Abstract
The inhibitor of Bruton tyrosine kinase γ (IBtkγ) is a negative regulator of the Bruton tyrosine kinase (Btk), which plays a major role in B-cell differentiation; however, the mechanisms of IBtkγ-mediated regulation of Btk are unknown. Here we report that B-cell receptor (BCR) triggering caused serine-phosphorylation of IBtkγ at protein kinase C consensus sites and dissociation from Btk. By liquid chromatography and mass-mass spectrometry and functional analysis, we identified IBtkγ-S87 and -S90 as the critical amino acid residues that regulate the IBtkγ binding affinity to Btk. Consistently, the mutants IBtkγ carrying S87A and S90A mutations bound constitutively to Btk and down-regulated Ca2+ fluxes and NF-κB activation on BCR triggering. Accordingly, spleen B cells from Ibtkγ−/− mice showed an increased activation of Btk, as evaluated by Y551-phosphorylation and sustained Ca2+ mobilization on BCR engagement. These findings identify a novel pathway of Btk regulation via protein kinase C phosphorylation of IBtkγ.
Introduction
Bruton tyrosine kinase (Btk) is a member of the Tec family of nonreceptor protein tyrosine kinases and is expressed in B cells, macrophages, and neutrophils.1 Btk sustains the developmental program of pre-B cells by limiting the pre-B cell expansion and by promoting B-cell differentiation.1,2 Consistently, mutations of BTK cause the human X-linked agammaglobulinemia and the murine X-linked immunodeficiency syndromes, which are characterized by increased susceptibility to recurrent bacterial infections as a consequence of the impaired generation of mature B cells and low production of immunoglobulin.3,4
Btk is a crucial component of the immunoglobulin B-cell receptor (BCR) signaling pathway. Evidence from Btk-deficient B cells (DT40)5 indicates that Btk is required for a proper tyrosine phosphorylation of phospholipase C-γ (PLC-γ), which in turn leads to inositol-3,4,5-triphosphate, a major mediator of [Ca2+]i mobilization, and to diacylglycerol, an activator of protein kinase C (PKC).6 These pathways activate specific transcription factors, including NF-κB,7,8 which regulate the gene transcription program required for B-cell survival and cell-cycle progression. Accordingly, DT40 Btk−/− chicken B cells show a drastic decrease in Ca2+ signaling and NF-κB activation on antigen stimulation,9 and Btk−/− mice have a significant reduction of B cells.10,11
Btk harbors the Pleckstrin homology domain (PH) and the Src homology domain 2 (SH2) and SH3, suggesting that its regulation occurs through protein-protein interaction.12 The PH domain mediates the binding of Btk to phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) resulting in the recruitment of Btk to the plasma membrane.13 The kinase activity of Btk is up-regulated by the Src-mediated phosphorylation of Y551 at the activation loop of the Btk kinase domain and by autophosphorylation of Y223 in the Src homology domain 3,14,15 whereas it is inactivated by the PKCβ-mediated phosphorylation of S180.16 Genetic evidence indicates that Syk, BLNK, and Btk are all required for the full activation of PLCγ and Ca2+ signaling on BCR triggering,17-20 which suggests the occurrence of a multimolecular complex for PLCγ activation.21,22
In this scenario, it was proposed that the BCR triggering causes the Syk-mediated tyrosine phosphorylation of the signaling adaptor BLNK, which recruits PLCγ and Btk by their SH2 domains23 and promotes the tyrosine phosphorylation and activation of PLCγ by Btk.24 Moreover, Syk binds directly to the SH2(N) domain of PLCγ and phosphorylates PLCγ at key regulatory tyrosine residues.25 Thus, PLCγ is recruited at the plasma membrane and is phosphorylated by both Syk and Btk to sustain the BCR-induced Ca2+ signaling. In addition to its kinase function, Btk was shown to interact with and activate the phosphatidylinositol-4-phosphate 5 kinase (PIP5K) at the cell membrane26 ; this pathway promotes the production of PI(4,5)P2, a substrate of both PLCγ and PI3K. Interestingly, the Btk-PH domain is required for the interaction with PIP5K.26 Thus, Btk works both as a kinase and a scaffold protein to amplify the Ca2+ signaling downstream of the antigen-stimulated BCR.27
We have previously identified the Inhibitor of Btk (IBtk) as a ≈25-kDa protein that bound to the PH domain and at a lesser extent to the SH2 domain of Btk and repressed Btk-mediated Ca+2 mobilization and NF-κB activation on BCR triggering.28 We have recently reported the physical and functional analysis of the IBTK locus, which encodes for the IBtk isoforms α, β, and γ.29 IBtkα (150.53 kDa) and IBtkβ (133.87 kDa) are ubiquitous and might exert regulatory functions beyond the Btk regulation by associating with other proteins.29 IBtkγ (26.31 kDa), which was the first identified isoform,28 arises from an independent promoter included within the intron 24 of the IBTK gene, resulting in a 240-amino acid protein.29 IBtkγ is mainly expressed in hematopoietic cells28,29 and might play a major role in Btk-mediated regulation of B cells by competing for the binding of Btk-PH-SH2 domain to other signaling molecules, such as BLNK, PIP3, and PIP5K.
In this study, we have addressed the role of IBtkγ in the regulation of Btk activity in response to the BCR triggering. The IBtkγ amino acidic sequence includes several PKC consensus sites, suggesting that IBtkγ may be regulated by serine-phosphorylation in response to B-cell signaling. To address this possibility, we evaluated the PKC-mediated phosphorylation of IBtkγ and its outcome on the stability of the Btk:IBtkγ complex and Btk activity. We found that IBtkγ is phosphorylated at serines 87 and 90 by PKC on BCR engagement; this phosphorylation causes the dissociation of the Btk:IBtkγ complex and allows Btk to translocate to the plasma membrane. These findings underscore a novel signaling pathway in which IBtkγ regulates the amplification of BCR signaling mediated by Btk.
Methods
Plasmids and antibodies
A description of plasmids and antibodies used in this study is reported in the supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell cultures, treatments, and luciferase assay.
Experimental details for cell cultures, treatments, and luciferase assay are described in supplemental Data.
Lentiviral production and transduction.
The generation of DeFew lymphoma cells stably expressing wild-type or mutant IBtkγ proteins was achieved by use of the pHIV-enhanced green fluorescent protein (EGFP) self-inactivating lentiviral vector30 containing an internal ribosome entry sequence (IRES) located upstream of the EGFP gene. Viral production and transduction were performed as previously described31 and reported in supplemental Data.
Western blotting and immunoprecipitation.
Experimental details for Western blotting and immunoprecipitation are reported in supplemental Data.
Subcellular fractionation and protein distribution.
Cells (1.8 × 108) were resuspended in RPMI medium (3.6 mL) and divided in aliquots to be stimulated with 15 μg/mL of anti-IgM F(ab′)2 for 5 or 20 minutes, or they were left untreated. Cells were lysed in 1.2 mL of the fractionation buffer (0.25M sucrose; 20mM tricine, pH 7.8; 1mM EDTA; protease inhibitors; and phosphatase inhibitors) by the use of a tight glass douncer (30 strokes). Lysates were clarified by centrifugation at 1000g for 10 minutes and supernatants were 1:10 diluted with fractionation buffer and subjected to ultracentrifugation by the use of an MLS-50 Beckmann rotor (80 minutes at 50 000 rpm). The pellet (membrane fraction) was resuspended in 1.2 mL of fractionation buffer. Membrane and cytosol samples were resuspended in NuPAGE sample buffer (Invitrogen), incubated for 15 minutes at 70°C, and analyzed by Western blotting.
Production of IBtkγ recombinant proteins.
In vitro kinase assays.
For Btk kinase assay, Btk was immunoprecipitated from cell extracts with anti-Btk Ab, and the Btk activity was evaluated by the use of an ELISA-based tyrosine kinase assay (cat. no. PTK101; Sigma-Aldrich), according to the manufacturer's protocol. Procedures for PKC kinase assay are described in supplemental Data.
In-gel tryptic digestion and mass spectrometry analysis.
After SDS-PAGE and staining, protein bands were processed according to Shevchenko et al.32 Further details are reported in supplemental Data.
Confocal microscopy.
Detailed procedures for confocal microscopy analysis are reported in supplemental Data.
Intracellular Ca2+ flux assay.
The intracellular Ca2+ fluxes were measured as previously described33 by flow cytometry with the FACSCalibur system 4-color, dual-laser (BD Biosciences). Cell staining and flow cytometry are described in supplemental Data.
Mice.
The generation of Ibtkγ−/− mice is described in supplemental Data. The Institutional Animal Care and Use Committee of the University “Magna Græcia” of Catanzaro approved the animal protocols, according to the guidelines of the National Institute of Health, Italy.
Statistical methods.
Statistical analysis was performed by the 2-tailed, unpaired Student t test. Data were reported as the means ± SEM. The differences between the means were accepted as statistically significant at the 95% level (P ≤ .05).
Results
BCR stimulation causes the PKC-mediated phosphorylation of IBtkγ and dissociation of the Btk:IBtkγ complex
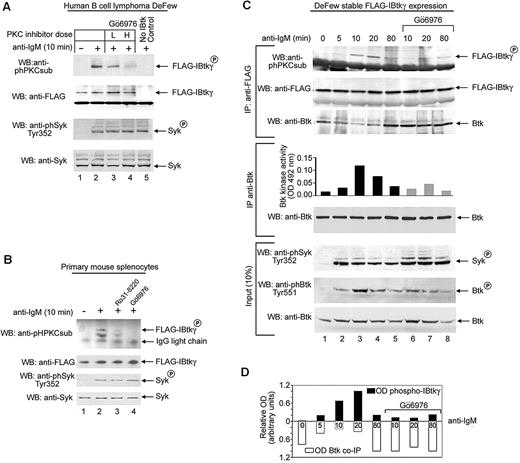
MotifScan-based analysis (http://scansite.mit.edu) of the amino acid sequence of IBtkγ (sequence submission: DQ005635) identified 5 canonical PKC sites at serines 87, 90, and 115 and at threonines 33 and 64 with a significant probability score for specific PKC isoforms (supplemental Figure 1). We tested whether the PKC-mediated phosphorylation of IBtkγ occurred in vivo on BCR activation. Because cell lines poorly express IBtkγ (supplemental Figure 2A), we first used lysates from DeFew cells, a human B lymphoma cell line,34 to phosphorylate in vitro the FLAG-IBtkγ immunoprecipitated from transfected HEK 293T cells. The PKC-specific phosphorylation was detected by use of the antiphosphoserine PKC substrate Ab (anti-phPKCsub Ab). The PKC-phosphorylation of IBtkγ was undetectable on incubation with lysates from unstimulated DeFew cells, whereas it occurred with lysates from anti–IgM-stimulated DeFew cells (Figure 1A top lanes 1 and 2). Consistent with BCR activation, phosphorylation of Syk on Tyr 352 was detected in stimulated cells (Figure 1A third panel lane 2). The PKC inhibitor Gö6976 decreased the PKC-specific phosphorylation of IBtkγ on anti-IgM stimulation in a dose-related manner (Figure 1A top lanes 3 and 4). Similar results were obtained with mouse spleen B lymphocytes stimulated with anti–mouse IgM (Figure 1B). These results indicate that the PKC-mediated phosphorylation of IBtkγ occurs on BCR triggering.
BCR triggering leads to the PKC-dependent phosphorylation of IBtkγ. (A) DeFew cells were pretreated for 20 minutes with the PKC inhibitor Gö6976 or control DMSO vehicle at low (L, 40nM) or high (H, 200nM) concentrations; then, cells were stimulated for 10 minutes with F(ab′)2 fragments of antihuman IgM (13 μg/mL), and lysates (3 × 106 cells) were incubated for 10 minutes with FLAG-IBtkγ linked to anti-FLAG agarose beads or control anti-FLAG–agarose beads. Proteins were analyzed by Western blotting with the indicated antibodies. As control of BCR triggering, Syk levels and Tyr352-phosphorylation of Syk were analyzed with specific antibodies. One representative of 3 independent experiments with similar results is shown. (B) Primary mouse splenocytes were pretreated for 10 minutes with either the indicated PKC inhibitors (200nM), or control DMSO vehicle, followed by a 10-minute stimulation with anti–mouse IgM F(ab′)2 (20 μg/mL). Lysates (8 × 106 splenocytes) were incubated with FLAG-IBtkγ linked to anti-FLAG agarose beads or control anti-FLAG-agarose beads; proteins were analyzed by Western blotting with the indicated antibodies. One representative of 3 independent experiments with similar results is shown. (C) DeFew cells stably expressing FLAG-IBtkγ were stimulated with F(ab′)2 fragments of anti–human IgM (13 μg/mL) or left untreated in the presence or absence of Gö6976 (80nM) for the indicated time; cells were lysed, immunoprecipitated with anti-FLAG, and analyzed by Western blotting with anti-phPKCsub and anti-FLAG antibodies (top). For in vitro Btk kinase activity, protein extracts were immunoprecipitated with anti-Btk and analyzed by an ELISA-based tyrosine kinase assay. One representative of 2 independent experiments with similar results is shown. (D) The optical density (OD) of phospho-FLAG-IBtkγ was normalized according to FLAG-IBtkγ (upper bars) or Btk coimmunoprecipitated (co-IP) with FLAG-IBtkγ (lower bars).
BCR triggering leads to the PKC-dependent phosphorylation of IBtkγ. (A) DeFew cells were pretreated for 20 minutes with the PKC inhibitor Gö6976 or control DMSO vehicle at low (L, 40nM) or high (H, 200nM) concentrations; then, cells were stimulated for 10 minutes with F(ab′)2 fragments of antihuman IgM (13 μg/mL), and lysates (3 × 106 cells) were incubated for 10 minutes with FLAG-IBtkγ linked to anti-FLAG agarose beads or control anti-FLAG–agarose beads. Proteins were analyzed by Western blotting with the indicated antibodies. As control of BCR triggering, Syk levels and Tyr352-phosphorylation of Syk were analyzed with specific antibodies. One representative of 3 independent experiments with similar results is shown. (B) Primary mouse splenocytes were pretreated for 10 minutes with either the indicated PKC inhibitors (200nM), or control DMSO vehicle, followed by a 10-minute stimulation with anti–mouse IgM F(ab′)2 (20 μg/mL). Lysates (8 × 106 splenocytes) were incubated with FLAG-IBtkγ linked to anti-FLAG agarose beads or control anti-FLAG-agarose beads; proteins were analyzed by Western blotting with the indicated antibodies. One representative of 3 independent experiments with similar results is shown. (C) DeFew cells stably expressing FLAG-IBtkγ were stimulated with F(ab′)2 fragments of anti–human IgM (13 μg/mL) or left untreated in the presence or absence of Gö6976 (80nM) for the indicated time; cells were lysed, immunoprecipitated with anti-FLAG, and analyzed by Western blotting with anti-phPKCsub and anti-FLAG antibodies (top). For in vitro Btk kinase activity, protein extracts were immunoprecipitated with anti-Btk and analyzed by an ELISA-based tyrosine kinase assay. One representative of 2 independent experiments with similar results is shown. (D) The optical density (OD) of phospho-FLAG-IBtkγ was normalized according to FLAG-IBtkγ (upper bars) or Btk coimmunoprecipitated (co-IP) with FLAG-IBtkγ (lower bars).
To improve the in vivo analysis of IBtkγ phosphorylation induced by BCR cross-linking, we generated DeFew B cells that stably expressed FLAG-IBtkγ. Then, IBtkγ was immunoprecipitated from FLAG-IBtkγ DeFew cells stimulated with anti-IgM and analyzed for association with Btk and PKC phosphorylation. By in vivo immunoprecipitation, we estimated that approximately 27% of total Btk was associated with IBtkγ in unstimulated cells (supplemental Figure 2B). The PKC phosphorylation of IBtkγ was observed as soon as 5 minutes on anti-IgM stimulus with a sustained increase up to 20 minutes posttreatment (Figure 1C top; 1D). The IBtkγ phosphorylation was inversely correlated with the binding of IBtkγ to Btk (Figure 1C top; 1D), whereas it directly correlated with the Btk activation, as evaluated by in vitro kinase activity (Figure 1C middle) and Tyr551 phosphorylation (Figure 1C bottom). Accordingly, the PKC inhibitor Gö6976, which significantly inhibited the PKC-phosphorylation of IBtkγ, caused an increased amount of Btk coimmunoprecipitated with IBtkγ (Figure 1C lanes 6-8 top; 1D), and a decreased activation of Btk on anti-IgM stimulation (Figure 1C lanes 6-8 middle and bottom). These results indicate that the PKC-mediated phosphorylation of IBtkγ induced by BCR triggering promotes the dissociation of the Btk:IBtkγ complex with the release of active Btk.
PKC directly phosphorylates IBtkγ
Next, we tested whether PKC directly phosphorylates IBtkγ. The recombinant GST-IBtkγ was produced in bacteria and incubated in a kinase assay with a mixture of PKC isoforms (PKC mix), the recombinant PKCβ, or PKCμ (also known as PKD). In the absence of PKC, GST-IBtkγ was unphosphorylated (Figure 2A lane 2), whereas it became phosphorylated in the presence of PKC mix, PKCβ, and PKCμ (Figure 2A lanes 3-5). Control GST alone was not phosphorylated (Figure 2A lane 1), whereas the PKCs isoforms were autophosphorylated (Figure 2A lanes 1,3-5). Furthermore, the native His-IBtkγ was in vitro phosphorylated by the PKCβ, a prototype member of PKC family protein that is mostly expressed in B cells,35 whereas the heat-denatured preparation remained unmodified (supplemental Figure 3).
Distinct PKC isoforms phosphorylate IBtkγ. (A) GST-IBtkγ (1 μg) was in vitro phosphorylated with purified PKCmix (25 ng), recombinant PKCβ (50 ng), or PKCμ (50 ng) in the presence of [γ-32P]ATP and analyzed by 4%–12% NuPAGE followed by autoradiography and Coomassie blue staining. (B) Schematic representation of GST-IBtkγ constructs with putative PKC phosphorylation sites. (C) GST or GST fused with N-terminus (aa 30-130) or C-terminus (aa 131-240) of IBtkγ (5 μg) were incubated with PKCmix (100 ng) in the presence of [γ-32P]ATP; proteins were analyzed by 4%–12% NuPAGE followed by autoradiography and Coomassie blue staining. (D) HEK 293T cells (1 × 106) were transfected with expression vectors of PKC isoforms or empty vector (1.5 μg) together with pCMV7.13xFLAG-IBtkγ or the relative empty vector (2 μg); 48 hours after transfection, cells were treated for 45 minutes with PMA, and lysates were analyzed by 10% SDS-PAGE followed by Western blotting with anti-phPKC substrate and anti-FLAG antibodies.
Distinct PKC isoforms phosphorylate IBtkγ. (A) GST-IBtkγ (1 μg) was in vitro phosphorylated with purified PKCmix (25 ng), recombinant PKCβ (50 ng), or PKCμ (50 ng) in the presence of [γ-32P]ATP and analyzed by 4%–12% NuPAGE followed by autoradiography and Coomassie blue staining. (B) Schematic representation of GST-IBtkγ constructs with putative PKC phosphorylation sites. (C) GST or GST fused with N-terminus (aa 30-130) or C-terminus (aa 131-240) of IBtkγ (5 μg) were incubated with PKCmix (100 ng) in the presence of [γ-32P]ATP; proteins were analyzed by 4%–12% NuPAGE followed by autoradiography and Coomassie blue staining. (D) HEK 293T cells (1 × 106) were transfected with expression vectors of PKC isoforms or empty vector (1.5 μg) together with pCMV7.13xFLAG-IBtkγ or the relative empty vector (2 μg); 48 hours after transfection, cells were treated for 45 minutes with PMA, and lysates were analyzed by 10% SDS-PAGE followed by Western blotting with anti-phPKC substrate and anti-FLAG antibodies.
The N-terminal sequence of IBtkγ from amino acid (aa) 30 to 130 includes 5 PKC consensus sites, whereas the C-terminal sequence from aa 131 to 240 lacks putative PKC sites (Figure 2B). Consistent with the mapping of PKC sites, GST-IBtkγ (aa 30-130) was in vitro phosphorylated by PKCβ, whereas GST-IBtkγ (aa 131-240) was unaffected (Figure 2C lanes 3 and 5). To identify the PKC isoforms that phosphorylate IBtkγ in vivo, HEK 293T cells were transfected with plasmids expressing FLAG-IBtkγ either alone, or together with specific HA-tagged PKC isoforms36 and stimulated with or without PMA, a PKC activator; then, FLAG-IBtkγ was immunoprecipitated and analyzed by immunoblotting with anti-PKC substrate Ab. In unstimulated cells, overexpression of the PKC isoforms α, β, γ, δ, ϵ, θ, and μ did not cause phosphorylation of FLAG-IBtkγ (Figure 2D lanes 6,8,10,12,14, 16,18). On PMA treatment, the phosphorylation of FLAG-IBtkγ occurred at lower level in PMA-treated control cells (Figure 2D lane 5) and was significantly increased upon over-expression of the PKC isoforms α, β, γ, δ, ϵ, and μ (Figure 2D lanes 7,9,13,15,17,19), with the exception of PKCθ, which did not cause the phosphorylation of IBtkγ (Figure 2D lane 11). These results demonstrate that IBtkγ is a specific substrate of most PKC isoforms in vitro and in vivo and is phosphorylated at the PKC consensus within the sequence from aa 30 to 130.
Identification of IBtkγ phospho-serines on PKC phosphorylation
To identify the phospho-serines generated by PKC-mediated phosphorylation of IBtkγ, GST-IBtkγ (aa 30-130) was in vitro phosphorylated with PKCβ, trypsinized, and subjected to liquid chromatography and mass-mass spectrometry (LC-MS/MS). This analysis identified the peptide 75-89 (PVNAWASSLHSVSSK) that includes the phosphoserine 81 or 87 (underlined), and the peptide 90-99 (SFRDFLLEEK) that shows the phosphoserine 90 (underlined; Figure 3A). The LC/MS-MS spectra are reported in supplemental Figure 4A through C. Serines 87 and 90 are located within canonical PKC consensus sites, whereas serine 81 is missed in a conventional PKC site (Figure 3B). To verify that the identified serines of IBtkγ are substrates of PKC, mutants GST-IBtkγ (aa 30-130), carrying base-pair substitutions of serines 81, 87, or 90 to alanines, were generated and subjected to phosphorylation by PKCβ in a kinase assay. IBtkγ S81A was phosphorylated to a comparable extent of the wild-type IBtkγ (Figure 3C lanes 2 and 3); differently, IBtkγ S87A and IBtkγ S90A were 60% and 40% phosphorylated, respectively, compared with wild-type IBtkγ (Figure 3C lanes 4 and 5). The double mutant IBtkγ S87/90A and the quintuple mutant IBtkγ S81/82/87/88/90A were 30% phosphorylated compared with the wild-type IBtkγ (Figure 3C lanes 6 and 9). These results indicate that S87 and S90 are the main targets of IBtkγ phosphorylation by PKC in vitro.
Identification of PKC phosphorylation sites of IBtkγ. (A) Schematic representation of the phosphopeptides detected by LC-MS/MS on in vitro phosphorylation of GST-IBtkγ (aa 30-130) (4 μg) with PKCβ (100 ng) followed by tryptic digestion. (B) Picture of IBtkγ N-terminal sequence with the putative PKC phosphorylation sites as predicted by Motifscan software; the PKC phosphorylation sites identified by LC-MS/MS are shown in red circles. (C) GST-IBtkγ (aa 30-130) wild-type or mutants carrying the indicated serine to alanine substitutions (1 μg) were in vitro phosphorylated with PKCmix (25 ng) for 30 minutes in the presence of [γ-32P]ATP; proteins were analyzed by 8% SDS-PAGE followed by autoradiography and Coomassie blue staining. The densitometry of the phospho-IBtkγ signal was performed in 3 independent experiments, and optical density of phospho-IBtkγ was normalized to Coomassie blue staining. Densitometry of IBtkγ mutants relatively to wild type was expressed as the mean values of 4 independent experiments ± SEM (bottom); the asterisk indicates a statistically significant difference between the IBtkγ mutant and the wild type according to the Student t test: (*P = .0003; **P = .0001; ***P = .0005; ****P = .0001; for n = 4). (D) DeFew cells (2.4 × 107 cells) stably expressing wild-type or mutants FLAG-IBtkγ-IRES-EGFP upon lentiviral transduction were stimulated for 10 minutes with F(ab′)2 fragments of anti–human IgM (13 μg/mL); cell lysates (1 mg) were immunoprecipitated with anti-phPKCsub Ab and analyzed by 10% SDS-PAGE and Western blotting with anti-FLAG Ab. A representative experiment of 2 independent experiments is shown. (E) Alignment of orthologous IBtkγ amino acid sequences from different species was performed with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/); the PKC sites are indicated in the square and include the S81 and S87 and S90 as identified by LC-MS/MS.
Identification of PKC phosphorylation sites of IBtkγ. (A) Schematic representation of the phosphopeptides detected by LC-MS/MS on in vitro phosphorylation of GST-IBtkγ (aa 30-130) (4 μg) with PKCβ (100 ng) followed by tryptic digestion. (B) Picture of IBtkγ N-terminal sequence with the putative PKC phosphorylation sites as predicted by Motifscan software; the PKC phosphorylation sites identified by LC-MS/MS are shown in red circles. (C) GST-IBtkγ (aa 30-130) wild-type or mutants carrying the indicated serine to alanine substitutions (1 μg) were in vitro phosphorylated with PKCmix (25 ng) for 30 minutes in the presence of [γ-32P]ATP; proteins were analyzed by 8% SDS-PAGE followed by autoradiography and Coomassie blue staining. The densitometry of the phospho-IBtkγ signal was performed in 3 independent experiments, and optical density of phospho-IBtkγ was normalized to Coomassie blue staining. Densitometry of IBtkγ mutants relatively to wild type was expressed as the mean values of 4 independent experiments ± SEM (bottom); the asterisk indicates a statistically significant difference between the IBtkγ mutant and the wild type according to the Student t test: (*P = .0003; **P = .0001; ***P = .0005; ****P = .0001; for n = 4). (D) DeFew cells (2.4 × 107 cells) stably expressing wild-type or mutants FLAG-IBtkγ-IRES-EGFP upon lentiviral transduction were stimulated for 10 minutes with F(ab′)2 fragments of anti–human IgM (13 μg/mL); cell lysates (1 mg) were immunoprecipitated with anti-phPKCsub Ab and analyzed by 10% SDS-PAGE and Western blotting with anti-FLAG Ab. A representative experiment of 2 independent experiments is shown. (E) Alignment of orthologous IBtkγ amino acid sequences from different species was performed with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/); the PKC sites are indicated in the square and include the S81 and S87 and S90 as identified by LC-MS/MS.
To explore the role of S87 and S90 in the in vivo phosphorylation of IBtkγ, we generated lentiviral vectors expressing the wild-type FLAG-IBtkγ-IRES-GFP and mutants FLAG-IBtkγ-IRES-GFP with selected serines to alanines substitutions. After transduction of DeFew cells, GFP-positive cells expressing FLAG-IBtkγ proteins were FACS-sorted and stimulated with anti-IgM to promote BCR activation; then, PKC-phosphorylated proteins were immunoprecipitated from cell extracts and analyzed for the presence of FLAG-IBtkγ proteins.
On BCR stimulation, the PKC phosphorylation was observed in both the wild-type IBtkγ and IBtkγ mutants S87A and S90A, whereas it was abolished in the double-mutant S87/90A and quintuple-mutant S81/82/87/88/90A (Figure 3D). These data point to the serines 87 and 90 as main targets of in vivo PKC phosphorylation of IBtkγ on BCR triggering. Of interest, the ClustalW2-based analysis (http://www.ebi.ac.uk/Tools/clustalw2/) of orthologus IBtkγ sequences in eukaryotes showed that S90 has the greatest degree of conservation among other serines (Figure 3E), suggesting its involvement in the IBtkγ regulation.
The PKC-dependent phosphorylation of the IBtkγ serines 87 and 90 causes the dissociation of the Btk:IBtkγ complex
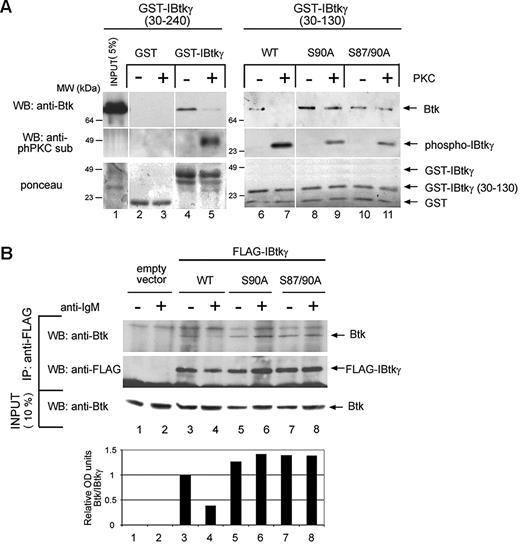
Because the stability of the Btk:IBtkγ complex inversely correlated with the PKC-dependent phosphorylation of IBtkγ (Figure 1C-D), we attempted to identify the crucial serine residues that regulate the association of IBtkγ to Btk. To this end, wild-type GST-IBtkγ, GST-IBtkγ (aa 30-130), and the derivatives S90A and S87/90A mutants were in vitro phosphorylated with PKC mix and incubated with cell extracts of pCDNA-BTK–transfected HEK 293T. After GST-pull down, Btk bound to unphosphorylated GST-IBtkγ (Figure 4A lane 4) but not to the PKC-phosphorylated form (Figure 4A lane 5). GST-IBtkγ (aa 30-130) behaved as the full-length GST-IBtkγ (Figure 4A lanes 6 and 7). The PKC phosphorylation did not affect the binding of the GST-IBtkγ S90A (aa 30-130) and GST-IBtkγ S87/90A (aa 30-130) mutants to Btk (Figure 4A lanes 8-11). These results indicated that the phosphorylation of serines 87 and 90 was required for the dissociation of IBtkγ from Btk.
PKC phosphorylation of IBtkγ at serine 90 reduces the affinity binding of IBtkγ to Btk. (A) Aliquots (5 μg) of GST, GST-IBtkγ (aa 30-240), GST-IBtkγ (aa 30-130), or the derivative mutants S90A, S87/90A were in vitro phosphorylated with PKCmix (150 ng) or left untreated, and then incubated with lysates of HEK 293T cells expressing Btk. After GST pull-down, proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-Btk and anti-phPKCsub Abs and Ponceau staining. A representative experiment of 2 independent experiments is shown. (B) DeFew cells (2.4 × 107) were transfected with wild-type FLAG-IBtkγ plasmids or mutants (120 μg) and 24 hours later were stimulated for 10 minutes with anti-IgM or left untreated; cell lysates were immunoprecipitated with anti-FLAG Ab and analyzed by 4%-12% NuPAGE followed by Western blotting anti-Btk and anti-FLAG antibodies. The levels of Btk bound to IBtkγ were measured by densitometry (bottom). A representative experiment of 2 independent experiments is shown.
PKC phosphorylation of IBtkγ at serine 90 reduces the affinity binding of IBtkγ to Btk. (A) Aliquots (5 μg) of GST, GST-IBtkγ (aa 30-240), GST-IBtkγ (aa 30-130), or the derivative mutants S90A, S87/90A were in vitro phosphorylated with PKCmix (150 ng) or left untreated, and then incubated with lysates of HEK 293T cells expressing Btk. After GST pull-down, proteins were separated by 10% SDS-PAGE and analyzed by immunoblotting with anti-Btk and anti-phPKCsub Abs and Ponceau staining. A representative experiment of 2 independent experiments is shown. (B) DeFew cells (2.4 × 107) were transfected with wild-type FLAG-IBtkγ plasmids or mutants (120 μg) and 24 hours later were stimulated for 10 minutes with anti-IgM or left untreated; cell lysates were immunoprecipitated with anti-FLAG Ab and analyzed by 4%-12% NuPAGE followed by Western blotting anti-Btk and anti-FLAG antibodies. The levels of Btk bound to IBtkγ were measured by densitometry (bottom). A representative experiment of 2 independent experiments is shown.
Next, we tested whether IBtkγ was phosphorylated by PKC when associated with Btk. To this end, we first isolated the protein complexes made of recombinant His-Btk with either GST-IBtkγ (aa 30-130) or GST-IBtkγ (aa 30-240), which were then phosphorylated with a PKC mix. The IBtkγ phosphorylation by PKC was unaffected and even slightly increased when the inhibitor was associated with His-Btk (supplemental Figure 5A). This evidence indicates that PKC could play an active role in disassembling the Btk:IBtkγ complex rather than simply preventing the Btk:IBtkγ association. Moreover, IBtkγ associated with Btk inhibited the PKC-mediated phosphorylation of Btk at S180 (supplemental Figure 5B), suggesting that the binding of IBtkγ to Btk precludes the access of PKC to the S180 Btk substrate.16
Next, we analyzed the role of S87/90 phosphorylation of IBtkγ in the stability of the Btk:IBtkγ complex in vivo. To this end, DeFew cells stably expressing wild-type FLAG-IBtkγ, the mutant FLAG-IBtkγ S90A, or IBtkγ S87/90A were stimulated with anti-IgM or left untreated and analyzed for the coimmunoprecipitation of FLAG-IBtkγ with the endogenous Btk. In unstimulated cells, Btk was immunoprecipitated with both the wild-type and IBtkγ mutants (Figure 4B lanes 3,5,7); the anti-IgM stimulation caused the loss of Btk association in the case of the wild-type IBtkγ (Figure 4B lane 4), whereas it did not affect the association of Btk with IBtkγ S90A or IBtkγ S87/90A (Figure 4B lanes 6-8). These results demonstrated that mutations of IBtk S87 and S90 were sufficient to prevent the dissociation of IBtkγ from Btk on BCR signaling, indicating that the phosphorylation of IBtkγ at S87 and S90 plays a crucial role in the regulation of the Btk:IBtkγ complex.
IBtkγ mutants lacking the S87 and S90 phosphorylation sites inhibit the Ca2+ mobilization and NF-κB activation after BCR triggering
Btk is crucial for Ca2+ release from the intracellular stores on antigen binding in DT40 chicken B cells9 and in human B-cell lines.37 We previously showed that IBtkγ negatively regulates the Btk activity and impairs the Btk-dependent Ca2+ fluxes in response to BCR triggering.28 To evaluate the role of IBtkγ serines 87 and 90 in Btk activity, we analyzed their impact on Ca2+ fluxes induced by BCR triggering.
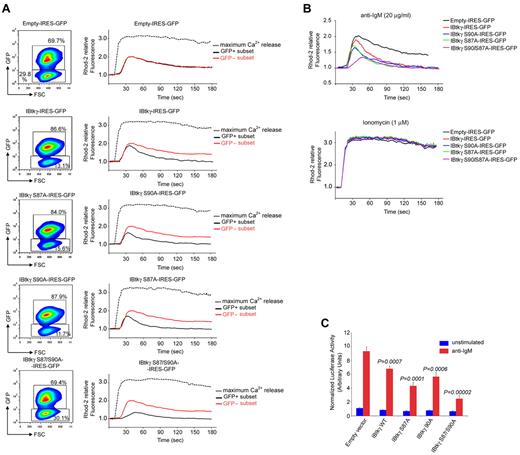
According to our previous data,28 DeFew cells stably expressing wild-type IBtkγ showed a reduced intracellular Ca2+ mobilization on anti-IgM stimulation compared with cells expressing the empty vector (Figure 5A-B). In cells expressing mutant IBtkγ S87A or IBtkγ S90A, a more vigorous inhibition of BCR-mediated Ca+2 mobilization was observed (Figure 5A-B). The Ca2+ mobilization on BCR triggering was even delayed and strongly reduced in cells expressing the double mutant IBtkγ S87/90A (Figure 5A-B), confirming a regulatory role of IBtkγ serines 87 and 90 on Btk-dependent activation of Ca2+ fluxes in response to BCR triggering. Similar results were obtained in DT40 chicken B cells (supplemental Figure 6).
IBtkγ S87/90A functions as super-repressor of Btk-dependent Ca2+ mobilization and NF-κB activation in response to BCR triggering. (A) Profile of Ca2+ mobilization in DeFew cells stably expressing the wild-type FLAG-IBtkγ- IRES-GFP or mutants. DeFew cells (1 × 107) were loaded with the Ca2+ indicator Rhod-2 and stimulated with anti–human IgM (20 μg/mL). The maximum Ca2+ release was measured after the addition of calcium ionophore ionomycin (1μM). The GFP and Rhod-2 fluorescences were acquired by FACS during 180-second time periods and analyzed by FlowJo software (TreeStar Inc). For each experimental point, either cytogram of GFP-positive and GFP-negative subsets, and histogram showing the profile of Ca2+ mobilization in the GFP subsets of each cell line, are reported. Ca2+ mobilization is shown as the Rhod-2 fluorescence intensity compared with the baseline (calculated as 1). A representative experiment of 3 independent experiments is shown. (B) Cumulative profiles of Ca2+ mobilization on anti-IgM (top), or ionomycin (bottom) for the GFP-positive gated populations of the experiment described in A. (C) NF-κB activation in response to BCR triggering in DeFew cells expressing FLAG-IBtkγ or mutants. DeFew cells (6 × 106) stably expressing the wild-type or mutant FLAG-IBtkγ-IRES-GFP were transfected with NF-κB-luc (15 μg) and renilla reporter plasmids (0.5 μg); 24 hours later, cells were treated for 18 hours with anti-IgM (20 μg/mL) or left untreated. The luciferase and renilla activities were measured in cells extracts at least in triplicate and luciferase activity was normalized as the ratio between the luciferase and renilla activity. Statistical significant difference between the empty vector and the vector encoding the wild-type or mutant IBtkγ was analyzed by Student t test. A representative experiment of 3 independent experiments is shown.
IBtkγ S87/90A functions as super-repressor of Btk-dependent Ca2+ mobilization and NF-κB activation in response to BCR triggering. (A) Profile of Ca2+ mobilization in DeFew cells stably expressing the wild-type FLAG-IBtkγ- IRES-GFP or mutants. DeFew cells (1 × 107) were loaded with the Ca2+ indicator Rhod-2 and stimulated with anti–human IgM (20 μg/mL). The maximum Ca2+ release was measured after the addition of calcium ionophore ionomycin (1μM). The GFP and Rhod-2 fluorescences were acquired by FACS during 180-second time periods and analyzed by FlowJo software (TreeStar Inc). For each experimental point, either cytogram of GFP-positive and GFP-negative subsets, and histogram showing the profile of Ca2+ mobilization in the GFP subsets of each cell line, are reported. Ca2+ mobilization is shown as the Rhod-2 fluorescence intensity compared with the baseline (calculated as 1). A representative experiment of 3 independent experiments is shown. (B) Cumulative profiles of Ca2+ mobilization on anti-IgM (top), or ionomycin (bottom) for the GFP-positive gated populations of the experiment described in A. (C) NF-κB activation in response to BCR triggering in DeFew cells expressing FLAG-IBtkγ or mutants. DeFew cells (6 × 106) stably expressing the wild-type or mutant FLAG-IBtkγ-IRES-GFP were transfected with NF-κB-luc (15 μg) and renilla reporter plasmids (0.5 μg); 24 hours later, cells were treated for 18 hours with anti-IgM (20 μg/mL) or left untreated. The luciferase and renilla activities were measured in cells extracts at least in triplicate and luciferase activity was normalized as the ratio between the luciferase and renilla activity. Statistical significant difference between the empty vector and the vector encoding the wild-type or mutant IBtkγ was analyzed by Student t test. A representative experiment of 3 independent experiments is shown.
The BCR signaling causes the activation of NF-κB by Btk-dependent and Btk-independent mechanisms.38 Thus, we tested whether wild-type IBtkγ and IBtkγ mutants could inhibit the pathway of the Btk-dependent activation of NF-κB after BCR stimulation. To this end, DeFew cells stably expressing the FLAG-IBtkγ proteins were transfected with NF-κB-luc reporter plasmid, followed by anti-IgM stimulation. On anti-IgM stimulation, the expression of wild-type IBtkγ reduced by 1.3-fold the NF-κB activation (Figure 5C). The expression of IBtkγ S87A or IBtkγ S90A further inhibited by 1.6- and 2.1-fold the NF-κB activity, respectively. Moreover, the NF-κB activation was abrogated by the double mutant S87/90A (Figure 5C). The inhibition of the BCR-induced Ca2+ mobilization and NF-κB activation by the IBtkγ proteins carrying serines 87 and 90 mutated to alanines was consistent with the constitutive binding of these mutants to Btk (Figure 4); in particular, these results indicate that IBtkγ S87/90A behaved as a super-repressor of Btk-dependent Ca2+ fluxes and NF-κB activation by impeding its dissociation from Btk in response to BCR signaling.
PKC-mediated phosphorylation of IBtkγ regulates the recruitment of Btk at the plasma membrane on BCR triggering
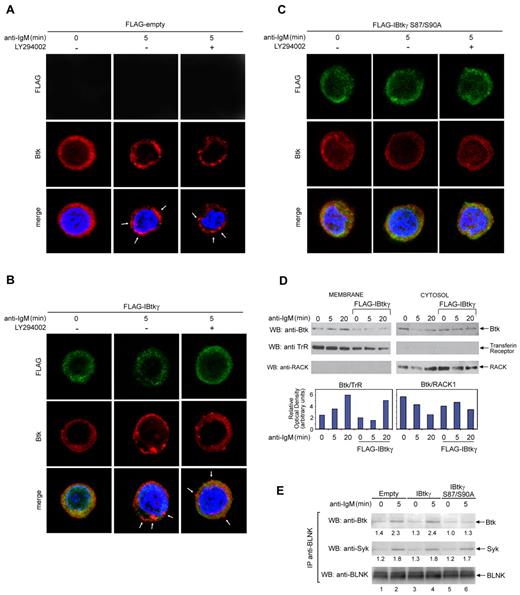
BCR triggering causes the rapid recruitment of Btk to the lipid rafts.39 In particular, PIP3 anchors Btk to the membrane through the PH domain.40 In this regard, the IBtkγ binding to the PH domain of Btk could compete for the binding of Btk to PIP3 and preclude the access of Btk to the membrane-signaling proteins. To test this possibility, DeFew cells expressing either wild-type FLAG-IBtkγ or mutant FLAG-IBtkγ S87/90A were treated with anti-IgM or left untreated, in the presence or absence of the PI3K inhibitor LY294002, and analyzed by confocal microscopy for the cellular distribution of the endogenous Btk. In unstimulated cells, Btk was uniformly diffused in the cytoplasm (Figure 6A left). The anti-IgM stimulation caused the punctuate aggregation of Btk at the plasma membrane in cells expressing the empty vector at 5 minutes (Figure 6A middle). At the same time points, the Btk recruitment to the plasma membrane was partially inhibited by the wild-type IBtkγ (Figure 6B middle) and was abolished by IBtkγ S87/90A (Figure 6C middle). Lack of Btk recruitment at the membrane as a consequence of IBtkγ expression was confirmed by immunoblotting of cytoplasmic and membrane fractions (Figure 6D). However, the membrane recruitment of Btk was unaffected by the PI3K inhibitor LY294002 (Figure 6A-C right), indicating that in our experimental conditions PIP3 production might be dispensable. In addition, we performed the immunoblotting analysis of the activation status of Btk as well as Akt, which is a well-known downstream target of PI3K in B-cell signal transduction.41 The BCR-mediated Tyr551 phosphorylation of Btk was unaffected by the PI3K inhibitor LY294002, whereas the Ser473 phosphorylation of Akt was completely inhibited (supplemental Figure 7). These results indicate that, on BCR stimulation, both membrane recruitment and activation of Btk were unaffected by PI3K inhibitors, as reported by other authors.42
IBtkγ S87/90A prevents the Btk translocation to the plasma membrane on BCR triggering. (A-C) IBtkγ prevents the Btk translocation to the plasma membrane on BCR triggering in a PI3K-independent manner. DeFew cells stably expressing empty vector (A), FLAG-IBtkγ (B), or FLAG-IBtkγ S87/90A (C) were stimulated with anti-IgM for 5 minutes in the presence or absence of the PI3K inhibitor LY294002 (25μM); then, cells were fixed and stained with either anti-Btk Ab followed by anti–rabbit Alexa 568 to detect endogenous Btk, and FITC-conjugated anti-FLAG Ab to detected FLAG-IBtkγ. Nuclei were stained with DAPI. Images shown are the distribution of transfected IBtkγ (green), endogenous Btk (red), and nuclei (blue) were along a single focal plane detected by confocal microscopy. Arrowheads indicate the punctuate aggregation of Btk at the plasma membrane. Bar = 10 μm. A representative experiment of 2 independent experiments is shown. (D) Immunoblotting analysis of the Btk cellular distribution. DeFew cells stably expressing FLAG-IBtkγ or control empty plasmid were stimulated with anti-IgM for 5 and 20 minutes, and cell lysates were subjected to subcellular fractionation. The Btk expression levels in the cytosolic and membrane fractions (10 μg) were analyzed by Western blotting with anti-Btk antibodies; RACK1 and transferrin receptor (TrR) were analyzed with the relative antibodies as markers of the cytosol and membrane, respectively. Densitometry of Btk expression levels in the cytosolic and membrane fractions was performed by the use of Scion Image; for each point, the Btk signal was normalized to the relative markers RACK1 and TrR. A representative experiment of 2 independent experiments is shown. (E) Association of Btk and BLNK in DeFew cells. DeFew cells stably expressing empty vector (A), FLAG-IBtkγ (B), or FLAG-IBtkγ S87/90A (C) were stimulated with anti-IgM for 5 minutes or left unstimulated. Cell lysates (1 mg) were immunoprecipitated with anti-BLNK Ab and analyzed by 4%-12% NuPAGE followed by immunoblotting with anti-Btk, anti-Syk, and anti-BLNK antibodies. A representative experiment of 2 independent experiments is shown.
IBtkγ S87/90A prevents the Btk translocation to the plasma membrane on BCR triggering. (A-C) IBtkγ prevents the Btk translocation to the plasma membrane on BCR triggering in a PI3K-independent manner. DeFew cells stably expressing empty vector (A), FLAG-IBtkγ (B), or FLAG-IBtkγ S87/90A (C) were stimulated with anti-IgM for 5 minutes in the presence or absence of the PI3K inhibitor LY294002 (25μM); then, cells were fixed and stained with either anti-Btk Ab followed by anti–rabbit Alexa 568 to detect endogenous Btk, and FITC-conjugated anti-FLAG Ab to detected FLAG-IBtkγ. Nuclei were stained with DAPI. Images shown are the distribution of transfected IBtkγ (green), endogenous Btk (red), and nuclei (blue) were along a single focal plane detected by confocal microscopy. Arrowheads indicate the punctuate aggregation of Btk at the plasma membrane. Bar = 10 μm. A representative experiment of 2 independent experiments is shown. (D) Immunoblotting analysis of the Btk cellular distribution. DeFew cells stably expressing FLAG-IBtkγ or control empty plasmid were stimulated with anti-IgM for 5 and 20 minutes, and cell lysates were subjected to subcellular fractionation. The Btk expression levels in the cytosolic and membrane fractions (10 μg) were analyzed by Western blotting with anti-Btk antibodies; RACK1 and transferrin receptor (TrR) were analyzed with the relative antibodies as markers of the cytosol and membrane, respectively. Densitometry of Btk expression levels in the cytosolic and membrane fractions was performed by the use of Scion Image; for each point, the Btk signal was normalized to the relative markers RACK1 and TrR. A representative experiment of 2 independent experiments is shown. (E) Association of Btk and BLNK in DeFew cells. DeFew cells stably expressing empty vector (A), FLAG-IBtkγ (B), or FLAG-IBtkγ S87/90A (C) were stimulated with anti-IgM for 5 minutes or left unstimulated. Cell lysates (1 mg) were immunoprecipitated with anti-BLNK Ab and analyzed by 4%-12% NuPAGE followed by immunoblotting with anti-Btk, anti-Syk, and anti-BLNK antibodies. A representative experiment of 2 independent experiments is shown.
On BCR stimulation, BLNK is localized to the plasma membrane and mediates the recruitment of Btk and PLCγ43 through association. Thus, we examined whether the dissociation of the Btk/IBtkγ complex could affect the association of Btk with BLNK. The anti-IgM stimulation increased the association of Btk with BLNK in DeFew B cells expressing the empty vector (Figure 6E lanes 1-2); a similar increase in Btk/BLNK association was observed in cells expressing FLAG-IBtkγ (Figure 6E lanes 3-4). Conversely, in DeFew B cells expressing the IBtkγ mutant S87/90A the BCR-induced association of Btk with BLNK was abrogated (Figure 6E lanes 5-6). In the same experimental points, the association of Syk with BLNK was unaffected by the expression of IBtkγ or IBtkγ mutant S87/90A (Figure 6E middle). Overall these results indicated that IBtkγ regulated the Btk recruitment to the plasma membrane in response to the BCR signaling. In particular, the IBtkγ mutant S87/90A, which constitutively bound to Btk, sequestered Btk in the cytoplasm and inhibited the generation of the Btk:BLNK complex at the plasma membrane.
Spleen B cells from Ibtkγ−/− mice show increased Btk activation and sustained intracellular Ca2+ fluxes in response to BCR engagement
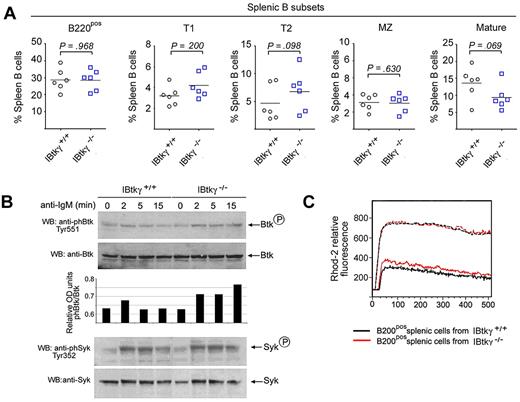
Next, we investigated the BCR signaling in Ibtkγ−/− mice (G.S., I.Q., manuscript in preparation). In the spleen of 5-week-old mice, the percentages of B-cell subsets were not significantly affected by the absence of IBtkγ (Figure 7A). The basal level of phosphorylated Btk-Y551 in spleen B cells of Ibtkγ+/+ and Ibtkγ−/− mice were similar (Figure 7B lanes 1,5); however, on BCR-engagement, spleen B cells of Ibtkγ−/− mice showed greater levels of Btk-Y551 phosphorylation up to 15 minutes, compared with Ibtkγ+/+ mice, indicating that an increased Btk activity occurred in the absence of IBtkγ (Figure 7B compare lanes 2-4 with lanes 6-8). Consistently, the Ca2+ fluxes of Ibtkγ−/− spleen cells on IgM stimulation were more sustained compared with wild-type cells (Figure 7C). These results are consistent with the inhibitory role of IBtkγ in Ca2+ mobilization.
Ibtkγ−/− spleen B cells show a sustained Btk-Y551 phosphorylation and intracellular Ca2+ signaling in response to BCR-engagement. (A) Percentage of the splenic B-cell subsets in 5-week-old mice. Spleen cell suspensions derived from 5-week-old mice were stained with the following antibodies: CD21-FITC, CD23-PE, AA4.1-APC, IgM-APC-Cy7, and analyzed by flow cytometry. Percentage for Ibtkγ+/+ and Ibtkγ−/− spleen cells for T1 (CD21negCD23negAA4.1posIgMpos), T2 (CD21posCD23posAA4.1posIgMpos), follicular mature (CD21posCD23posAA4.1negIgMpos), and marginal zone (MZ; CD21highCD23negAA4.1negIgMpos) type B cells are reported (n = 5). Statistical analysis was performed with Prism v.4.0 (GraphPad software) and included the unpaired 2-tailed t test. (B) Ibtkγ−/− spleen B cells show a sustained Btk-Y551 phosphorylation on BCR engagement. Purified splenic B cells from either wild-type or Ibtkγ−/− 5-week-old mice were stimulated with F(ab′)2 fragments of anti–mouse IgM (10 μg/mL) for the indicated time and then analyzed by Western blotting with the indicated antibodies. The amounts of phosphorylated Btk are reported as relative optical density and evaluated according to the intensity of the phospho-Btk signal normalized to the corresponding Btk signal. A representative experiment of 3 independent experiments is shown. (C) Profile of Ca2+ mobilization in splenic B cells from wild-type or Ibtkγ−/− mice. Purified spleen B cells (1 × 107) from either wild-type or Ibtkγ−/− 5-week-old mice were loaded with the Rhod-2 Ca2+ indicator and stimulated with F(ab′)2 fragments of anti–mouse IgM (10 μg/mL). The maximum Ca2+ release was measured after the addition of calcium ionophore ionomycin (1μM). Ca2+ mobilization is shown as the Rhod-2 fluorescence intensity compared with the baseline (calculated as 1). A representative experiment of 3 independent experiments is shown.
Ibtkγ−/− spleen B cells show a sustained Btk-Y551 phosphorylation and intracellular Ca2+ signaling in response to BCR-engagement. (A) Percentage of the splenic B-cell subsets in 5-week-old mice. Spleen cell suspensions derived from 5-week-old mice were stained with the following antibodies: CD21-FITC, CD23-PE, AA4.1-APC, IgM-APC-Cy7, and analyzed by flow cytometry. Percentage for Ibtkγ+/+ and Ibtkγ−/− spleen cells for T1 (CD21negCD23negAA4.1posIgMpos), T2 (CD21posCD23posAA4.1posIgMpos), follicular mature (CD21posCD23posAA4.1negIgMpos), and marginal zone (MZ; CD21highCD23negAA4.1negIgMpos) type B cells are reported (n = 5). Statistical analysis was performed with Prism v.4.0 (GraphPad software) and included the unpaired 2-tailed t test. (B) Ibtkγ−/− spleen B cells show a sustained Btk-Y551 phosphorylation on BCR engagement. Purified splenic B cells from either wild-type or Ibtkγ−/− 5-week-old mice were stimulated with F(ab′)2 fragments of anti–mouse IgM (10 μg/mL) for the indicated time and then analyzed by Western blotting with the indicated antibodies. The amounts of phosphorylated Btk are reported as relative optical density and evaluated according to the intensity of the phospho-Btk signal normalized to the corresponding Btk signal. A representative experiment of 3 independent experiments is shown. (C) Profile of Ca2+ mobilization in splenic B cells from wild-type or Ibtkγ−/− mice. Purified spleen B cells (1 × 107) from either wild-type or Ibtkγ−/− 5-week-old mice were loaded with the Rhod-2 Ca2+ indicator and stimulated with F(ab′)2 fragments of anti–mouse IgM (10 μg/mL). The maximum Ca2+ release was measured after the addition of calcium ionophore ionomycin (1μM). Ca2+ mobilization is shown as the Rhod-2 fluorescence intensity compared with the baseline (calculated as 1). A representative experiment of 3 independent experiments is shown.
Discussion
IBtkγ was first described as a cytoplasmic inhibitor of Btk that binds to Btk and inhibits its activation on BCR signaling.28 To date, a comprehensive picture of the regulation of the IBtkγ activity is missing. In this study, we show that the BCR triggering modulates the binding affinity of IBtkγ to Btk. Indeed, we observed a strict correlation between the PKC-phosphorylation of IBtkγ at serines 87 and 90 and the dissociation of IBtkγ from Btk with the release of active Btk for further signaling. The involvement of PKC in the IBtkγ phosphorylation was assessed in vivo by the use of PKC inhibitors and by exogenous expression of distinct PKC isoforms followed by in vitro kinase assay. Accordingly, PMA, a PKC activator, induced the PKC-mediated phosphorylation of IBtkγ at PKC consensus sites followed by the dissociation of IBtkγ from Btk.
We identified serines 87 and 90 as crucial amino acid residues for signaling-dependent regulation of the IBtkγ activity. In fact, although S81, S87, and S90 of IBtkγ were identified by mass spectrometry as targets of in vitro phosphorylation by PKC, S87 and S90 were the only amino acid residues that regulated the stability of the Btk:IBtkγ complex on B-cell stimulation with anti-IgM or PMA. This evidence was assessed by generating an array of IBtkγ mutants carrying base-pair substitutions of serine to alanine within the PKC consensus sites, which were tested in binding and functional assays to evaluate their interaction with Btk. Either the IBtkγ S87A, and IBtkγ S90A mutants, which could not be phosphorylated at S87 and S90, respectively, did not dissociate from Btk on BCR triggering or PMA treatment; consistently, the S87/90A double mutant acted as a super-repressor of Btk as measured by increased inhibition of the Btk-dependent Ca2+ fluxes and NF-κB activation (Figure 5). These results do not rule out that other IBtkγ serines are phosphorylated in vivo and may be involved in IBtkγ regulation.
In support of this possibility, IBtkγ S87/90A mutant and even the quintuple mutant IBtkγ S81/82/87/88/90A still showed a significant degree of phosphorylation. However, in vitro binding and functional assays with the IBtkγ mutants indicated that phosphorylation of serines 87 and 90 regulated the stability of the Btk:IBtkγ complex. Furthermore, the analysis of IBtkγ for conserved amino acids among different species showed that S90 is highly conserved in eukaryotes, which underlines the role of S90 phosphorylation as a main regulator of the IBtkγ activity. Moreover, the sequence of IBtkγ from amino acid 66-203 was required for the inhibition of Btk,28 further supporting that S87 and S90, which are located within the inhibitory domain of IBtkγ and are PKC-phosphorylated, regulate the physical and functional interaction between the 2 proteins.
The BCR-induced membrane recruitment of Btk required the dissociation of Btk from IBtkγ. In fact, on anti-IgM stimulation, the wild-type IBtkγ significantly reduced the accumulation of Btk at the plasma membrane, whereas the transdominant-negative mutant IBtkγ S87/90A constitutively retained Btk in the cytoplasm. The lack of Btk membrane recruitment on BCR triggering could explain the inhibition of Ca2+ fluxes and Btk-dependent activation of NF-κB by IBtkγ S87/90A. Accordingly, spleen B cells from Ibtkγ−/− mice29 expressed sustained levels of Btk-Y551 phosphorylation and intracellular Ca2+ signaling in response to BCR engagement.
On BCR triggering, the membrane recruitment and activation of Btk were unaffected by the PI3K inhibitor LY294002, indicating that the PIP3 production was dispensable for Btk activation, which is consistent with a previous report.42 Indeed, the Btk recruitment to the plasma membrane may be mediated by the scaffold protein BLNK.24 In this regard, we showed that the dissociation of IBtkγ from Btk was required for the generation of the Btk:BLNK complex, as the double mutant IBtkγ S87/S90A, which bound constitutively to Btk, abrogated the BCR-induced association of Btk with BLNK. It is worthwhile to note that both IBtkγ and BLNK bind to the SH2 domain of Btk,28,44 which may explain the reason why IBtkγ prevents the association of Btk with BLNK.
Our results are consistent with a model of Btk regulation in B cells in which the interaction of IBtkγ with Btk is timely regulated by PKC-mediated phosphorylation of IBtkγ at serines 87 and 90. In unstimulated B lymphocytes, IBtkγ binds to Btk and precludes its membrane recruitment and activation exerted by BCR downstream signaling molecules, such as Syk. The active Btk translocating to the plasma membrane shortly on BCR triggering would depend on the amount of Btk released from the binding of IBtkγ, which in turn is strictly correlated with the ratio of S87/90-phosphorylated to unphosphorylated IBtkγ. According to this model, the mutant IBtkγ S87/90A, which binds constitutively to Btk because it is unphosphorylated by PKC at the crucial serines 87 and 90, prevents the translocation of Btk at the plasma membrane and its interaction with BLNK on BCR triggering, and thus constitutively inhibits the Btk-dependent Ca2+ signaling and NF-κB–dependent gene transcription (supplemental Figure 8). In the early step of BCR signaling, Syk phosphorylates and activates PLCγ,25 leading to a low level of Ca2+ signaling and PKC activation in a Btk-independent manner. Indeed, a low but consistent Ca2+ flux was observed in DT40 Btk−/− on BCR triggering (supplemental Figure 6B); accordingly, Takata and Kurosaki9 reported a low but consistent PLCγ activation at early time points of BCR stimulation in DT40 Btk−/− cells. Thus, the Btk-independent activation of the PLCγ/PKC pathway could contribute to the early signaling that activates Btk via IBtkγ phosphorylation by PKC, which would result in a positive autoregulatory loop of Btk activity.27,28,45
Our findings provide new insights into the complex regulation of Btk activity that occurs via allosteric protein interactions and posttranslational changes. In fact, Btk is inhibited by physical association with IBtkγ at the PH-TH domain (aa 25-173) and, to a less extent, at the SH2 domain (aa 281-372).28 Moreover, Btk is inhibited by the PKCβ-mediated phosphorylation of S180,16 activated by the Src-mediated phosphorylation of Y55114 and autophosphorylation of Y223.15 We have shown a novel mechanism of regulation of Btk via PKC-mediated S87/90-phosphorylation of IBtkγ, which causes the dissociation of the Btk:IBtkγ complex with the release of active Btk. Moreover, the Btk:IBtkγ complex does not undergo to S180-Btk phosphorylation by PKC, indicating that IBtkγ precludes the access of PKC to the S180 Btk substrate. Altogether, these results underscore a positive regulatory role of PKC in Btk regulation.
Collectively, our data underscore a novel regulatory role of IBtkγ that timely regulates the threshold activation level of the BCR immunoreceptor complex. Our preliminary analysis of Ibtkγ−/− mice indicates that the lack of IBtkγ interferes with B cell development. In fact, in BM, Ibtkγ−/− mice showed a reduced number of both immature and mature B cells, which was likely because of an increased pre-BCR signaling driving the proliferative activity of the pre-B compartment with a concomitant block of B-cell development (G.S., I.Q., manuscript in preparation).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Jae-Won Soh (Inha University) for expression plasmids of PKC isoforms; Dr Vivek Malhotra (University of California, San Diego) for the PKD expression plasmid; Dr Brian Welm (University of California, San Francisco) for pHIV-EGFP lentiviral vector; Dr Francesca Accattato and Dr Marta Greco (Italsistemi Biotechnology Institute) for help in GST-proteins production; Dr Tiziana Mega and Dr Michela Lupia (University “Magna Graecia” of Catanzaro) for help in lentivirus production; and Dr Luigi Racioppi (Duke University) for revising the manuscript.
This work was supported by grants from MIUR to C.P., I.Q., and G.S., and FIRB to I.Q. and G.S. We acknowledge the support of the Italian Association for Cancer Research (AIRC) to G.S.
Authorship
Contribution: E.J. performed the analysis of IBtkγ phosphorylation and Btk association in response to BCR signaling and devised the confocal microscopy experiments; A.P., M.N., and E.V. performed research and assisted with data analysis; M.P., C.F., and A.D.L., assisted with the FACS experiments; G.F., E.D.S., A.R., and D.d.N. assisted with lentiviral production; E.I., A.G., D.B., and L.L. assisted with the mouse experiments; M.G. performed MS analysis; C.P. performed the FACS analysis, assisted with data analysis, and reviewed the manuscript; and I.Q. and G.S. conceived this study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ileana Quinto, Department of Experimental and Clinical Medicine, University of Catanzaro “Magna Graecia,” 88100 Catanzaro, Italy; e-mail: quinto@unicz.it; Giuseppe Scala, Department of Experimental and Clinical Medicine, University of Catanzaro “Magna Graecia,” 88100 Catanzaro, Italy; e-mail: scala@unicz.it.
References
Author notes
E.J. and C.P. contributed equally to this work.

![Figure 2. Distinct PKC isoforms phosphorylate IBtkγ. (A) GST-IBtkγ (1 μg) was in vitro phosphorylated with purified PKCmix (25 ng), recombinant PKCβ (50 ng), or PKCμ (50 ng) in the presence of [γ-32P]ATP and analyzed by 4%–12% NuPAGE followed by autoradiography and Coomassie blue staining. (B) Schematic representation of GST-IBtkγ constructs with putative PKC phosphorylation sites. (C) GST or GST fused with N-terminus (aa 30-130) or C-terminus (aa 131-240) of IBtkγ (5 μg) were incubated with PKCmix (100 ng) in the presence of [γ-32P]ATP; proteins were analyzed by 4%–12% NuPAGE followed by autoradiography and Coomassie blue staining. (D) HEK 293T cells (1 × 106) were transfected with expression vectors of PKC isoforms or empty vector (1.5 μg) together with pCMV7.13xFLAG-IBtkγ or the relative empty vector (2 μg); 48 hours after transfection, cells were treated for 45 minutes with PMA, and lysates were analyzed by 10% SDS-PAGE followed by Western blotting with anti-phPKC substrate and anti-FLAG antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-09-308080/4/m_zh89991171970002.jpeg?Expires=1769227671&Signature=rB2n1WNL4DKXRoFGHlH4WF3WGR4oVgggaYYwF20RhLOHILmenerKCaSMHxFJNKBSGI5jn~8qmGfFbWc5SjGX7wugWelH~Ek1gU7biyDQ2yTHeLnarAHfU9~cJnL3Ms4BhBZ~AnMEJoLJc-HTpejPQtj23VvQPU68CfazduWQ6dF-jT-xQQM1b-T~X-s4TNnKWF-X3AkLATPK2Z7ZXgiSAiZ~ndfRzXU3m08utO5IlUnVv3WSa3c-7aBa9H~UuogEHEoS2TLqN9sdvAIlZNVUntJ2fJ6920UOmdUUIFjACSGo1ga0ekwemQS~60LG4CEMzh4VGCtzA9twU3maOS5~mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Identification of PKC phosphorylation sites of IBtkγ. (A) Schematic representation of the phosphopeptides detected by LC-MS/MS on in vitro phosphorylation of GST-IBtkγ (aa 30-130) (4 μg) with PKCβ (100 ng) followed by tryptic digestion. (B) Picture of IBtkγ N-terminal sequence with the putative PKC phosphorylation sites as predicted by Motifscan software; the PKC phosphorylation sites identified by LC-MS/MS are shown in red circles. (C) GST-IBtkγ (aa 30-130) wild-type or mutants carrying the indicated serine to alanine substitutions (1 μg) were in vitro phosphorylated with PKCmix (25 ng) for 30 minutes in the presence of [γ-32P]ATP; proteins were analyzed by 8% SDS-PAGE followed by autoradiography and Coomassie blue staining. The densitometry of the phospho-IBtkγ signal was performed in 3 independent experiments, and optical density of phospho-IBtkγ was normalized to Coomassie blue staining. Densitometry of IBtkγ mutants relatively to wild type was expressed as the mean values of 4 independent experiments ± SEM (bottom); the asterisk indicates a statistically significant difference between the IBtkγ mutant and the wild type according to the Student t test: (*P = .0003; **P = .0001; ***P = .0005; ****P = .0001; for n = 4). (D) DeFew cells (2.4 × 107 cells) stably expressing wild-type or mutants FLAG-IBtkγ-IRES-EGFP upon lentiviral transduction were stimulated for 10 minutes with F(ab′)2 fragments of anti–human IgM (13 μg/mL); cell lysates (1 mg) were immunoprecipitated with anti-phPKCsub Ab and analyzed by 10% SDS-PAGE and Western blotting with anti-FLAG Ab. A representative experiment of 2 independent experiments is shown. (E) Alignment of orthologous IBtkγ amino acid sequences from different species was performed with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/); the PKC sites are indicated in the square and include the S81 and S87 and S90 as identified by LC-MS/MS.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-09-308080/4/m_zh89991171970003.jpeg?Expires=1769227671&Signature=4bYml4t1WC6cAwvlZ0TOfXkQ7QNlDa3ggEoTAgPWX8TCmWkQUeOU9aA2Jn3lY2EbJXUToCWesK0KhDRlTnihgVtUugwuRvWklCLzkHsy4IQpkgY7CWrKRP1fv~PkOCb8t4tJZ0eDwU-RtRRJbCN5q5iG7SIh6Ui3I2rB24bVYedZFdxe1YEgLMtr9mKdoWvp3xjdn-Bsg1ZjH6ryjPHj-Y5PaOrB6pJL8adCmAhRuOuPetySYzSls7sq9BZ7aYSHEWWaQv37hWLNBotX7pnLAu9P5ORVz-Xu~rzgc5anEpg7YZSYINO5HzmGbNP4gdldVCY44vFGNj2BWxeSfygjQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)