Abstract
The small GTPase Rac1 is involved in the activation of the reduced NAD phosphate oxidase complex resulting in superoxide production. We recently showed that Bcl-2 overexpression inhibited apoptosis in leukemia cells by creating a pro-oxidant intracellular milieu, and that inhibiting intracellular superoxide production sensitized Bcl-2–overexpressing cells to apoptotic stimuli. We report here that silencing and functional inhibition of Rac1 block Bcl-2–mediated increase in intracellular superoxide levels in tumor cells. Using confocal, electron microscopy and coimmunoprecipitation, as well as glutathione S-transferase–fusion proteins, we provide evidence for a colocalization and physical interaction between the 2 proteins. This interaction is blocked in vitro and in vivo by the BH3 mimetics as well as by synthetic Bcl-2 BH3 domain peptides. That this interaction is functionally relevant is supported by the ability of the Bcl-2 BH3 peptide as well as the silencing and functional inhibition of Rac1 to inhibit intracellular superoxide production as well as overcome Bcl-2–mediated drug resistance in human leukemia cells and cervical cancer cells. Notably, the interaction was observed in primary cells derived from patients with B-cell lymphoma overexpressing Bcl-2 but not in noncancerous tissue. These data provide a novel facet in the biology of Bcl-2 with potential implications for targeted anticancer drug design.
Introduction
Tumorigenesis is a function of an imbalance between the rates of cell proliferation and death, and as such a number of tumor suppressors function by altering the balance in favor of cell death, whereas tumor promoters, such as Ras and Bcl-2, facilitate survival in part by blocking death execution signals. Bcl-2 was first identified as a proto-oncogene in B-cell lymphomas as a result of chromosomal translocation t(14,18).1 Since then, a host of proteins structurally related to Bcl-2 have been grouped together under the Bcl-2 family,2 with members that either facilitate apoptosis (proapoptotic) or inhibit execution (antiapoptotic). These proteins share structural domains termed as Bcl-2 homology (BH) domains; antiapoptotic Bcl-2-like members (eg, Bcl-2, Bcl-xL, Bcl-w, and Mcl-1) have 4 BH domains (BH1-BH4), proapoptotic members (eg, Bax, Bak, and Bok) lack BH4 domain, and a group of proapoptotic members known as Bcl-2 BH3-only proteins (eg, Bid, Bim, Bad, Bmf, Noxa, and Puma) contain only BH3 domain. These BH domains are involved in homodimerization or heterodimerization with another family member or with other proteins.3 Because of their contrasting effects on apoptotic signaling, a critical ratio of proapoptotic and antiapoptotic Bcl-2 proteins is paramount in tumor cells' response to death stimuli.
Since the discovery of Bcl-2 as an antiapoptotic protein and its association with clinical chemo-resistance,4-6 the underlying mechanism(s) for the latter is extensively being studied. We previously demonstrated a novel mechanism by which Bcl-2 expression regulated apoptosis in human leukemia cells; overexpression of Bcl-2 resulted in a slight pro-oxidant state (an increase in intracellular superoxide [O2∸] levels), which inhibited apoptosis.7 These data corroborated our earlier findings linking a slight increase in intracellular O2∸ on expression of a constitutively active small GTPase, Rac1, to apoptotic inhibition in human melanoma cells. On the contrary, introduction of a dominant negative Rac1 (RacN17) not only decreased intracellular O2∸ but also restored cell sensitivity to apoptosis.8
Rac1 belongs to Rho family of GTPases, which include Cdc42 and RhoA,9 and is implicated in many cellular functions, such as cell trafficking, actin polymerization, gene transcription, and cell proliferation.10 Interestingly, pro-oxidant activity of Bcl-2 and its antiapoptotic effect were neutralized on transient transfection with RacN17; however, the mechanism remained elusive.7 Stimulated by these findings, we investigated the possibility of a physical interaction between Bcl-2 and Rac1, which could explain, at least in part, the mechanism of their similar functional biology.
Bcl-2 binds to nonhomologous proteins, such as Ras, p53, Raf-1, Beclin 1, and others.11-13 Different binding partners seem to regulate Bcl-2's activity by distinct mechanisms at outer mitochondrial membrane. For example, Bcl-2 interacts with Raf-1 through its BH4 domain and targets Raf-1 to mitochondria where it phosphorylates Bad, thereby preventing its interaction with Bcl-2 and inhibiting apoptosis.11 Alternatively, p53 sequesters Bcl-2 at mitochondrial membranes, thus preventing its binding to Bax and providing a favorable milieu for Bax-induced mitochondrial outer membrane permeabilization.12,14
Using primary tissues derived from B-cell lymphoma patients as well as a variety of human cancer cell lines, we provide evidence for Bcl-2–Rac1 interaction, which could be disrupted in vitro and in vivo by Bcl-2 BH3 domain-targeting peptides. Furthermore, interrupting Bcl-2–Rac1 interaction with BH3 domain peptides resulted in a decrease in Bcl-2-mediated mitochondrial O2∸ and restored tumor cell sensitivity to drug-induced apoptosis.
Methods
Primary cells from patients and cell lines
Peripheral blood was obtained from healthy volunteers and primary tumor tissues from patients at National University Hospital in accordance with approved Institutional Review Board protocol. Lymphocytes were obtained as described previously.15
Raji, Jurkat, CEM, NIH3T3, and MRC5 were cultured in RPMI 1640. HeLa, IMR90, and L6 were grown in Dulbecco modified Eagle medium. CEM, Jurkat, and HeLa cells stably transfected with pcDNA3.1 containing neomycin or human Bcl-2 gene and rat cells stably transfected with Bcl-2-Acta were selected with G418. All cells were cultured at 37°C under 5% CO2.
Recombinant proteins and plasmids
Recombinant human Bcl-2 and glutathione S-transferase (GST)-Rac1 were purified as previously.16 Plasmid for GST-Rac1 was provided by Dr Edward Manser. Vector for recombinant Bcl-2 was provided by Dr J.C. Reed (Burnham Institute). Plasmids carrying GST-Ras, GST-Ral A, and GST-Cdc42 were provided by Dr De Gunzburg (Institute Curie). Recombinant Bax (mouse) and Bcl-xL (human) were purchased from Protein X Lab. The Bcl-2 mutant (T69A/S70A/T87A) was obtained from Addgene.
Coimmunoprecipitation and GST-pulldown assays
Anti-Rac1 or anti-Bcl-2 (4 μg) was added to whole cell lysates or fractionated lysates (1000 μg) for immunoprecipitation. Subcellular fractions were obtained as previously.17 The immunocomplexes were analyzed by Western blotting as previously18,19 and probed with anti-Rac1 (Upstate Biotechnology), anti-pan Ras, anti-Bax, anti–Bcl-xL, or anti–Bcl-2 (Santa Cruz Biotechnology). For GST-pulldown assays, GST-Rac1 (10 μg) was incubated with 40 μL glutathione Sepharose-4B beads (30°C; 1 hour) followed by incubation with recombinant proteins or cell extracts (4°C; overnight).
Immunofluorescence by confocal microscopy
In vitro peptide inhibition assay
Bax BH3 (KKLSECLKRIGDELDS), Bcl-2 BH3 (PVVHLTLRQAGDDFSR), Bcl-2 BH4 (TGYDNREIVMKYIHYKLSQRGYEW), and Bcl-xL BH4 (SNRELVVDFLSYKLSQKGYS) peptides were synthesized by SynPep Corp. Recombinant Bcl-2 (5 μg) was preincubated with synthetic peptides (4°C; 2 hours) followed by incubation with GST-Rac1 (4°C; overnight). Bound complexes were analyzed by Western blotting.
In cellulo peptide inhibition assay
Cells were incubated with Bax BH3, Bcl-2 BH3, and control (ALILTLV) peptides fused with an internal sequence of TAT protein (RKKRRQRRR) (37°C; 4 hours) and fixed for confocal microscopy.
RNA interference
Transient transfection with 30nM siRac1 or scrambled siRNA (Ambion/Applied Biosystems) were carried out using siPORT NeoFX Transfection Agent (Ambion).
Determination of O2∸
Determination of cell viability
Statistical analysis
Statistical analysis was performed using paired Student t test, and P < .05 was considered as significant.
Results
Inhibition of Rac1 alleviates Bcl-2-induced increase in O2∸
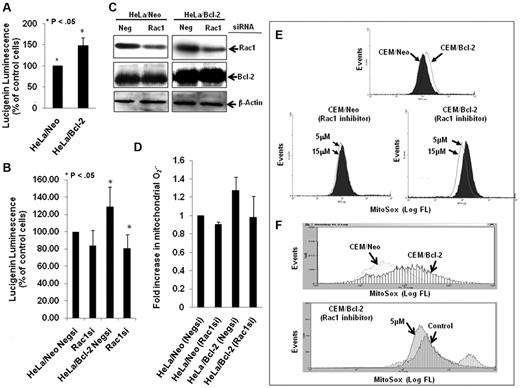
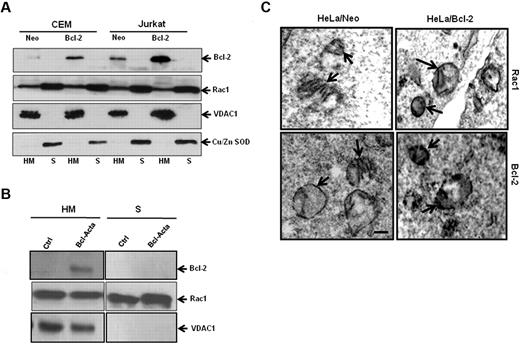
In accordance with our earlier findings,7,18 Bcl-2 overexpression resulted in a moderate elevation in intracellular O2∸ in HeLa human cervical cancer cells (Figure 1A), which was blocked on siRNA-mediated gene silencing of Rac1 (Figure 1B), a critical mediator of the membrane reduced NAD phosphate (NADPH) oxidase assembly and reactive oxygen species production.20 Interestingly, the effect of Rac1 silencing (Figure 1C) was also observed on the increase in mitochondrial O2∸ afforded by Bcl-2 overexpression in HeLa/Bcl-2 cells versus HeLa/Neo cells (Figure 1D). Of note, silencing Rac1 did not affect Bcl-2 expression in our system. To provide further evidence for the involvement of Rac1 in Bcl-2-induced O2∸ production, the effect of pharmacologic inhibition of Rac1 (NSC23766) was tested in another model of Bcl-2 overexpression (CEM leukemia cells). Indeed, functional inhibition of Rac1 resulted in a significant decrease in mitochondrial O2∸ in CEM/Bcl-2 cells compared with CEM/Neo cells (Figure 1E-F).
Silencing or pharmacologic inhibition of Rac1 reduces intracellular and intramitochondrial O2∸ levels in Bcl-2-overexpressing cells. (A) Intracellular O2∸ levels were determined in HeLa/Neo and HeLa/Bcl-2 cells using lucigenin-based chemiluminescence. (B) Intracellular levels were determined in HeLa/Neo and HeLa/Bcl-2 cells silenced with control scrambled siRNA (Negsi) Rac1 siRNA using lucigenin-based chemiluminescence assay. (B-C) Error bars represent the mean ± SD (n = 4). (C) Western blot analysis of Rac1, Bcl-2, and β-actin expression in HeLa/Neo and HeLa/Bcl-2 cells transfected with control scrambled siRNA (Neg) or siRNA against Rac1. (D-F) Intramitochondrial O2∸ levels were determined after cells (D-E) or isolated mitochondria (F) loading with MitoSOX as described in “Determination of O2∸.” The mean fluorescence intensity values are expressed as mean ± SE. The histograms are representative of 3 independent experiments.
Silencing or pharmacologic inhibition of Rac1 reduces intracellular and intramitochondrial O2∸ levels in Bcl-2-overexpressing cells. (A) Intracellular O2∸ levels were determined in HeLa/Neo and HeLa/Bcl-2 cells using lucigenin-based chemiluminescence. (B) Intracellular levels were determined in HeLa/Neo and HeLa/Bcl-2 cells silenced with control scrambled siRNA (Negsi) Rac1 siRNA using lucigenin-based chemiluminescence assay. (B-C) Error bars represent the mean ± SD (n = 4). (C) Western blot analysis of Rac1, Bcl-2, and β-actin expression in HeLa/Neo and HeLa/Bcl-2 cells transfected with control scrambled siRNA (Neg) or siRNA against Rac1. (D-F) Intramitochondrial O2∸ levels were determined after cells (D-E) or isolated mitochondria (F) loading with MitoSOX as described in “Determination of O2∸.” The mean fluorescence intensity values are expressed as mean ± SE. The histograms are representative of 3 independent experiments.
Enhanced interaction between endogenous Bcl-2 and Rac1 in clinical biopsies from B-cell lymphoma patients
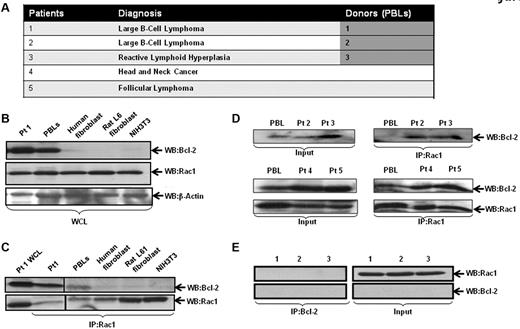
Intrigued by our findings on the effect of inhibiting Rac1 on Bcl-2-induced O2∸ production, we set out to investigate whether this effect was a function of a physical interaction between the 2 oncoproteins. As Bcl-2 is up-regulated in 80% of follicular lymphomas through a t(14;18)(q32;q21) chromosomal translocation,21 we first addressed this in primary biopsy tissues derived from patients diagnosed with B-cell lymphoma, reactive lymphadenopathy, and peripheral blood leukocytes (PBLs) from volunteers (Figure 2A). As a negative control, lysates from IMR90 human lung fibroblasts, Rat L6 muscle cells, and NIH3T3 cells were used. Cell lysates from tissues or cell lines were immunoprecipitated and probed for interaction by Western blotting. Our results show that most of the clinical samples had high Bcl-2 expression, which strongly correlated with the interaction level with Rac1 (Figure 2C-D). In contrast, whereas Rac1 was abundantly expressed in all the fibroblast lines (IMR90, Rat L6, and NIH3T3) and PBLs derived from 3 normal donors, the expression of Bcl-2 was undetectable, and no Rac1-Bcl-2 interaction was detected (Figure 2B-C,E). It should be pointed out that presence of the interaction in the 2 PBL samples from volunteers was because of the increased Bcl-2 expression observed in these cells (Figure 2B-D).
Interaction of endogenous Bcl-2 with Rac1 in primary tumor tissue. (A) Table representing the different patient samples used in the analysis. (B) Whole cell lysates (WCL) from primary tissue derived from patient 1 (B-cell lymphoma), PBLs from healthy donor, IMR90 human fibroblasts, rat L6 smooth muscle cell fibroblasts, and mouse NIH3T3 fibroblasts were analyzed for the expression of Rac1 and Bcl-2 (C-2, Santa Cruz Biotechnology). (C) Cell lysates were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2 and anti-Rac1 (as internal control). (D) Cell lysates from clinical material (patients 2-5) and PBLs were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2 using Western blot analysis. (E) Cell lysates from PBLs from 3 healthy donors were immunoprecipitated with anti–Bcl-2 and probed with anti-Rac1.
Interaction of endogenous Bcl-2 with Rac1 in primary tumor tissue. (A) Table representing the different patient samples used in the analysis. (B) Whole cell lysates (WCL) from primary tissue derived from patient 1 (B-cell lymphoma), PBLs from healthy donor, IMR90 human fibroblasts, rat L6 smooth muscle cell fibroblasts, and mouse NIH3T3 fibroblasts were analyzed for the expression of Rac1 and Bcl-2 (C-2, Santa Cruz Biotechnology). (C) Cell lysates were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2 and anti-Rac1 (as internal control). (D) Cell lysates from clinical material (patients 2-5) and PBLs were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2 using Western blot analysis. (E) Cell lysates from PBLs from 3 healthy donors were immunoprecipitated with anti–Bcl-2 and probed with anti-Rac1.
To provide further clinical evidence, we used fluorescence imaging for Bcl-2–Rac1 colocalization using confocal microscopy on sections derived from 3 B-cell lymphoma patients, 1 chronic lymphocytic leukemia patient, and 2 benign lymph nodes. Indeed, strong colocalization of Bcl-2 and Rac1 was observed in sections from diffuse large B-cell lymphoma patients, whereas minimal interaction was detected in benign lymph nodes (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These data demonstrated the first evidence for the colocalization of Bcl-2 and Rac1 in clinically diagnosed lymphomas with Bcl-2 overexpression.
Rac1 is a novel binding partner of Bcl-2
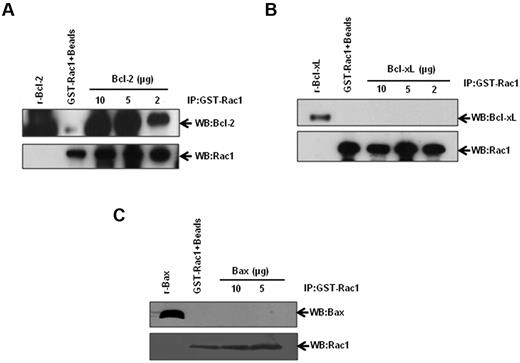
Stimulated by our results demonstrating Bcl-2–Rac1 interaction in primary tissues from lymphoma patients, we set out to corroborate and further investigate the existence of a physical interaction between these 2 proteins using a variety of established human cancer cell lines. We first used an in vitro GST-pulldown assay using GST-Rac1 and recombinant Bcl-2. Incubation of GST-Rac1 with increasing concentrations of recombinant Bcl-2 resulted in a dose-dependent increase in Bcl-2–Rac1 interaction (Figure 3A). In contrast, Bcl-xL, the closest functional homolog of Bcl-2,22 and Bax, a proapoptotic member of Bcl-2 family, failed to interact with GST-Rac1 (Figure 3B-C). Corroborating these findings, there was no detectable interaction between Rac1 and Bcl-xL or Bax in lysates from CEM/Neo, CEM/BCL-2, Jurkat, or Raji cells (supplemental Figure 2A-B). Furthermore, transient overexpression of Bcl-xL in HeLa cells did not result in an increase in intracellular O2∸ (supplemental Figure 2C-D), thereby indicating that the pro-oxidant effect was specific to Bcl-2. These data provided strong evidence to support a specific interaction between Rac1 and Bcl-2. It should be pointed out that interaction between Bcl-2 and Ras has been previously demonstrated11,23,24 and was confirmed by our data indicating that other GTPases, Cdc42, Ras, and Ral A interacted with Bcl-2 (supplemental Figure 3).
Identification of Rac1-Bcl-2 interaction. Various amounts of recombinant Bcl-2 (A), Bcl-xL (B), and Bax (C) were incubated with GST-Rac1. The membranes were probed with the respective antibodies (anti-Bcl-2, anti–Bcl-xL, or anti-Bax). Recombinant proteins alone were used as positive controls. GST-Rac1 alone was used as a negative control. The data are representative of 4 independent experiments.
Identification of Rac1-Bcl-2 interaction. Various amounts of recombinant Bcl-2 (A), Bcl-xL (B), and Bax (C) were incubated with GST-Rac1. The membranes were probed with the respective antibodies (anti-Bcl-2, anti–Bcl-xL, or anti-Bax). Recombinant proteins alone were used as positive controls. GST-Rac1 alone was used as a negative control. The data are representative of 4 independent experiments.
Bcl-2–Rac1 interaction is enhanced on Bcl-2 overexpression
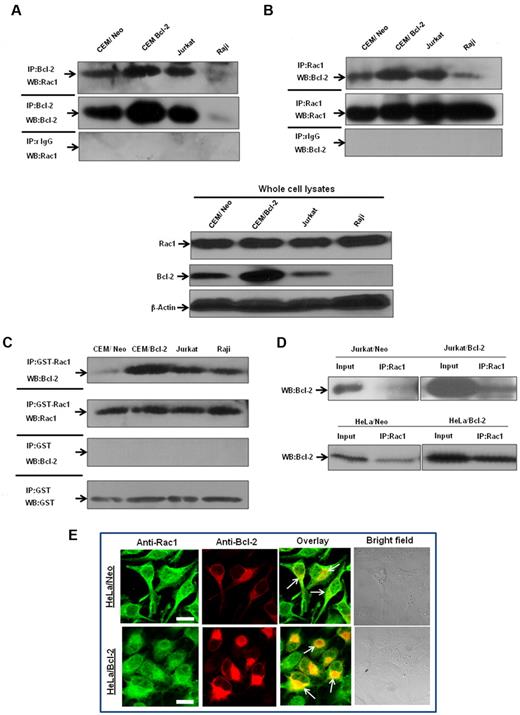
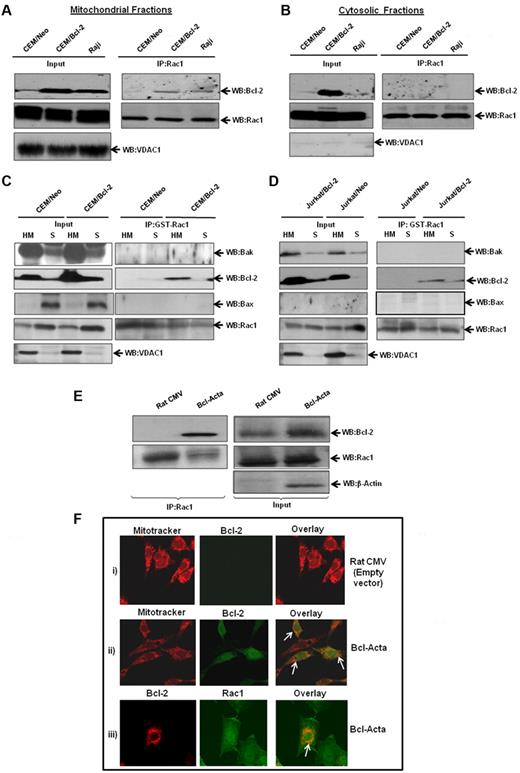
To verify that endogenous Bcl-2 and Rac1 interacted in vivo, coimmunoprecipitation experiments were performed in a variety of human tumor cell lines, CEM chronic myeloid leukemia cells, and its Bcl-2-overexpressing variant (CEM/Bcl-2), Jurkat T-cell leukemia cells, and Raji B-cell lymphoma cells. Endogenous Rac1 coprecipitated with endogenous Bcl-2 in lysates from all cell lines tested, but not with negative control isotype-matched antibodies (Figure 4A). Conversely, endogenous Bcl-2 coprecipitated with endogenous Rac1, but not with isotype-matched control (Figure 4B). In addition, the interaction strongly correlated with endogenous Bcl-2 expression levels; CEM/Bcl-2 and Jurkat cells with the highest Bcl-2 expression levels showed significantly stronger Rac1–Bcl-2 association. Furthermore, immunoprecipitation of lysates from a variety of human tumor cell lines with GST-Rac1 or anti-Rac1 and probing with anti–Bcl-2 confirmed existence of a direct interaction (Figure 4C-D). Once again, the interaction level was proportional to expression levels of Bcl-2, which was also observed in 2 other Bcl-2–overexpressing cells (data not shown).
Interaction between endogenous Bcl-2 and Rac1 is enhanced on Bcl-2 overexpression. (A) Whole cell lysates from various tumor cell lines were immunoprecipitated with anti-Bcl-2 and probed with anti-Rac1. Immunoprecipitation with control rabbit anti-IgG antibodies was used as a negative control. (B) Whole cell lysates from tumor cell lines were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2. Immunoprecipitation with control mouse anti-IgG2b antibodies was used as a negative control. Whole cell lysates probed with anti-Rac1, anti–Bcl-2, and β-actin are shown on the bottom panel. (C) Endogenous Bcl-2 was pulled down with purified recombinant GST-Rac1 using cell lysates from CEM/Neo, CEM/Bcl-2, Jurkat, and Raji cell lines. The proteins were immunoblotted with anti–Bcl-2 (first panel) and anti-Rac1 (second panel). Pulldown with GST was used as a negative control. (D) Lysates from Jurkat/Neo, Jurkat/Bcl-2, HeLa/Neo, and HeLa/Bcl-2 were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2. (E) Representative confocal microscopy of Bcl-2 expression and Rac1 expression in HeLa/Bcl-2 cells. Rac1 was probed with anti-Rac1 and fluorescein isothiocyanate-conjugated secondary mouse IgG, whereas Bcl-2 was probed with anti–Bcl-2 and rhodamine-conjugated secondary rabbit IgG. The panels were merged using Fluoview (FV10-ASW.1.3 viewer) software to detect areas of colocalization of Rac1 and Bcl-2 proteins. Bar represents 20 μm. All experiments were performed at least 3 or more times independently.
Interaction between endogenous Bcl-2 and Rac1 is enhanced on Bcl-2 overexpression. (A) Whole cell lysates from various tumor cell lines were immunoprecipitated with anti-Bcl-2 and probed with anti-Rac1. Immunoprecipitation with control rabbit anti-IgG antibodies was used as a negative control. (B) Whole cell lysates from tumor cell lines were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2. Immunoprecipitation with control mouse anti-IgG2b antibodies was used as a negative control. Whole cell lysates probed with anti-Rac1, anti–Bcl-2, and β-actin are shown on the bottom panel. (C) Endogenous Bcl-2 was pulled down with purified recombinant GST-Rac1 using cell lysates from CEM/Neo, CEM/Bcl-2, Jurkat, and Raji cell lines. The proteins were immunoblotted with anti–Bcl-2 (first panel) and anti-Rac1 (second panel). Pulldown with GST was used as a negative control. (D) Lysates from Jurkat/Neo, Jurkat/Bcl-2, HeLa/Neo, and HeLa/Bcl-2 were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2. (E) Representative confocal microscopy of Bcl-2 expression and Rac1 expression in HeLa/Bcl-2 cells. Rac1 was probed with anti-Rac1 and fluorescein isothiocyanate-conjugated secondary mouse IgG, whereas Bcl-2 was probed with anti–Bcl-2 and rhodamine-conjugated secondary rabbit IgG. The panels were merged using Fluoview (FV10-ASW.1.3 viewer) software to detect areas of colocalization of Rac1 and Bcl-2 proteins. Bar represents 20 μm. All experiments were performed at least 3 or more times independently.
Bcl-2–Rac1 colocalization was further investigated in Bcl-2–overexpressing HeLa cells (HeLa/Bcl-2) using immunofluorescence microscopy. Results confirmed subcellular distribution of these proteins (ie, diffuse and some granular pattern for Rac1) being a cytosolic as well as a membrane protein,25 whereas a distinctly granular pattern for Bcl-2, indicative of its membrane localization26 (Figure 4E). More importantly, merging the images in the overlay panel showed strong colocalization of Rac1 and Bcl-2 (Figure 4E). Taken together, these data further provide evidence for a direct interaction between Rac1 and Bcl-2, which is accentuated in a Bcl-2 expression-dependent manner.
Rac1 is present in mitochondrial membranes and is unchanged on Bcl-2 overexpression
Because Bcl-2 is primarily a mitochondrial protein, we next asked whether Rac1 was also present in mitochondrial membranes. Subcellular fractionation was carried out using CEM and Jurkat cells. To verify purity of mitochondria-enriched fractions (HM) and cytosolic fractions (S), fraction-specific proteins were probed. Voltage-dependent anion channel 1 (VDAC1), a major outer mitochondrial protein was exclusively detected in mitochondrial fractions, whereas copper zinc superoxide dismutase (Cu/Zn SOD), a cytosolic protein, was found in cytosolic fractions (Figure 5A). Bcl-2, being a mitochondrial protein, is retained in HM fractions with an increased expression at mitochondrial membranes of CEM/Bcl-2 and Jurkat/Bcl-2 cells as opposed to CEM/Neo and Jurkat/Neo cells (Figure 5A). Interestingly, we show, for the first time, the presence of Rac1 in mitochondria-enriched fractions (HM), in addition to its cytosolic (S) localization. To confirm these findings, we made use of a rat cell line tailored to specifically express Bcl-2 in mitochondria (Bcl-Acta). Indeed, presence of Bcl-2 as well as Rac1 was clearly shown in the HM fraction of Rat-Acta cells (Figure 5B). To further verify the localization of Rac1 at mitochondrial membranes, electron microscopy was carried out using HeLa/Neo and HeLa/Bcl-2 cells. Electron micrographs clearly showed localization of Rac1 to mitochondrial membranes, which correlated with Bcl-2 expression (Figure 5C). These data clearly demonstrate colocalization of Rac1 and Bcl-2 at mitochondria. It should be pointed out that localization of Rac1 to the mitochondria was also detected in nontransformed fibroblast lines IMR90 and MRC5; however, no detectable Bcl-2 expression was seen (supplemental Figure 4), thereby providing evidence for the presence of Rac1 in mitochondria.
Rac1 localizes to the mitochondrial membranes. (A) Jurkat and CEM cells overexpressing Bcl-2 (30 × 106) were lysed and fractionated into purified heavy membrane (HM) and cytosolic (S) fractions. The fractions were then probed for VDAC1, Bcl-2, Rac1, and Cu/Zn SOD. (B) Rat cell lines overexpressing Bcl-2 targeted to the mitochondria (30 × 106) were lysed and fractionated into purified heavy membrane (HM) and cytosolic (S) fractions. The fractions were then probed for VDAC1, Bcl-2, and Rac1. (C) Representative electron micrographs of HeLa/Neo and HeLa/Bcl-2 cells. The cells were stained with anti-Rac1 and anti–Bcl-2 antibodies and prepared for electron microscopy as described in “Immunochemical staining and transmission electron microscopy.” Arrows point to immunoreactive regions on the mitochondria showing the presence of Rac1 and Bcl-2 on the inner and outer mitochondrial membranes of HeLa/Neo and HeLa/Bcl-2. Bar represents 1 μm. Data presented are representative of at least 3 independent experiments.
Rac1 localizes to the mitochondrial membranes. (A) Jurkat and CEM cells overexpressing Bcl-2 (30 × 106) were lysed and fractionated into purified heavy membrane (HM) and cytosolic (S) fractions. The fractions were then probed for VDAC1, Bcl-2, Rac1, and Cu/Zn SOD. (B) Rat cell lines overexpressing Bcl-2 targeted to the mitochondria (30 × 106) were lysed and fractionated into purified heavy membrane (HM) and cytosolic (S) fractions. The fractions were then probed for VDAC1, Bcl-2, and Rac1. (C) Representative electron micrographs of HeLa/Neo and HeLa/Bcl-2 cells. The cells were stained with anti-Rac1 and anti–Bcl-2 antibodies and prepared for electron microscopy as described in “Immunochemical staining and transmission electron microscopy.” Arrows point to immunoreactive regions on the mitochondria showing the presence of Rac1 and Bcl-2 on the inner and outer mitochondrial membranes of HeLa/Neo and HeLa/Bcl-2. Bar represents 1 μm. Data presented are representative of at least 3 independent experiments.
Rac1-Bcl-2 interaction at mitochondria is enhanced on Bcl-2 overexpression
Based on our findings that Bcl-2 and Rac1 colocalized to mitochondrial membranes, we next set out to investigate whether the interaction occurred predominantly at mitochondria. Therefore, subcellular fractions from CEM/Neo, CEM/Bcl-2, and Raji cells were assessed for specific interaction between Bcl-2 and Rac1. Indeed, Bcl-2 was detected in mitochondria-enriched fractions immunoprecipitated with anti-Rac1, whereas no interaction was observed in cytosolic fractions (Figure 6A-B). Furthermore, the interaction with Rac1 was directly related to Bcl-2 expression levels at mitochondria: minimal in CEM/Neo cells but definitely detectable in both CEM/Bcl-2 and Raji cells that have a much higher mitochondrial Bcl-2 expression. It should be pointed out that Bcl-2 overexpression in CEM cells resulted in a significant Bcl-2 expression in the cytosolic fraction; however, it failed to interact with Rac1. This was explored further using Raji cells that selectively express Bcl-2 in the mitochondria, and results showed no interaction between the 2 proteins in the cytosolic fraction (Figure 6B).
Increased interaction between endogenous Bcl-2 and Rac1 at the mitochondrial membranes. (A) Mitochondria-enriched fractions from CEM/Neo, CEM/Bcl-2, and Raji cells were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2 by Western blotting. The mitochondrial input was probed with anti-VDAC1. (B) Cytosolic fractions from CEM/Neo, CEM/Bcl-2, and Raji cells were immunoprecipitated with anti-Rac1. The proteins were then immunoblotted with anti–Bcl-2 (first panel) and anti-Rac1 (second panel) antibodies. The input shows the presence of Rac1 in the cytosolic fractions of CEM/Neo, CEM/Bcl-2, and Raji cells. (C-D) GST-pulldown was done using mitochondria-enriched (HM) and cytosolic fractions (S) from CEM/Neo, CEM/Bcl-2, Jurkat/Neo, and Jurkat/Bcl-2 using GST-Rac1. The membranes were then immunoblotted with anti-Bak (first panel), anti–Bcl-2 (second panel), and anti-Bax (third panel) antibodies. The input was probed for VDAC1 to show purity of mitochondrial fractions. (E) Lysates from rat cytomegalovirus (CMV; control plasmid) or Bcl-Acta were immunoprecipitated with anti-Rac1 and probed for Bcl-2. Inputs are shown in the right panel. (F) Rat CMV or Bcl-Acta cells were loaded with Mitotracker and anti–Bcl-2 or anti-Rac1 as described in “Immunofluorescence by confocal microscopy” and analyzed for colocalization by fluorescence confocal microscopy. Data presented are representative of at least 3 independent experiments.
Increased interaction between endogenous Bcl-2 and Rac1 at the mitochondrial membranes. (A) Mitochondria-enriched fractions from CEM/Neo, CEM/Bcl-2, and Raji cells were immunoprecipitated with anti-Rac1 and probed with anti–Bcl-2 by Western blotting. The mitochondrial input was probed with anti-VDAC1. (B) Cytosolic fractions from CEM/Neo, CEM/Bcl-2, and Raji cells were immunoprecipitated with anti-Rac1. The proteins were then immunoblotted with anti–Bcl-2 (first panel) and anti-Rac1 (second panel) antibodies. The input shows the presence of Rac1 in the cytosolic fractions of CEM/Neo, CEM/Bcl-2, and Raji cells. (C-D) GST-pulldown was done using mitochondria-enriched (HM) and cytosolic fractions (S) from CEM/Neo, CEM/Bcl-2, Jurkat/Neo, and Jurkat/Bcl-2 using GST-Rac1. The membranes were then immunoblotted with anti-Bak (first panel), anti–Bcl-2 (second panel), and anti-Bax (third panel) antibodies. The input was probed for VDAC1 to show purity of mitochondrial fractions. (E) Lysates from rat cytomegalovirus (CMV; control plasmid) or Bcl-Acta were immunoprecipitated with anti-Rac1 and probed for Bcl-2. Inputs are shown in the right panel. (F) Rat CMV or Bcl-Acta cells were loaded with Mitotracker and anti–Bcl-2 or anti-Rac1 as described in “Immunofluorescence by confocal microscopy” and analyzed for colocalization by fluorescence confocal microscopy. Data presented are representative of at least 3 independent experiments.
To confirm these findings, we made use of GST-Rac1 pulldown system using mitochondria-enriched (HM) and cytosolic (S) fractions from CEM/Bcl-2 and Jurkat/Bcl-2 cells. Results showed that GST-Rac1 was able to pull down Bcl-2 in HM fractions from both cell lines (Figure 6C-D). Furthermore, this interaction was specific to Bcl-2, as 2 proapoptotic Bcl-2 family proteins Bak and Bax did not show binding to GST-Rac1. Similar experiments were performed using Bcl-Acta rat cell line tailored to specifically express Bcl-2 in the mitochondria. First, we show that Bcl-2 coimmunoprecipitated with Rac1 in Bcl-Acta cell lysate (Figure 6E). Second, fluorescence confocal microscopy using a mitochondrial specific probe, MitoTracker Red, showed colocalization of Bcl-2 at mitochondria in Bcl-Acta cells (Figure 6F). More importantly, Rac1 colocalized with Bcl-2 at mitochondria (Figure 6F), thus providing further evidence for the presence of Rac1 at mitochondria as well as its interaction with mitochondrial targeted Bcl-2.
Bcl-2–Rac1 interaction shows a BH3 domain dependency in vitro and in vivo
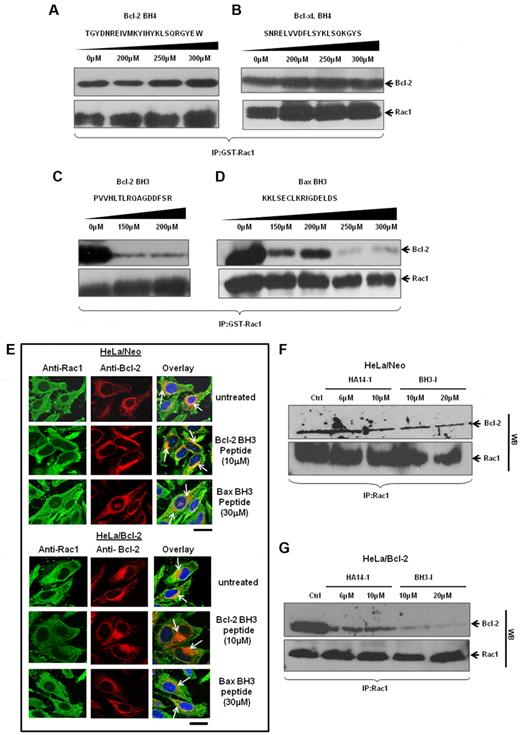
Thus far, our data provide strong evidence for a direct interaction between Rac1 and Bcl-2, especially at mitochondria. As Bcl-2 family proteins harbor specific homology domains, the BH domains, for interactions with proteins within and outside of the family, we next set out to identify Rac1 interacting domain(s) within Bcl-2. To do that, synthetic peptides matching BH3 domain (Bcl-2 or Bax) or BH4 domain (Bcl-2 or Bcl-xL) were obtained and used for in vitro GST-pulldown assays involving GST-Rac1 and Bcl-2. Results showed that incubation with up to 300μM Bcl-2 BH4 or Bcl-xL BH4 peptide did not disturb the interaction between Rac1 and Bcl-2 (Figure 7A-B). However, incubation with either Bcl-2 BH3 or Bax BH3 peptide27,28 significantly blocked the ability of GST-Rac1 to pull down Bcl-2 (Figure 7C-D). Because the in vitro data implicated Bcl-2 BH3 domain in its interaction with Rac1, we further assessed involvement of BH3 domain in vivo using TAT-tagged Bcl-2 BH3 or Bax BH3 peptides (TAT sequence enables BH3 peptides to be cell-permeable29 ). HeLa/Neo and HeLa/Bcl-2 cells were preincubated with respective BH3 peptides and subjected to confocal microscopy. Immunofluorescence staining of HeLa/Neo cells showed slight colocalization of Rac1 and Bcl-2 because of the lower Bcl-2 expression in these cells, which was minimally affected by 10μM TAT–Bcl-2 BH3 or 30μM TAT-Bax BH3 peptide (Figure 7E). In contrast, TAT–Bcl-2 BH3 peptide significantly inhibited the strong colocalization of Bcl-2 with Rac1 in HeLa/Bcl-2 cells, whereas a moderate inhibitory effect was observed with TAT-Bax BH3 peptide (Figure 7F). The fact that the BH4 peptide blocked the interaction between Ras and Bcl-2 without affecting Rac1–Bcl-2 interaction (supplemental Figure 5) argues in favor of a BH3 domain dependence of this interaction. These data indicate that Bcl-2 BH3 domain appears to be necessary for Bcl-2–Rac1 interaction. To substantiate these findings, HeLa/Neo and HeLa/Bcl-2 cells were treated with various doses of 2 well-known pharmacologic inhibitors of Bcl-2, HA14-I30 or BH3-I.31 These inhibitors bind to BH3 domain of Bcl-2 and inhibit its antiapoptotic function by displacing Bax and BH3 proapoptotic molecules. Indeed, preincubation of cells with Bcl-2 inhibitors before immunoprecipitation showed the ability of both HA14-1 and BH3-I to block the interaction (Figure 7G). This inhibitory effect was also more pronounced in Bcl-2–overexpressing cells. We also provide evidence that this effect of Bcl-2 inhibitors on Bcl-2–Rac1 interaction was not a function of a change in the expression of either Rac1 or Bcl-2 (supplemental Figure 6). These data provide strong experimental evidence for a Bcl-2 BH3 domain dependency of Bcl-2–Rac1 interaction.
Rac1 and Bcl-2 interaction shows a BH3 domain dependency in vitro and in vivo. (A-D) The effect of increasing concentrations of synthetic peptides spanning the BH3 and BH4 domains on the ability of GST-Rac1 to capture Bcl-2 was assessed using Western blotting. Only BH3 peptides inhibited the interaction between GST-Rac1 and Bcl-2. Membranes were also probed with anti-Rac1 to show equal loading of GST-Rac1 for all the Western blots. (E) Representative confocal microscopy images of Rac1 and Bcl-2 expression in HeLa/Neo and HeLa/Bcl-2 cells incubated with various doses of TAT-tagged Bcl-2 BH3 and Bax BH3 peptides for 2 hours. The green fluorescence indicates Rac1; and red fluorescence, Bcl-2. The nucleus was stained with Hoechst. Areas of orange fluorescence indicate strong colocalization of Rac1 and Bcl-2. Bar represents 20 μm. (F-G) HeLa/Neo and HeLa/Bcl-2 cells were incubated with various doses of HA14-I and BH3-I for 4 hours and lysates were harvested for coimmunoprecipitation with anti-Rac1 antibodies. The first panel was immunoblotted with anti–Bcl-2, and the second panel was immunoblotted with anti-Rac1 to show equal loading of Rac1. Data presented are representative of at least 3 independent experiments.
Rac1 and Bcl-2 interaction shows a BH3 domain dependency in vitro and in vivo. (A-D) The effect of increasing concentrations of synthetic peptides spanning the BH3 and BH4 domains on the ability of GST-Rac1 to capture Bcl-2 was assessed using Western blotting. Only BH3 peptides inhibited the interaction between GST-Rac1 and Bcl-2. Membranes were also probed with anti-Rac1 to show equal loading of GST-Rac1 for all the Western blots. (E) Representative confocal microscopy images of Rac1 and Bcl-2 expression in HeLa/Neo and HeLa/Bcl-2 cells incubated with various doses of TAT-tagged Bcl-2 BH3 and Bax BH3 peptides for 2 hours. The green fluorescence indicates Rac1; and red fluorescence, Bcl-2. The nucleus was stained with Hoechst. Areas of orange fluorescence indicate strong colocalization of Rac1 and Bcl-2. Bar represents 20 μm. (F-G) HeLa/Neo and HeLa/Bcl-2 cells were incubated with various doses of HA14-I and BH3-I for 4 hours and lysates were harvested for coimmunoprecipitation with anti-Rac1 antibodies. The first panel was immunoblotted with anti–Bcl-2, and the second panel was immunoblotted with anti-Rac1 to show equal loading of Rac1. Data presented are representative of at least 3 independent experiments.
As the BH3 domain of Bcl-2 is also involved in sequestering of proapoptotic proteins, such as Bax, we investigated whether the interaction of Rac1 with Bcl-2 could compromise the ability of Bcl-2 to bind Bax. Here we show that a mutant (T69A/S70A/S87A) of Bcl-2 was unable to interact with Rac1 while retaining the ability to bind Bax (supplemental Figure 7). These data indicate that Bcl-2 engages a region within the BH3 domain or adjacent loop region to bind Rac1; however, the hydrophobic groove that binds Bax remains unaffected.
Bcl-2 BH3 peptide and functional inhibition or silencing of Rac1 decrease mitochondrial O2∸ levels and enhance apoptosis sensitivity
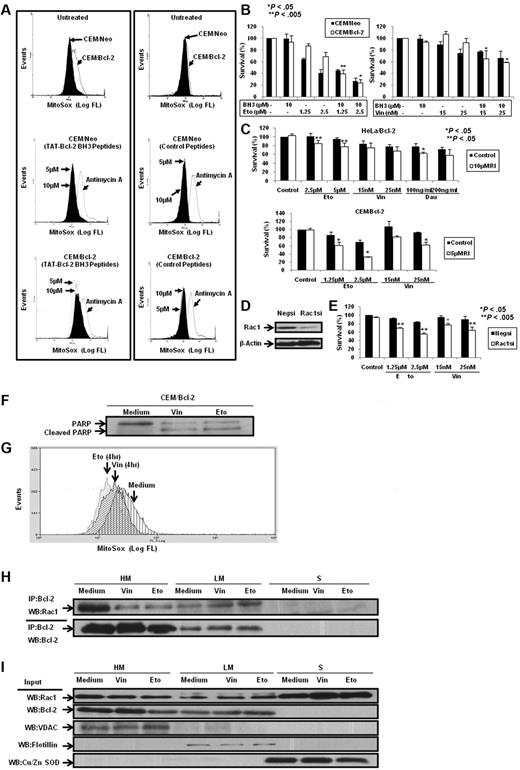
Having demonstrated that BH3 peptides and Bcl-2 inhibitors disrupted Bcl-2–Rac1 interaction in Bcl-2–overexpressing cells in vitro and in vivo, we next asked whether inhibition of this interaction affected Bcl-2-induced increase in mitochondrial O2∸. To test this, CEM/Neo and CEM/Bcl-2 cells were incubated with TAT-Bcl-2 BH3 peptides and mitochondrial O2∸ was measured using MitoSOX. Consistent with our published findings7,18 and data shown in this report, overexpression of Bcl-2 resulted in a significant increase in mitochondrial O2∸ production, which was unaffected by the control (unrelated to BH3) peptide. However, incubation with TAT-Bcl-2 BH3 peptide significantly reduced mitochondrial O2∸ levels in CEM/Bcl-2 cells but not in CEM/Neo cells (Figure 8A). Similar results were obtained with HeLa/Bcl-2 cells (data not shown).
Bcl-2 BH3 peptides and silencing or functional inhibition of Rac1 inhibit intramitochondrial O2∸ levels and enhance sensitivity in Bcl-2-overexpressing cells to chemotherapeutic agents. (A) CEM/Neo and CEM/Bcl-2 cells were incubated with various doses of Bcl-2 BH3 peptides and control peptides for 2 hours. MitoSOX dye was used to assess mitochondrial O2∸ levels as described in “Determination of O2∸.” (B) CEM/Neo and CEM/Bcl-2 cells were preincubated for 1 hour with 10μM Bcl-2 BH3 peptide followed by a 24-hour incubation with various doses of etoposide (Eto) or vincristine (Vin). Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide assay as described in “Determination of cell viability.” Data are mean ± SD of at least 3 independent experiments performed in triplicate. P < .05 and P < .005 compared with the drug treatment alone. (C) HeLa/Bcl-2 and CEM/Bcl-2 cells were preincubated with Rac1 inhibitor (RI) for 1 hour followed by 24-hour incubation with various doses of etoposide (Eto), vincristine (Vin), or daunorubicin (Dau). Cell viability was assessed as described in “Determination of cell viability.” Data are mean ± SD of at least 3 independent experiments performed in triplicate. P < .05 and P < .005 compared with the drug treatment alone. (D) Western blot analysis of Rac1 and β-actin expression in HeLa/Bcl-2 cells transfected with nonspecific scrambled siRNA (Negsi) or siRNA against Rac1 (Rac1si). (E) HeLa/Bcl-2 cells silenced with nonspecific scrambled siRNA (Negsi) or siRNA against Rac1 (Rac1si) were treated with various doses of etoposide (Eto) or vincristine (Vin) for 24 hours. Cell viability was assessed by the crystal violet assay as described in “Determination of cell viability.” Data are mean ± SD of at least 3 independent experiments performed in triplicate. P < .005 and P < .05 compared with the drug treatment alone. (F) Western blot analysis for poly(ADP-ribose) polymerase (PARP; both full length and cleaved) on exposure to 50nM vincristine (Vin) or 5μM etoposide (Eto) was performed to show the proapoptotic phenotypes of CEM/Bcl-2 cells. (G) Intramitochondrial O2∸ levels were determined following cell loading with MitoSOX as described in “Determination of O2∸.” The histograms are representative of 3 independent experiments. (H) CEM cells overexpressing Bcl-2 (60 × 106) were treated with 50nM vincristine (Vin) or 5μM etoposide (Eto) for 16 hours, lysed, and fractionated into purified heavy membrane (HM), LM, and cytosolic (S) fractions. The fraction lysates were then immunoprecipitated with anti-Bcl-2 and probed with anti-Rac1. (I) The fraction lysates were probed for Rac1, Bcl-2, VDAC1, Flotillin, and Cu/Zn SOD.
Bcl-2 BH3 peptides and silencing or functional inhibition of Rac1 inhibit intramitochondrial O2∸ levels and enhance sensitivity in Bcl-2-overexpressing cells to chemotherapeutic agents. (A) CEM/Neo and CEM/Bcl-2 cells were incubated with various doses of Bcl-2 BH3 peptides and control peptides for 2 hours. MitoSOX dye was used to assess mitochondrial O2∸ levels as described in “Determination of O2∸.” (B) CEM/Neo and CEM/Bcl-2 cells were preincubated for 1 hour with 10μM Bcl-2 BH3 peptide followed by a 24-hour incubation with various doses of etoposide (Eto) or vincristine (Vin). Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide assay as described in “Determination of cell viability.” Data are mean ± SD of at least 3 independent experiments performed in triplicate. P < .05 and P < .005 compared with the drug treatment alone. (C) HeLa/Bcl-2 and CEM/Bcl-2 cells were preincubated with Rac1 inhibitor (RI) for 1 hour followed by 24-hour incubation with various doses of etoposide (Eto), vincristine (Vin), or daunorubicin (Dau). Cell viability was assessed as described in “Determination of cell viability.” Data are mean ± SD of at least 3 independent experiments performed in triplicate. P < .05 and P < .005 compared with the drug treatment alone. (D) Western blot analysis of Rac1 and β-actin expression in HeLa/Bcl-2 cells transfected with nonspecific scrambled siRNA (Negsi) or siRNA against Rac1 (Rac1si). (E) HeLa/Bcl-2 cells silenced with nonspecific scrambled siRNA (Negsi) or siRNA against Rac1 (Rac1si) were treated with various doses of etoposide (Eto) or vincristine (Vin) for 24 hours. Cell viability was assessed by the crystal violet assay as described in “Determination of cell viability.” Data are mean ± SD of at least 3 independent experiments performed in triplicate. P < .005 and P < .05 compared with the drug treatment alone. (F) Western blot analysis for poly(ADP-ribose) polymerase (PARP; both full length and cleaved) on exposure to 50nM vincristine (Vin) or 5μM etoposide (Eto) was performed to show the proapoptotic phenotypes of CEM/Bcl-2 cells. (G) Intramitochondrial O2∸ levels were determined following cell loading with MitoSOX as described in “Determination of O2∸.” The histograms are representative of 3 independent experiments. (H) CEM cells overexpressing Bcl-2 (60 × 106) were treated with 50nM vincristine (Vin) or 5μM etoposide (Eto) for 16 hours, lysed, and fractionated into purified heavy membrane (HM), LM, and cytosolic (S) fractions. The fraction lysates were then immunoprecipitated with anti-Bcl-2 and probed with anti-Rac1. (I) The fraction lysates were probed for Rac1, Bcl-2, VDAC1, Flotillin, and Cu/Zn SOD.
Our earlier findings have highlighted the critical role of a slight pro-oxidant state in tumor cell resistance to apoptotic stimuli, including the death inhibitory activity of Bcl-2.7,8 Prompted by the ability of TAT-Bcl-2 BH3 peptide to decrease mitochondrial O2∸ in Bcl-2–overexpressing cells, we next investigated whether this decrease in mitochondrial O2∸ could over-ride death inhibitory activity of Bcl-2. To do so, cells were exposed to the commonly used chemotherapeutic agent, etoposide, in the presence or absence of TAT-Bcl-2 BH3 peptide. Indeed, 10μM TAT-Bcl-2 BH3 peptide significantly (P < .005) increased sensitivity of CEM/Bcl-2 cells to low-dose exposure (1.25μM) of etoposide that does not by itself affect cell viability in Bcl-2–overexpressing cells (Figure 8B). A similar effect of TAT-Bcl-2 BH3 peptide was observed on vincristine-induced apoptosis of CEM/Neo and CEM/Bcl-2 cells (Figure 8B). Bcl-2 BH3 peptide disrupted Bcl-2–Rac1 interaction in the sensitization experiments (data not shown). It should be noted that, unlike TAT-Bax BH3 peptide, the TAT-Bcl-2 BH3 peptide did not affect cell viability on its own (supplemental Figure 8A-B). Peptide with protein sequence of no homology to human sequence was also included to serve as a negative control, and exposure to this nonspecific peptide did not affect cellular response to etoposide or vincristine (supplemental Figure 9A-B). Furthermore, the effect of the BH3 peptide on subcellular localization of the Bcl-2–Rac1 complex was also studied. Results indicate that BH3 peptide significantly reduced the interaction at the mitochondria, whereas the complex as well as Rac1 localized to the plasma membrane-enriched fraction in CEM/Bcl-2 cells (supplemental Figure 10A-B).
In addition, we also investigated the effect of either inhibiting the activity of Rac1 or silencing the expression of Rac1 on the sensitivity of Bcl-2–overexpressing cells to vincristine, etoposide, or daunorubicin. Results show that pharmacologic inhibition of Rac1 activity or siRNA-mediated gene knockdown of Rac1 significantly increased the sensitivity of Bcl-2–overexpressing cells to these drugs (Figure 8C-E).
Having shown that the disruption of the interaction between Bcl-2 and Rac1 sensitized Bcl-2–overexpressing cells to apoptotic stimuli, we next asked whether the cellular localization of this complex was altered in cells undergoing apoptosis. To do so, we used concentrations of vincristine and etoposide that generated an apoptotic phenotype in CEM/Bcl-2 cells (Figure 8F) and assessed the effect on intramitochondrial O2∸ and subcellular localization of the complex. Interestingly, exposure to apoptotic concentrations of vincristine or etoposide significantly reduced intramitochondrial O2∸ (Figure 8G) as well as the interaction in the mitochondria-enriched fractions (HM), whereas a reciprocal increase was detected in the light membrane fractions (LM; Figure 8H). In addition, we observed an increase in the plasma membrane localization of Rac1 in cells exposed to vincristine and etoposide (Figure 8I).
Finally, intrigued by the effect of inhibiting the interaction between Bcl-2 and Rac1 on intracellular O2∸, we asked whether the interaction itself was dependent on NADPH oxidase activity. We observed a significant decrease in the interaction at the mitochondria (HM) in cells on exposure to the pharmacologic inhibitor of NADPH oxidase diphenyleneiodonium and an increase in the complex at the LM (supplemental Figure 11A). Interestingly, we also see an increased relocalization of Rac1 to the LM fractions in cells on inhibition of the NADPH oxidase (supplemental Figure 11B). As for the localization of the NADPH oxidase subunits to the mitochondria, there was no detectable expression of the p47phox or p91phox; however, weak expression of Nox4 was observed in the mitochondria of CEM/Bcl-2 cells (supplemental Figure 11C).
Discussion
We have previously shown that Bcl-2 expression in tumor cells resulted in a pro-oxidant intracellular milieu, in particular an increase in intracellular O2∸, and linked this to the antiapoptotic activity of Bcl-2.7 In further support of this, we recently reported that pro-oxidant activity of Bcl-2 was a function of an increase in mitochondrial oxygen consumption and cytochrome c oxidase activity with evidence for a better cytochrome c oxidase assembly in Bcl-2–overexpressing cells.18,19 In an effort to understand the regulation of cellular redox status by Bcl-2, we were intrigued by our earlier findings that transfection with a dominant negative small GTPase Rac1 (RacN17) overcame Bcl-2-mediated resistance to apoptosis in human leukemia cells.7 Corroborating that, here we report that siRNA-mediated gene silencing of Rac1 or its pharmacologic inhibition abrogated the increase in intracellular as well as intramitochondrial O2∸ in Bcl-2-overexpressing cells. Of particular note, we provide strong evidence using a variety of experimental strategies to support physical interaction between Rac1 and Bcl-2. This novel interaction was observed in primary tissues derived from B-cell lymphoma patients and a variety of human tumor cell lines overexpressing Bcl-2, thereby suggesting a functional significance in vivo. Interestingly, increased expression of Bcl-2 did not change subcellular localization of Rac1, which was detected in outer mitochondrial membrane and colocalized with Bcl-2. This is consistent with the findings that Bcl-2 is known to translocate Raf kinase to mitochondria,13 and also binds to activated Ras in the mitochondria.11 Of note, similar to what was shown in the report describing interaction of Bcl-2 with N-Ras,32 our data indicate that Bcl-2 expression level is the rate-limiting step in the interaction with Rac1.
Bcl-2-BH3 domain is important for interaction with Rac1
Rac1 is a regulatory component of the plasma membrane NADPH oxidases in phagocytic cells.33 In nonphagocytic counterparts, Rac1 is known to activate NADPH oxidase-like complexes triggering reactive oxygen species production. Interestingly, localization of Rac1 to mitochondria-enriched fractions was previously reported; however, unlike its role at plasma membrane, its function in the mitochondria has not been extensively studied.34,35 To that end, a recent report suggested a link between activated Rac1, mitochondrial oxidative stress, and disruption of mitochondrial transmembrane potential,36 and a second report demonstrated that inhibition of Rac1 in neuronal cells triggered Bim-mediated mitochondrial apoptosis.37 These reports provide evidence to the indirect impact of Rac1 at mitochondria, through second messengers, such as ceramide or JNK. Our report describes a novel binding partner of Rac1 in the mitochondria and provides evidence for a functional relevance in transformed cells.
Despite the relatively high degree of structural similarity between various members of Bcl-2 family, and functional redundancy within the antiapoptotic group, interaction of Rac1 was exclusive to Bcl-2; Rac1 did not coprecipitate with Bcl-xL or Bax. The 3-dimensional structures of Bcl-2 and Bcl-xL have been elucidated.38,39 Primary sequences of Bcl-2 and Bcl-xL are 60% homologous; however, differences in the amino acid residues could account for the topologic as well as electrostatic differences between Bcl-2 and Bcl-xL, which might explain the specificity of Rac1 for Bcl-2.22 From another standpoint, the fact that Rac1 belongs to the Ras superfamily and that a number of the Ras and Rho members interact with Bcl-2 suggest a degree of conservation with probable functional relevance and or redundancy.
In our quest for the functional domain within Bcl-2 responsible for interaction with Rac1, we made use of a number of synthetic peptides targeting various BH domains within Bcl-2. Synthetic peptides of BH3 domain of Bcl-2 strongly disrupted the interaction between recombinant Bcl-2 and Rac1. This is rather interesting as BH3 domain is usually used for interaction with members of the proapoptotic Bcl-2 family, such as Bax. However, here we provide evidence that a mutant of Bcl-2 (AAA) retained the ability to bind Bax but completely lost the ability to interact with Rac1. These data suggest a scenario whereby a different region around the BH3 domain (such as the loop region) might play a critical role in the interaction with Rac1, whereas the hydrophobic groove still retains Bax binding activity. The in vitro peptide inhibition results were further corroborated by tracking the interaction in vivo using Bcl-2 BH3 and Bax BH3 peptides that were fused to protein transduction domain (TAT) of HIV. Indeed, cell-permeable TAT-tagged Bcl-2 BH3 peptides efficiently inhibited Bcl-2–Rac1 interaction in Bcl-2–overexpressing cells displacing Rac1 from Bcl-2. Furthermore, BH3 mimetics, such as HA14-140 and BH3-I,31 yielded similar results, thus strongly implicating the BH3 domain in Bcl-2–Rac1 interaction. It should be pointed out that the earlier report describing interaction of Bcl-2 with Ras had identified BH4 domain as the interacting motif within Bcl-2.11 We do not see an effect of BH4 targeted peptides on Bcl-2–Rac1 interaction, whereas the interaction between Ras and Bcl-2 is inhibited. It is highly probable that, despite the homology between Rac1 and Ras family, the presence of the additional insert region (amino acids 124-135) that is distinct from the Ras family41 may contribute to this domain specific interaction with Bcl-2.
BH3 domain is critical in pro-oxidant activity of Bcl-2
We have previously shown a novel mechanism of death inhibition by Bcl-2 implicating its ability to create a slight pro-oxidant intracellular milieu vis-à-vis O2∸ anion,7 and more recently linked this activity to an effect on mitochondrial respiration and O2∸ production.18,19 Having observed a strong Bcl-2–Rac1 interaction involving the BH3 domain, it was logical to investigate whether inhibiting this domain-specific interaction could alleviate the mitochondrial pro-oxidant activity of Bcl-2. Indeed, Bcl-2 BH3 peptides not only disrupted Bcl-2–Rac1 interaction but also significantly inhibited the increase in mitochondrial O2∸ affected by Bcl-2 overexpression. These data provide 2 important clues vis-à-vis the relevance of BH3 domain-mediated interaction with Rac1. First, the inhibitory effect of BH3 synthetic peptides on the interaction at mitochondria suggests that Bcl-2 BH3 domain is critical in the interaction with Rac1. Second, inhibition of Bcl-2–mediated increase in mitochondrial O2∸ provides evidence for the involvement of BH3 domain in pro-oxidant activity of Bcl-2, and in this regard the functional relevance of Rac1, being an important component of oxidase assembly and activation, becomes critical. In light of recent reports demonstrating that BH3 mimetics decreased oxygen consumption in Bcl-2–overexpressing cells,18,42 the functional relevance of Bcl-2–Rac1 interaction within the context of mitochondrial bioenergetics is plausible and remains a focus of our ongoing investigations.
Bcl-2–Rac1 interaction could be an important therapeutic target
Bcl-2 is overexpressed in 80% of follicular lymphomas,43 and is a major hindrance in therapeutic management of the disease. Having established that Bcl-2 interacted with Rac1 and that this interaction could be the mechanism underlying the pro-oxidant activity of Bcl-2, we set out to investigate functional relevance of this vis-à-vis the antiapoptotic activity of Bcl-2. To that end, we present evidence that interaction between Bcl-2 and Rac1 may indeed be an important determinant in the death inhibitory activity of Bcl-2 as Bcl-2 BH3 synthetic peptide as well as the functional inhibition or silencing of Rac1-restored death signaling in response to the conventionally used chemotherapeutic agents vincristine, etoposide, or daunorubicin in Bcl-2-overexpressing cells. It should be pointed out that this was not a function of nonspecific cytotoxicity of Bcl-2 BH3 peptide that, unlike Bax BH3 peptide, had no effect of its own without an apoptotic trigger. To further assess the clinical relevance of these data, we tested the hypothesis that Bcl-2 overexpression in clinical settings should be associated with a significantly increased colocalization of and interaction between Bcl-2 and Rac1. Indeed, data from patients with diffuse B-cell lymphoma, head and neck carcinoma, and follicular lymphoma provide strong evidence for the presence of Bcl-2–Rac1 interaction as well as colocalization, whereas such interaction was not detected in noncancerous tissues/cells. Although the clinical data are from a small number of primary samples, the strong association of Bcl-2 overexpression with its ability to interact with Rac1 provides support to a functional relevance of this interaction from the standpoint of Bcl-2 biology. By implication, this interaction presents a novel drug target for cancers where Bcl-2 overexpression presents a therapeutic challenge.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Charanjit Kaur, Professor Bay Boon Huat, and Chan Yee Gek from the Department of Anatomy, National University of Singapore, for rendering generous technical assistance and advisory support.
This work was supported by the Biomedical Research Council and the National University of Singapore Academic Research Fund (S.P.) and the National Medical Research Council (program grant; S.P., M.-V.C.).
Authorship
Contribution: R.V. and J.K. performed the experiments and wrote part of the manuscript; J.L.H. performed the clinical sample analysis and work; T.L. provided the clinical materials and surgical specimen; B.C.G. provided clinical material and partial funding for the work; M.L. helped in the generation of the GST-fusion constructs and recombinant proteins; C.B. helped in the generation of the recombinant proteins and TAT-tagged BH peptides; M.-V.C. provided generation of the recombinant proteins and critical comments and wrote the manuscript; and S.P. served as the lead principal investigator, conceived the study, provided funding for the work, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shazib Pervaiz, Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, 2 Medical Dr, Building MD9 #01-05, Singapore 117597; e-mail: phssp@nus.edu.sg.
References
Author notes
R.V. and J.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal