted phase-contrast microscopy (×100; *P < .05, **P < .01). (D) PTK7 expression changes in healing wound breaks were measured by immunoblotting. After wound breaks were created by pipette tips in confluent cell layers, cell samples were serially collected over the next 36 hours and immunoblotting was performed as described. Band densities were measured and statistically analyzed (*P < .05, **P < .01). (E) Cells were stained using anti–mouse PTK7 monoclonal antibody during the wound break assay. MS1 cells were stained 6 hours (left and middle panels) and 18 hours (right panel) after wounding. Enhanced localized PTK7 expression was observed in the wound margin (white arrow; ×200, except bottom right [×400]; green: PTK7; blue: DAPI). Arrowhead indicates lamellipodia and filopodia staining with PTK7 antibody.
In the cell proliferation assay, proliferation was reduced to 38.9% of shNeg in shKDR (P < .0001), and 73.5% of shNeg in shFlt-1 (P = .047; Figure 5B). In the case of siPTK7 cotransduction, the proliferation activity of shFlt-1 condition was not changed by siPTK7 transduction (P = .112). However, interestingly, cell migration was significantly inhibited in siPTK7-transduced cells. Cell migration in shFlt-1 with siPTK7 condition showed 32.5% of shNeg (P < .001) and 44.2% of shFlt-1 only (P = .002; Figure 5C). In addition, in wild-type MS1, the cell migration was significantly inhibited by siPTK7 transduction (supplemental Figure 3).
As the above results may imply that PTK7 is specifically associated with cell migration, the expression pattern of PTK7 in wound healing was determined. By immunoblotting, facilitated PTK7 expression was observed during wound break assay in a time-dependent manner in VECs (Figure 5D). PTK7 induction began to rise at 6 hours and the enhanced expression continued to 36 hours after making scratch wound in confluent monolayer VECs.
To determine the PTK7 distribution pattern in cell migration, PTK7 immunostaining during wound break assay was performed. Interestingly, PTK7 was found to be more highly expressed in the cells located at the edge of the wound and the expression gradually decreased from the wound margin to the confluent cell area (Figure 5E). From 6 hours after wound break, PTK7 expression changes were noted and continued (arrow) until complete wound healing. In addition, lamellipodia or filopodia of migratory cells expressed a higher PTK7 level than other parts of the cells (arrowhead).
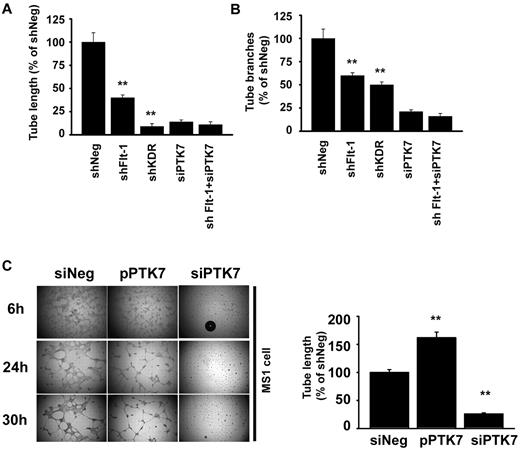
The effect of PTK7 on in vitro tube formation was also investigated. Capillary tube length and branch numbers were significantly down-regulated both in shFlt-1– and shKDR-transduced cells (Figure 6A-B; supplemental Figure 4). The tube length was more impaired in shKDR cells than shFlt-1, but the tube branching impairment was similar between shFlt-1 and shKDR. Interestingly, additional siPTK7 transduction in shFlt-1 cells caused more significant suppression in tube branching (48.3%) than in tube length (15.2%).
Facilitation of in vitro angiogenesis by PTK7 overexpression. (A-B) Endothelial tube formation was compared in shFlt-1, shKDR, and siPTK7 transfection conditions. Cells were seeded at 4 × 104/mL into Matrigel-coated wells of a 48-well plate. A camera-equipped inverted microscope was used for photography. Total tube length (A) and branches (B) were counted at 3 high-magnification images from different areas of each condition; the tube lengths were measured with an image-analysis program (Image Grabbor Version 1.4, Scion Corp; **P < .01 compared with shNeg). All the experiments were performed 3 times with triplicated samples. (C) In vitro tube formation was compared using cells transfected with mock, pPTK7, or PTK7-silenced vascular endothelial cells. Twenty-four hours after transductions, cells were collected and seeded at 4 × 104/mL into Matrigel-coated wells of a 48-well plate. Analysis of tube length was the same method used in panel A. All the experiments were done 3 times with triplicated samples.
Facilitation of in vitro angiogenesis by PTK7 overexpression. (A-B) Endothelial tube formation was compared in shFlt-1, shKDR, and siPTK7 transfection conditions. Cells were seeded at 4 × 104/mL into Matrigel-coated wells of a 48-well plate. A camera-equipped inverted microscope was used for photography. Total tube length (A) and branches (B) were counted at 3 high-magnification images from different areas of each condition; the tube lengths were measured with an image-analysis program (Image Grabbor Version 1.4, Scion Corp; **P < .01 compared with shNeg). All the experiments were performed 3 times with triplicated samples. (C) In vitro tube formation was compared using cells transfected with mock, pPTK7, or PTK7-silenced vascular endothelial cells. Twenty-four hours after transductions, cells were collected and seeded at 4 × 104/mL into Matrigel-coated wells of a 48-well plate. Analysis of tube length was the same method used in panel A. All the experiments were done 3 times with triplicated samples.
Next, we constructed a PTK7-overexpression vector (pPTK7) to increase PTK7 expression in endothelial cells and to induce PTK7 synthesis in 3T3 fibroblast cells that normally express low levels of PTK7. We optimized transduction conditions and obtained enhanced and induced PTK7 expression in MS1 cells and 3T3 fibroblasts, respectively (supplemental Figure 5A). At first, we introduced pPTK7 in 3T3 fibroblasts, cultured on Matrigel coated 48-well dishes for 24 hours. Interestingly, cell-cell connections for making tubelike structures were significantly up-regulated under pPTK7 transduction conditions (supplemental Figure 5B). From 6 hours onward, fibroblasts with pPTK7 transduction formed finely organized tubelike networks. However, the mock-transduced cells failed to make mature organized structures until 48 hours after cell inoculation.
The pattern of capillary network formation in MS1 on Matrigel showed marked difference among siNeg, siPTK7, and pPTK7 transfection conditions. In siPTK7-treated conditions, the elongation of cytoplasmic poles was not observed up to 3 days, causing failure of tubelike network formation. However, in case of the control vector–transduced condition (mock), tubelike structures began to form 4 hours after seeding of cells on the Matrigel surface and the peak response was observed at 16-24 hours. The finest and most delicate capillary tubes were observed in the pPTK7 transduction condition. The total length of vascular network was significantly higher in the mock than the siPTK7 conditions (Figure 6C).
In vivo angiogenesis using micropocket assay
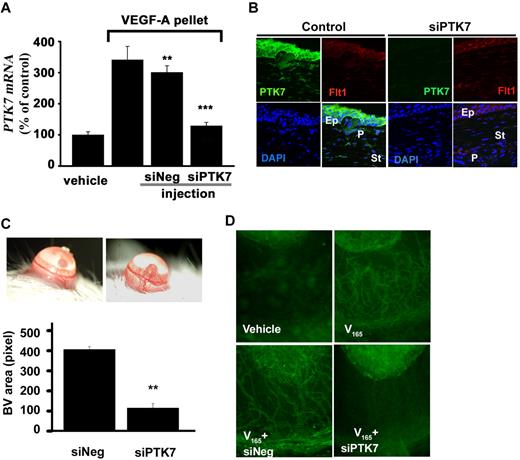
Last, we investigated the role of PTK7 in mediating VEGF-A–induced angiogenesis in vivo. Mice receiving 160 ng of VEGF-A in a corneal micropocket were randomly assigned to 2 groups, and subconjunctivally injected with control siRNA or PTK7-silencing siRNA 0, 1, 3, 5, and 7 days after surgery. To determine the effect of siPTK7 in vivo, PTK7 mRNA levels were determined in the mouse cornea by real-time RT-PCR. Compared with the siNeg, siPTK7 subconjunctival injection showed significant down-regulation in PTK7 mRNA expression (Figure 7A). Suppression of PTK7 expression by siPTK7 was further confirmed by microscopy. PTK7 expression by the epithelium and anterior stromal keratocyte was significantly reduced by subconjunctival siPTK7 injection (Figure 7B).
Inhibition of VEGF-A induced neovascularization by siPTK7. (A) Eight days after corneal pellet insertion, total RNA was extracted from the corneas, and then expression of PTK7 mRNA was measured by real-time RT-PCR. (B) Confocal microscopic images from postoperative day 7 cornea (green: PTK7; red: Flt-1; blue: DAPI; Ep: epithelium; P: pellet; St: cornea stroma) magnification ×200 using a TCS SP2 microscope (Leica). (C) Slit lamp corneal photographs were taken 7 days later and vascularized areas were measured using image-analysis software (Image Grabbor Version 1.4; Scion Corp). The total vascularized area of PTK7 siRNA injection group was suppressed 73% compared with the control siRNA–treated group (**P < .01). (D) Corneas from both groups were exposed, stained with anti-CD31-FITC antibody, and observed under fluorescence microscopy (×100; Nikon Eclipse TE200 instrument).
Inhibition of VEGF-A induced neovascularization by siPTK7. (A) Eight days after corneal pellet insertion, total RNA was extracted from the corneas, and then expression of PTK7 mRNA was measured by real-time RT-PCR. (B) Confocal microscopic images from postoperative day 7 cornea (green: PTK7; red: Flt-1; blue: DAPI; Ep: epithelium; P: pellet; St: cornea stroma) magnification ×200 using a TCS SP2 microscope (Leica). (C) Slit lamp corneal photographs were taken 7 days later and vascularized areas were measured using image-analysis software (Image Grabbor Version 1.4; Scion Corp). The total vascularized area of PTK7 siRNA injection group was suppressed 73% compared with the control siRNA–treated group (**P < .01). (D) Corneas from both groups were exposed, stained with anti-CD31-FITC antibody, and observed under fluorescence microscopy (×100; Nikon Eclipse TE200 instrument).
Angiogenesis in eyes treated with subconjunctival injections of PTK7-silencing siRNA was significantly decreased, compared with controls (Figure 7C). The total vascularized area of the PTK7-silenced group was suppressed 73% compared with the siNeg-treated group (P < .0001). In the PTK7-silenced group, initial new vessel budding from the preexisting blood vessels, as well as mature new vessels, was less than that seen in the siNeg-treated group (Figure 7C). Immunohistochemical staining for CD31, in PTK7-silenced eyes showed significantly decreased vascularized areas, smaller blood vessel diameter, and less blood vessel branching than siNeg (Figure 7D).
Discussion
Although PTK7 function has been studied for several years,4,6,16,21,22 the precise mechanisms of action of PTK7 have remained poorly understood. Here, we show that PTK7 interacts with Flt-1, but not with Flk-1/KDR or Flt-4, during angiogenesis, using both immunoprecipitation and physical (SPR; BIAcore) methods. Moreover, Flt-1 phosphorylation is inhibited in PTK7-silenced endothelial cells. These findings suggest that PTK7 is essential for activation and regulation of Flt-1.
The biologic effects of VEGF-A are mediated by 2 high-affinity receptor tyrosine kinases expressed on endothelial cells, specifically, Flk-1 (VEGFR2) and Flt-1 (VEGFR1). VEGF-A signaling through Flk-1 is known to positively regulate endothelial cell proliferation and migration, whereas the precise function of Flt-1 is still less clear than that of Flk-1. Flt-1 was originally known as an “inhibitory regulator for angiogenesis” (for reviews see Ferrara et al10 and Shibuya23 ). However, recent studies also suggest that Flt-1 could function as a positive regulator of endothelial cell migration and blood vessel formation. Kappas and colleagues have reported that Flt-1 modulates Flk-1/KDR signaling and positively regulates both VEC migration orientation and blood vessel branching24 and that blood vessel disorganization seen in the Flt-1 knockdown condition was rescued by transfer of the Flt-1 gene. In addition, Flt-1 can activate and convey signals for increased DNA synthesis and cell proliferation, and to activate tyrosine phosphorylation of PLCγ and phosphoinositol 3 phosphate kinase (PI3K), which may amplify the angiogenic response to VEGF.10,11,25,26 Moreover, Nishi and coworkers have reported that Flt-1 regulates Akt signaling, which plays a critical role in maintenance of endothelial integrity necessary for blood vessel stability.27 Taken together, these studies support the concept that Flt-1 and its downstream signals may be essential not only in maintaining the integrity of healthy blood vessels but also in promoting cell migration in angiogenesis. Our data suggest that PTK7 may play the role of an essential coreceptor for inducing angiogenic signaling from Flt-1.
It is notable that VEGF-A enhances the interaction between Flt-1 and PTK7. In the absence of VEGF-A, the Ka of interaction is 3.7 ± 1.1 (105M−1s−1). However, the Ka value is increased to 19.3 ± 3.6 when VEGF-A is expressed. In addition, the Kd rises in the presence of VEGF-A, implying that the VEGF-A binding site of Flt-1 may not physically overlap with the binding site for PTK7. Structurally, Flt-1 and PTK7 share similar extracellular regions, with 7 immunoglobulin-like domains; thus the receptors may form heterodimers in a manner not unlike the dimerization required for tyrosine kinase receptor activation. An NMR structural biology study would help better define this relationship and further assist in identification of protein segments important in Flt-1 control functions.
We also demonstrate herein cell migration inhibition after PTK7-silencing and enhanced in vitro tube formation in Matrigel when PTK7 is overexpressed. Interestingly, during the wound break assay, cells migrating to the break area, or at the wound margin, show higher PTK7 expression than cells farther away from the margin (Figure 5C). The enhanced PTK7 expression on migrating cells and their filopodia or lamellipodia may provide a hint as to the essential role of PTK7 in cell migration. The uneven PTK7 distribution among the cells in the migratory area may affect the activation level of Flt-1 in each cell and eventually, to induction of particular pattern of cell migration and tube formation. Recently, it has been shown that heterogeneous Flt-1 activation induces mosaic patterns of VEC proliferation and migration, which may induce tubelike or blood vessel formation.24 Therefore, PTK7 may control the migration direction or polarity of VECs by regulating Flt-1 activation and help to form the normal vascular structure.
The role of PTK7 in cell migration and polarity has been shown in an embryogenic study. PTK7 knockout in mice is lethal in early gestation because of defective neural tube closure caused by migration failure.6 Notably, Flt-1 knockout mice have a similar pattern of loss of cell migration polarity, similar to that seen in the PTK7 knockout. An earlier study showed that Flt1−/− mice have fully developed endothelial cells but they are not organized into vascular channels.13 Excessive endothelial proliferation without mature blood vessel formation may indicate a role for Flt-1 in endothelial cell migration polarity and maturation of blood vessels.28,29 When these previous mouse knockout studies and our present work on the need for the interaction between Flt-1 and PTK7 for successful endothelial cell migration and tube formation are taken together, we can deduce that Flt-1-PTK7 crosstalk is essential for endothelial cell migration and tube formation and may endow a cell with a migration direction. The fact that PTK7 function for cell migration can be effectively inhibited by soluble PTK7 suggests that the extracellular domain of PTK7 is essential for its interaction with Flt-1.16,30
In conclusion, our data suggest that PTK7 is critical in regulation of Flt-1 signal transduction and is essential for endothelial cell migration. A better understanding of the mechanisms underlying these phenomena may help to more precisely define the activities and regulatory nature of Flt-1 in angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We sincerely appreciate the assistance of Dr Andrius Kazlauskas, Dr Bjorn Olson, and Dr Eun Young Park for helpful discussions and for providing materials for plasmid construction and amplification.
This work was supported by the National Institutes of Health (NIH/NEI RO1-12963).
National Institutes of Health
Authorship
Contribution: H.K.L. designed and performed the experiments and wrote the manuscript; S.C. contributed to data analysis and writing and assisted in experiments; E.-D.K. contributed vital new reagents and assisted in data analysis; and R.D. contributed to the underlying hypothesis, manuscript writing, and funding of the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Reza Dana, Schepens Eye Research Institute, 20 Staniford St, Boston, MA 02114; e-mail: reza.dana@schepens.harvard.edu.


![Figure 5. PTK7 expression and its role in vascular endothelial cell migration. (A) Expressions of Flt-1 and KDR mRNA were determined in short hairpin RNA for Flt-1(shFlt-1) or KDR (shKDR) transduction conditions by real-time RT-PCR. (B) shRNA-transduced cells were plated at a density of 2 × 104/well in a 6-well plate and cultured with VEGF-A (20 ng/mL). Then cell numbers were counted on each time point (**P < .01 vs shNeg). (C) Cell migration after wound breakage was determined by cell restitution assay. Some shFlt-1–transduced cells were transfected with PTK7-silencing siRNA (○). VEGF-A (20 ng/mL) was added to serum-free medium and cell migration was observed over the next 36 hours. Migratory cells were counted using inverted phase-contrast microscopy (×100; *P < .05, **P < .01). (D) PTK7 expression changes in healing wound breaks were measured by immunoblotting. After wound breaks were created by pipette tips in confluent cell layers, cell samples were serially collected over the next 36 hours and immunoblotting was performed as described. Band densities were measured and statistically analyzed (*P < .05, **P < .01). (E) Cells were stained using anti–mouse PTK7 monoclonal antibody during the wound break assay. MS1 cells were stained 6 hours (left and middle panels) and 18 hours (right panel) after wounding. Enhanced localized PTK7 expression was observed in the wound margin (white arrow; ×200, except bottom right [×400]; green: PTK7; blue: DAPI). Arrowhead indicates lamellipodia and filopodia staining with PTK7 antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/21/10.1182_blood-2010-09-306928/4/m_zh89991171680005.jpeg?Expires=1764998278&Signature=SYD7U0qdvK7gUsVXwQfjr2Xv3M9AOS28GGYZQ-zfec6jb8~92pOpeY1VbHNrarnmLVV7Z3qGsjeixKiBdjaZITUBEueIQPrSz~nDGRvnF76seitNP-cbMUV4BLQxLsEqrbH7WMOqAvLjx2cClu5AL9Yz7UqZd1NWFLIATyuq9jBNtbzyi9Gwir-BE8eZa8omFkPmZdRgmYYQuuGZgpgewlkV4kdzChT0ZvwMIuCBomJfcw4-GI7e~C62ZFfi36lbrwrWhKmaf7z6wX-rs6x9bqUUa8XFKJAXLLsJh5ng8LfnB99lbU2yC4RHS8Edh3MRd6CfrOh~2Ohk7OE~ZkQWtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal