Abstract
As the result of intense clinical and basic research, acute promyelocytic leukemia (APL) has progressively evolved from a deadly to a curable disease. Historically, efforts aimed at understanding the molecular bases for therapy response have repeatedly illuminated APL pathogenesis. The classic model attributes this therapeutic success to the transcriptional reactivation elicited by retinoic acid and the resulting overcoming of the differentiation block characteristic of APL blasts. However, in clinical practice, retinoic acid by itself only rarely yields prolonged remissions, even though it induces massive differentiation. In contrast, as a single agent, arsenic trioxide neither directly activates transcription nor triggers terminal differentiation ex vivo, but cures many patients. Here we review the evidence from recent ex vivo and in vivo studies that allow a reassessment of the role of differentiation in APL cure. We discuss alternative models in which PML-RARA degradation and the subsequent loss of APL cell self-renewal play central roles. Rather than therapy aimed at inducing differentiation, targeting cancer cell self-renewal may represent a more effective goal, achievable by a broader range of therapeutic agents.
Introduction
Since the discovery, 20 years ago, of the PML-RARA fusion, the oncogene responsible for > 95% of acute promyelocytic leukemia (APL) forms,1,2 clinical management of the disease has shown spectacular improvements and has now reached unprecedented efficacy. Indeed, recent trials combining the use of specific APL-targeted drugs, retinoic acid (RA) and arsenic trioxide, to various regimens of regular anthracycline-based chemotherapy have resulted in major long-term antileukemic effects and achieved high rates of survival, with > 90% patients disease-free and off treatment after 5 years.3-6 Some cures have even been obtained without using DNA-damaging chemotherapies.
However, the molecular and cellular mechanisms involved in APL pathogenesis and responsiveness to treatment are still somehow controversial. As shown by Breitman7 in the 1980s, RA induces the rapid differentiation of immature primary APL blasts into terminally differentiated granulocytes ex vivo. This observation, combined with RA clinical efficacy, logically led to the assumption of a direct link between differentiation and response to therapy. APL has therefore been considered as a malignancy amenable to “differentiation therapy.” At the molecular level, RA signals through the RAR family of nuclear receptors that regulate transcription. The historical model has proposed that PML-RARA is a potent transcriptional repressor in APL cells and that it becomes an activator on RA treatment. Such transcriptional switch induces the maturation of APL blasts, which ultimately leads to clearance of the disease. Yet, some recent evidence questions this model, and the causal links between transcriptional activation, differentiation, and eradication of APL blasts have never been clearly established. In particular, arsenic trioxide has emerged as an even more potent anti-APL agent than RA, although the former is unable to directly reactivate PML-RARA-dependent transcription. Clearly, this must be explained mechanistically. This “Perspectives” article aims at clarifying and discussing the current knowledge on the role and significance of differentiation in response to APL treatment.
APL pathogenesis and the differentiation model
Disease characteristics
APL is characterized by the proliferation of leukemia blasts blocked at the promyelocyte stage of differentiation. Although this clonal expansion provokes the progressive disappearance of normal hematopoiesis, it is rarely associated with a large circulating tumor burden. The oncogenic transformation originates from a chromosomic translocation fusing most of the RARA gene with other genes encoding proteins with self-aggregation motifs.8 The most common translocation, t(15;17), gives rise to the PML-RARA fusion protein, which, in addition to a differentiation block, confers major self-renewal and growth properties on the leukemia clone. This is the only detectable genomic abnormality in most cases. Other alterations linked to disease progression and hyperleukocytosis include MYC amplification, Fms-like tyrosine kinase 3 activation, or RAS mutations. Accordingly, the sole expression of the PML-RARA protein is able to transform murine myeloid progenitors ex vivo9 and to recapitulate APL features in transgenic mice, although with incomplete penetrance.10,11
PML-RARA and the APL model
PML-RARA, which binds DNA via its RARA domain, is a potent transcriptional repressor. It can multimerize through the coiled-coil domain of PML12-14 and form large protein complexes by recruiting various partners.15 These include DAXX, a transcriptional corepressor recruited onto sumoylated PML K160 and required for immortalization by PML-RARA,9 nuclear receptor corepressors, such as silencing mediator for retinoid and thyroid hormone receptors (SMRT) or nuclear receptor corepressor (NCOR),16 histone deacetylases (HDAC3),17,18 polycomb group proteins, and DNA-methylating complexes,19,20 which all cooperate to enforce transcriptional and epigenetic repression through enhanced binding onto PML-RARA multimers. Accordingly, PML-RARA complexes blunt RA-induced activation of many canonical RARA target genes2 in a dominant-negative manner (reviewed by Melnick and Licht8 ). Because RARA signaling is implicated in the control of myeloid differentiation,21 such transcriptional repression by PML-RARA should result in a differentiation block. However, neither the down-regulation of RARA primary target genes by PML-RARA nor the direct link between transcriptional repression and differentiation block has ever been formally established in a physiologically relevant system.
In addition, PML-RARA clearly displays highly relaxed DNA-binding properties and consequently affects the transcription of many more genes than RARA targets, in particular those controlled by other nuclear receptors.22-25 This major gain of function reflects both PML-RARA multimerization and the presence of other DNA-binding proteins in the complex. RXRA, the universal partner of type II nuclear receptors, indeed favors the recognition of noncanonical RARA-binding sites by the oncogenic complex.23,25 Accordingly, genetic experiments have demonstrated that RXRA binding is required for cell transformation by RARA fusions.26,27 Importantly, in the case of the AML1-ETO fusion, auto-aggregation of the protein similarly allows relaxed binding site specificity.28,29 So, loosening of the DNA recognition specificity through multimerization appears to be a common and critical feature of transcription factor-derived oncogenic fusion proteins in acute myeloid leukemias and may be implicated in the arrest of differentiation.
The molecular bases of differentiation therapy
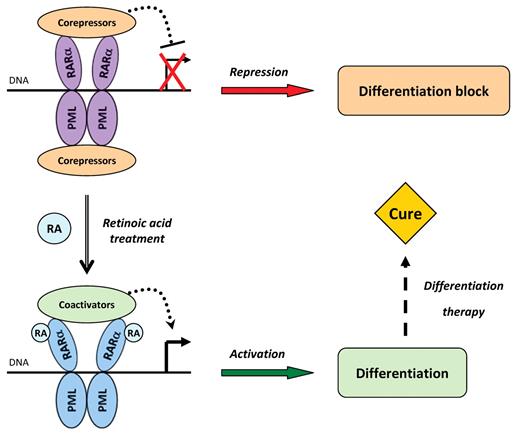
RA induces a profound change in the phenotype of APL cells, as they quickly shift from immature promyelocytes to short-lived, terminally differentiated, granulocytes, ex vivo or in vivo.30,31 Pharmacologic RA doses trigger a conformational change of the fusion protein, causing the release of corepressors and the recruitment of coactivators. This switch allows active transcription of the target genes initially repressed by PML-RARA, resulting in rapid cell differentiation in vitro and in vivo and ultimately leading to disease remission (Figure 1). This massive differentiation correlates with a sharp proliferation arrest in patients and in APL cellular models.32,33 In murine models, RA evokes full differentiation within 48 hours, and the complete loss of APL cells within a week is compensated by the regrowth of normal bone marrow cells.34
Historical model of PML-RARA-mediated transcriptional repression, RA-induced activation, and differentiation therapy. In the nucleus of APL cells, PML-RARA forms dimers through the coiled-coil domain of PML and binds DNA via its RARA moiety. The complex tightly associates with corepressors, resulting in transcriptional repression of target genes and in differentiation arrest. On exposure to pharmacologic doses of RA, PML-RARA switches to an active conformation and the corepressors are replaced by coactivators, thus enforcing the transcription of target genes, triggering the differentiation process and ultimately leading to cure.
Historical model of PML-RARA-mediated transcriptional repression, RA-induced activation, and differentiation therapy. In the nucleus of APL cells, PML-RARA forms dimers through the coiled-coil domain of PML and binds DNA via its RARA moiety. The complex tightly associates with corepressors, resulting in transcriptional repression of target genes and in differentiation arrest. On exposure to pharmacologic doses of RA, PML-RARA switches to an active conformation and the corepressors are replaced by coactivators, thus enforcing the transcription of target genes, triggering the differentiation process and ultimately leading to cure.
Historically, because of this relationship between the differentiation of leukemia blasts and tumor regression, RA treatment has been considered as the first example of differentiation therapy, as well as a striking illustration of transcription-based strategies.6,35 This straightforward model has been somehow downplayed by a number of unexpected observations. In patients, RA treatment generally results only in transient disease remission but cannot entail definitive cure unless RA is combined to regular chemotherapy.36 Only very rare patients respond durably to standard-dose RA as single agent,37 even though complete differentiation of the tumor is always observed. Accordingly, recent studies have argued that differentiation may not be the principal mechanism underlying the definitive clearance of APL cells.
Limits of the differentiation paradigm
Differentiation without cure
RA-induced differentiation has been clearly uncoupled from remission induction in both APL patients and murine models.34,38,39 For example, 3-day low-dose RA treatment of APL mice elicits full differentiation of leukemia cells in the marrow but a very limited antitumor effect as assessed by overall survival and secondary transplant assays.34 Along this line, patients with acquired resistance to treatment resulting from suboptimal RA concentrations exhibit ongoing differentiation of the leukemia blasts but sustained cell proliferation and tumor growth.40,41
Actually, in vivo, much higher RA concentrations are required for curing APL than for the reactivation of transcription or induction of differentiation. Whereas complete differentiation of the leukemia clone is achieved even at low doses of RA, APL clearance (as assessed by survival or loss of clonogenic cells in transplantation assays) correlates almost linearly with the blood RA concentration (J.A., unpublished observations, 2010). This probably explains why, in patients, only liposomal RA, which yields much higher intracellular concentrations than the standard RA oral regimen, has led to some cures as a single agent42 (Table 1).
The response to treatment in different APL models
| . | PML-RARα . | PLZF-RARα . | |||||
|---|---|---|---|---|---|---|---|
| RAlow . | RAmedium . | RAhigh . | As2O3 . | RAmedium/As2O3 . | RAlow . | RAhigh . | |
| Ex vivo | |||||||
| Differentiation | + | ++ | +++ | − | + | + | +++ |
| Degradation | − | +++ | +++ | +++ | +++ | − | +++ |
| LIC loss | − | +++ | +++ | +++ | +++ | − | +++ |
| In vivo | |||||||
| Differentiation | ++ | +++ | +++ | + | ++ | ++ | +++ |
| Degradation | − | ++ | +++ | ++ | +++ | − | ++ |
| LIC loss | − | ++ | +++ | +* | +++ | − | + |
| In patients | |||||||
| Differentiation | +++ | +++ | +++ | ++ | +++ | ++ | ++ |
| Cure | − | + | +++ | +++ | +++ | − | −/+† |
| . | PML-RARα . | PLZF-RARα . | |||||
|---|---|---|---|---|---|---|---|
| RAlow . | RAmedium . | RAhigh . | As2O3 . | RAmedium/As2O3 . | RAlow . | RAhigh . | |
| Ex vivo | |||||||
| Differentiation | + | ++ | +++ | − | + | + | +++ |
| Degradation | − | +++ | +++ | +++ | +++ | − | +++ |
| LIC loss | − | +++ | +++ | +++ | +++ | − | +++ |
| In vivo | |||||||
| Differentiation | ++ | +++ | +++ | + | ++ | ++ | +++ |
| Degradation | − | ++ | +++ | ++ | +++ | − | ++ |
| LIC loss | − | ++ | +++ | +* | +++ | − | + |
| In patients | |||||||
| Differentiation | +++ | +++ | +++ | ++ | +++ | ++ | ++ |
| Cure | − | + | +++ | +++ | +++ | − | −/+† |
The loss of LICs is assessed by serial replating ex vivo or transplantation in vivo.
Ex vivo indicates PML-RARA-transformed murine hematopoietic progenitor cells in methylcellulose cultures; in vivo, APL transgenic murine models.
As2O3 displays attenuated effects in mice due to dosage or pharmacokinetics issues.
Rare patients have been reported to respond to RA treatment by itself.
In addition, in a murine model, a PML-RARA mutation at S873 (a phosphorylation site modulating RA-triggered degradation) sharply impairs the efficacy of RA treatment regarding leukemia regression, whereas the effect on APL blast differentiation remains comparable with that observed in wild-type PML-RARA APL.34 This key result uncovers the genetic uncoupling of the 2 effects of RA on APL cells (differentiation and disease clearance). A similar uncoupling is noted in PLZF-RARA-driven APLs (see “The concept of LICs”).
Finally, in addition to the response to therapy, examination of the disease onset in transgenic mice has revealed that abnormal expansion of the myeloid compartment always precedes the differentiation block and that during the latency period PML-RARA-expressing cells are morphologically granulocytes.11 Thus, the transcriptional repression imposed by PML-RARA does not simply result in differentiation arrest, and the impairment of differentiation fails to appear as the sole driver of APL leukemogenesis.
The case of PLZF-RARA
The second most common translocation in APL leads to expression of the PLZF-RARA fusion protein, which is associated with a clinically RA-resistant variant of the disease.43,44 Initial studies suggested that resistance might stem from stronger repression of RARA target genes because of the ability of the PLZF moiety of the fusion to bind corepressors, hence blunting RA-induced transcriptional activation.16-18 However, it was recently demonstrated that PLZF-RARA leukemia cells actually fully differentiate on RA treatment both ex vivo and in vivo and, accordingly, exhibit a clear-cut activation of target genes identical to that noted in PML-RARA-transformed cells.34,45 Moreover PLZF-RARA transgenic mice lacking the reciprocal RARA-PLZF translocation develop a terminally differentiated and yet fully penetrant leukemia.46 This again suggests that the differentiation block observed in APL cells might not be the primary driving force of leukemogenesis.
Nevertheless, clinically, PLZF-RARA APLs display much lower sensitivity to RA than PML-RARAs,34,45 illustrated by the much lesser RA-sensitive clonogenic activity of leukemia blasts as assessed in secondary transplantations (only long-term treatment with high-dose RA can achieve efficient APL clearance in these mice). Several recent proposals have been put forward to explain such clinical RA resistance of the disease, notably stable recruitment of polycomb complex47 and induction of CRABP1 expression.48 Yet, some RA responsiveness, notably in combination therapies, has been reported in patients.34,49,50 Thus, uncoupling of disease eradication from blast differentiation is particularly striking in PLZF-RARA-driven APLs (Table 1).
The paradox of arsenic trioxide
Unlike RA, arsenic trioxide induces limited transcriptional changes in APL cells. In particular in NB4 cells, it does not seem to affect PML-RARA-enforced repression of RARA target genes51 and indeed regulates much fewer genes, and in a much less profound manner than RA.52 Yet it has proved extremely efficient at eradicating leukemia blasts and, even as single agent,53-56 at curing patients with PML-RARA-driven APL. Such curative effects in the absence of immediate transcriptional activation are clearly at variance with the classic model of differentiation therapy. Furthermore, ex vivo arsenic treatment elicits significant cell death but only very limited differentiation of APL blasts unless cytokines or cyclic adenosine monophosphate is added to the culture.41,57-59 In vivo, however, a slow blast differentiation generally follows the initial burst of apoptosis, but with delayed kinetics compared with that observed with RA, suggesting that tumor differentiation may not constitute the primum movens of arsenic-induced cures.
Should transcriptional activation be the actual goal of APL therapy?
Recently, several lines of evidence have cast some doubts on the key role of transcription-activating therapeutic strategies. First, the model of transcriptional repression and epigenetic silencing induced by PML-RARA remains a working model. Very few studies have addressed the issue of the role of PML-RARA on the basal levels of transcription in primary cells. Indeed, PML-RARA may even activate the basal transcription of a subset of target genes, including p21.60 Conversely, some primary PML-RARA target genes appear to be repressed on RA treatment,25,61 challenging the notion that RA merely switches PML-RARA from a repressor to an activator of transcription. These data point to the necessary reevaluation of PML-RARA-mediated transcriptional control.
Second, the actual mechanisms underlying transcriptional repression, enhanced corepressor binding, and either PML-RARA-induced transformation or the differentiation block observed in APL need reassessment. On the one hand, the presence of RXRA in the complex, although absolutely required for leukemic transformation, does not increase PML-RARA binding affinity for corepressors26 (even though it may allow 2 corepressor molecules to bind to the complex). Conversely, tethering of corepressors onto RARA does not appear sufficient for leukemogenesis, as dominant-negative or even self-dimeric RARA and RARA-HDAC fusion fails to efficiently initiate APL.62-64 On the other hand, cyclic adenosine monophosphate signaling disrupts the interaction between RARA or PML-RARA and the corepressors65 but does not induce terminal maturation of leukemia cells.41 Similarly, HDAC inhibitors provoke apoptosis but not terminal differentiation of APL blasts.66,67 Finally, in the presence of cyclic adenosine monophosphate, RXRA ligands (rexinoids) activate PML-RARA-dependent transcription and promote differentiation, even in RA-resistant APLs,23,41 but fail to significantly prolong survival in mice.
Collectively, the molecular bases of therapy-induced differentiation appear unsolved, and the terminal maturation of leukemia blasts does not seem essential for the cure of APL. This probably explains why differentiation therapy, although extremely successful in the case of APL, has so far failed to be extended to other leukemias.68 Actually, PML-RARA does not only impair differentiation but also massively boosts the self-renewal capacity of transformed progenitors (“The concept of LICs”). Note that acquisition of self-renewal may have a transcriptional basis. Therefore, treatments should not necessarily aim at achieving full differentiation of APL blasts but may rather have to focus on other properties of leukemia cells, such as their ability to self-renew.
Other models for the cure of APL
The concept of LICs
Several studies have suggested that all leukemia cells are not equal: there is a hierarchy in myeloid leukemias as in normal hematopoiesis.69 Only very few leukemia cells are able to give rise to a new full-grown tumor on transplantation in vivo or to yield colonies in ex vivo cultures. The abundance of these clonogenic leukemia-initiating cells (LICs) depends on the tumor type. In murine APL, LICs seem to represent approximately 1% of blasts. They display an enhanced self-renewal capacity, although, phenotypically, they are committed myeloid progenitors.70,71 Thus, like several myeloid transcription factor-derived oncoproteins, PML-RARA confers some stem cell features, notably self-renewal, onto more differentiated cells.
The introduction of the LIC concept in APL raises a very significant issue: what is the endpoint to be used to assess the effect of a given treatment? The global response of the tumor may conceal a more heterogeneous picture, with distinct responses in specific cell compartments. For instance, one could propose that the discrepancy between all-trans retinoic acid and arsenic in terms of APL cure might be the result of the targeting of different cell populations. Clearly, as illustrated by the case of PLZF-RARA-driven APLs, differentiation of the tumor bulk and transient tumor regression do not allow predicting the clinical outcome: LICs are the cells to be targeted for tumor eradication. Indeed, the persistence of LICs and the subsequent emergence of resistant leukemia clones on treatment are responsible for relapses in APL patients exactly as in CML patients, notably through mutations that abrogate PML-RARA ability to bind RA.72 One convenient way to assess LIC abundance after therapy in vivo has been transplantation of marrow cells into untreated secondary hosts; and, importantly, the inability to transplant APL from treated mice always correlates with the cure of the primary recipient.
Oncoprotein degradation
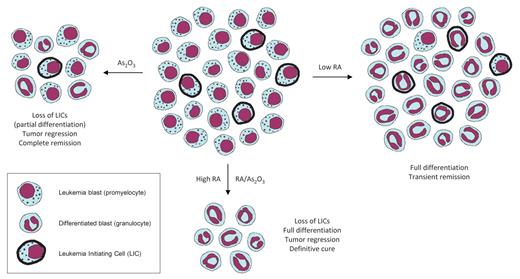
Because APL largely appears as an oncogene-driven monogenic disease, the destruction of the PML-RARA protein alone may prove sufficient to eradicate the leukemia. It was unexpectedly observed that the 2 clinically active drugs, RA and arsenic, both degrade PML-RARA.73 RA induces PML-RARA degradation via its RARA moiety,74 whereas arsenic acts on the PML part of the fusion51,75-77 through recently elucidated mechanisms.78-81 Because RA and arsenic target PML-RARA in 2 distinct ways, one may predict that they would synergistically degrade the fusion protein and, hence, cooperate to cure APL. Data from murine models have indeed demonstrated their dramatic synergy for clearing the disease.82 Along this line, the RA/arsenic combination cures most patients even in the absence of genotoxic chemotherapy4,7,83 (Table 1; Figure 2). Finally, although PML-RARA degradation by RA and arsenic was initially considered as a mere consequence of differentiation, recent murine evidence strongly supports its primordial role in the eradication of LICs and disease remission.34,38,39 In addition to PML-RARA degradation, other mechanisms may cooperate in the remarkable curative effect of arsenic as a single agent, either through PML itself84 or via other putative arsenic targets implicated in LIC self-renewal.
A revised model of treatment outcomes in APL. Low RA doses (right) induce differentiation of the bulk of the tumor without affecting the LICs, leading to transient clinical responses but no cure in patients. Arsenic treatment (left) entails some differentiation and substantial loss of LICs, resulting in tumor regression and durable remissions with a high proportion of definitively cured patients. The combination of arsenic and RA, as well as high RA concentrations (bottom) elicit both complete differentiation of leukemia blasts and eradication of LICs, thus leading to strong tumor regression and ultimately to cure in most patients.
A revised model of treatment outcomes in APL. Low RA doses (right) induce differentiation of the bulk of the tumor without affecting the LICs, leading to transient clinical responses but no cure in patients. Arsenic treatment (left) entails some differentiation and substantial loss of LICs, resulting in tumor regression and durable remissions with a high proportion of definitively cured patients. The combination of arsenic and RA, as well as high RA concentrations (bottom) elicit both complete differentiation of leukemia blasts and eradication of LICs, thus leading to strong tumor regression and ultimately to cure in most patients.
Actually, loss of the driving oncoprotein may even directly elicit differentiation processes. Indeed, a substantial level of spontaneous blast differentiation can be obtained by the artificial down-regulation of PML-RARA by shRNAs in different models of APL (J.A., unpublished observations, 2010), suggesting that maturation of leukemia cells can result from the mere lifting of PML-RARA transcriptional control. Note that this is suggestive for the absence of irreversible chromatin marks, which would preclude gene reactivation and subsequent differentiation. One may also propose that the induction of the rapid and complete differentiation of leukemia blasts by RA may at least partially occur through the reactivation of the endogenous RARA pathway after derepression of PML-RARA target genes, and not through direct PML-RARA-mediated transcriptional activation (Figure 3). In line with these observations, PML-RARA degradation by arsenic entails the transcriptional derepression of a subset of PML-RARA target genes.52,53 Accordingly, the proliferation of differentiating APL blasts in the blood of treated patients (known as “differentiation syndrome”) has been reported with both RA and arsenic therapies.85 Collectively, these observations could suggest that derepression of PML-RARA targets resulting from its degradation may suffice to trigger differentiation.
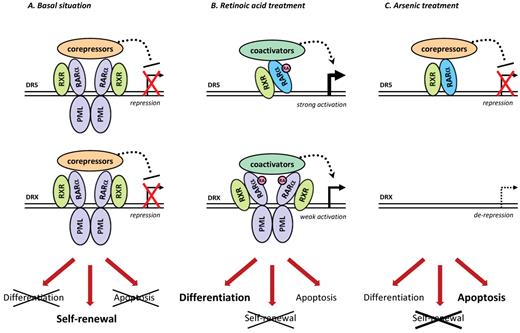
A system biology model of the molecular mechanisms involved in APL. (A) In the absence of treatment, PML-RARA homodimers associated with RXR bind to specific sequences in the promoter region of target genes. The bound DNA sequences are either RARA-binding sequences (eg, DR5) or more relaxed repeat motifs (referred to as DRX). The direct or indirect regulation of target genes is responsible for the differentiation block, aberrant self-renewal, and impairment of apoptosis observed in APL blasts. (B) On RA treatment, PML-RARA activates the transcription of target genes. One may also propose that PML-RARA degradation by RA allows RARA-binding to DR5-containing promoters. (C) Arsenic triggers PML-RARA degradation, resulting in the derepression of specific PML-RARA target genes (with DRX-containing promoters), whereas RARA can replace PML-RARA on DR5 sequences. The differential modulation of these 2 subsets of target genes by RA or arsenic may account for the different cellular aspects of response in vivo.
A system biology model of the molecular mechanisms involved in APL. (A) In the absence of treatment, PML-RARA homodimers associated with RXR bind to specific sequences in the promoter region of target genes. The bound DNA sequences are either RARA-binding sequences (eg, DR5) or more relaxed repeat motifs (referred to as DRX). The direct or indirect regulation of target genes is responsible for the differentiation block, aberrant self-renewal, and impairment of apoptosis observed in APL blasts. (B) On RA treatment, PML-RARA activates the transcription of target genes. One may also propose that PML-RARA degradation by RA allows RARA-binding to DR5-containing promoters. (C) Arsenic triggers PML-RARA degradation, resulting in the derepression of specific PML-RARA target genes (with DRX-containing promoters), whereas RARA can replace PML-RARA on DR5 sequences. The differential modulation of these 2 subsets of target genes by RA or arsenic may account for the different cellular aspects of response in vivo.
Differentiation block versus self-renewal
It appears that PML-RARA-induced differentiation arrest of leukemia blasts and increased self-renewal of progenitors are 2 distinct features of APL, probably driven by different gene programs.34,86 At the transcriptional level, subsets of PML-RARA target genes could be differentially regulated through variable specificities and affinities for DNA-binding sequences, which would depend on the nature of the binding sequence (eg, type of AGGTCA repeats) and might be finely tuned by post-translational modifications (phosphorylation, sumoylation) on either PML-RARA or other members of the oncogenic complex. The possibility of 2 epigenetic signatures, distinguishing the genes involved in self-renewal from those responsible for the differentiation block, cannot be ruled out. Indeed, RARA fusions interacts with several chromatin-remodeling complexes, including polycomb,47,87 which play critical roles in lineage commitment and stem cell maintenance.88 In this respect, DAXX has recently been identified as an important histone chaperone implicated in the deposition of histone variant H3.3, a mark of active chromatin.89
One may also envision that indirect or nongenomic effects of PML-RARA can play a significant role in leukemic transformation, for example, via PML-RARA-mediated titration of important proteins, such as RXRA, involved in the control of myeloid lineage determination,90 or DAXX, whose titration could modulate not only apoptosis but also cell fate.91 One of the titrated partners deserves special mention: PML itself. Indeed, PML-RARA forms heterodimers with PML and disrupts PML nuclear bodies.92-95 Hence, PML-RARA probably interferes with PML functions in a transcription-independent manner. Although the exact roles of PML in a cell are still poorly understood, it seems to control pathways involved in resistance to apoptosis96 and stem cell biology in both normal and leukemic contexts.84,97 The proposed regulation of P53 function by PML could turn out to be a reasonable clue, as PML seems to mediate the impairment of P53 activation by PML-RARA.67,98 PML also modulates the v-akt murine thymoma viral oncogene homolog 1/phosphatase and tensin homolog (AKT/PTEN) axis through the sequestration of DAXX, HAUSP, and PP2A in nuclear bodies.99 PML-RARA-induced disruption of nuclear bodies could therefore be associated with the loss of negative control on self-renewal. All these effects naturally depend on PML-RARA protein levels, which explains why only complete degradation of the oncoprotein results in the loss of clonogenicity.
These progresses in our understanding of the molecular determinants of the leukemia phenotype in APL raise hopes for the complete dissection of its pathogenesis. The availability of various well-characterized therapies on the one hand, and of murine models bearing mutant PML-RARA on the other, should allow system biology approaches to explore the remaining APL uncharted regions (Figure 3). Indeed, at least 3 situations can be opposed and compared: (1) differentiation without loss of clonogenicity, as obtained with low-dose RA treatments but also with the phosphorylation-deficient S873A PML-RARA mutant; (2) differentiation with simultaneous loss of clonogenicity, which results for instance from high RA doses or the RA/arsenic combination; and (3) loss of clonogenicity accompanied by delayed differentiation observed on arsenic treatment (Figure 2). Gene expression analysis in these conditions may unveil the nature of the pathways regulating terminal differentiation and, critically, of the self-renewal mechanism(s) implicated in APL.
In conclusion, a large spectrum of convergent evidence, gathered from in vivo and ex vivo settings, now allows reassessing the model of differentiation therapy in APL. Both the genetic and therapeutic uncoupling of tumor differentiation and disease clearance have been disclosed in murine APL models (Table 1), demonstrating that terminal differentiation of leukemia blasts is not only insufficient but also unnecessary for definitive cure and therefore unlikely to account for the clinical successes of APL therapy.
At the cellular and molecular levels, it appears that the complete and rapid differentiation observed on RA treatment may be explained by the reversion of PML-RARA–enforced transcriptional control, whereas loss of self-renewal reflects full PML-RARA degradation34,38,39 and results in LIC eradication. Future studies should decipher whether therapy-induced loss of self-renewal may be considered as a restricted differentiation program. This new working model fits with major observations from the past 20 years, notably that the combination of RA and arsenic, because the 2 act via distinct pathways, yields the most complete degradation of the fusion protein and has proved highly effective in clearing disease in both transgenic mice82 and patients.4,7,83 It also enlightens the physiopathologic bases of therapy response of the disease and gives new clues for a deeper understanding of the basic mechanisms of oncogenesis in APL.
This model thus opens a new era for oncoprotein-targeting therapies, which may be of much wider clinical relevance than differentiation therapy. Indeed, targeting the driving oncoprotein may emerge as an efficient general strategy in cancer treatment. It could be readily applicable to oncogenic fusion proteins other than PML-RARA (provided both parts of the fusion can be therapeutically targeted for degradation), but might also demonstrate activity in other cancers in which the cells are addicted to the presence of a master oncogene.100
Acknowledgments
The authors thank J. C. Gluckman for careful editing of the manuscript and J. Godet and the Comité des Yvelines de la Ligue contre le Cancer for their continuous trust and support.
J.A. is supported by the French Ministry of Research. The laboratory is supported by the Ligue Nationale contre le Cancer, Inserm, Centre National de la Recherche Scientifique, University Paris Diderot (formerly known as Paris-7), the Institut Universitaire de France, EPITRON (an integrated project funded by the European union under the 6th framework program (LSHC-CT-2005-518417), and Canceropole programs.
Authorship
Contribution: J.A. and H.d.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hugues de The, Centre National de la Recherche Scientifique Unité Mixte de Recherche7212, Inserm U944, Hôpital St Louis, 1 Av C. Vellefaux, 75475 Paris Cedex 10, France; e-mail: dethe@univ-paris-diderot.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal