Abstract
B-cell receptor (BCR) signaling is aberrantly activated in chronic lymphocytic leukemia (CLL). Bruton tyrosine kinase (BTK) is essential to BCR signaling and in knockout mouse models its mutation has a relatively B cell–specific phenotype. Herein, we demonstrate that BTK protein and mRNA are significantly over expressed in CLL compared with normal B cells. Although BTK is not always constitutively active in CLL cells, BCR or CD40 signaling is accompanied by effective activation of this pathway. Using the irreversible BTK inhibitor PCI-32765, we demonstrate modest apoptosis in CLL cells that is greater than that observed in normal B cells. No influence of PCI-32765 on T-cell survival is observed. Treatment of CD40 or BCR activated CLL cells with PCI-32765 results in inhibition of BTK tyrosine phosphorylation and also effectively abrogates downstream survival pathways activated by this kinase including ERK1/2, PI3K, and NF-κB. In addition, PCI-32765 inhibits activation-induced proliferation of CLL cells in vitro, and effectively blocks survival signals provided externally to CLL cells from the microenvironment including soluble factors (CD40L, BAFF, IL-6, IL-4, and TNF-α), fibronectin engagement, and stromal cell contact. Based on these collective data, future efforts targeting BTK with the irreversible inhibitor PCI-32765 in clinical trials of CLL patients is warranted.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent adult leukemia with an immunophenotype expressing the T-cell marker CD5 together with CD19, CD20, CD23, and dim-surface immunoglobulin.1 Although immunophenotypically similar to the normal B1 lymphocytes, CLL cells have a distinct mRNA gene expression profile that most approximates a postgerminal memory B cell.2 For many years CLL has been viewed as a nonproliferating leukemia based on the nonproliferating blood compartment; however, as with normal B cells, it has come to be recognized that CLL cell proliferation probably occurs in sites where microenvironmental stimulation occurs such as the lymph nodes and spleen. In such sites, proliferation centers are observed with a high proportion of dividing CLL cells expressing survivin that are often surrounded by either T cells or accessory stromal cells capable of providing cytokine costimulation.3,4 Studies administering heavy water allow accurate measurement of all body compartments of CLL and assess the birth rate of CLL tumor cells in vivo.5 These studies have demonstrated a broad range of proliferation of CLL cells that varies based on disease state and also immunoglobulin heavy chain variable (IVGH) mutational status.5,6 In particular, a higher tumor birth rate is noted in CLL patients with IVGH unmutated disease and ZAP-70 expression. Multiple studies have documented evidence of enhanced B-cell receptor (BCR) signaling in patients with IVGH unmutated disease or those with increased ZAP-70 expression.7-9 Thus, accessory cytokines, cell-cell contact in the microenvironment, and also BCR-signaling coupled to B-cell proliferation appear sentinel to CLL progression and pathogenesis.
While understanding of CLL biology has improved dramatically, until very recently integration of these findings to treatment interventions has been lacking. Specifically, treatment has included alkylators, nucleoside analogs, and their combination where small advances in improved response and progression-free survival (PFS) have been noted.10,11 However, these therapies have had very little impact on overall survival of CLL. The addition of the chimeric CD20 antibody, rituximab, perhaps represents the most significant advance in CLL therapy. Rituximab single agent activity12 and phase 2 studies combining it with fludarabine (FR) or fludarabine and cyclophosphamide (FCR) have demonstrated improved overall survival (OS) over historical controls.13,14 A randomized trial of FCR versus fludarabine or cyclophosphamide alone15 demonstrated significant improvement in response; PFS and OS. While the presumptive mechanism of rituximab in CLL has been assumed to be immunologic (reviewed in Jaglowski and Byrd16 ), a recent study demonstrated a direct effect on BCR-signaling in both normal and malignant B cells via perturbation of membrane rafts by CD20 antibody engagement.17 Given the survival benefit of rituximab as part of chemoimmunotherapy in CLL, this provides even more evidence for therapeutics directed at BCR-signaling and the proliferating component of CLL promoted by cytokines and cell-cell contact in the microenvironment.
Targeting different components of the BCR pathway using pharmacologic agents can occur through a variety of different pathways including inhibition of proximal kinases such as Lyn,18,19 Syk,20-22 and PI3K23,24 that each are constitutively active in CLL. Inhibition of both Syk21 and the PI3K pathway24,25 prevents CLL cells from interacting with the microenvironment and inhibition of Lyn,18 Syk20-22 and PI3K23-25 all promote proapoptotic signals. Clinical use of both the Syk inhibitor fostamatinib disodium26 and the PI3K-δ isoform specific inhibitor CAL-10127 have shown clinical activity in refractory CLL. Given the success of therapeutic agents targeting BCR, identification of a proximal downstream kinase involved in both BCR and CLL proliferation induced by microenvironmental cytokines and cellular contact would offer the potential to deliver more selective therapy.
Bruton tyrosine kinase (BTK) is a member of the Tec family kinases with a well-characterized role in BCR-signaling and B-cell activation. BTK is activated upstream by Src-family kinases and leads to downstream activation of essential cell survival pathways such as NF-κB and MAPK. Although BTK is expressed in multiple hematopoietic cells, the primary defect in knockout mice is B cell–specific, suggesting a more selective B-cell function. BTK mutations in humans give rise to X-linked agammaglobulinemia, an inherited disorder that is characterized by severe B cell–specific defects including severely decreased levels of immunoglobulin production and the absence of B cells; further suggesting the importance and selectivity of BTK to B cells. BTK was recently identified in a siRNA screen as an essential kinase for survival in a subset of diffuse large-cell lymphomas driven by activated BCR where an irreversible BTK inhibitor, PCI-32765, was shown to promote apoptosis.28 A second study of PCI-32765 recently noted in vivo clinical responses in dogs with aggressive B-cell lymphomas.29
Based on the promise of BTK in aggressive lymphoma and the importance of BCR-signaling in CLL, we hypothesized that the BTK inhibitor PCI-32765 would have multiple influences on disrupted apoptosis, proliferation, and microenvironment stimuli. Herein, we describe a detailed study demonstrating that PCI-32765 promotes apoptosis, inhibits proliferation, and also prevents CLL cells from responding to survival stimuli provided by the microenvironment. Collectively, these studies provide significant support for development of PCI-32765 as a therapeutic agent for the treatment of CLL and related diseases.
Methods
Patient sample processing and cell culture
Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board (IRB) of The Ohio State University (OSU; Columbus, OH). All patients examined in this series had immunophenotypically defined CLL30 and had been without prior therapy for a minimum of 30 days. CLL cells were isolated using ficoll density gradient centrifugation (Ficoll-Plaque Plus; Amersham Biosciences). Enriched CLL fractions were prepared using the Rosette-Sep kit from StemCell Technologies according to the manufacturer's instructions. Isolated cells were incubated in RPMI 1640 media supplemented with 10% heat-inactivated human serum (HS; Valley Biomedical), 2mM l-glutamine (Invitrogen), and 100 U/mL penicillin/100 μg/mL streptomycin (Sigma-Aldrich) at 37°C in an atmosphere of 5% CO2. The purity of enriched populations of CLL was routinely checked using CD19-phycoerthrin (PE) staining by flow cytometry. Normal cells were obtained from Red Cross partial leukocyte preparations, and B cells or T cells were negatively selected using the appropriate Rosette-Sep kits. The Hs5 cell line was obtained from ATCC.
Reagents and antibodies
PCI-32765 was provided by Pharmacyclics. PE-labeled isotype control mouse IgG1, CD19, CD3, FITC-labeled annexin-V and propidium iodide (PI) were purchased from BD Pharmingen. Human IL-6, IL-10, and TNF-α Quantikine ELISA Kits, z-VAD-fmk, rhIL-4, rhIL-6, and rhBAFF were purchased from R&D Systems. Recombinant human soluble CD40L was purchased from PeproTech. Ac-DEVD-AFC was purchased from Enzyme Systems Products. Anti-CD3 T-cell activation plates and fibronectin coated multiwell plates were purchased from BD Biosciences. TNF-α was purchased from Sigma-Aldrich. CpG685 was obtained from the OSU Cytogenetics Core Lab. 4G10 agarose conjugated IP beads were purchased from Millipore.
Immunoblot analysis
Whole cell extracts were prepared as previously described by our group with the addition of phosphatase inhibitor cocktail 1 and 2, protease inhibitor cocktail P8340 and 1mM phenylmethylsulfonyl fluoride (all from Sigma-Aldrich) to the lysis buffer.31 Equivalent amounts of protein were separated on polyacrylamide gels and transferred onto nitrocellulose membranes. After antibody incubations, proteins were detected with chemiluminescent substrate (SuperSignal; Pierce). The following antibodies were used for detection, anti-PARP, anti–phospho-ERK1/2 (Thr202/Tyr204), anti-ERK1/2, anti–phospho-AKT (Ser473), and anti-AKT (Cell Signaling Technologies), anti-BTK (Epitomics), anti-actin (Santa Cruz Biotechnologies), and anti-GAPDH (Millipore).
Quantitative RT-PCR
RNA was extracted from a minimum of 2 × 107 cells using TRIzol reagent (Invitrogen). cDNA was prepared using a SuperScript First-Strand Synthesis System (Invitrogen) as previously described.32 Real-Time PCR was performed using predesigned TaqMan Gene Expression Assays and ABI Prism 7700 sequence detection system (Applied Biosystems).
Viability and flow cytometric studies
An MTT (3′[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assay was performed to determine cytotoxicity as previously described by our group.24 In addition, cell viability was also measured at various time points using annexin-V/PI flow cytometry (Beckman-Coulter) as previously described.24 Stromal coculture was done as previously described24 by plating a 75 cm2 flask (80%-100% confluent) per 6-well plate 24 hours before the addition of CLL cells.
Enzymatic caspase assay
Detection of cytokine production by ELISA assay
Cytokine production was measured using Quantikine ELISA assays (R&D Systems). CD3 stimulation was done using anti-CD3 T-cell activation plates (BD Biosciences). CD3+ T cells were drugged for 4 and 24 hours with vehicle only and various concentrations of PCI-32765. After drugging, cells were added to anti-CD3 or untreated plates, the supernatant was collected and an ELISA assay was performed according to the manufacturer's directions.
EMSA
NF-κB DNA binding was assessed as previously reported.34 Briefly, 32P-labeled probe containing an NF-κB consensus binding site (5′ AGT TGA GGG GAC TTT CCC AGG C 3′; Santa Cruz Biotechnologies) was labeled using the Nick Translation System (Invitrogen). A total of 3 μg of nuclear protein was incubated for 30 minutes at room temperature in 1X binding buffer (5X: 50mM Tris-HCl, pH 7.5, 5mM EDTA, 20% Ficoll, 5mM dithiothreitol, 375mM KCl) plus 250 ng of poly (dI-dC; Sigma-Aldrich). Complexes were separated on a 6% polyacrylamide gel in Tris borate EDTA buffer (89mM Tris-base, 89mM boric acid, 2mM EDTA). The gels were then dried and autoradiographed. For shift experiments, antibodies to the p65 or p50 subunit (sc-372X and SC114X; Santa Cruz Biotechnologies) of NF-κB were incubated with nuclear extract for 10 minutes before the addition of 32P-labeled probe.
Proliferation assay
Proliferation of CLL cells was determined by tritiated thymidine incorporation. Briefly, 5 × 105 CLL cells in quadruplicate were stimulated with CpG685 for 120 hours in 96-well culture plates in RPMI 1640 containing 10% HS. Cells were treated with various concentrations of PCI-32765 and were pulsed with [3H]-thymidine (1.0 μCi/well) for 15 hours. The cells were harvested using Packard Filtermate 196, plates were dried overnight and [3H]-thymidine incorporation was measured using Packard Micro plate Scintillation and Luminescence Counter.
Statistical analysis
As many of the measurements used samples from the same patients, linear mixed-effect models were used for analysis to take into consideration the dependency of these observations. For RT-PCR data, the raw Ct value was normalized to internal control, and the standardized data were analyzed using linear mixed-effect models. For independent group comparisons or correlations test, analysis of variance or spearman correlation tests were used. The Holm procedure was used to correct for multiple comparisons when appropriate.35 Type I error is strongly controlled at α = 0.05 for single comparisons and after adjustment for multiple comparisons or multiple endpoints.
Results
BTK Protein but not mRNA expression is highly variable among CLL patients
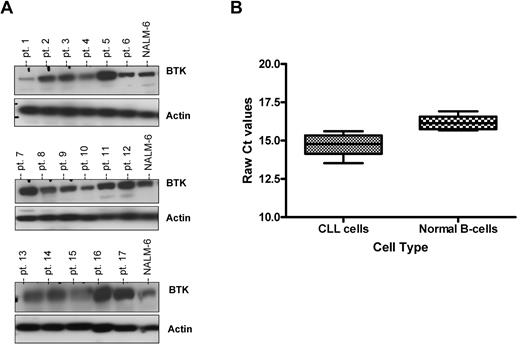
Given the identification of BTK as an essential signaling kinase for survival in large-cell lymphoma cells dependent on BCR-signaling36 and similar activation found in CLL cells, we sought to determine the expression profile of BTK in primary CLL cells. Expression of BTK was examined in cells from 30 CLL patients. All CLL cells evaluated demonstrated expression of BTK protein; however, the expression of BTK was highly variable among patient samples (Figure 1A and data not shown). Given the variable expression of BTK protein levels among different patients, we next attempted to correlate if such expression had any association with clinical features including age, sex, prior treatment status, IVGH mutational status, ZAP-70 expression, or specific genetic aberrations. We found that there was no significant association between BTK protein expression and any of the aforementioned variables (P > .21 for all tested variables).
BTK protein but not mRNA expression is highly variable among CLL patients. (A) CD19+ cells from CLL patients (N = 30) were examined for BTK expression by immunoblot. Results are shown from 3 of 6 experiments. (B) RNA was extracted and converted to cDNA from CD19+ cells from CLL patients (N = 18) and CD19+ normal B cells (N = 5). RT-PCR analysis was done to determine quantities of BTK mRNA. Ct values are relative to 18S. Higher relative Ct values represent lower gene expression.
BTK protein but not mRNA expression is highly variable among CLL patients. (A) CD19+ cells from CLL patients (N = 30) were examined for BTK expression by immunoblot. Results are shown from 3 of 6 experiments. (B) RNA was extracted and converted to cDNA from CD19+ cells from CLL patients (N = 18) and CD19+ normal B cells (N = 5). RT-PCR analysis was done to determine quantities of BTK mRNA. Ct values are relative to 18S. Higher relative Ct values represent lower gene expression.
Given the variable expression of BTK protein in CLL cells and the known regulation by transcription factors, we next sought to determine whether BTK mRNA was also variably expressed in CLL cells using normal B cells derived from healthy volunteers as a control. A subset of the previous 30 CLL patients (N = 18) were evaluated by RT-PCR for BTK gene expression. We found that all CLL patient cells expressed BTK mRNA, with only modest variability between different patients, but was significantly higher than that observed in normal B cells (P = .0001; Figure 1B). We saw no correlation between mRNA expression and protein expression of BTK in CLL patients (P = .32 with a correlation coefficient of −0.28) suggesting that the deregulation of BTK protein expression must be occurring at a posttranscriptional level (data not shown). This contrasts with normal B cells that display very little variability in BTK protein or mRNA expression (data not shown).
PCI-32765 induces selective cytotoxicity in CLL cells independent of IVGH mutational status or interphase cytogenetics
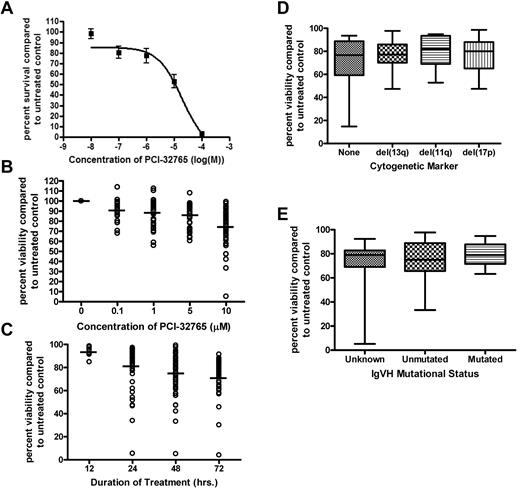
PCI-32765 is an irreversible BTK inhibitor that has been previously described.37 Given the promising results associated with BTK inhibition in human36 and canine37 large B-cell lymphoma, we sought to determine the in vitro activity of PCI-32765 against CLL patient cells. CLL cells from 10 patients were treated with increasing concentrations of PCI-32765 on a log scale (ranging from 0.01μM to 100μM) for 48 hours. We found that PCI-32765 exhibited a significant dose-dependent induction of cytotoxicity in CLL cells (Figure 2A) as measured by MTT incorporation (P < .0001). Cell death was examined separately for the appearance of annexin-V–staining after treatment. We found evidence of both early (annexin-V-positive only) and late (annexin-V/PI dual positive) apoptosis in cells treated with 10μM PCI-32765 (data not shown). Apoptosis induced by PCI-32765 in primary CLL cells was significant compared with vehicle treatment alone at 10μM PCI-32765 (P < .001; Figure 2B). Although the cytotoxic effect was modest, PCI-32765 treatment as low as 0.1μM was still significant compared with vehicle treatment alone (P < .001). PCI-32765–induced cell death increased in a dose-dependent and time-dependent manner (Figure 2B-C). Evidence of apoptosis was observed as early as 12 hours and continued to increase up to 72 hours (Figure 2C).
PCI-32765 induces selective cytotoxicity in CLL cells independent of IVGH mutational status or interphase cytogenetics. (A) CD19+ cells from CLL patients (N = 10) were incubated with or without increasing concentrations of PCI-32765 (0.01μM-100μM) for 48 hours. Viability was determined by MTT assay and was calculated relative to time-matched untreated controls. (B) CD19+ cells from CLL patients (N > 60) were incubated with or without PCI-32765 (0.1μM-10μM) for 48 hours. Viability was determined by annexin-V/PI flow cytometry. Dark lines represent averages. (C) CD19+ cells from CLL patients (N > 60) were incubated with or without 10μM PCI-32765 for 12-72 hours. Dark lines represent averages. (D) CD19+ cells from CLL patients (N > 60; minimum 10 per group) were incubated with or without 10μM PCI-32765 for 48 hours. Cytogenetics was determined independently of our laboratory. (E) CD19+ cells from CLL patients (N > 60; minimum 12 per group) were incubated with or without 10μM PCI-32765 for 48 hours. Mutational status was determined independently of our laboratory. In panels B through E viability was determined by annexin-V/PI flow cytometry, and was calculated relative to time-matched untreated controls.
PCI-32765 induces selective cytotoxicity in CLL cells independent of IVGH mutational status or interphase cytogenetics. (A) CD19+ cells from CLL patients (N = 10) were incubated with or without increasing concentrations of PCI-32765 (0.01μM-100μM) for 48 hours. Viability was determined by MTT assay and was calculated relative to time-matched untreated controls. (B) CD19+ cells from CLL patients (N > 60) were incubated with or without PCI-32765 (0.1μM-10μM) for 48 hours. Viability was determined by annexin-V/PI flow cytometry. Dark lines represent averages. (C) CD19+ cells from CLL patients (N > 60) were incubated with or without 10μM PCI-32765 for 12-72 hours. Dark lines represent averages. (D) CD19+ cells from CLL patients (N > 60; minimum 10 per group) were incubated with or without 10μM PCI-32765 for 48 hours. Cytogenetics was determined independently of our laboratory. (E) CD19+ cells from CLL patients (N > 60; minimum 12 per group) were incubated with or without 10μM PCI-32765 for 48 hours. Mutational status was determined independently of our laboratory. In panels B through E viability was determined by annexin-V/PI flow cytometry, and was calculated relative to time-matched untreated controls.
Although significant cytotoxicity was observed with PCI-32 765 treatment, the variability in patient response was evident. Treatment with 10μM PCI-32765 resulted in a range of cell death from 6.5% to 94.5%. Because of this large variability in cell death, we sought to determine whether traditional prognostic factors would predict response to PCI-32765. We found that there were no significant differences in PCI-32765–induced cell death based on interphase cytogenetic analysis (Figure 2D). IVGH mutational status was also examined for differences in response to PCI-32765 as it has a strong influence on not just chemotherapy response but also on progression-free survival associated with standard therapies used to treat CLL.38 Similar to our findings with cytogenetic analysis, we found no significant differences in sensitivity to PCI-32765 in patients with mutated IVGH compared with those with unmutated IVGH (P = .24; Figure 2E). Together this suggests that PCI-32765 works independent of traditional laboratory predictors of response. In an attempt to explain the variation in patient responses, we compared BTK protein expression to PCI-32765 drug response and found no significant correlation (P = .50 with a correlation coefficient of −0.19); suggesting that response is not dependent solely on BTK expression but more likely on cellular dependence on microenvironmental signals (data not shown).
PCI-32765 cytotoxicity against CLL cells is dependent on caspase pathway activation
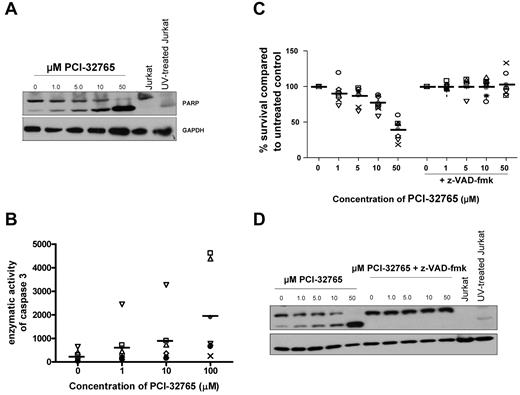
Next, we sought to determine whether treatment with PCI-32765 promotes cell death through a caspase-dependent or caspase-independent pathway. CLL patient cells were treated with various concentrations of PCI-32765 for 8 hours and assessed for cleavage of PARP protein (a caspase-3 substrate). We found that PCI-32765 induced PARP cleavage in a dose-dependent manner (Figure 3A). To confirm activation of caspase-3 after PCI-32765 treatment, enzymatic activity of caspase-3 was determined after 8 hours of PCI-32765 treatment where we found a significant dose-dependent induction of caspase-3 enzymatic activity after treatment (P < .001; Figure 3B). To determine whether PCI-32765 induced apoptosis was dependent on active caspase processing we treated CLL cells with PCI-32765 in the presence or absence of the pan-caspase inhibitor z-VAD-fmk. We found that inhibition of caspase activity by z-VAD-fmk completely prevented the induction of apoptosis provided by PCI-32765 (P < .001; Figure 3C) concurrent with a decrease in cleaved PARP expression and a decrease in enzymatic activity of caspase-3 (Figure 3D and data not shown). These studies show that PCI-32765 not only induces caspase pathway activation, but is dependent on this activation for the induction of cell death observed in CLL cells.
PCI-32765 cytotoxicity against CLL cells is dependent on caspase pathway activation. (A) CD19+ cells from CLL patients (N = 4) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) for 8 hours and PARP cleavage was assessed by immunoblot. Results are shown from 1 of 4 experiments. (B) CD19+ cells from CLL patients (N = 7) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) for 8 hours. Cells were lysed and caspase activity was determined by the amino trifluoromethyl coumarin assay. Results were calculated relative to micrograms of protein. Each symbol represents an individual patient and dark lines represent averages. (C) CD19+ cells from CLL patients (N = 10) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) and 100μM z-VAD-fmk for 48 hours. Viability was determined by annexin-V/PI flow cytometry, and is shown relative to time-matched untreated controls. Each symbol represents an individual patient and dark lines represent averages. (D) CD19+ cells from CLL patients (N = 3) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) and 100μM z-VAD-fmk for 8 hours. PARP cleavage was assessed by immunoblot. Results are shown from 1 of 4 experiments.
PCI-32765 cytotoxicity against CLL cells is dependent on caspase pathway activation. (A) CD19+ cells from CLL patients (N = 4) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) for 8 hours and PARP cleavage was assessed by immunoblot. Results are shown from 1 of 4 experiments. (B) CD19+ cells from CLL patients (N = 7) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) for 8 hours. Cells were lysed and caspase activity was determined by the amino trifluoromethyl coumarin assay. Results were calculated relative to micrograms of protein. Each symbol represents an individual patient and dark lines represent averages. (C) CD19+ cells from CLL patients (N = 10) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) and 100μM z-VAD-fmk for 48 hours. Viability was determined by annexin-V/PI flow cytometry, and is shown relative to time-matched untreated controls. Each symbol represents an individual patient and dark lines represent averages. (D) CD19+ cells from CLL patients (N = 3) were incubated with or without various concentrations of PCI-32765 (1μM-50μM) and 100μM z-VAD-fmk for 8 hours. PARP cleavage was assessed by immunoblot. Results are shown from 1 of 4 experiments.
PCI-32765 induces selective cytotoxicity in B cells compared with T cells, but alters activation-induced T-cell cytokine production
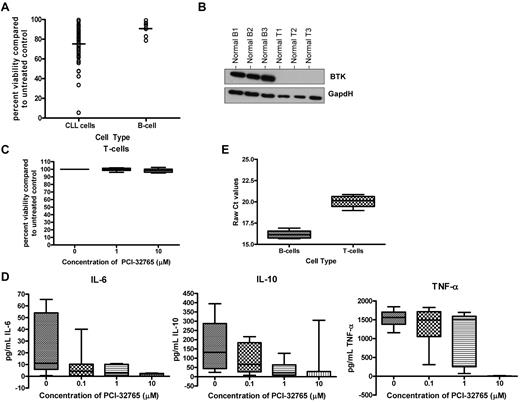
Given the expression of BTK protein in B cells but not T cells, we sought to determine whether PCI-32765–induced cytotoxicity was indeed selective to B cells. We first sought to compare the effect of PCI-32765 on CLL cells compared with normal B cells. We evaluated the cytotoxicity of PCI-32765 on CD19+ normal B cells from healthy volunteers and found that PCI-32765 at a high concentration (10μM) did produce modest apoptosis (95% CI; 8.3-23.5) that was significantly increased (P < .001) compared with vehicle treatment alone as shown in Figure 4A. However, treatment with PCI-32765 induced significantly more apoptosis in CLL cells compared with normal B cells (P < .001; Figure 4A). These data suggest that transformed B cells are more sensitive to PCI-32765 treatment than normal B cells.
PCI-32765 induces selective cytotoxicity in B cells compared with T cells, but alters activation induced T-cell cytokine production. (A) CD19+ cells from CLL patient cells (N > 60) and CD19+ cells from normal B cells (N = 10) were incubated with 10μM PCI-32765 for 48 hours. Viability was determined by annexin-V/PI flow cytometry, and was calculated relative to time-matched untreated controls. Dark lines represent averages. (B) CD3+ T cells (N = 3) and CD19+ B cells (N = 3) from normal volunteers were examined for BTK expression by immunoblot. (C) CD3+ T cells (N = 10) from normal volunteers were incubated with or without increasing concentrations of PCI-32765 (0.1μM-10μM) for 48 hours. Viability was determined by annexin-V/PI flow cytometry, and was calculated relative to time-matched untreated controls. (D) CD3+ T cells (N = 7) from normal volunteers were incubated with or without increasing concentrations of PCI-32765 (0.1μM-10μM) for 48 hours. Cells were stimulated using an anti-CD3 T-cell activation plate for 24 hours, and IL-6, IL-10, and TNF-α production were measured by ELISA. (E) RNA was extracted and converted to cDNA from CD19+ normal B cells and CD3+ normal T cells (N = 5; each). RT-PCR analysis was done to determine quantities of BTK mRNA.
PCI-32765 induces selective cytotoxicity in B cells compared with T cells, but alters activation induced T-cell cytokine production. (A) CD19+ cells from CLL patient cells (N > 60) and CD19+ cells from normal B cells (N = 10) were incubated with 10μM PCI-32765 for 48 hours. Viability was determined by annexin-V/PI flow cytometry, and was calculated relative to time-matched untreated controls. Dark lines represent averages. (B) CD3+ T cells (N = 3) and CD19+ B cells (N = 3) from normal volunteers were examined for BTK expression by immunoblot. (C) CD3+ T cells (N = 10) from normal volunteers were incubated with or without increasing concentrations of PCI-32765 (0.1μM-10μM) for 48 hours. Viability was determined by annexin-V/PI flow cytometry, and was calculated relative to time-matched untreated controls. (D) CD3+ T cells (N = 7) from normal volunteers were incubated with or without increasing concentrations of PCI-32765 (0.1μM-10μM) for 48 hours. Cells were stimulated using an anti-CD3 T-cell activation plate for 24 hours, and IL-6, IL-10, and TNF-α production were measured by ELISA. (E) RNA was extracted and converted to cDNA from CD19+ normal B cells and CD3+ normal T cells (N = 5; each). RT-PCR analysis was done to determine quantities of BTK mRNA.
Given that PCI-32765 induces modest apoptosis of normal B cells, we sought to determine whether T cells, which lack BTK expression would be affected by PCI-32765 treatment. We first confirmed the lack of BTK protein expression in naive normal T cells compared with normal B cells (Figure 4B). Treating naive normal T cells with PCI-32765 as described previously, we did not observe significant cell death even at a dose of 15μM (P = .54; Figure 4C and data not shown). This result suggests that PCI-32765 lacks significant cytotoxicity toward T cells compared with that observed in normal B cells or CLL cells.
Although no significant cytotoxicity was seen in normal T cells we sought to determine whether PCI-32765 might inhibit other targets by evaluating cytokine production by T cells. We assessed the ability of PCI-32765 to disrupt T-cell cytokine production by measuring IL-6, IL-10, and TNF-α production after CD3 ligation. We found that PCI-32765 could inhibit T-cell production of IL-6, IL-10, and TNF-α in a dose-dependent manner (P < .0007 for all cytokines; Figure 4D). As T cells lack BTK protein, we sought to determine whether activation of T cells via CD3 ligation lead to the induction of BTK protein expression. We first evaluated naive CD3+ T cells from normal volunteers and found that in all samples (N = 5) BTK mRNA was expressed but to a significantly lesser extent than in normal B cells (P < .001; Figure 4E). However, we found that CD3 ligation did not lead to detectable BTK protein expression (data not shown). These studies demonstrate that although PCI-32765 lacks direct cytotoxic properties in T cells, it can inhibit production of inflammatory cytokines such as IL-6, IL-10, and TNF-α; suggesting that PCI-32765 probably is influencing an alternative kinase(s) expressed in T cells.
PCI-32765 antagonizes BCR dependent signaling pathways
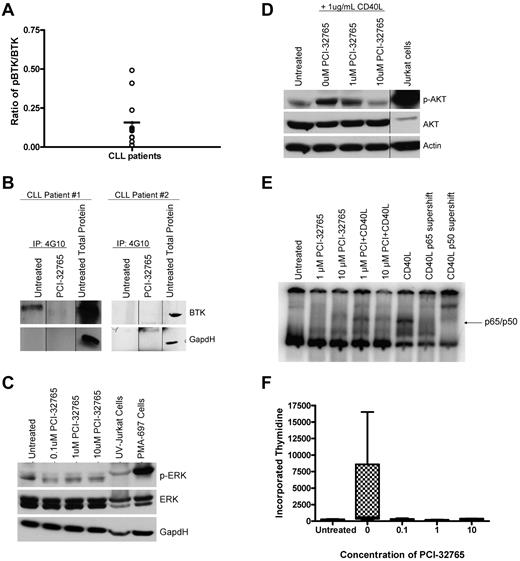
BTK contributes significantly to BCR-signaling prompting us to determine whether BTK was constitutively active in CLL cells. BTK is activated by Src kinase-induced phosporylation at Tyr551 and autophosphorylation at Tyr223. Identification of tyrosine phosphorylated BTK is therefore considered a surrogate marker for kinase activity. We therefore performed an immunoprecipitation assay using a 4G10 (global tyrosine) agarose conjugated beads followed by Western blot analysis of BTK and found constitutive phosphorylation of BTK in basal CLL cells varied by patient (Figure 5A-B). As expected, when no constitutive phosphorylation of BTK was observed, we saw no change in phosphorylation of BTK after treatment with 10μM PCI-32765 (Figure 5B). When constitutive phosphorylation of BTK was observed, treatment with 10μM PCI-32765 reduced the levels of phosphorylated BTK; suggesting inhibition of kinase activity (Figure 5B).
PCI-32765 antagonizes BCR dependent signaling pathways and cell proliferation. (A) CD19+ cells from CLL patients (N = 12) were immunoprecipitated for 4G10. BTK phosphorylation at tyrosine sites was then evaluated by immunoblot analysis. Quantification was done using the Alpha Innotech FluorChemQ MultiImage III system. Dark line represents average. (B) CD19+ cells from CLL patients (N = 4) were treated with and without 10μM PCI-32765 for 2 hours. Cells were immunoprecipitated with 4G10 phospho-tyrosine antibody. BTK phosphorylation at tyrosine sites was then evaluated by immunoblot analysis. Results are shown from 1 of 4 experiments. (C) CD19+ cells from CLL patients (N = 7) were incubated with various concentrations of PCI-32765 for 1 hour. ERK phosphorylation at Thr202/Tyr204 was assessed by immunoblot. Results are shown from 1 of 3 experiments. (D) CD19+ cells from CLL patients (N = 4) were incubated with 1 or 10μM PCI-32765 and/or 1 μg/mL CD40L for 1 hour. AKT phosphorylation at ser473 was assessed by immunoblot. Results are shown from 1 of 3 experiments. (E) CD19+ cells from CLL patients (N = 3) were incubated with 10μM PCI-32765 and/or 1 μg/mL CD40L for 1 hour. EMSA analysis was done with nuclear extract using a radio-labeled oligonucleotide containing a consensus NF-κB binding site. Antibody shifts were performed from CD40L treated sample incubated with antibodies specific to the p65 or p50 subunits of NF-κB. The p65/p50 complex is indicated. Results are shown from 1 of 3 experiments. (F) CD19+ cells from CLL patients (N = 7) were incubated with or without 10μM PCI-32765 and 3.2μM CpG685. Proliferation was assessed by tritiated thymidine incorporation.
PCI-32765 antagonizes BCR dependent signaling pathways and cell proliferation. (A) CD19+ cells from CLL patients (N = 12) were immunoprecipitated for 4G10. BTK phosphorylation at tyrosine sites was then evaluated by immunoblot analysis. Quantification was done using the Alpha Innotech FluorChemQ MultiImage III system. Dark line represents average. (B) CD19+ cells from CLL patients (N = 4) were treated with and without 10μM PCI-32765 for 2 hours. Cells were immunoprecipitated with 4G10 phospho-tyrosine antibody. BTK phosphorylation at tyrosine sites was then evaluated by immunoblot analysis. Results are shown from 1 of 4 experiments. (C) CD19+ cells from CLL patients (N = 7) were incubated with various concentrations of PCI-32765 for 1 hour. ERK phosphorylation at Thr202/Tyr204 was assessed by immunoblot. Results are shown from 1 of 3 experiments. (D) CD19+ cells from CLL patients (N = 4) were incubated with 1 or 10μM PCI-32765 and/or 1 μg/mL CD40L for 1 hour. AKT phosphorylation at ser473 was assessed by immunoblot. Results are shown from 1 of 3 experiments. (E) CD19+ cells from CLL patients (N = 3) were incubated with 10μM PCI-32765 and/or 1 μg/mL CD40L for 1 hour. EMSA analysis was done with nuclear extract using a radio-labeled oligonucleotide containing a consensus NF-κB binding site. Antibody shifts were performed from CD40L treated sample incubated with antibodies specific to the p65 or p50 subunits of NF-κB. The p65/p50 complex is indicated. Results are shown from 1 of 3 experiments. (F) CD19+ cells from CLL patients (N = 7) were incubated with or without 10μM PCI-32765 and 3.2μM CpG685. Proliferation was assessed by tritiated thymidine incorporation.
Activation of BTK in B cells and other cell types has been shown to lead to downstream activation of survival pathways including the PI3K, MAPK, and NF-κB pathways. We therefore sought to determine whether PCI-32765 could inhibit constitutive and activation-induced signaling through these pathways. MAPK signaling was interrogated by evaluation of ERK1/2 phosphorylation. We found that basal levels of ERK1/2 phosphorylation varied among CLL patients, but in all patients evaluated treatment with PCI-32765 resulted in a reduction of basal ERK1/2 phosphorylation (Figure 5C). Next, we sought to determine whether the PI3K pathway was being altered by PCI-32765 treatment; this was determined by evaluating induced AKT phosphorylation (as basal levels of AKT are below the limit of detection). We found that CD40L stimulation induced the phosphorylation of AKT in a manner that was completely reversible by treatment with even low doses of PCI-32765 (Figure 5D). Lastly, we sought to determine whether PCI-32765 was altering NF-κB DNA binding in CLL. We found that CD40L treatment induced DNA binding to an NF-κB consensus binding site that was dose-dependently reduced by PCI-32765 treatment (Figure 5E). These data suggest that PCI-32765 treatment can alter signaling via the MAPK, PI3K, and NF-κB pathways thereby providing multiple mechanisms for altering cell survival.
PCI-32765 inhibits proliferation of CLL cells
In addition to disrupted apoptosis, proliferation of CLL cells in vivo in proliferation centers with clonal evolution and disease progression has been well characterized. We next sought to determine whether PCI-32765 could effectively inhibit CLL cell proliferation following treatment with well-characterized stimulating agents. We chose to use CpG oligonucleotides that are well documented to promote in vitro CLL cell proliferation39 to assess the ability of PCI-32765 to antagonize this process. As shown in Figure 5F, CLL cells without stimulation have a very low tritiated thymidine uptake that is greatly enhanced after 120 hours of treatment with CpG865. Treatment of CLL cells with even low doses of PCI-32765 (0.1μM) demonstrates the ability of this agent to inhibit CLL cell proliferation (Figure 5F). Overall, these in vitro studies provide support that PCI-32765 antagonizes the ability of CLL cells to proliferate after TLR signaling.
PCI-32765 antagonizes microenvironment stimuli
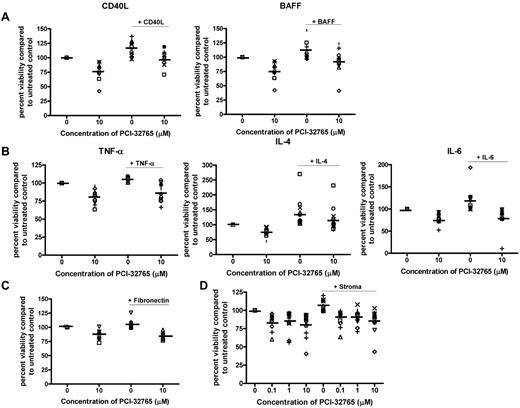
Given the importance of microenvironmental stimuli on survival and activation of CLL cells as well as response to therapy, we next sought to evaluate the role of PCI-32765 in regulating microenvironmental stimuli. The TNF family members CD40L and BAFF have been shown to effectively prevent spontaneous apoptosis and induce activation of key signaling pathways in CLL patient cells.40 We found that both CD40L and BAFF significantly reduced the spontaneous apoptosis associated with CLL cells (P < .001 for both; Figure 6A). Cotreatment with PCI-32765 abrogated the protection induced by both CD40L and BAFF (P = .23 and .086, respectively; Figure 6A). Similar to TNF family members, cytokines such as TNF-α, IL-6, and IL-4 released from T cells as well as other cells that make up the CLL microenvironment also protect CLL cells from spontaneous apoptosis and tend to be elevated in CLL patients.41-44 Again we found that these cytokines prevented the spontaneous apoptosis associated with CLL cells (P < .001 for all evaluated cytokines; Figure 6B). Cotreatment with PCI-32765 reduced the protection induced by TNF-α, IL-6, and IL-4 stimulation (P > .15 for all evaluated cytokines; Figure 6B). To confirm that these results were not limited to soluble factors we next evaluated the effect of PCI-32765 on fibronectin (ligand for CD49 days) adhesion. We again found that fibronectin adhesion protected CLL cells from spontaneous apoptosis (although protection was modest, P < .001), and PCI-32765 treatment again reversed this effect (P = .33; Figure 6C). These findings suggest that PCI-32765 can disrupt signaling from the microenvironment that leads to in vivo CLL cell survival and potentially drug resistance.
PCI-32765 antagonizes microenvironment stimuli. (A) CD19+ cells from CLL patients (N = 10) were incubated with or without 10μM PCI-32765 and 1 μg/mL CD40L or 50 ng/mL BAFF for 48 hours. (B) CD19+ cells from CLL patients (N = 5-10) were incubated with or without 10μM PCI-32765 and 20 ng/mL TNF-α, 40 ng/mL IL-6 or 800 U/mL IL-4 for 48 hours. (C) CD19+ cells from CLL patients (N = 7) were incubated with or without 10μM PCI-32765 on and off fibronectin coated plates for 48 hours. (D) CD19+ cells from CLL patients (N = 8) were isolated from peripheral blood and incubated with or without various concentration of PCI-32765 (0.1μM-10μM) in suspension or on an Hs5 cell layer for 48 hours. (A-D) Viability was determined by annexin-V/PI flow cytometry, and is shown relative to time-matched untreated controls for each group. Dark lines represent averages.
PCI-32765 antagonizes microenvironment stimuli. (A) CD19+ cells from CLL patients (N = 10) were incubated with or without 10μM PCI-32765 and 1 μg/mL CD40L or 50 ng/mL BAFF for 48 hours. (B) CD19+ cells from CLL patients (N = 5-10) were incubated with or without 10μM PCI-32765 and 20 ng/mL TNF-α, 40 ng/mL IL-6 or 800 U/mL IL-4 for 48 hours. (C) CD19+ cells from CLL patients (N = 7) were incubated with or without 10μM PCI-32765 on and off fibronectin coated plates for 48 hours. (D) CD19+ cells from CLL patients (N = 8) were isolated from peripheral blood and incubated with or without various concentration of PCI-32765 (0.1μM-10μM) in suspension or on an Hs5 cell layer for 48 hours. (A-D) Viability was determined by annexin-V/PI flow cytometry, and is shown relative to time-matched untreated controls for each group. Dark lines represent averages.
The survival benefit of CLL in vivo is not only influenced by soluble factors, such as those previously discussed, but also by co-contact with a variety of cells composing the bone marrow and lymph node microenvironment.45,46 Of major importance to drug resistance and induction of cytotoxicity to chemotherapeutic agents is the effect of both direct contact and the communications between CLL cells and bone marrow stroma. We thus sought to determine whether coincubation with CLL cells on a stromal cell line (Hs5 cells) would affect the cytotoxic properties of PCI-32765. Direct treatment of the Hs5 stromal cells with 10μM of PCI-32765 had no effect on viability, cell morphology, or cell growth patterns (data not shown). Coculture of CLL cells for 48 hours on the Hs5 stromal cell line resulted in a marked reduction of spontaneous apoptosis suggesting a strong protective effect was elicited (P < .001; Figure 6D). Treatment of CLL cells under coculture conditions resulted in cell death comparable with that seen under normal suspension conditions (Figure 6D). Together these data suggest that the cytotoxic effect elicited by PCI-32765 will not be significantly diminished by the presence of an in vivo microenvironment.
Discussion
Herein, we have presented to our knowledge the first justification for clinical development of the irreversible BTK inhibitor PCI-32765 in CLL. Our study demonstrates that BTK mRNA and protein expression is present in all CLL patients. Of interest, mRNA expression of BTK was similar among all CLL patients whereas protein expression varied dramatically and was unrelated to mRNA levels. This may be because of the regulation of BTK protein by transcription factors such as NF-κB. Treatment of CLL cells with PCI-32765 is more cytotoxic compared with normal B cells. This modest effect of PCI32765 is probably a consequence of minimal-to-absent constitutive BTK tyrosine phosphorylation derived from the peripheral blood CLL cells. However, activation of these cells through BCR or CD40 ligand, similar to that mimicked in vivo in proliferation centers in lymph nodes and bone marrow, demonstrated tyrosine phosphorylation of BTK, a surrogate marker of increased kinase activity (data not shown). The influence of BTK inhibition was further demonstrated by showing the influence of downstream targets influenced by BTK inhibition with PCI-32765 in stimulated CLL cells including MAPK, PI3K, and NF-κB signaling. Our studies demonstrated evidence of both ERK and AKT signaling alterations as well as changes in NF-κB binding after PCI-32765 treatment, suggesting that treatment decreases BCR-induced survival signals. In addition, we have shown that PCI-32765 completely abrogated CpG oligonucleotide-induced proliferation of CLL cells. Furthermore, treatment with PCI-32765 prevented CLL cells from deriving the protective effect elicited by microenvironmental stimuli including CD40L, BAFF, TNF-α, IL-4, and IL-6. Similarly, coculture on a stromal cell line significantly decreased the spontaneous apoptosis of CLL cells. Again, treatment with PCI-32765 was able to overcome stromal protection and induce apoptosis comparable with cells cultured in suspension. Together, these data suggest that PCI-32765 may act directly on CLL cells to induce apoptosis, but may also act on the surrounding microenvironment to inhibit external sources of proliferation and also help prevent the creation of a survival niche for the CLL cells.
While our mechanistic studies clearly demonstrate that PCI-32765 is actively inhibiting BTK in CLL cells, other data presented suggests that this inhibitor has alternative targets potentially relevant to CLL that to this point remain unidentified. This is best demonstrated by the T-cell studies where we demonstrate as previously reported that BTK mRNA is very minimally expressed and protein is not detectable in both resting and activated cells. Despite this finding, treatment with PCI-32765 demonstrates no T-cell cytotoxicity but does greatly diminish production of several anti-inflammatory cytokines including IL-6 and IL-10 that have been shown to enhance CLL cell survival.47,48 The impact of such inhibition therefore could be quite positive, as several reports have noted the important contribution of T cells to CLL proliferation and survival. Blocking production of these different cytokines in vivo by T cells in CLL patients could potentially affect the survival of CLL cells. Several kinases with homology to BTK in the TEC kinase family including BMX and ITK have similar cysteine residues that might also be reversibly or irreversibly inhibited by PCI-32765. Our laboratory is currently pursuing studies to identify alternative target kinases of PCI-32765. It is indeed possible that one of these targets could be in-part responsible for the preclinical activity observed with PCI-32765.
To date, introduction of other kinase inhibitors that influence BCR signaling such as the PI3K-δ inhibitor CAL-10149 and the Syk inhibitor fostamatinib disodium50 have demonstrated interesting clinical activity with profound reduction in nodal disease and relatively profound early increases in peripheral lymphocyte counts that in a subset of patients slowly diminish with time over several months. Very preliminary results from a single agent continuous-dosing study of PCI-32765 in CLL have shown similar results in CLL patients.51 Our finding of modest apoptosis with PCI-32765 in vitro with both disrupted ability to actively respond to microenvironmental stimulatory and survival signals along with inhibition of proliferation provides a potential model for how these different therapeutics targeting BCR signaling are potentially working in different CLL compartments. Specifically, while very modest response in blood tumor cell reduction is noted promptly as with other therapeutics used in CLL, the apoptotic threshold is altered more differentially in the bone marrow and blood compartments where cells are actively stimulated by both stromal contact and cytokine/chemokine stimulation. In addition, proliferation of the CLL clone in this same compartment is effectively antagonized by these agents. Over time, treatment with these different therapeutic agents that target BCR are effective in actively controlling the disease. Proving this change in compartmentalization and differential response in vivo will require application of animal models such as the TCL1 transgenic mouse model of CLL that our group is now pursuing. Given the strong dependence of normal B cells on BTK function as suggested by the knockout mouse phenotype, and the finding that BTK is an essential kinase for survival in an siRNA lethal screening study of large B-cell lymphoma dependent on BCR signaling,36 PCI-32765, which our data demonstrates potently inhibits this kinase, represents an outstanding candidate to actively develop as a therapeutic directed at this new treatment paradigm in CLL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Leukemia & Lymphoma Society, P50-CA140158, PO1-CA95426, PO1 CA81534, 1K12 CA133250, and The D. Warren Brown Foundation. A.J.J. is a Paul Calabresi Scholar.
National Institutes of Health
Authorship
Contribution: S.E.M.H. planned the research, performed experiments, analyzed data, drafted the first and subsequent drafts of the paper, and approved the final version of the paper; A.L.G., E.H., and A.R. were involved in planning components of the research, performed experiments, and reviewed drafts and approved the final version of the paper; X.Z. was involved in planning components of the research, did all the statistical analysis, reviewed drafts, and approved the final version of the paper; S.J., J.F., J.J., and K.A.B. accrued patients to the CLL clinical trial, reviewed drafts of the paper, and approved the final version of the paper; J.J.B. and A.H. were involved in planning components of the research, provided necessary reagents essential to the hypothesis of this paper, reviewed drafts, and approved the final version of the paper; and A.J.J. and J.C.B. planned every aspect of the proposal, supervised the research, analyzed data, reviewed and modified drafts, obtained funding for the research work, and approved the final version of the paper.
Conflict-of-interest disclosure: J.J.B. and A.H. are employees of Pharmacyclics Inc and both have financial interests in PCI-32765 development. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, MD, B302 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu; or Amy J. Johnson, PhD, OSUCCC Bldg Rm 455C, 410 West 12th Ave, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.
References
Author notes
A.J.J. and J.C.B. are senior authors and contributed equally to this work.