Abstract
Arsenic had been used in treating malignancies from the 18th to mid-20th century. In the past 3 decades, arsenic was revived and shown to be able to induce complete remission and to achieve, when combined with all-trans retinoic acid and chemotherapy, a 5-year overall survival of 90% in patients with acute promyelocytic leukemia driven by the t(15;17) translocation-generated promyelocytic leukemia–retinoic acid receptor α (PML-RARα) fusion. Molecularly, arsenic binds thiol residues and induces the formation of reactive oxygen species, thus affecting numerous signaling pathways. Interestingly, arsenic directly binds the C3HC4 zinc finger motif in the RBCC domain of PML and PML-RARα, induces their homodimerization and multimerization, and enhances their interaction with the SUMO E2 conjugase Ubc9, facilitating subsequent sumoylation/ubiquitination and proteasomal degradation. Arsenic-caused intermolecular disulfide formation in PML also contributes to PML-multimerization. All-trans retinoic acid, which targets PML-RARα for degradation through its RARα moiety, synergizes with arsenic in eliminating leukemia-initiating cells. Arsenic perturbs a number of proteins involved in other hematologic malignancies, including chronic myeloid leukemia and adult T-cell leukemia/lymphoma, whereby it may bring new therapeutic benefits. The successful revival of arsenic in acute promyelocytic leukemia, together with modern mechanistic studies, has thus allowed a new paradigm to emerge in translational medicine.
Introduction
Arsenic is the 20th most abundant element in the earth's crust.1 It is a chemical analog of phosphorus and lies directly below P in the periodic table. A unique feature of arsenic is its extremely paradoxical abilities: it is toxic to humans, animals, and plants, but is used instead of phosphorus by a recently isolated bacterium2 ; it kills people, but saves lives; it can cause some cancers but cures others such as the acute promyelocytic leukemia (APL)3 ; it is one of the oldest drugs in the world but was progressively revived between the 1970s and 1990s, because of its striking efficacy on APL, which represented the most malignant type of acute leukemia.3 These paradoxical effects of arsenic reflect its unique metabolism and multiple properties.4 In this review, we address how arsenic rebuilds its reputation from a notorious poison to a new lease of life by mechanistic molecular and translational medicine studies.
Arsenic in nature
Arsenic is the 33rd element in the periodic table and exists ubiquitously in either inorganic or organic forms, pure metallic arsenic being rarely found in nature. Arsenic occurs in 5 different valence states, +V (arsenate), +III (arsenite), +I (arsonium metal), 0 (arsenic), and −III (arsine).5 Most arsenic compounds have no smell or special taste and are white or colorless powders that do not evaporate. Arsenic gets into air when contaminated materials are burned. Inorganic arsenic occurs naturally in soils and sedimentary rocks such as minerals and ores containing copper or lead, and it occurs in combination with many other elements especially oxygen, chlorine, and sulfur. There are 3 inorganic arsenic forms, namely, red arsenic (As4S4, also known as realgar), yellow arsenic (As2S3, also known as orpiment), and white arsenic (arsenic trioxide, ATO; As2O3). ATO is made by burning realgar or orpiment.6 Organic arsenic is arsenic compounds containing carbon that can be found in nature in water, natural gas, and shale oil.5 Examples of organic arsenic are methylarsine (CH3AsH2), dimethylarsine [(CH3)2AsH], trimethylarsine [(CH3)3As], monomethylarsonic acid [CH3AsO(OH)2, MMAV], monomethylarsenous acid [CH3As(OH)2, MMAIII], dimethylarsinic acid [(CH3)2AsO(OH), DMAV], dimethylarsenous acid [(CH3)2AsOH, DMAIII], trimethylarsinic oxide [(CH3)3AsO, TMAO], tetramethylarsonium ion [(CH3)4As+, TMA+], arsenobetaine [(CH3)3As+CH2COO−, AB], arsenocholine [(CH3)As+CH2 CH2OH, AC], and others.5,7-9 Darinaparsin is an organic arsenic composed of dimethylated arsenic linked to glutathione.10
Regarding arsenic metabolism, an arsenic methyltransferase, encoded by a gene that was originally annotated as Cyt19 and subsequently As3mt, was identified in rat that catalyzes the methylation of arsenite, with S-adenosyl-L-methionine as the methyl donor.11,12 Inorganic arsenic is metabolized by a sequential process involving a 2-electron reduction of pentavalent arsenic to trivalent arsenic, followed by oxidative methylation to pentavalent organic arsenic.13,14 The postulated scheme is as follows: iAsV → iAsIII → MAsV → MAsIII → DMAsV → DMAsIII → TMAsV → TMAsIII.13-16 Of note, derivatives of MAsIII and DMAsIII are more toxic than either iAsV or iAsIII.16 Methylarsine oxide (MAsIIIO) and to a lesser extent iododimethylarsine are more potent growth inhibitors and apoptotic inducers than iAsIII in leukemia cells, and apoptosis is associated with greater hydrogen peroxide accumulation and inhibition of glutathione peroxidase activity. In vivo hepatic methylation of iAsIII may contribute to ATO-induced apoptosis but not differentiation of APL cells.15
Arsenic is toxic to human health in that it can induce skin lesions, hemorrhagic gastroenteritis, cardiac arrhythmia, psychiatric disease, and cancers.9,17,18 Generally, inorganic arsenic species are more toxic than organic forms to living organisms, and arsenite is usually more toxic than arsenate. Exposure to ATO by ingestion of 70-80 mg has been reported to be fatal for humans.7,19 The World Health Organization set the first International Drinking Water Standard for arsenic concentration at 200 μg/L in 1958, recommended lowering the standard to 50 μg/L in 1963, and further lowered the standard to 10 μg/L in 1993.20 Yet millions of people worldwide ingest drinking water contaminated with arsenic at levels > 100 μg/L.5,7,20
The toxicity of trivalent arsenic is related to its high affinity for the sulfhydryl groups of biomolecules such as glutathione and lipoic acid and the cysteinyl residues of many proteins and enzymes.7,21 Arsenic up-regulates glutathione-related genes and enzyme activities and binds to sulfhydryl groups.22,23 The formation of AsIII-sulfur bonds results in various harmful effects by inhibiting the activities of enzymes such as glutathione reductase, glutathione peroxidases, thioredoxin reductase, and thioredoxin peroxidase.4,7,21 Because all of these enzymes regulate cellular redox status by providing antioxidant defense, arsenic exposure leads to production of reactive oxygen species (ROS).4 Similarly, flavin enzymes such as NAD(P)H oxidase and NO synthase isozymes have been proposed to be involved in the generation of ROS associated with arsenic exposure.4 Arsenic also alters global histone H3 methylation.24 Consequently, arsenic affects many signal transduction cascades (eg, activation of the epidermal growth factor receptor [EGFR] signal pathway25 ) and activates (or inactivates) transcription factors such as activated protein-126 and nuclear factor–erythroid 2-related factor 2.27,28 Biomarkers of arsenic exposure include the total arsenic in urine,29 clastogenicity in peripheral lymphocytes, micronuclei in oral mucosa and bladder cells, and induction of heme oxygenase. A potential susceptibility biomarker is variability in arsenic metabolism, which reflects polymorphisms in the genes that encode the arsenic-metabolizing enzymes.29
Arsenic as an old remedy
Arsenic is one of the oldest drugs in the world. It was first mentioned by Hippocrates (460-370 BC) who used realgar and orpiment pastes to treat ulcers in Western medicine. In China, arsenic pills for the treatment of periodic fever were recorded in the Chinese Nei Jing Treaty (263 BC).30 Si-Miao Sun (581-682 AD) purified a medicine composed of realgar, orpiment, and ATO in treating malaria,31 whereas Shi-Zhen Li (1518-1593 AD)32 in the Ming Dynasty described the use of ATO as a remedy for a variety of diseases in his pharmacopedia. Arsenic therapy was introduced to Europe by Avicennes (980-1037 AD) and Paracelsus (1493-1541 AD). In 1774, Lefébure introduced an arsenic-containing paste proposed to be an “established remedy to radically cure all cancers.”6 Fowler solution (1% potassium arsenite, KAsO2) was first described in 1845 and was used to treat anemia and rheumatism, psoriasis, eczematous eruptions, dermatitis herpetiformis, asthma, cholera, and syphilis. In 1865, Fowler solution was the first chemotherapeutic agent used in the treatment of leukemia that produced some transient improvement.33-35 In 1931 Forkner and Scott, at Boston City Hospital, “rediscovered” Fowler solution for the treatment of chronic myeloid leukemia (CML), and arsenicals and irradiation remained the treatment of choice until busulphan was introduced in 1953.33-35
Arsenic in treating APL
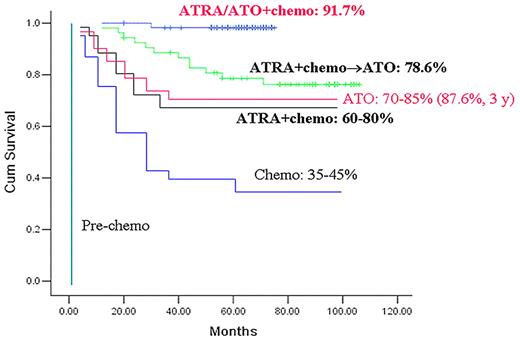
APL, the M3 subtype of acute myeloid leukemia (AML M3), is characterized by the accumulation of abnormal promyelocytes in blood and bone marrow, the occurrence of fibrinogenopenia and disseminated intravascular coagulation, and the specific chromosomal translocation t(15;17)(q22;q21).36-38 The t(15;17) fuses the retinoic acid receptor α (RARα) gene on chromosome 17 to the promyelocytic leukemia (PML) gene on 15q, yielding the PML-RARα fusion protein that is the key driver of APL leukemogenesis.36-38 APL was firstly described by Hillestad in 19573 and was considered at the time the most fatal type of acute leukemia.3,36,38 The past 5 decades have witnessed tremendous advances in improving APL outcome from highly fatal to highly curable (Figure 1).35,36,38,39 Although chemotherapy (anthracyclines) shines the first light of hope,40 all-trans retinoic acid (ATRA), which triggers terminal differentiation of APL cells, achieves a complete remission (CR) rate of 90%38,41-44 (and references within these articles). Although ATRA alone rarely cures APL, its combination with anthracyclines allowed a significant number of cures. ATO further prolongs survival of patients with APL, especially those with relapsed or refractory disease, and cures a number of them as a single agent. Moreover, combined use of ATRA and ATO not only markedly enhances clearance of PML-RARα transcript, but allows the 5-year overall survival (OS) to reach 91.7%38,39,45 (Figure 1). Mechanistically, both ATRA and ATO trigger catabolism of the PML-RARα fusion protein. However, ATO has no effect on APL driven by promyelocytic leukemia zinc finger (PLZF)–RARα, which is generated by t(11;17) and accounts for 1%-2% of patients with APL.46-51 Thus, the story of APL can serve as a model for the development of targeted therapies and curative approaches for malignant disease.51
A historical view of the treatment outcome (represented by 5-year overall survival) of APL.
A historical view of the treatment outcome (represented by 5-year overall survival) of APL.
Clinical efficacy
In the early 1970s, a group from Harbin Medical University in northeastern China tested Ailing-1 containing 1% ATO and a trace amount of mercury chloride in a variety of cancers by intravenous administration. In the 1990s, Sun et al52 showed that Ailing-1 induced CR in 21 of 32 patients with APL with an impressive 10-year survival rate of 30%. The efficacy of pure ATO in treating relapsed APL was then reported by Shanghai Institute of Hematology (SIH) in 1996-1999.53,54 Shen et al54 evaluated the therapeutic effect of ATO in the treatment of 15 patients with APL at relapse after ATRA-induced and chemotherapy-maintained CR. ATO was administered intravenously at the dose of 10 mg/d. They showed that CR was achieved in 9 of 10 patients (90%) treated with ATO alone and in the remaining 5 patients with ATO in combination with low-dose chemotherapeutic drugs or ATRA. No bone marrow depression was encountered during ATO treatment.54 Niu et al53 reported that clinical CR was obtained in 8 of 11 newly diagnosed cases (72.7%) and in 40 of 47 relapsed patients (85.1%). They recommended that ATRA is used as first choice for remission induction in newly diagnosed APL cases, whereas ATO can be either used as a rescue for relapsed cases or included into multidrug consolidation/maintenance clinical trials. Furthermore, after CR achieved with the use of ATO alone, a molecular remission is obtainable in a relatively high proportion of the patients, from 72%55 to 91%56 in different multicenter studies, showing that ATO is an effective drug for APL. With the use of ATO as a single agent, a good long-term remission can be obtained, as evidenced by a 5-year event-free survival (EFS) of 69%57 to 72.7%58 in 2 recent reports (Table 1).
Outcome of patients with APL treated with ATO-based regimens since 2006
| Year . | Author . | No. . | Regimen . | CR, % . | EFS, %* . | DFS, %* . | OS, %* . |
|---|---|---|---|---|---|---|---|
| 2010 | Powell et al59 | 244 (237 standard controls) | Induction: ATRA/CT; consolidation: ATRA/CT/ATO | 90 | 80 (3 y) | 90 (3 y) | 86 (3 y) |
| 2010 | Zhou et al58 | 19 (age ≤ 15) | ATO | 89.5 | 72.7 | NR | 83.9 |
| 2010 | Mathews et al57 | 72 | ATO | 86.1 | 69 | 80 | 74.2 |
| 2009 | Dai et al60 | 90 | ATO/ATRA | 93.3 | 92.2 (3 y) | ||
| 2006 | Ghavamzadeh et al61 | 111 | ATO | 85.6 | NR | 63.7 (2 y) | 87.6 (3 y) |
| 2008 | Hu et al39 | 85 | ATO/ATRA/CT | 94.1 | 89.2 | NR | 91.7 |
| 2007 | Wu et al62 | 114 | As4S4/ATRA or As4S4/CT | 94 (4 y) |
| Year . | Author . | No. . | Regimen . | CR, % . | EFS, %* . | DFS, %* . | OS, %* . |
|---|---|---|---|---|---|---|---|
| 2010 | Powell et al59 | 244 (237 standard controls) | Induction: ATRA/CT; consolidation: ATRA/CT/ATO | 90 | 80 (3 y) | 90 (3 y) | 86 (3 y) |
| 2010 | Zhou et al58 | 19 (age ≤ 15) | ATO | 89.5 | 72.7 | NR | 83.9 |
| 2010 | Mathews et al57 | 72 | ATO | 86.1 | 69 | 80 | 74.2 |
| 2009 | Dai et al60 | 90 | ATO/ATRA | 93.3 | 92.2 (3 y) | ||
| 2006 | Ghavamzadeh et al61 | 111 | ATO | 85.6 | NR | 63.7 (2 y) | 87.6 (3 y) |
| 2008 | Hu et al39 | 85 | ATO/ATRA/CT | 94.1 | 89.2 | NR | 91.7 |
| 2007 | Wu et al62 | 114 | As4S4/ATRA or As4S4/CT | 94 (4 y) |
NR indicates not reported.
Five years, unless otherwise indicated.
The efficacy of As4S4 in APL was also investigated. In 1995, Huang et al63 reported remarkable results of the Realgar-Indigo Naturalis Formula, in which As4S4 is the principle ingredient, in treating 60 patients with APL, including 43 newly diagnosed cases. In 2002, Lu et al64 showed that of the 129 patients with APL receiving pure As4S4,103 cases (79.8%) achieved CR. In the newly diagnosed group (19 patients), the estimated disease-free survival rates for 1 and 3 years were 86.1% and 76.6%, respectively, with a median follow-up time of 13.5 months. They further showed that in 114 patients receiving As4S4 in combination with ATRA or chemotherapy (mitoxantrone or hydroxyurea or both), the estimated 4-year disease-free survival was 94%.62
Mechanisms of action
PML-RARα as a direct arsenic target.
That ATO exerts drastic therapeutic effects against APL, but not other subtypes of AML (including variant APL driven by the PLZF-RARα fusion), suggests a crucial link between its mechanism of action and PML-RARα, a potent transcriptional regulator that alters expression of ATRA or non-ATRA target genes.38,65-67 Indeed, it was rapidly shown that arsenic efficiently triggers the degradation of PML-RARα through its PML moiety.68-70
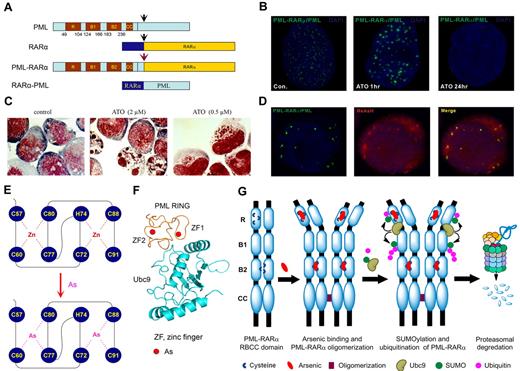
The wild-type PML and the PML moiety in fusion protein harbor the RBCC domain,69,70 which contains 1 RING and 2 B boxes (B box 1 and B box 2) motifs capable of binding metal (physiologically zinc) ions, and a coiled-coil (CC) motif mediating homodimer formation.71,72 Figure 2A is a schematic representation of major domains of PML, RARα, and PML-RARα. In normal cells, PML proteins are the main components of spherical nuclear organelles designated nuclear bodies (NBs), which play a key role in regulation of apoptosis, epigenetic control of chromatin, and transcriptional expression, as well as storage/modulation of certain nuclear proteins.73-75 NB structures are disrupted in APL cells, because of formation of PML/PML-RARα heterocomplex. This yields a much larger number of tiny dots by immunofluoresence analysis, showing the disorganization of this nuclear domain.76 The ATRA and arsenic-triggered degradation process is intimately coupled to changes in PML/PML-RARα localization.46,68,70,77-79 Indeed, in ATO-treated APL cells or cells transfected with PML, PML-RARα,79 both PML-RARα and wild-type PML are quickly translocated to the nuclear matrix, sumoylated, ubiquitinated, and subsequently degraded by the proteasome. In parallel, in arsenic-treated APL cells, dots of PML-containing proteins are aggregated to form larger particles at the nuclear matrix, before their ultimate disappearance (Figure 2B) and the cells are committed to apoptosis or partial differentiation (Figure 2C).
Effects of arsenic on APL cells. (A) Schematic represents the structure of PML, RARα, and PML-RARα. (B) The NB4 cells were treated with 1μM ATO for indicated time points and were assessed by immunofluorescence staining with an anti-PML antibody (green). Stainings were analyzed using a Leica TCS SP5 confocal microscope equipped with a 63×/1.4 NA oil objective (Leica Microsystems). Images were processed using Leica AF lite software. (C) Arsenic induces dual effects on APL cells. The NB4 cells were treated with indicated concentration for 48 hours and stained with the Wright stain. Stainings were analyzed using an Olympus BX51 research microscope equipped with a 100×/1.30 NA oil objective (Olympus). Images were processed using Adobe Photoshop CS (Adobe Systems). Original magnification, ×1000. (D) Colocalization of PML and PML-RARα with the fluorescent organic arsenical ReAsH in NB4 cells. Images were visualized and processed using the equipment described for panel B. (E) The schematic diagram of the structure of PML RING coordinated with zinc or arsenic. (F) Predicted structure of PML RING/UBC9 complex. (G) A working model of the mechanism by which arsenic controls the fate of PML and PML-RARα.
Effects of arsenic on APL cells. (A) Schematic represents the structure of PML, RARα, and PML-RARα. (B) The NB4 cells were treated with 1μM ATO for indicated time points and were assessed by immunofluorescence staining with an anti-PML antibody (green). Stainings were analyzed using a Leica TCS SP5 confocal microscope equipped with a 63×/1.4 NA oil objective (Leica Microsystems). Images were processed using Leica AF lite software. (C) Arsenic induces dual effects on APL cells. The NB4 cells were treated with indicated concentration for 48 hours and stained with the Wright stain. Stainings were analyzed using an Olympus BX51 research microscope equipped with a 100×/1.30 NA oil objective (Olympus). Images were processed using Adobe Photoshop CS (Adobe Systems). Original magnification, ×1000. (D) Colocalization of PML and PML-RARα with the fluorescent organic arsenical ReAsH in NB4 cells. Images were visualized and processed using the equipment described for panel B. (E) The schematic diagram of the structure of PML RING coordinated with zinc or arsenic. (F) Predicted structure of PML RING/UBC9 complex. (G) A working model of the mechanism by which arsenic controls the fate of PML and PML-RARα.
Because the wild-type PML and the PML moiety in the fusion protein harbor a number of adjacently located cysteine residues with metal-binding ability in their RBCC domain,71,72 we hypothesized that ATO might target PML/PML-RARα at this domain. We tested this possibility by biotin-arsenic/streptavidin pull-down affinity assay and a red fluorescent organic arsenic compound ReAsH/immunofluorescent analysis. Zhang et al79 and Jeanne et al46 showed that ReAsH colocalized with PML/PML-RARα (Figure 2D) and that arsenic could directly bind the wild-type and fusion proteins. Consistently, deletion of the RING domain in PML abolished the ReAsH colocalization signal. The PML RING peptide containing C3HC4 (aa 57-91) zinc finger (ZF) was expressed and purified for refined arsenic-binding analyses. With the use of matrix-assisted laser desorption ionization– time of flight mass spectrometry, it was found that 1 PML RING molecule without metal ions (apo-PML RING) could bind 2 arsenics at ATO concentrations of 1-2μM.79 Arsenic bound PML RING through thiol groups of cysteines with the formation of arsenic-sulphur bonds, as evidenced by near-ultraviolet absorbance spectrometry assays. With the use of x-ray absorption spectra assay, including extended x-ray absorption fine structure and x-ray absorption near-edge structure, the local structures of PML RING around metal ions within ∼ 6 Å were obtained, and it was found that trivalent arsenics could coordinate to PML RING each by 3 conserved cysteines, in ZF1 with C60, C77, and C80 and in ZF2 with C72, C88, and C91, compared with the coordination by zinc in ZF1 with C57, C60, C77, C80 and in ZF2 with C72, H74, C88, and C91 (Figure 2E). In addition, the PML B box 2 domain also directly bound arsenic in vitro, whereas its deletion led to a significant reduction of ReASH colocalization signals in cells.46,79 Of note, arsenic was able to competitively replace zinc in PML RING coordinated with zinc (zinc-PML RING) according to the nuclear magnetic resonance heteronuclear single-quantum coherence spectra.79 On binding to arsenic, PML RING underwent conformational changes and aggregation, most probably because of the formation of homodimer by intermolecular As-S bonds, followed by oligomerization among these homodimers.46,79 In in vitro reconstituted conditions and mammalian 2-hybrid assay in cells, arsenic binding to PML facilitated its interaction with the unique small ubiquitin-like protein (SUMO) E2 conjugase UBC9, leading to sumoylation at K65 and K160.80 K160 sumoylation also mediated subsequent recruitment of 11S proteasome.78,81 A 3-dimensional structure modeling shows that the 2 ZFs in PML RING motif locate at the interface with UBC9, providing a structural basis for the formation of PML/UBC9 complex in the presence of arsenic (Figure 2F). Recent studies indicate that RNF4, a ubiquitin E3 ligase containing SUMO interaction motifs,77,82 could recruit sumoylated PML/PML-RARα and promote their proteasomal degradation.77,82 These results show that ATO controls the fate of the PML-RARα by directly binding PML (Figure 2G) and, at least partially, explaining why ATO is effective for APL.79
In addition to formation of arsenic-cysteine bonds that favor aggregation, arsenic-induced ROS also initiate intermolecular disulfide formation.46 Disulfide-linked PML or PML-RARα multimers become nuclear matrix-associated and form NBs. Thus, PML oxidation regulated NB biogenesis. In that respect, non–arsenical oxidants also elicited PML-RARα multimerization, NB association, degradation, and leukemia response in vivo. Critically, oxidants did not affect PLZF-RARα–driven APL, a genetic demonstration that PML is the key target.46 Arsenic can also bind other proteins, including the ubiquitin E3 ligases c-CBL (Casitas B-lineage lymphoma) and SIAH1,83 both harboring RING finger motifs (Table 2).
Arsenic binding proteins
| Proteins and references . | Disease or cell lines . | Category . | Functions . |
|---|---|---|---|
| PML79 | APL | Phosphoprotein; scaffold protein | Tumor suppressor; probable transcription factor |
| c-CBL83 | CML, K562 cells | Ubiquitin E3 ligase | Proteolysis |
| SIAH183 | CML, K562 cells | Ubiquitin E3 ligase | Proteolysis |
| Trx R84 | MCF-7 cells | Oxidoreductase | Redox regulation |
| GSR85 | Arsenic toxication | Oxidoreductase | Redox regulation |
| TPX-2 II86 | Ovary cells | Peroxidase | Redox regulation |
| PDI87 | Fibrosarcoma cells | Oxidoreductase | Redox regulation |
| MTs88 | Arsenic detoxication | Metallothioneins | Binding heavy metals |
| MTF189 | Arsenic detoxication | Transcription factor | Activation of metallothionein transcription |
| Keap127 | hepa1c1c7 cells | Phosphoprotein | Transcription regulation |
| Tubulins90-92 | K562 cells | Cell skeleton proteins | Structural subunit of microtubules |
| β-Actin91-93 | K562 and MCF-7 cells | Cell skeleton proteins | Cytoskeleton |
| PPM1D94 | Malignancies | Phosphatase; oncoprotein | Protein serine/threonine phosphatase; regulation of cell cycle |
| JNK phosphatase26 | Carcinogenesis | Dual specificity protein phosphatase | Cellular signaling |
| Iκ B95 | Inflammation and carcinogenesis | Protein kinase | Regulation of NFκB pathway |
| Galectin-186,96 | Ovary cells | Pyruvate kinase | Regulation of NFκB pathway |
| PKM291 | MCF-7 cells | Phosphoprotein | Glycolysis |
| Hemoglobin97 | Red blood cells | Globin family | Oxygen transport |
| Proteins and references . | Disease or cell lines . | Category . | Functions . |
|---|---|---|---|
| PML79 | APL | Phosphoprotein; scaffold protein | Tumor suppressor; probable transcription factor |
| c-CBL83 | CML, K562 cells | Ubiquitin E3 ligase | Proteolysis |
| SIAH183 | CML, K562 cells | Ubiquitin E3 ligase | Proteolysis |
| Trx R84 | MCF-7 cells | Oxidoreductase | Redox regulation |
| GSR85 | Arsenic toxication | Oxidoreductase | Redox regulation |
| TPX-2 II86 | Ovary cells | Peroxidase | Redox regulation |
| PDI87 | Fibrosarcoma cells | Oxidoreductase | Redox regulation |
| MTs88 | Arsenic detoxication | Metallothioneins | Binding heavy metals |
| MTF189 | Arsenic detoxication | Transcription factor | Activation of metallothionein transcription |
| Keap127 | hepa1c1c7 cells | Phosphoprotein | Transcription regulation |
| Tubulins90-92 | K562 cells | Cell skeleton proteins | Structural subunit of microtubules |
| β-Actin91-93 | K562 and MCF-7 cells | Cell skeleton proteins | Cytoskeleton |
| PPM1D94 | Malignancies | Phosphatase; oncoprotein | Protein serine/threonine phosphatase; regulation of cell cycle |
| JNK phosphatase26 | Carcinogenesis | Dual specificity protein phosphatase | Cellular signaling |
| Iκ B95 | Inflammation and carcinogenesis | Protein kinase | Regulation of NFκB pathway |
| Galectin-186,96 | Ovary cells | Pyruvate kinase | Regulation of NFκB pathway |
| PKM291 | MCF-7 cells | Phosphoprotein | Glycolysis |
| Hemoglobin97 | Red blood cells | Globin family | Oxygen transport |
Elimination of leukemia-initiating cells.
Leukemia-initiating cells (LICs) are pluripotent, self-renewing, phenotypically primitive and mitotically quiescent cells that have been identified in acute and chronic myeloid and lymphoid leukemia subtypes. Their noncycling status and inherent or acquired drug resistance mechanisms allow them to escape conventional and targeted therapies that effectively kill proliferating leukemia cells.98 In APL, PML-RARα is required, and even a minute amount of the oncoprotein allows LICs self-renewal in vivo.99 Zheng et al100 showed that in Sca1+/lin− murine hematopoietic stem cells retrovirally transduced with PML-RARα and LICs from PML-RARα mice, ex vivo treatment with arsenic overcomes the aberrant stem cell capacity of PML-RARα+ LICs. Whereas transcriptional activation of PML-RARα on effect of ATRA probably controls differentiation, only the catabolism of the fusion protein triggers LIC eradication and long-term remission of mouse APL.99 ATRA induces differentiation of PLZF-RARα–driven mouse APL, but neither LIC clearance nor disease remission was achieved, explaining the clinical ATRA resistance of this rare APL subtype. Importantly, the ATRA/ATO combination rapidly clears PML-RARα+ LICs, resulting in APL eradication in murine models and patients.99 Because anthracyclines101 produce ROS, the ATRA and idarubicin regimen102 may induce PML-RARα degradation and hence promote LIC clearance, resulting in dramatically prolonged survival.102
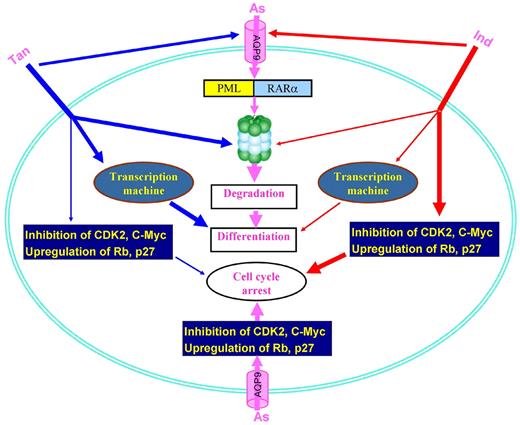
Arsenic targeting of PML may also be important in the non-APL setting. Indeed, Ito et al103 showed that PML was required for hematopoietic stem cell maintenance, and in CML it appeared to be the factor that enabled LICs to maintain their quiescence—the inert state that prevented them from being destroyed by cancer therapies. Interestingly, ATO could reversibly decrease PML expression in LICs, suggesting that this agent may be of broader interest than previously thought. In gliomas, ATO seemed able to inhibit the Notch pathway and deplete the cancer stem-like cell population.104 ATO was shown to antagonize the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector.105 ATO also represses NFκB106 and β-catenin,107 facilitating elimination of LICs (Figure 3).
Arsenic targets critical pathways for the leukemia-initiating cells. Arsenic induces generation of ROS, perturbation of some signal pathways, and modulation of transcriptional factors. Arsenic also activates MEK1/ERK pathway, whereas combination of MEK1 inhibitor (MEKi, red filled circle) and arsenic results in synergistic antitumor effects.
Arsenic targets critical pathways for the leukemia-initiating cells. Arsenic induces generation of ROS, perturbation of some signal pathways, and modulation of transcriptional factors. Arsenic also activates MEK1/ERK pathway, whereas combination of MEK1 inhibitor (MEKi, red filled circle) and arsenic results in synergistic antitumor effects.
Cell differentiation, apoptosis, and autophagy.
At the cellular level, ATO exerts dose-dependent dual effects on APL cells70 in that it induces apoptosis through activating the mitochondria-mediated intrinsic apoptotic pathway at high concentrations (1-2μM) and promotes cell differentiation at low concentrations (0.25-0.5μM; Figure 2C). The mechanisms of proapoptotic activity of ATO were scrutinized by many groups at gene/protein levels,38,108 and a large body of information has been gathered, including histone H3 phosphoacetylation at the CASP-10109 ; the involvement of JNK signaling110 ; anion exchanger 2111 and GSTP1-1112 ; the suppression of human telomerase reverse transcriptase (hTERT), C17, and C-MYC through Sp1 oxidation113 ; the repression of NFκB activation106 ; and the down-regulation of the WT1 gene.114 A pathway composed of ataxia telangiectasia mutated and Rad3-related, PML, CHK2, and p53 has been proposed to mediate ATO-induced apoptosis.115 ATO up-regulates a set of genes such as NADPH oxidase116 and 8-hydroxy-2′-deoxyguanosine (8-OHdG),117 and causes generation of ROS, which play a role as a mediator to induce apoptosis through release of cytochrome c to cytosol and activation of caspases. However, although all those mechanisms probably contribute to chronic or acute arsenic toxicity, it is unclear what is their contribution to arsenic response in APL, because they are unlikely to be specific for APL cells.
Recent studies further showed that in APL cells both ATRA and ATO induce autophagy by the mammalian target of rapamycin (mTOR)118 or MEK/ERK pathway,119 and autophagic degradation contributes significantly to proteolysis of PML-RARα. Inhibitors of autophagy or molecular targeting of BECN1 or ATG7 results in reversal of the suppressive effects of ATO on leukemic cells.119
Arsenic-based combinatory therapeutic regimens
ATO/ATRA combination.
Rationales.
Combination treatment regimens have been carefully designed to conquer APL.38 In a PML-RARα mouse model and a human NB4 APL cell line–based ascites/leukemia mouse model, ATRA/ATO combination dramatically prolongs survival or even eradicates the disease.120,121 Both ATO99,100 and ATRA99 induce LIC loss in PML-RARα mouse APL and the combination of the two agents results in synergistic clearance of LICs through cooperative PML-RARα degradation. Moreover, ATRA was shown to be able to increase the expression of the cell membrane arsenic transporter AQP9, which facilitated arsenic uptake.122
Although ATO alone induces partial differentiation,70 it synergizes with cAMP analog 8-CPT-cAMP in inducing full maturation of ATRA-sensitive and ATRA-resistance cells.123,124 However, activation of cAMP signaling was shown to enhance LIC loss by ATRA.99,125,126 Interestingly, ATRA could rapidly trigger a marked increase in the intracellular cAMP level and cAMP-dependent protein kinase (PKA) activity.127 Therefore, a cross talk may exist between ATO and ATRA signaling pathways through the cAMP/PKA node.
Moreover, ATRA potentiates ATO-induced RXRα phosphorylation and cooperates with ATO to induce apoptosis,128 and it was shown that ATRA induced degradation, whereas ATO antagonized catabolism of IκB.106 In the maturation-resistant NB4-R1 cells, ATRA exhibited antiproliferative properties through down-regulation of telomerase,129 and ATO enhanced this effect.130 When transcriptome/proteome approaches were used, Zheng et al131 reported that, although ATRA mainly caused transcriptional remodeling, ATO induced a deeper change of proteome pattern. ATRA/ATO combination amplified RA signaling, as highlighted by molecules involving IFN, calcium, cAMP/PKA, MAPK/JNK/p38, G-CSF, and TNF pathways. ATRA/ATO combination strongly activated the ubiquitin-proteasome pathway and significantly repressed genes/proteins promoting cell cycle or enhancing cell proliferation. Interestingly, the ATRA/ATO combination did not enhance the expression of stress response–related genes, including HSPA8, HSPCA, and AHSAI.131 Taken together, these results suggest that the ATRA/ATO combination may cause a synergy in therapeutic efficacy but not adverse effects.
Clinical efficacy.
A randomized clinical trial comparing ATRA/ATO combination and monotherapy was conducted in SIH from April 2001 to February 2003.38,45,132 Sixty-one APL cases were randomly assigned into 3 groups treated, respectively with ATRA, ATO, and the combination of the 2. It was reported in 2004 that the 3 groups achieved the same results in terms of CR rates (≥ 90%), although the combination therapy group needed the shortest time duration for remission induction. An obvious advantage of the combination therapy was that it generated much less minimal residual disease after consolidation than the 2 other therapeutic approaches as measured with real-time quantitative RT-PCR for PML-RARα. After a follow-up of 8-30 months all patients in the combination therapy group were in good clinical remission, whereas 7 of 37 cases in the 2 monotherapy groups relapsed (P < .05), showing the superiority of the combination therapy.45 Under this circumstance, the investigators from the SIH decided, from the ethical point of view, a termination of the randomized grouping and only the arm of combination therapy should be extended. In 2009, SIH reported the results of 85 patients administrated ATRA/ATO with a median follow-up of 70 months. Eighty patients (94.1%) entered CR.39 Kaplan-Meier estimates of the 5-year EFS and OS for all patients were 89.2% ± 3.4% and 91.7% ± 3.0%, respectively, and the 5-year relapse-free survival and OS for patients who achieved CR (n = 80) were 94.8% ± 2.5% and 97.4% ± 1.8%, respectively. On ATRA/ATO, prognosis was not influenced by initial white blood cell count, distinct PML-RARα types, or FLT3 mutations. The toxicity profile was mild and reversible (see “Adverse effects of arsenic”). The results were confirmed by recent long-term follow-up studies.133,134 Powell et al59 reported that of the 244 patients who received ATRA/chemotherapy as induction and ATRA/chemotherapy plus ATO as consolidation therapies, 195 cases (80%) achieved a 3-year EFS. Compared with the SIH trial that used ATRA/ATO/chemotherapy as induction therapy,39 the slightly lower EFS rate of this study might be because of the multicenter nature of the trial or could reflect the advantage of incorporating ATO into induction remedy for newly diagnosed APL. Taken together, ATRA/ATO/chemotherapy combinatory regimen transforms APL from a highly fatal to a highly curable disease.
Realgar-Indigo Naturalis Formula.
ATRA/As4S4 combination also showed enhanced therapeutic efficacy in APL.62 In traditional Chinese medicine, combination therapy containing multiple drugs with distinct but related mechanisms has been advocated for > 2500 years by prescriptions called formulas to amplify therapeutic efficacies of each agent and to minimize adverse effects.30,135 On the basis of traditional Chinese medicine theories, a patented Realgar-Indigo Naturalis Formula (RIF) was designed in the 1980s,63 in which a mined ore realgar was the principle element, whereas indigo naturalis, Salvia miltiorrhiza, and radix pseudostellariae were adjuvant components to assist the effects of realgar. Multicenter clinical trials showed that a CR rate of 96.7%136 to 98%63 and a 5-year OS rate of 86.88%137 were achieved in patients with APL receiving RIF, with moderate gastrointestinal discomfort and rash as the main adverse effects. Realgar in combination with indigo also exhibited an extent of anti-APL activity.138 The mechanisms of action of RIF were carefully dissected with the use of As4S4 (A), indirubin (I), and tanshinone IIA (T) as representatives of realgar, indigo naturalis, and Salvia miltiorrhiza, respectively,139 and it was shown that ATI combination yielded enhanced therapeutic efficacies against APL in murine model. ATI combination caused synergic effects and resulted in a much more profound differentiation of APL cells, potentiated ubiquitination and degradation of PML-RARα oncoprotein, stronger reprogramming of myeloid differentiation regulators, and enhanced G1/G0 arrest compared with cells treated with monoagents or biagents. Furthermore, T and I up-regulated AQP9 and facilitated transportation of arsenic into malignant promyelocytes, which in turn intensified arsenic-mediated PML-RARα degradation and therapeutic efficacies (Figure 4). These results open a new window for a better understanding of the therapeutic strategies of other traditional formulas.
Mechanisms of action of representative components of RIF in treating APL. As, As4S4; Ind, indirubin; Tan, tanshinone IIA.
Mechanisms of action of representative components of RIF in treating APL. As, As4S4; Ind, indirubin; Tan, tanshinone IIA.
ATO in combination with MEK1 inhibition.
Studies have shown that activation of ERK1/2 as well as of the kinases immediately upstream of ERK, known as MAP/ERK kinases or MEKs can confer a drug-resistant phenotype to cancer cells. For example, rapamycin and its analogs activate the MAPK pathway in solid tumor,140 imatinib increases the activity of p42/44 MAPK in CML CD34+ cells that contributes to incomplete elimination of CML progenitors,141 and FLT3 inhibitor-resistant cells show continued activation of PI3K/AKT and/or RAS/MEK/MAPK signaling pathways.142 Accordingly, MAPK/MEK inhibitors may be helpful to overcome drug resistance in leukemic cells. Altman et al143 showed that in leukemia cells on ATO treatment, the AKT kinase is phosphorylated/activated to regulate downstream engagement of mTOR and its effectors. Targeted disruption of AKT1/AKT2 genes or inhibition of mTOR strongly enhances ATO's effects on leukemia cells.143 Treatment with ATO induces a MAPK-mediated PML phosphorylation144 that is associated with subsequent ubiquitination and proteasomal degradation.46 Lunghi et al145 reported that APL cells exploited the RAS-MAPK pathway to inactivate the proapoptotic protein BAD by phosphorylation at Ser112 and to delay ATO-induced apoptosis. MEK1 inhibitors suppressed ERK1/2, dephosphorylated BAD, and inhibited the ATO-induced increase of Bcl-xL, resulting in enhanced apoptosis and overcoming drug resistance.145 Combined use of ATO and MEK1 inhibitors leads to induction of the p53AIP1 (p53-regulated apoptosis-inducing protein 1) in NB4 and K562 cell lines146 and primary cells from patients with AML,137 and inhibition of tumor growth and elongation of survival in a human xenograft multiple myeloma model. These studies provide the framework for testing MEK1 inhibitor/ATO combination in patients with hematologic malignancies.148
Arsenic in treating CML
CML, a malignant myeloproliferative disease originated from pluripotential hematopoietic stem cells, is characterized by the Philadelphia chromosome formed by translocation t(9;22)(q34;q11) that generates a chimeric fusion protein BCR-ABL with constitutively activated tyrosine kinase activity.149 Imatinib mesylate (IM; or Gleevec, Glivec, or STI571), a rationally designed BCR-ABL inhibitor, has shown remarkable clinical efficacy that achieved an estimated 5-year OS of 89% in 553 patients with CML.150 However, IM151,152 and dasatinib152,153 do not deplete LICs, whereas a proportion of patients develop IM resistance,152,154-156 and patients with advanced stage disease respond initially but then relapse.152,157 Moreover, cardiotoxicity of IM was also reported.158,159
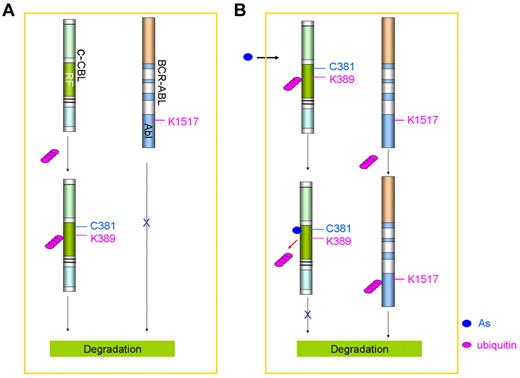
Historically, ATO therapy was the first chemotherapeutic intervention for CML. Fowler solution was used to treat CML in the 19th century and became the mainstream therapeutic reagent for leukemia.160 In the 1930s, the efficacy of arsenic in the treatment of CML established it as a primary therapeutic agent for this disease.160 Until the advent of modern chemotherapy, arsenic and radiation were the mainstays of treatment for patients with CML. Arsenic was shown to be able to target PML and to eradicate quiescent LICs in CML.103 ATO inhibited translation of mRNA of BCR/ABL, resulting in attenuation of BCR/ABL levels and apoptosis of human leukemia cells.161 Zhang et al162 reported that arsenic targets BCR-ABL by ubiquitination of key lysine residues, leading to its proteasomal degradation. Recently, Mao et al83 showed that arsenic could directly bind c-CBL, the E3 ligase of BCR-ABL, by the conserved cysteines, including C381 at RING finger domain (Figure 5), resulting in inhibition of c-CBL's self-ubiquitination at K389 and subsequent proteasomal degradation. Consistent with these results, substitution of cysteine at 381 or lysine at 389 by alanine abrogated arsenic binding and c-CBL's self-ubiquitination, respectively. Consequently, elevated c-CBL promoted ubiquitination of BCR-ABL at K1517, leading to degradation of the aberrant kinase (Figure 5).
Effects of arsenic on c-CBL and BCR-ABL in CML cells. (A) Without arsenic treatment, c-CBL is self-ubiquitinated and degraded in proteasome. (B) Arsenic treatment inhibits c-CBL self-ubiquitination and proteasomal degradation, and triggers ubiquitination of BCR-ABL at K1517 followed by degradation in the proteasome.
Effects of arsenic on c-CBL and BCR-ABL in CML cells. (A) Without arsenic treatment, c-CBL is self-ubiquitinated and degraded in proteasome. (B) Arsenic treatment inhibits c-CBL self-ubiquitination and proteasomal degradation, and triggers ubiquitination of BCR-ABL at K1517 followed by degradation in the proteasome.
Arsenic exerts synergistic effects with IM in inducing apoptosis of CML cells and in prolonging survival of mice inoculated with CML cells.162-164 It was shown that arsenic and IM induce cell cycle arrest at G2/M and G1 phases, respectively. Arsenic and IM synergistically activate the endogenous and exogenous endoplasmic reticulum (ER) stress, leading to enhanced cell apoptosis.162,163,165 These discoveries provide rationales for a clinical trial to test the arsenic/IM combination therapy in CML.
Arsenic in treating other malignancies
Arsenic has been used in treating multiple myeloma, myelodysplasia syndrome, and lymphoid malignancies, including non-Hodgkin lymphoma, and has displayed beneficial effects in some cases (Table 3). In adult T-cell leukemia/lymphoma (ATL)–derived cells, ATO reportedly synergized with IFNα to induce cell-cycle arrest and apoptosis176 through down-regulation of the HTLV-1 oncoprotein Tax and inactivation of NF-κB.177,178 Clinically, arsenic/IFN therapy exhibited some efficacy in 7 patients with refractory aggressive ATL,170 whereas in 10 cases of newly diagnosed chronic ATL arsenic/IFN/zidovudine combination showed an impressive 100% response rate.171 Recent animal studies in Lck-Tax transgenics that develop an ATL-like disease have recapitulated the therapeutic action of the arsenic/IFNα association, strongly suggesting that the latter is actually targeting Tax for degradation.179 Moreover, transplantation studies have shown that Tax degradation is accompanied by loss of leukemia-initiating activity, but not short-term growth, providing a striking parallel with APL and suggesting that arsenic may promote catabolism of specific classes of oncoproteins.
Clinical studies of arsenic in treating other malignancies
| Disease . | Year . | Authors . | No. . | Regimen . | Response . |
|---|---|---|---|---|---|
| MM | 2006 | Berenson et al166 | 65 | ATO + AA + melphan | 2 CR; 15 PR; 14 MR |
| 2006 | Abou-Jawde et al167 | 20 | ATO + AA + dexamethasone | 6 PR | |
| 2006 | Wu et al168 | 20 | ATO + AA + dexamethasone | 2 PR; 6 MR | |
| 2007 | Berenson et al169 | 22 | ATO + AA + bortezomib | 2 PR; 4 MR | |
| ATL | 2004 | Hermine et al170 | 7 (Relapsed/refractory) | ATO + IFN | 1 CR; 3 PR |
| 2009 | Kchour et al171 | 10 (Newly diagnosed) | ATO + IFN + zidovudine | 9 CR; 1 PR | |
| MDS | 2006 | Schiller et al172 | 76 | ATO | 1 CR; 13 HI |
| 2006 | Vey et al173 | 115 | ATO | 1 CR; 1 PR; 22 HI | |
| 2008 | Zheng et al174 | 21 | ATO + RA + thalidomide | 1 CR; 1 PR; 3 HI | |
| Lymphoid malignancies | 2009 | Chang et al175 | 16 | ATO + AA | 1 response |
| Disease . | Year . | Authors . | No. . | Regimen . | Response . |
|---|---|---|---|---|---|
| MM | 2006 | Berenson et al166 | 65 | ATO + AA + melphan | 2 CR; 15 PR; 14 MR |
| 2006 | Abou-Jawde et al167 | 20 | ATO + AA + dexamethasone | 6 PR | |
| 2006 | Wu et al168 | 20 | ATO + AA + dexamethasone | 2 PR; 6 MR | |
| 2007 | Berenson et al169 | 22 | ATO + AA + bortezomib | 2 PR; 4 MR | |
| ATL | 2004 | Hermine et al170 | 7 (Relapsed/refractory) | ATO + IFN | 1 CR; 3 PR |
| 2009 | Kchour et al171 | 10 (Newly diagnosed) | ATO + IFN + zidovudine | 9 CR; 1 PR | |
| MDS | 2006 | Schiller et al172 | 76 | ATO | 1 CR; 13 HI |
| 2006 | Vey et al173 | 115 | ATO | 1 CR; 1 PR; 22 HI | |
| 2008 | Zheng et al174 | 21 | ATO + RA + thalidomide | 1 CR; 1 PR; 3 HI | |
| Lymphoid malignancies | 2009 | Chang et al175 | 16 | ATO + AA | 1 response |
MM indicates multiple myeloma; AA, ascorbic acid; CR, complete response; PR, partial response; MR, minor response; ATL, adult T-cell leukemia/lymphoma; MDS, myelodysplasia syndrome; HI, hematologic improvement; and RA, retinoic acid.
There are 111 recently completed or ongoing clinical trials listed on www.clinicaltrials.gov that evaluate ATO alone or in combination with other agents for treatment of cancers, excluding APL. ATO is under investigation as treatment for a variety of solid tumors, including lung cancer, hepatocellular carcinoma, and colorectal cancer. Limited clinical activity as a single agent has been reported in a small number of patients with hepatocellular carcinoma, melanoma, and renal cell carcinoma; ATO in combination with chemotherapy has shown promising activity in osteosarcoma and Ewing sarcoma180 (and references in this review article).
Adverse effects of arsenic
Although arsenic seems to be synonymous with poison, nearly all recent clinical trial results suggest that arsenic at therapeutic concentrations is generally well tolerated. No bone marrow depression and chemotherapy-associated secondary malignancy were observed with arsenic treatment.52,54
Sudden death was recorded in one study,181 and severe liver impairment was documented.53 These toxicities might be because of a genetic basis with exceptional susceptibilities to arsenic toxicity in rare patients, exposure to anthracyclines or other cardiotoxic agents before ATO therapy, and abnormal electrolyte levels, or other unidentified factors.53,57,181,182 Hyperleukocytosis, a retinoic acid syndrome (or differentiation syndrome)–like clinical entity was also reported and was shown to be driven by chemokine production induced by ATO or ATRA as a single agent or in combination.183 In long-term studies,39,57,58,182 the toxicity profile of arsenic was mild, and 24 months after the last dose of ATRA/ATO patients had urine arsenic concentrations well below the safety limit. Mathews et al57 reported that despite counseling against pregnancy after therapy, in view of the absence of data on effect of prior ATO therapy and teratogenicity, 7 patients (4 women and 3 men) have had 8 normal babies.
Perspectives
As a traditional poison, inappropriate use of arsenic may kill people; as one of the oldest drugs in the world, its appropriate application cures some cancer types and saves lives. These facts clearly suggest that when considering how to control an emerging “bad factor,” one might try to find out its other side and the safe translation.
Arsenic is the most potent single agent against APL. The revival of arsenic by its application in treating APL is a unique story in cancer research. It also highlights some of the essential concepts in pharmacology, such as the key importance of the therapeutic ratio between normal cells and the target. It illustrates the power of combinations. Indeed, in APL, ATO/ATRA combination has exhibited drastically enhanced therapeutic efficacy compared with either single agent, transforming the fate of an otherwise highly fatal disease. In treating other types of malignancies, including solid tumors, rational combinatory regimens could be designed to improve clinical outcome. For example, because arsenic binds and activates c-CBL that controls signaling of EGFR, the therapeutic efficacy of ATO in combination with EGFR inhibitor could be tested in non–small cell lung cancer and other related human malignancies.
Acknowledgments
We apologize to our many colleagues whose work could not be cited because of space restrictions. We thank Prof Zhen-Yi Wang at SIH for his long term support, Dr Laurent Degos from Hospital Saint Louis in Paris, and Dr Samuel Waxman from Mount Sinai Medical Center in New York for friendly long-term collaboration.
This work was supported in part by the Chinese National Key Program for Basic Research (973; 2010CB529200) and National High Tech Program (863), National Natural Science Foundation of China, Shanghai Municipal Commission for Science and Technology, Shanghai Municipal Commission for Education, and Samuel Waxman Cancer Research Foundation.
Authorship
Contribution: S.-J.C., G.-B.Z., X.-W.Z., J.-H.M., H.d.T., and Z.C. all contributed to the writing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sai-Juan Chen, Shanghai Institute of Hematology, Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 197 Rui Jin Rd II, Shanghai, 200025, China; e-mail: sjchen@stn.sh.cn; or Zhu Chen, Shanghai Institute of Hematology, Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 197 Rui Jin Rd II, Shanghai, 200025, China; e-mail: zchen@stn.sh.cn.