Abstract
TLRs expressed on dendritic cells (DCs) differentially activate DCs when activated alone or in combination, inducing distinct cytokines and costimulatory molecules that influence T-cell responses. Defining the requirements of DCs to program T cells during priming to become memory rather than effector cells could enhance vaccine development. We used an in vitro system to assess the influence of DC maturation signals on priming naive human CD8+ T cells. Maturation of DCs with lipopolysaccharide (LPS; TLR4) concurrently with R848 (TLR7/8) induced a heterogeneous population of DCs that produced high levels of IL12 p70. Compared with DCs matured with LPS or R848 alone, the DC population matured with both adjuvants primed CD8+ T-cell responses containing an increased proportion of antigen-specific T cells retaining CD28 expression. Priming with a homogenous subpopulation of LPS/R848–matured DCs that were CD83Hi/CD80+/CD86+ reduced this CD28+ subpopulation and induced T cells with an effector cytokine signature, whereas priming with the less mature subpopulations of DCs resulted in minimal T-cell expansion. These results suggest that TLR4 and TLR7/8 signals together induce DCs with fully mature and less mature phenotypes that are both required to more efficiently prime CD8+ T cells with qualities associated with memory T cells.
Introduction
The generation of memory and effector T cells from primary T-cell responses in vivo relies on antigen presentation by dendritic cells (DCs), a population of highly specialized APCs.1 In addition to providing T cells with recognizable antigens by processing and presenting acquired proteins in the form of peptide-MHC complexes, activated DCs express costimulatory ligands and produce the cytokines necessary for the initiation of functional T-cell responses.2 Triggering of costimulatory molecules by ligands present on DCs can influence T-cell expansion, generation of effector functions, and T-cell survival, although the exact contribution of each ligand alone and together in mediating these outcomes is not explicitly clear.3,4 In addition, cytokines produced by activated DCs during priming provide a necessary third signal that further influences T-cell expansion, development of effector functions, and response duration.5,6
Differential programming of T-cell responses by signals provided during priming has been shown to not only affect the magnitude of responses,7 but also the generation of T-cell memory precursors and the shaping of the eventual quality of T-cell memory early during the effector stage of the response.8-10 Modulating the signal strength during activation, such as reducing the duration of antigenic stimulation,10 failing to provide costimulation through CD28,11 and/or reducing the inflammatory environment,12 have all been shown to influence the magnitude and kinetics of the memory response,13 and each of these signals could potentially be mediated through changes in DC functions. Changes in antigen processing, costimulatory ligand expression, and cytokine production occur during DC maturation and result from ligation of CD40 by CD40L or through triggering of innate pattern-recognition receptors such as TLRs by ligands commonly provided by pathogens.14,15 DCs have unique maturation responses to the triggering of different TLRs, resulting in qualitative and quantitative differences in the surface expression of T-cell costimulatory molecules and the cytokines synthesized, which can translate into the induction of different T-cell responses.15,16 In addition, ligation of multiple TLRs concurrently or in sequence, particularly TLR3 or TLR4 together with TLR7, TLR8, or TLR9, has been shown to induce a synergistic increase in the production of multiple cytokines by DCs, including IL12p70.17 This synergistic activation/maturation of DCs can result in enhanced CD4+ T-cell Th1 polarization and increased generation of effector CD8+ T cells compared with maturation by each TLR agonist individually.17,18 Therefore, a better understanding of how DC maturation through distinct TLR pathways influences the characteristics of T-cell responses and memory development is necessary for the effective use of adjuvants with vaccines.19
The antigen-experienced CD8+ T-cell memory population can be subdivided into central and effector memory cells. These subsets are commonly discriminated by the expression of the lymph node homing receptors CCR7 and CD62L on central memory T cells and poised cytolytic function in effector memory T cells,20 but heterogeneity within these populations suggests that distinctions of memory populations based solely on these characteristics is not entirely definitive.21,22 The costimulatory molecules CD27 and CD28 are expressed on naive CD8+ T cells, and maintenance of their expression has been associated with cells in the less differentiated memory pool (central memory T cells), whereas loss of expression is associated with terminal differentiation and development of effector functions (effector memory T cells).23,24 The retention of CD28 expression is functionally correlated with the ability to produce IL2 and to mediate autocrine proliferation in response to antigens,25,26 and with increased survival due in part to the induction of bcl-xl expression.27 This suggests that CD28 may serve as a phenotypic and functional indicator of antigen-specific CD8+ T cells best suited for long-term survival in vivo. The persistence of CD8+ T cells expressing CD28 in HIV-infected individuals is correlated with enhanced control of HIV viral replication,28 and CD8+ T cells that persist in the long term after adoptive immunotherapy usually express CD28.29
In the present study, we sought to characterize the influence of specific TLR agonists on DC maturation and to determine how differences in TLR-induced DC maturation affect the priming of human naive CD8+ T cells. The high frequency of naive Melan-A26-35 specific CD8+ T cells in healthy HLA-A*0201+ individuals30 allowed us to evaluate directly in vitro the influence of APC maturation signals on the response of naive human CD8+ T cells during priming. Our results demonstrate that concurrent maturation of immature DCs with both TLR4 and TLR7/8 agonists synergize to promote the production of IL12p70, but induce a heterogeneous population of DCs, including subsets differing phenotypically in the expression of markers of maturation. Priming T cells with DCs matured with both TLR4 and TLR7/8 signals resulted in the enhanced generation of responding CD8+ T cells retaining CD28 expression compared with DCs matured via either TLR pathway alone. In contrast, stimulation with a homogenous subpopulation of CD83Hi/CD80+/CD86+ mature DCs purified from DCs matured with both TLR4 and TLR7/8 signals resulted in the reduced generation of CD8+ T cells that retained CD28 expression, suggesting that priming a naive CD8+ T-cell population with TLR-matured DCs expressing differing degrees of maturation markers may favor the expansion of less terminally differentiated effector responses and provide for improved in vivo persistence and establishment of T-cell memory.
Methods
Cell lines and cell culture
PBMCs from healthy A*0201 donors were harvested by leukapheresis and cryopreserved after informed consent in accordance with the Declaration of Helsinki according to Fred Hutchinson Cancer Center institutional review board–approved protocols. T2 (ATCC) is an HLA-A*0201+ human transporter associated with antigen processing (TAP)–deficient hybridoma. T cells were maintained as described previously.31 In brief, cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% normal human serum, HEPES, l-glutamine, penicillin, streptomycin (Invitrogen), and β-mercaptoethanol. DC cultures were maintained in serum-free DC medium (CellGenix) supplemented with 1% normal human serum. T2 cells were maintained in a medium described previously.31
Peptides and pentamer
The Melan-A26-35A*0201–restricted variant peptide (ELAGIGILTV) and the allophycocyanin-conjugated pentamer were obtained from ProImmune.
Antibodies and flow cytometry
Maturation of DCs was assayed at the time of priming using flow cytometry incorporating the following antibodies: HLA-ABC-FITC, CD80-PE, CCR7-PE-Cy7, and CD83-allophycocyanin (from BD Pharmingen); HLA-DR-ECD (Beckman-Coulter); and CD86-Pacific Blue (BioLegend). DCs were gated according to forward (FSC) and side (SSC) scatter profile and dead cells were excluded using Live/Dead fixable stain (Invitrogen). Phenotyping of Melan-A specific T cells was determined 9 days after priming using flow cytometry incorporating pentamer staining in conjunction with the following antibodies: CD45RA-FITC, CD28-PE, and CD8-PerCP-Cy5.5 (BD Pharmingen); CD27-allophycocyanin-Cy7 (BioLegend); and CD45RO-ECD (Beckman-Coulter).
For intracellular granzyme analysis, cells were surface phenotyped and subsequently permeabilized with Cytofix/Cytoperm (BD Pharmingen) and then stained with the following antibodies under permeabilizing conditions: granzyme B–Alexa Fluor 700 (BD Pharmingen) and granzyme A–Pacific Blue (BioLegend). Isotype antibodies for granzyme antibodies were used to establish gating.
For cytokine analysis, T cells were incubated for 4.5 hours with T2 cells pulsed with Melan-A peptide or an irrelevant peptide (10 μg/mL) in the presence of 10 μg/mL of brefeldin A (Sigma-Aldrich). After incubation, cells were stained with CD8-FITC, followed by intracellular staining as detailed above with the following antibodies: IL2-PE, IFNγ-allophycocyanin, and TNF–Alexa Fluor 700 (BD Pharmingen). Cytokine-producing cells were gated according to lymphocyte FSC/SSC profile and CD8+ cells. Reported cytokine frequencies were corrected by subtraction of values from the irrelevant peptide for each sample. Samples were fixed in 1% paraformaldehyde and stored at 4°C after staining. All flow cytometry data were collected on an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software Version 8.8.6 (TreeStar) and the Pestle Version 1.6.2 and Spice Version 5.2 software suite (M. Roederer, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD).
Preparation and maturation of DCs
Monocyte-derived DCs were generated from Histopaque-1077 (Sigma-Aldrich)–separated PBMCs using anti-CD14 microbeads (Miltenyi Biotec), followed by culture for 6 days in DC medium supplemented with recombinant human GM-CSF (800 IU/mL; Berlex) and recombinant human IL4 (1000 IU/mL; R&D Systems). Media and cytokines were replenished at day 3, and on day 6 nonadherent DCs were harvested and matured with the indicated maturation agent and Melan-A peptide (10 μg/mL) in DC medium supplemented with GM-CSF and IL4. DCs were matured with one or more of the following reagents: a cytokine mixture32 of recombinant human TNFα (10 ng/mL), IL1β (10 ng/mL), IL6 (10 ng/mL; R&D Systems), and PGE2 (1 μg/mL; MP Biomedicals); ultra-pure Salmonella minnesota LPS (10 ng/mL; List Biologic); and R848 (5 μg/mL; Axxora). After maturation for 18-20 hours, DCs and culture supernatant were harvested and DCs were washed and used for T-cell priming. Concentrations for TLR adjuvants were chosen based on the maximal increase in CD80 expression.
IL12 p70 ELISA analysis
Supernatants from DC maturation cultures were collected and frozen at −80°C during DC harvest. IL12p70 cytokine was quantitated using the OptEIA IL12p70 kit (BD Biosciences) according to the manufacturer's instructions.
T-cell preparation and in vitro priming
Preparation of T cells and in vitro priming were as described previously.31 Naive (CD45ROneg) CD8+ T cells were purified from Histopaque-1077–separated PBMCs by negative selection using anti-CD45RO microbeads (Miltenyi Biotec), followed by positive enrichment using anti-CD8 microbeads (Miltenyi Biotec), as described previously.31 For priming, purified naive CD8+ T cells were combined with autologous matured DCs at a 10:1 responder-to-stimulator ratio (T cells:DCs) and were conduced in either 24-well (1 × 106 CD8+ T cells) or 48-well (5 × 105 CD8+ T cells) plates. Medium was supplemented with recombinant human IL7 and IL15 (5 ng/mL; R&D Systems) on day 3 after priming and provided every 3 days thereafter in fresh culture medium. Re-expression of TCR chains occurred between days 8 and 9 and was coincident with the peak response after in vitro priming (data not shown). Nine days after priming, T cells were aliquoted for quantitation, phenotypic, and functional analysis, with each priming well analyzed independently.
Statistical analysis
To assess statistical significance between groups, we performed an unpaired t test (Figure 3), a Mann-Whitney nonparametric test (Figure 2), or a Krustal-Wallis test followed by a Dunn Multiple Comparison Test (Figures 4–5) using Prism 5 software (GraphPad). P values ≤ .05 were considered significant.
Results
Heterogeneous maturation of DCs by TLR agonists as reflected by the expression of maturation and costimulatory molecules
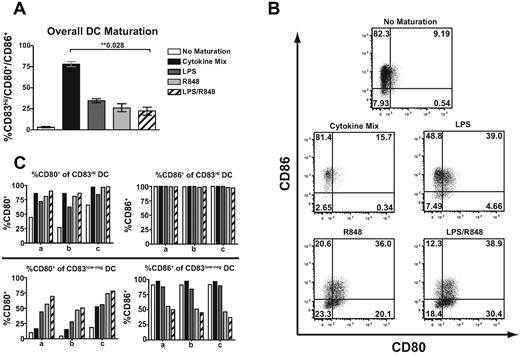
Increased expression of the costimulatory molecules CD80 and CD86 and the Ig superfamily member CD83 are used as distinguishing markers of DCs that have undergone maturation, making them competent to induce functional T-cell responses.1 Although it is assumed that mature DCs expressing the highest levels of all 3 markers are the most effective APCs for priming naive T cells, the expression of each marker can be variable on individual DCs. To characterize the effectiveness of TLR4 and/or TLR7/8 adjuvants for inducing DC maturation and expression of costimulatory molecules, monocyte-derived DCs were matured with LPS (TLR4), R848 (TLR7/8), or both adjuvants together, and the expression of CD80, CD86, and CD83 were evaluated and compared with a defined cytokine cocktail (IL6, TNFα, IL1β, and PGE2) commonly used to efficiently induce DC maturation in vitro.32
We evaluated the percentage of DCs displaying coincident surface expression of all 3 markers as a surrogate indicator of TLR ligand effectiveness as adjuvants to promote DC maturation. Multiple concentrations of LPS and R848 were evaluated, but for standardization purposes, individual ligand doses that maximally induced CD80 expression on immature DCs were used for all studies. All TLR ligands induced maturation of DCs to express higher levels of the 3 molecules relative to DCs cultured in the absence of maturation agents, but with differing efficiencies (Figure 1A). However, the expression of these molecules was heterogeneous on the induced DC populations. After maturation with either LPS or R848, approximately 25%-35% of DCs were CD83Hi/CD80+/CD86+ mature DCs, and these TLR ligands were much less efficient at inducing expression of all 3 maturation markers than the cytokine cocktail (78%). The addition of both TLR ligands together did not increase the fraction of mature DCs expressing all 3 markers (22%). The marker most disparate between cytokine-induced and TLR-induced DC maturation was CD83, which was very inefficiently up-regulated by TLR-induced maturation (83% for Cytokine Mix vs 24% for LPS/R848, data not shown). Because of the limited understanding of the function of CD83 in T-cell activation, we also evaluated the induction of the costimulatory molecules CD80 and CD86 by DCs independently of CD83 by gating on DCs that were low/negative for CD83 after TLR ligand exposure (Figure 1B). Maturation with R848 increased the percentage of DCs expressing CD80 and low levels of CD83 compared with the cytokine cocktail or LPS, and this percentage was slightly increased when LPS was used together with R848. However, despite increasing CD80 expression, R848 induced less CD86 expression in the CD83 low/negative population relative to the other maturation signals, and CD86 expression was further reduced if the DCs received an LPS maturation signal concurrent with R848 (Figure 1B-C). This was not the case for the CD83Hi population, which uniformly expressed high levels of CD86. These results suggest that maturation with R848 and LPS together, which supports the development of a large population of DCs lacking CD83 and expressing the costimulatory molecule CD80 in the absence of CD86, might provide differential costimulation during priming.
Maturation of monocyte-derived DCs with TLR agonists results in differential surface expression of DC maturation markers. Monocyte-derived DCs were activated with TLR4 and/or TLR7/8 agonists or with a defined mixture of cytokines (IL1β, TNFα, IL6, and PGE2) known to efficiently promote maturation. (A) Percentage of DCs demonstrating surface expression of all of the CD83/CD80/CD86 molecules within the FSC/SSC DC gate. Bars represent the means of 4 donors ± SEM. (B) Representative plots of the differential surface expression of CD80 and CD86 within the CD83low/neg DC population. (C) Percentage of DCs displaying CD80 or CD86 within the CD83Hi DC gate (top panel) or the CD83low/neg gate (bottom panel) from 3 donors (a, b, and c). **Statistical significance with P values shown.
Maturation of monocyte-derived DCs with TLR agonists results in differential surface expression of DC maturation markers. Monocyte-derived DCs were activated with TLR4 and/or TLR7/8 agonists or with a defined mixture of cytokines (IL1β, TNFα, IL6, and PGE2) known to efficiently promote maturation. (A) Percentage of DCs demonstrating surface expression of all of the CD83/CD80/CD86 molecules within the FSC/SSC DC gate. Bars represent the means of 4 donors ± SEM. (B) Representative plots of the differential surface expression of CD80 and CD86 within the CD83low/neg DC population. (C) Percentage of DCs displaying CD80 or CD86 within the CD83Hi DC gate (top panel) or the CD83low/neg gate (bottom panel) from 3 donors (a, b, and c). **Statistical significance with P values shown.
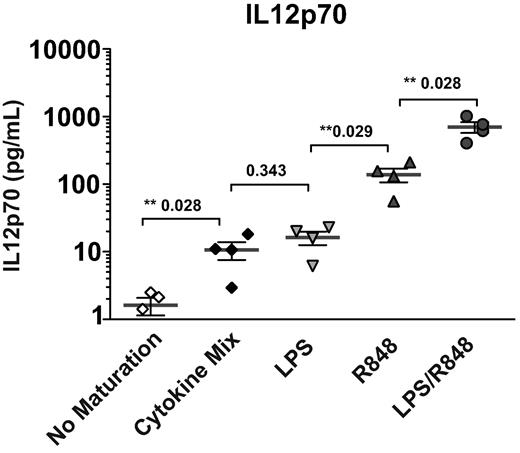
In addition to costimulatory molecules, DCs undergoing maturation also secrete cytokines that can influence priming of CD8+ T-cell responses and affect the outcome of the response. After exposure to each of the maturation signals, immature DCs were induced to secrete IL12p70 (Figure 2). Maturation with R848 resulted in the release of significantly more IL12p70 than the cytokine cocktail or LPS alone. Moreover, maturation with both R848 and LPS together resulted in a synergistic increase in IL12p70 production. Similar results were obtained with DCs derived from multiple donors (Figure 2). These results suggest that these TLR ligands induce DC maturation with distinct expression patterns of T-cell costimulatory molecules, but that induction of high amounts of IL12p70 does not correlate with the extent of expression of maturation markers by DCs.
Use of TLR4 and TLR7/8 agonists in combination results in the synergistic release of IL12p70 during DC maturation. Immature DCs were stimulated with the indicated maturation reagents and IL12p70 production was quantified by ELISA of DC culture supernatants. Data points indicate values for separate donors performed in independent experiments and lines indicate means ± SEM. **Statistical significance with P values shown.
Use of TLR4 and TLR7/8 agonists in combination results in the synergistic release of IL12p70 during DC maturation. Immature DCs were stimulated with the indicated maturation reagents and IL12p70 production was quantified by ELISA of DC culture supernatants. Data points indicate values for separate donors performed in independent experiments and lines indicate means ± SEM. **Statistical significance with P values shown.
Enhanced generation of CD28+CD8+ T cells among the responding antigen-specific population primed with DCs matured with TLR agonists
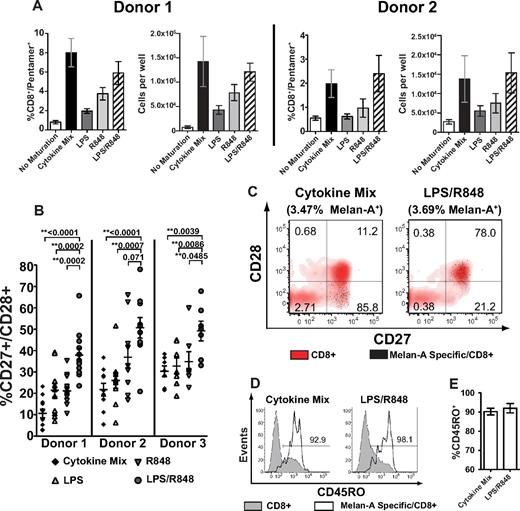
Delineating how efficiently and differentially DCs activated by distinct vaccine adjuvants prime CD8+ T-cell responses can provide insights for the use of candidate adjuvants in human vaccines. To investigate the TLR ligands used for maturation in Figures 1 and 2 as model adjuvants for priming CD8+ T-cell responses, we evaluated antigen-specific responses after a single stimulation of naive human CD8+ T cells in an in vitro system that permits dissection of outcomes and modification of individual variables. Monocyte-derived DCs were matured for 18-20 hours with the cytokine cocktail, TLR4 or TLR7/8 ligands alone, or TLR4 and TLR7/8 ligands together in the presence of Melan-A26-35 peptide, and used to prime autologous HLA-A*0201+ naive CD8+ T cells. DCs matured with the cytokine cocktail and DCs matured with R848 and LPS together induced responses by Melan-A specific T cells of similar frequencies and magnitudes, as determined by flow cytometry and enumeration of cells 9 days after priming in vitro (Figure 3A). In contrast, DCs matured with either R848 or LPS alone induced reduced responses relative to both adjuvants together.
DCs matured with TLR4 and TLR7/8 agonists together prime an antigen-specific response containing an increased number of CD28+ CD8+ T cells. Monocyte-derived DCs were matured with LPS, R848, LPS + R848, or the cytokine cocktail together with Melan-A26-35 peptide, and subsequently cultured with purified naive (CD45ROneg) autologous CD8+ T cells. Quantitation and phenotyping of Melan-A specific CD8+ T cells was performed 9 days after priming. (A) Representative data from 2 donors showing the mean frequency and absolute number of CD8+/Melan-A pentamer+ cells after priming by the indicated DC population (± SEM). (B) Representative data from 3 donors showing the percentage of CD27+/CD28+ of CD8+/Melan-A pentamer+ cells primed by Cytokine Mix, LPS, R848, or LPS/R848 matured DCs. Data points indicate individual priming wells measured from 3 (donor 1) or 2 (donors 2 and 3) independent experiments, and lines indicate means ± SEM. (C) Representative flow cytometric analysis of CD27/CD28 surface expression on Melan-A pentamer+ CD8+ cells superimposed on CD8+ pentamer-negative cells within the same priming well. Percentages represent the proportion of each subset within CD8+/Melan-A+ cells. (D) Representative CD45RO surface expression on Melan-A pentamer+ CD8+ cells overlaid on CD8+ pentamer-negative cells with the percentage of CD45RO+/CD8+/Melan-A+ cells indicated. (E) Mean percentage of CD45RO+ of CD8+/Melan-A+ cells from 3 donors primed by the indicated DCs ± SEM. **Statistically significant differences with P values shown.
DCs matured with TLR4 and TLR7/8 agonists together prime an antigen-specific response containing an increased number of CD28+ CD8+ T cells. Monocyte-derived DCs were matured with LPS, R848, LPS + R848, or the cytokine cocktail together with Melan-A26-35 peptide, and subsequently cultured with purified naive (CD45ROneg) autologous CD8+ T cells. Quantitation and phenotyping of Melan-A specific CD8+ T cells was performed 9 days after priming. (A) Representative data from 2 donors showing the mean frequency and absolute number of CD8+/Melan-A pentamer+ cells after priming by the indicated DC population (± SEM). (B) Representative data from 3 donors showing the percentage of CD27+/CD28+ of CD8+/Melan-A pentamer+ cells primed by Cytokine Mix, LPS, R848, or LPS/R848 matured DCs. Data points indicate individual priming wells measured from 3 (donor 1) or 2 (donors 2 and 3) independent experiments, and lines indicate means ± SEM. (C) Representative flow cytometric analysis of CD27/CD28 surface expression on Melan-A pentamer+ CD8+ cells superimposed on CD8+ pentamer-negative cells within the same priming well. Percentages represent the proportion of each subset within CD8+/Melan-A+ cells. (D) Representative CD45RO surface expression on Melan-A pentamer+ CD8+ cells overlaid on CD8+ pentamer-negative cells with the percentage of CD45RO+/CD8+/Melan-A+ cells indicated. (E) Mean percentage of CD45RO+ of CD8+/Melan-A+ cells from 3 donors primed by the indicated DCs ± SEM. **Statistically significant differences with P values shown.
After priming with matured DCs, previously naive Melan-A specific CD8+ T cells acquired a CD45RO+/CD45RAneg/CD27+ phenotype, which is consistent with antigen encounter (data not shown). However, CD8+ T cells primed by DCs matured via TLR ligands contained an increased percentage of cells retaining surface expression of CD28 compared with T cells primed with DCs matured with the cytokine cocktail (Figure 3B). Moreover, priming by DCs matured with R848 and LPS together resulted in a significantly higher percentage of T cells retaining CD28 relative to cytokine-matured DCs or DCs matured with either ligand alone. This value averaged from 35%-50% between multiple donors (data not shown). Therefore, priming with LPS/R848 resulted in a larger magnitude of cells retaining CD28. The surfaceexpression level of CD28 retained by this population was similar to the expression on CD8+ tetramer-negative cells despite acquisition of a CD45RO+ phenotype comparable to priming by cytokine-matured DCs (Figure 3C-E). These results suggest that the priming environment created by LPS/R848–matured DCs expands more antigen-specific T cells retaining markers consistent with precursors of central memory T cells compared with DC maturation with cytokines or with the TLR adjuvants individually. Moreover, expansion of this precursor population was achieved without reducing the magnitude of the total response.
CD8+ T cells primed by TLR4- and TLR7/8–matured DCs display similar cytokine and granzyme expression at the response peak
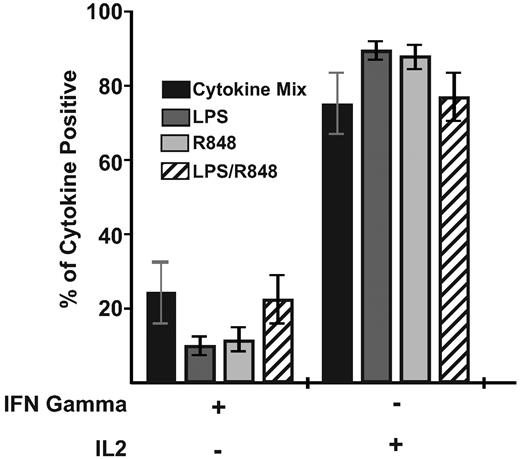
Although evaluation of the phenotype of responding T cells after priming provides insight into the effectiveness of adjuvants in generating potential memory precursors, analysis of the functional capabilities of the primed response is necessary to provide insights regarding both the immediate and potentially long-term protective nature of the T-cell responses generated. Therefore, we also evaluated the functions acquired by Melan-A specific CD8+ T cells 9 days after priming. Responding cells were restimulated with the Melan-A26-35 peptide for analysis by multiparameter flow cytometry of their capacity to produce IFNγ and IL2. After priming with any of the mature DC populations, the majority of Melan-A specific cells assessed at the response peak retained similar proportions of IL2-producing cells regardless of the DC-maturation mechanism (Figure 4). In addition, all DC-maturation conditions induced similar proportions of IFNγ+/IL2neg responding effector T cells. Therefore, independently of the magnitude or phenotype of the response, T cells primed by the differently matured DCs exhibited proportionally similar capacities to make cytokines at the response peak.
Priming by DCs matured with either TLR adjuvants or the cytokine cocktail generates similar frequencies of cytokine-responsive Melan-A specific T cells. CD8+ T cells primed by the indicated DC population were restimulated with 10 μg/mL of Melan-A26-35 peptide 9 days after priming, and intracellular IL2 and IFNγ were analyzed simultaneously. Bars indicate the mean proportion (± SEM) of total responding CD8+ T cells producing the cytokines indicated. Data are combined from 2 donors generated in independent experiments. Statistical analysis showed no significant differences between groups.
Priming by DCs matured with either TLR adjuvants or the cytokine cocktail generates similar frequencies of cytokine-responsive Melan-A specific T cells. CD8+ T cells primed by the indicated DC population were restimulated with 10 μg/mL of Melan-A26-35 peptide 9 days after priming, and intracellular IL2 and IFNγ were analyzed simultaneously. Bars indicate the mean proportion (± SEM) of total responding CD8+ T cells producing the cytokines indicated. Data are combined from 2 donors generated in independent experiments. Statistical analysis showed no significant differences between groups.
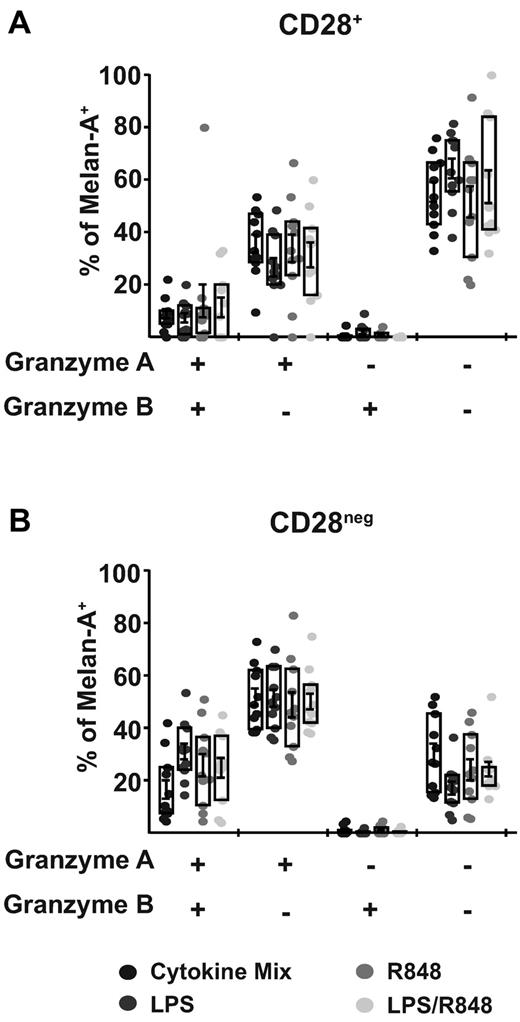
To further assess the acquisition of effector functions, we also evaluated the expression of cytolytic granules by measuring the proteases granzyme A and B. DCs matured with cytokines or TLR signals all induced populations of antigen-specific T cells displaying expression of one or both granzymes in both the CD28+ (Figure 5A) and the CD28neg (Figure 5B) subsets. Consistent with the characteristics of differentiated effector T cells, the CD28neg population displayed a higher frequency of T cells expressing both granzyme A and B and granzyme A alone relative to the CD28+ population. In addition, there were no significant differences in this expression pattern regardless of the nature of the DCs that stimulated the response. None of the adjuvant conditions resulted in the induction of perforin detectable by flow cytometry (data not shown), suggesting a limited potential for these T cells primed under limited inflammatory conditions to mediate direct cytolysis. These results suggest that the CD28+ population expanded by the LPS/R848 priming condition acquired limited effector functions, which is consistent with a reduced level of differentiation characteristic of potential memory precursors.
CD28neg population primed by DCs matured with either TLR or cytokines contains a higher proportion of granzyme-expressing, Melan-A specific CD8+ T cells. T cells primed by the indicated DCs were stained for the denoted surface molecules and then permeabilized for intracellular staining of granzyme A and B 9 days after priming. The expression of granzyme A and/or B is shown on the x-axis and bars indicate the mean percentage of Melan-A pentamer+/ CD8+ T cells expressing the granzymes (± SEM). Intracellular granzyme expression was evaluated in Melan-A pentamer+/CD8+ T cells segregated into CD28+ (A) or CD28neg (B) populations. Boxes indicate the interquartile range (± SEM) and points indicate individual priming wells. Data are combined from 2 donors generated in independent experiments and a total of 11 individual priming wells per condition. Statistical analysis showed no significant differences between groups.
CD28neg population primed by DCs matured with either TLR or cytokines contains a higher proportion of granzyme-expressing, Melan-A specific CD8+ T cells. T cells primed by the indicated DCs were stained for the denoted surface molecules and then permeabilized for intracellular staining of granzyme A and B 9 days after priming. The expression of granzyme A and/or B is shown on the x-axis and bars indicate the mean percentage of Melan-A pentamer+/ CD8+ T cells expressing the granzymes (± SEM). Intracellular granzyme expression was evaluated in Melan-A pentamer+/CD8+ T cells segregated into CD28+ (A) or CD28neg (B) populations. Boxes indicate the interquartile range (± SEM) and points indicate individual priming wells. Data are combined from 2 donors generated in independent experiments and a total of 11 individual priming wells per condition. Statistical analysis showed no significant differences between groups.
Heterogeneous DC subpopulations displaying different levels of maturation contribute to priming an enhanced number of CD28+CD8+ T cells
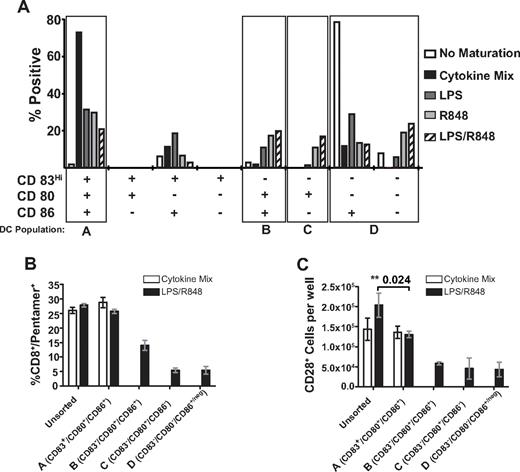
TLR maturation of DCs resulted in the generation of a mixture of DCs heterogeneous for expression of the costimulatory molecules CD80 and CD86, and expression of these molecules appeared to occur independently of the induction of CD83 expression (Figure 1 and Figure 6A). To better understand the impact of DC phenotype on the generation of CD8+ T cells retaining CD28 expression, we analyzed the phenotype of DCs matured with the different agonists, and selected for comparison subpopulations of fully mature DCs (CD83Hi/CD80+/CD86+), immature DCs (CD83low/neg/CD80−/CD86+/neg), and 2 other DC subsets that appeared in increased frequency in DCs matured by the TLR4 and TLR7/8 agonist combination (CD83low/neg/CD80+/CD86+ and CD83low/neg/CD80+/CD86neg; Figure 6A). We used flow cytometric cell sorting to isolate these distinct DC subsets and used the purified subpopulations for in vitro priming. The 4 different populations of DCs used are denoted as: DC-A (CD83Hi/CD80+/CD86+), DC-B (CD83low/neg/CD80+/CD86+), DC-C (CD83low/neg/CD80+/CD86neg), and DC-D (CD83low/neg/CD80neg/CD86+/neg; Figure 6A).
DCs sorted by expression of maturation and costimulatory molecules differentially contribute to the frequency and phenotype of the primary Melan-A specific CD8+ T-cell response. (A) Frequency of DC subpopulations expressing combinations of the CD83, CD80, and CD86 molecules after maturation with the indicated reagent. DC subpopulations from one representative experiment are shown with DCs gated by FSC/SSC, and the specific populations sorted for subsequent experiments are indicated by the boxes. Similar results were obtained from 5 independent donors. (B-C) Data are from one donor representative of 3 independent sorting experiments showing the mean percentage of CD8+/Melan-A pentamer+ cells (B) and the absolute number of Melan-A specific CD8+ T cells displaying dual expression of CD27 and CD28 (C) after priming by sorted DC subpopulations (± SEM). Quantitation of cell numbers and the phenotype of Melan-A specific cells were determined 9 days after priming. **Statistically significant differences with P values shown.
DCs sorted by expression of maturation and costimulatory molecules differentially contribute to the frequency and phenotype of the primary Melan-A specific CD8+ T-cell response. (A) Frequency of DC subpopulations expressing combinations of the CD83, CD80, and CD86 molecules after maturation with the indicated reagent. DC subpopulations from one representative experiment are shown with DCs gated by FSC/SSC, and the specific populations sorted for subsequent experiments are indicated by the boxes. Similar results were obtained from 5 independent donors. (B-C) Data are from one donor representative of 3 independent sorting experiments showing the mean percentage of CD8+/Melan-A pentamer+ cells (B) and the absolute number of Melan-A specific CD8+ T cells displaying dual expression of CD27 and CD28 (C) after priming by sorted DC subpopulations (± SEM). Quantitation of cell numbers and the phenotype of Melan-A specific cells were determined 9 days after priming. **Statistically significant differences with P values shown.
Priming with the unsorted DC population or the sorted DC-A fraction (CD83Hi/CD80+/CD86+) matured with LPS/R848 or the same DC populations matured with cytokines induced responses with similar frequencies of Melan-A specific T cells (Figure 6B). However, despite similar frequencies of Melan-A specific T cells, unsorted LPS/R848–matured DCs generated a greater number of T cells retaining CD28 than the unsorted cytokine-matured DCs, as observed previously, or the DC-A subpopulation induced by LPS/R848 (Figure 6C). Priming with the DC-B, DC-C, and DC-D subpopulations induced by LPS/R848 maturation signals generated a higher frequency of CD28+ cells relative to the DC-A subpopulation (data not shown), but the actual total number of antigen-specific T cells that were CD28+ was reduced relative to the unsorted and LPS/R848 DC-A fractions (Figure 6C). Therefore, each of these subpopulations of DCs alone appears to be less capable of inducing expansion of antigen-specific T cells, and the expansion of large numbers of CD28+ Melan-A specific T cells by LPS/R848–matured DCs requires the DC-A subpopulation together with other populations showing incomplete expression of maturation markers.
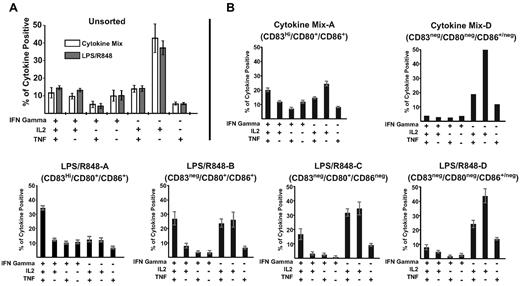
To evaluate the influence of priming with different homogeneous DC subpopulations on T-cell function, we measured cytokine production by restimulated T cells primed by sorted DC subpopulations. Compared with unsorted DCs (Figure 7A), DCs expressing high levels of all maturation markers (DC-A: CD83Hi/CD80+/CD86+) induced a higher frequency of T cells that produced the effector cytokine IFNγ alone or in conjunction with IL2 and TNF, and a lower frequency of cells that produced only IL2 (Figure 7B). This was observed for the DC-A subpopulation matured with either cytokines or R848/LPS, but generation of multifunctional responses (IFNγ+, IL2+, and TNF+) appeared to be more prominent with the LPS/R848 DC-A population than with the Cytokine Mix DC-A population (35% vs 20%). Priming T cells with DCs that showed progressively lower levels of costimulatory molecule expression (DC-B, DC-C, and DC-D) resulted in a progressively reduced generation of T cells capable of producing IFNγ and an increased generation of cells that produced IL2 alone or IL2 together with TNF. These results suggest that subpopulations of DCs exposed to activation signals but expressing lower levels of maturation markers contribute to priming antigen-specific responses retaining CD28 and producing fewer effector cytokines, but that these DC subpopulations alone induce a response of lesser magnitude than unfractionated DCs. Therefore, priming by a heterogeneous population of phenotypically mature and “semi-mature” DCs induces a response that does not reduce the total number of antigen-specific T cells primed by mature DCs but includes more T cells retaining CD28 expression.
DCs matured with LPS/R848 agonists displaying reduced expression of CD83, CD80, and/or CD86 prime a response with reduced ability to produce effector cytokines. CD8+ T cells primed by the indicated DC subpopulations were restimulated with 10 μg/mL of Melan-A26-35 peptide 9 days after priming, and intracellular IL2, IFNγ, and TNF production were analyzed simultaneously. Bars indicate the mean proportion of total responding CD8+ T cells producing the cytokines indicated (± SEM). Data are combined from 2 donors generated in independent experiments (A) and representative of 2 independent sorting experiments (B). However, for the DC-D phenotype, the limited number of DCs generated after cytokine maturation permitted establishment of only one priming well, so SEM could not be calculated for this population.
DCs matured with LPS/R848 agonists displaying reduced expression of CD83, CD80, and/or CD86 prime a response with reduced ability to produce effector cytokines. CD8+ T cells primed by the indicated DC subpopulations were restimulated with 10 μg/mL of Melan-A26-35 peptide 9 days after priming, and intracellular IL2, IFNγ, and TNF production were analyzed simultaneously. Bars indicate the mean proportion of total responding CD8+ T cells producing the cytokines indicated (± SEM). Data are combined from 2 donors generated in independent experiments (A) and representative of 2 independent sorting experiments (B). However, for the DC-D phenotype, the limited number of DCs generated after cytokine maturation permitted establishment of only one priming well, so SEM could not be calculated for this population.
Discussion
The availability of new adjuvant compounds targeting components of the innate immune system has increased the need to elucidate how adjuvants such as TLR agonists function in humans and how they can be used to improve and shape T-cell immune responses. In this study, we used a human in vitro priming system to assess directly the impact of TLR agonists on the priming of CD8+ T cells and to determine how different agonists alone or together influence the quantity and quality of CD8+ T cells generated after priming. Most studies evaluating TLR agonist–mediated maturation of DCs have examined expression of the maturation/costimulatory molecules CD83, CD80, and CD86 as individual parameters,33,34 but have not evaluated the importance of differential expression levels of these molecules on subpopulations of DCs. We evaluated the influence of TLR agonists on DC costimulatory/maturation molecule expression, and found that doses of TLR ligands that individually induced maximal CD80 expression on immature monocyte-derived DCs did not induce uniform expression of all maturation markers, but rather that individual DCs displayed variable levels of each of the 3 molecules. In addition, DC subpopulations expressing lower levels of maturation molecules influenced T-cell priming and, together with fully mature CD83Hi/CD80+/CD86+ DCs, primed a significantly larger population of CD8+ T cells that retained the CD28 costimulatory marker.
Our results highlight 2 important findings regarding the use of TLR agonists as adjuvants for DC maturation. The first is the distinction between inducing expression of maturation and costimulatory molecules and inducing cytokine synthesis after activation of DCs. Similar to initial studies that evaluated TLR4 and TLR7/8 ligands in combination,17 we observed that the ligands together induced a synergistic increase in IL12p70 production. However, although a modest increase in the percentage of CD80+ DCs was detected after administration of the TLR4 and TLR7/8 combination, combining these ligands did not result in an increase in the overall percentage of DCs expressing all 3 classic maturation markers (CD83/CD80/CD86), but rather resulted in a trend toward decreased overall maturation based on expression of these molecules. Previous studies have demonstrated that TLR synergy operates principally at the level of cytokine up-regulation, and have suggested that cytokine induction and up-regulation of CD80 and CD86 in DCs may involve separate processes.17,35 Therefore, it appears that the principle synergistic influence of TLR4 and TLR7/8 on DCs is increased cytokine expression in the absence of significantly increased maturation, at least as reflected by the expression of classic maturation markers.
Our second finding regarding DC maturation with TLR adjuvants is that the DC population generated is heterogeneous rather than uniform with regard to expression of costimulatory and maturation molecules. Moreover, DC subpopulations that express lower levels of maturation and costimulatory molecules appeared to be necessary, but not sufficient, to induce greater numbers of primed CD8+ T cells retaining CD28, and elimination of such DC subpopulations during T-cell priming resulted in changes in the phenotype and function of the response. The importance of CD80 versus CD86 in priming T-cell responses is currently not resolved, with some studies suggesting that CD80 provides a stronger costimulatory signal36 and others suggesting an overlapping function for both molecules.37 Priming with CD83low/neg/CD80+/CD86+ and CD83low/neg/CD80+/CD86neg DC subpopulations was associated with no differences in expansion of antigen-specific T cells relative to CD83low/negCD80neg/ CD86+. This suggests that expression of CD80 together with CD86 in CD83low/neg DCs was not sufficient to enhance T-cell expansion, and that expression of either molecule had indistinguishable impacts on response magnitude or function. Interpretation of this outcome is complicated by the fact that CD80 displays a higher affinity for the CTLA4 inhibitory molecule,38 and therefore additional costimulation provided by expression of CD80 with CD86 on DCs may be offset by negative regulation through ligation of CTLA4 on T cells. The impact of the CD83low/neg DC subpopulations displaying CD80 and/or CD86 in enhancing the generation of CD8+ T cells that retain CD28 expression is only observed when present together with CD83Hi DCs, which may provide additional factors necessary to promote T-cell expansion independently of CD80 or CD86.
The CD83 molecule has traditionally been used to identify mature DCs presumed to be responsible for inducing productive immune responses.1 The role of CD83 in supporting T-cell priming is not entirely clear, but studies have suggested that it may have a direct function in supporting T-cell proliferation.39,40 In the present study, CD83Hi/CD80+/CD86+ DCs induced greater T-cell expansion than CD83low/neg/CD80+/CD86+ DCs (Figure 6), suggesting that CD83 expression may identify a subpopulation of DCs more efficient at mediating CD8+ T-cell expansion. The ability of CD83low/neg subpopulations to predominantly influence the phenotype rather than the total number of T cells generated in the presence of CD83Hi DCs, and the limited capacity of CD83low/neg DCs to promote T-cell expansion in the absence of CD83Hi DCs, suggest that the influence of this CD83low/neg DC subpopulation during priming could operate indirectly in a paracrine means through differential cytokine secretion, directly by providing reduced activation signals, or by a combination of both of these factors. Immature DCs are generally perceived as inducing T-cell nonresponsiveness to maintain tolerance,41 but CD83neg DCs expressing costimulatory molecules, similar to the CD83low/neg DC subpopulations evaluated in our study, have been shown to prime antigen-specific T cells that could develop effector functions after secondary stimulation.42 Analysis of DC subpopulations expressing reduced levels of costimulatory molecules in mice has demonstrated that such immature DCs induce less T-cell proliferation and limited effector differentiation after priming, but that these deficiencies are largely corrected when the concentration of antigen is increased.43 These studies are consistent with our observations that despite displaying reduced levels of maturation markers, phenotypically less mature DCs can still influence priming.
In studies demonstrating suboptimal expansion by immature DCs in vitro and in vivo, the expanded T cells become CD44high, retain CD62L and CCR7 expression, and exhibit reduced cytotoxicity, indicative of the acquisition of a phenotype consistent with precursors of central memory cells and failure to fully differentiate to effector cells.43,44 Studies evaluating the influence of antigen signaling duration rather than DC maturation state have demonstrated that CD8+ T cells receiving a brief antigenic stimulus similarly acquire a central memory phenotype and produce significantly more IL2 compared with T cells receiving prolonged antigen stimulation, which developed cytotoxic activity and a reduced ability to produce IL2.45 This reduced differentiation of primed T cells is in agreement with a progressive differentiation model in which differences in costimulation or cytokines provided in the local milieu result in varying signal strength provided to individual naive T cells, promoting a response composed of a spectrum of T cells that have acquired different amounts of effector functions and differentiation.46
Our results suggest that the enhanced number of CD8+ T cells acquiring limited effector functions and retaining CD28 generated by priming with LPS/R848–matured DCs may result from T cells primed in the presence of the incompletely mature subpopulations of DCs that provide a reduced signal for expansion, resulting in a reduced differentiation of T cells. Incompletely mature DC subpopulations are not sufficient to generate a large response alone, because this requires the presence of CD83Hi DCs. Sorting mature CD83Hi/CD80+/CD86+ DC populations allowed us to select DCs that would theoretically provide a higher strength signal during priming, at least in part because of increased costimulation, and this resulted in enrichment for an IFNγ+ effector response that lacked the expanded population of CD28+ T cells. Generating a CD8+ T-cell response of large magnitude containing a large subpopulation of cells retaining CD28 expression appears to require priming with heterogeneous DC populations that include both phenotypically fully mature and incompletely mature subpopulations. Further studies are necessary to determine the mechanistic contributions of each of these DC subpopulations to promoting the expansion of the CD28+ population.
The results of the present study provide further insights into strategies for using TLR-based adjuvants to prime CD8+ T-cell immune responses. We found that TLR agonists induce populations of DCs that can differentially contribute to priming CD8+ T-cell responses, and that the DCs displaying incomplete acquisition of maturation markers may promote priming of less differentiated antigen-specific T cells, whereas DCs expressing more complete maturation markers may bias the primed response toward predominantly differentiated effector cells. Our assay focuses on priming CD8+ T cells directly by TLR-matured DCs independently of the potential contributions of a concurrent antigen-specific CD4+ T-cell response. Studies in mice have demonstrated that CD8+ T cells at the peak of the in vivo primary response to pathogens exhibit similar phenotypes and functions in the absence of CD4+ T cells, with the role of CD4 help in vivo becoming evident after response contraction during the formation/maintenance of functional memory.47 Although we have not evaluated the influence of T-cell help in our assay, recruitment of CD4+ T-cell help could provide additional signals to DCs displaying incomplete acquisition of maturation markers or directly to CD8+ T cells,48 potentially priming a more differentiated effector response. Central memory T cells displaying reduced effector differentiation have been shown to be superior to effector memory cells for protection from viral challenge49 and for mediating tumor regression.50 This suggests that the use of adjuvants that promote the expansion of a larger magnitude of antigen-specific cells retaining central memory markers and showing reduced effector differentiation may be advantageous for future vaccine and immunotherapy strategies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Colette Chaney, RN, and Natalie Duerkopp (Fred Hutchinson Cancer Research Center) for providing leukapheresis samples and donor recruitment, and Mario Roederer (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) for providing the Pestle and Spice software suite.
This research was conducted as part of the Collaboration for AIDS Vaccine Discovery and was supported by a grant from the Bill & Melinda Gates Foundation. It was supported in part by National Institutes of Health grants LLS 7008-09, P01 CA18029, and R01 CA 33084.
National Institutes of Health
Authorship
Contribution: J.S.P. designed, performed, and analyzed experiment results and drafted the manuscript; M.C., L.S.R., and E.A.-N. performed experiments; E.A.-N. and M.J.M. provided vital reagents; J.S.P. and P.D.G analyzed experiment results and revised the manuscript; and M.W. instituted the in vitro assay. All authors edited and approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeff Pufnock, Department of Clinical Research, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: jpufnock@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal