In this issue of Blood, Joyal and colleagues make the insightful finding that Semaphorin 3A (Sema3A) is secreted by hypoxic neurons in ischemic/avascular retina, thereby inhibiting vascular regeneration of the retina and enhancing pathologic preretinal neovascularization.1
Ischemic retinopathies are a major cause of blindness in both the working-age and pediatric population. They include a wide array of conditions including diabetic retinopathy, retinal vein occlusions, and retinopathy of prematurity. These conditions are characterized by an initial phase of retinal vascular drop-out and/or growth cessation. Ischemia of the retina results in up-regulation of proangiogenic growth factors that promote preretinal neovascularization, which can lead to blindness. It is puzzling why revascularization of the ischemic retina does not occur (or only modestly occurs), when there is robust pathologic neovascularization into the vitreous, a region of the eye normally devoid of blood vessels. Joyal and colleagues provide an important mechanism accounting for this discrepancy, demonstrating that neuronal secretion of the semaphorin Sema3a poses a barrier preventing revascularization of the ischemic retina.
The deficient revascularization of ischemic retina in the setting of markedly up-regulated proangiogenic growth factors led the investigators to surmise the existence of a vasorepulsive force arising from the ischemic retina. This force would act to repel newly forming vessels away from the ischemic retina, toward the vitreous.1 The investigators viewed class 3 semaphorins as very plausible candidates for this vasorepulsive influence, specifically Sema3A. These molecules have been implicated as axon guidance cues but are also known to have a role in the regulation of blood vessels.2 Sema3A in particular participates in angiogenesis during development3 and has more recently been implicated as an inhibitor of tumor angiogenesis.4
Joyal and colleagues investigated Sema3A as a vasorepulsive molecule using the murine oxygen-induced retinopathy (OIR) model, a widely used model for the study of ischemic retinopathies, in which hyperoxic exposure is used to induce retinal vaso-obliteration in neonatal mice5,6 Revascularization of the vaso-obliterated retina eventually occurs in thismodel, but at a relatively slow rate, and pathologic preretinal neovascularization therefore develops because of the up-regulation of proangiogenic growth factors by the ischemic retina. Tremendous insights have been gained regarding the factors leading to vaso-obliteration and preretinal neovascularization.6,7 Relatively less is known about the factors governing revascularization in this model, although inroads have been made, including the role of inflammatory mediators.8,9
Joyal and colleagues characterized expression and function of Sema3A in the OIR model. Consistent with its hypothesized role as a vasorepulsive force, retinal expression of Sema3A was increased after vascular obliteration and persisted during the phase of preretinal neovascularization. Importantly, this increase in expression was localized to the avascular zone of retina, primarily the ganglion cell layer.1 This localized induction of Sema3A expression was dependent on inflammatory cytokines, specifically IL-1β.1 To establish the role of Sema3A in vascular regulation, knockdown of Sema3A mRNA was performed, using small hairpin interfering RNA (shRNA) encoded in lentiviral vectors. Knockdown of Sema3A resulted in a significant decrease in vaso-obliteration and a significant acceleration of revascularization of the avascular retina. This was accompanied by a reduction in pathologic preretinal neovascularization, as one would expect given the reduction in ischemic retinal area. Importantly, knockdown of Sema3A was also associated with marked improvement in neuroretinal function assessed by electroretinography.1
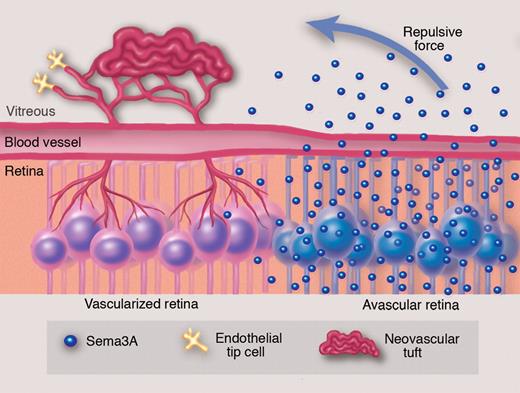
To elaborate on their in vivo findings, the investigators explored the function of Sema3A at the cellular level. They used an in vitro model of retinal ganglion cells (RGCs) exposed to hypoxia, to approximate the in vivo retinal hypoxic environment. After 40 hours of hypoxia, these RGCs substantially increased their expression of Sema3A. Conditioned media from the hypoxic RGCs inhibited proliferation and migration of cultured endothelial cells and vessel sprouting from aortic explants. These effects were abrogated when Sema3A knockdown was performed in the hypoxic RGCs.1 Of particular relevance to their overall hypothesis of vasorepulsion, new vessels from aortic explants deviated from zones coated with recombinant Sema3A, instead invading vehicle-coated regions.1 This adds to a previous study that supported a repelling effect of Sema3A for cultured endothelial cells.10 These experiments support the idea that RGCs secrete Sema3A in a hypoxic environment, which serves to inhibit vascularization (see figure).
It has long been appreciated that there is a deficiency in revascularization of ischemic retina even though there is robust pathologic neovascularization into the vitreous, a region of the eye normally devoid of blood vessels. Experiments by Joyal and colleagues suggest that ganglion cells in the avascular retina secrete Semaphorin 3A, which acts as a repulsive force against revascularization of the ischemic retina and directs neovessels toward the vitreous to form pathologic neovascular tufts. Professional illustration by Marie Dauenheimer.
It has long been appreciated that there is a deficiency in revascularization of ischemic retina even though there is robust pathologic neovascularization into the vitreous, a region of the eye normally devoid of blood vessels. Experiments by Joyal and colleagues suggest that ganglion cells in the avascular retina secrete Semaphorin 3A, which acts as a repulsive force against revascularization of the ischemic retina and directs neovessels toward the vitreous to form pathologic neovascular tufts. Professional illustration by Marie Dauenheimer.
Given the antiangiogenic and vasorepulsive role of Sema3A proposed by the authors, one might predict that intravitreal administration of Sema3A would inhibit pathologic preretinal neovascularization, limiting invasion of the vitreous by the pathologic new vessels. Indeed, the investigators found this to be the case, demonstrating that intravitreal administration of recombinant Sema3A significantly inhibited pathologic preretinal neovascularization in the OIR model.1 The result raises the possibility of Sema3A as a therapy for pathologic retinal neovascularization, with the caveat that there might be effects on the normal retinal vasculature as well.
The study by Joyal and colleagues represents a highly significant advance in the investigation of ischemic retinopathies. It addresses an important gap in the understanding of these conditions, providing a novel mechanism that explains the mystery of why there is deficient revascularization of ischemic tissue when there is robust pathologic neovascularization of adjacent regions. The mechanism provided by the investigators provides a framework for understanding this discrepancy, and also sheds further light on why neovascularization has a penchant for occurring at the borders of ischemic and nonischemic retina. The study also provides a novel link between inflammation and semaphorins. The observation that IL-1β can increase local Sema3A expression serves as a guide for future studies regarding the possible role of semaphorins in other pathologic settings. In linking hypoxia and inflammation to semaphorin expression, the study also adds further clarification to the sequence of events underlying the pathophysiology of the oxygen-induced retinopathy model, with potential implications for ischemic retinopathies in general. This could enhance the identification of suitable points of intervention in clinical conditions. Finally, the study points to new strategies directed at promoting revascularization of ischemic retinal tissue, in lieu of (or in addition to) direct inhibition of downstream pathologic neovascularization.
The findings of this study raise multiple important questions. It is certainly interesting to speculate on the potential role of other semaphorins, or indeed other classes of guidance molecules, in OIR. It will be of great interest to determine how these findings in the oxygen-induced retinopathy model might pertain to ischemic retinopathies in general. Indeed, as discussed by the investigators, there may well be direct relevance to the understanding of ischemic strokes, a condition in which Sema3A production has already been reported. Joyal and colleagues provide a most valuable conceptual framework for future investigations, one that could provide new avenues for therapy of these important conditions.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal