Innate immunity is the host defensive weapon against pathogens and this issue of Blood offers an interesting new bullet for the immune system gun by studying the role of Toll-like receptor 2 (TLR2) in megakaryocytic function; a field previously not explored. Beaulieu and colleagues provide evidence that inflammation, through TLR2, can increase megakaryocyte maturation and modulate its phenotype, contributing to our understanding of the interrelationship between inflammation and hemostasis.1
It is fascinating to imagine that, potentially, systemic infection could enter the bone marrow, be recognized by megakaryocytic TLR2, activate this signaling pathway to alter cellular function and, therefore, influence platelet formation.
TLRs are activated upon interaction with different pathogen-associated molecular patterns. In particular, TLR2 recognizes a large number of infectious-derived stimuli such as triacylated and diacylated lipoproteins, those from gram-positive bacteria, from mycobacteria, glycosylphosphatidylinositol anchors, phenol-soluble modulin, zymosan from fungi, glycolipids, and LPS from non-enterobacteria.2 Upon stimulation of TLR2, several different signaling proteins are stimulated, resulting in NFκB pathway activation.2 In innate immune cells, this signal cascade leads to increased expression and release of inflammatory cytokines such as interleukins and tumor necrosis factor-α.2 The inflammatory pattern induced by TLR2 activation is related to both immunologic and atherosclerotic disease. Atherosclerosis mouse models demonstrate an association between TLR2 and atherosclerotic lesion development.3 More interestingly, evidence supports a role for TLR2 in atherosclerosis and ischemic coronary artery disease in humans4 as well as in asthma and atopic diseases.2
There is limited information directly linking TLRs and megakaryocytes in infectious- and thrombotic-related cardiovascular diseases. TLR4, the most studied TLR until now,2 has been associated with hematopoietic progenitor cells and appears to contribute to increased apoptosis in myelodysplastic syndromes.5 Nevertheless, no direct association has been shown with athero-thrombotic disease. However, platelet TLR2 and related innate immune transcripts have been associated with cardiovascular disease and its risk factors.6
The interplay between TLR2, inflammation, and platelets has only been partially defined. In addition to hemostasis, platelets mediate inflammation and bacterial clearance from the bloodstream. Stimulation of the immune TLR2 on the platelet surface activates phosphoinositide-3-kinase (PI3K) and causes platelet activation and platelet-dependent thrombosis.7 Inflammatory conditions are known to increase thrombopoietin (TPO) levels, thereby increasing the number of circulating platelets.7 It has been reported that TPO levels and platelet counts increase after 24 hours of exposure to low-grade endotoxemia8 ; a possible pathway could be related to TLR2 activation.7 Previous studies have shown megakaryocyte expression of TLRs, in particular, 1, 2, 4 and 6.1 The purpose of these receptors and the role of inflammation on megakaryocyte maturation and platelet development is still a matter of debate to which the study by Beaulieu and colleagues significantly contributes.
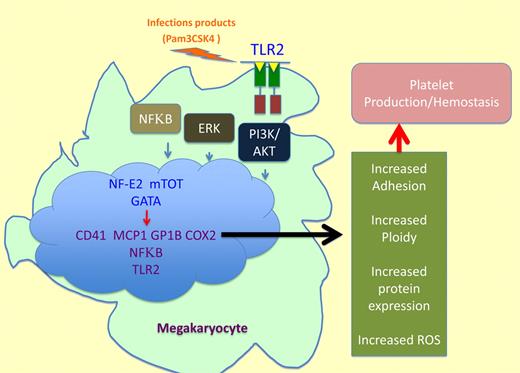
Beaulieu and colleagues suggest that the stimulation of megakaryocyte TLR2 by a specific, synthetic ligand, Pam3CSK4, results in an increase in phosphorylation of NFκB p65 subunit, Akt, and ERK1/2. This phosphorylation results in altered gene expression, protein levels, and megakaryocytic function. Therefore, it is hypothesized that inflammation, through TLR2, can affect megakaryopoiesis, promoting increased platelet development. More specifically, Beaulieu and colleagues demonstrate that on activation of these signaling pathways factors that control megakaryocyte maturation, polyploidization, and platelet production, including transcription factors GATA-1, NF-E2, and mTOR, are up-regulated (see figure). The up-regulation of the above transcription factors is associated with megakaryocyte maturation in this model. In addition, Beaulieu and colleagues found megakaryocytic up-regulation of thrombotic-related genes, GP1b and CD41, increased DNA content, ROS production, and adhesion to extracellular matrices. Overexpression of these inflammatory-related genes not only affects megakaryocyte/platelet development but also differentiation of surrounding cells in the bone marrow suggesting a complex interplay among inflammatory stimuli, megakaryocyte maturation, platelet formation and immune defenses.
A new link between immunity and megakaryocyte function. TLR2 activation by a specific ligand such as Pam3CSK4 induces the following cascade: (1) NFκB, ERK-MAPK, and PI3K/Akt pathways activation. (2) Increased levels of transcription factors associated with megakaryocyte maturation such as GATA-1, NF-E2, and mTOR. (3) Enhanced gene expression and augmented inflammatory related protein levels of GP1b, CD41, MCP-1, COX2, NFκB1, and TLR2. (4) ROS production, increased adhesion ploidy, and protein expression resulting in platelet production.
A new link between immunity and megakaryocyte function. TLR2 activation by a specific ligand such as Pam3CSK4 induces the following cascade: (1) NFκB, ERK-MAPK, and PI3K/Akt pathways activation. (2) Increased levels of transcription factors associated with megakaryocyte maturation such as GATA-1, NF-E2, and mTOR. (3) Enhanced gene expression and augmented inflammatory related protein levels of GP1b, CD41, MCP-1, COX2, NFκB1, and TLR2. (4) ROS production, increased adhesion ploidy, and protein expression resulting in platelet production.
TLR2 stimulation also increased inflammatory-related markers such as MCP-1, COX2, TLR2, and NFκB1 (see figure). It is possible that MCP-1 secretion by megakaryocytes in this setting could increase differentiation of immune related cells. In addition, MCP-1 secreted by platelets could stimulate the immune response in circulation. Finally, megakaryocyte TLR2 stimulation increased adhesion to fibrinogen and fibronectin, and polyploidization in the presence of TPO. Overall, the data from Beaulieu and colleagues suggest that, through TLR2, inflammatory processes can increase megakaryocytic maturation potentially influencing platelet function and thrombosis.
This study extends our understanding of immune receptors found on cells traditionally believed to be dedicated to the process of thrombosis, specifically, of TLR2-mediated megakaryocyte function and phenotypic modification. Thus, this field is extending the previously established role for TLR2 involvement in immunologic and inflammatory related diseases. The next step is to clarify the clinical relevance of this new information and to find new strategies to study TLR2 as a therapeutic target.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal