Abstract
Despite the promising introduction of the proteasome inhibitor bortezomib in the treatment of mantle cell lymphoma (MCL), not all patients respond, and resistance often appears after initial treatment. By analyzing a set of 18 MCL samples, including cell lines with constitutive or induced resistance to bortezomib, we found a high correlation between loss of sensitivity to the proteasome inhibitor and up-regulation of the prosurvival chaperone BiP/Grp78. BiP/Grp78 stabilization was ensured at a posttranscriptional level by an increase in the chaperoning activity of heat shock protein of 90 kDa (Hsp90). In bortezomib-resistant cells, both BiP/Grp78 knockdown and cell pretreatment with the Hsp90 inhibitor of the ansamycin class, IPI-504, led to synergistic induction of apoptotic cell death when combined with bortezomib. Cell exposure to the IPI-504–bortezomib combination provoked the dissociation of Hsp90/BiP complexes, leading to BiP/Grp78 depletion, inhibition of unfolded protein response, and promotion of NOXA-mediated mitochondrial depolarization. The IPI-504–bortezomib combination also prevented BiP/Grp78 accumulation, thereby promoting apoptosis and inhibiting the growth of bortezomib-resistant tumors in a mouse model of MCL xenotransplantation. These results suggest that targeting unfolded protein response activation by the inhibition of Hsp90 may be an attractive model for the design of a new bortezomib-based combination therapy for MCL.
Introduction
Mantle cell lymphoma (MCL) represents ∼ 5%-10% of all non-Hodgkin lymphomas. It is characterized by the expansion of mature B-clonal lymphocytes harboring the t(11;14)(q13;q32) translocation, which induces overexpression of cyclin D1, and consequent cell cycle deregulation. In addition, MCL tumor cells carry a high number of secondary chromosomal and molecular alterations affecting proteins involved in cell cycle progression, senescence, and cellular response to DNA damage.1 The prognosis for this disease is in most cases extremely poor, with a median survival of 5-7 years.2 Conventional chemotherapeutic regimens have been the standard treatment of MCL. However, all these strategies are rapidly confronted with the onset of resistance, and allogenic bone marrow transplantation represents the only potential curative approach in young patients.3
Recently, new therapeutic approaches have been entered into the clinic, and the proteasome inhibitor bortezomib was approved for the treatment of relapsed/refractory MCL.4 Bortezomib induces a p53-independent cytotoxicity, mediated by the proapoptotic BH3-only protein NOXA, and the generation of reactive oxygen species in MCL cells.5 Further studies have shown a synergism between bortezomib and the BH3-mimetics, obatoclax and ABT-737, highlighting the relevance of NOXA induction to counteract the protective effect of MCL-1 accumulation in proteasome-compromised cells.6,7 Hypotheses to explain how MCL cells can acquire resistance to bortezomib include the overexpression and/or accumulation of antiapoptotic BCL-2 proteins, the rise of a bortezomib-resistant nuclear factor-κB activity, and an increased proteasomal activity.8-10 Despite these studies, the precise mechanism by which bortezomib-resistant MCL cases could lose the capacity to undergo NOXA-mediated mitochondrial apoptosis remains unclear.
A key defect might reside within the endoplasmic reticulum (ER) stress pathway, because its activation in MCL cells exposed to bortezomib has been recently shown to elicit NOXA transcription.11 ER homeostasis is controlled by the immunoglobulin heavy chain binding protein (BiP), also referred as 78-kDa glucose-regulated protein (Grp78). BiP/Grp78 forms a large multiprotein complex with a set of other ER molecular chaperones, including the heat shock protein of 90 kDa (Hsp90) ER homolog, Grp94, protein disulfide isomerase, calcium binding protein, and cyclophilin B.12 Under nonstressed conditions, BiP/Grp78 binds to and maintains in an inactive monomeric state the ER transmembrane PKR-like ER kinase, inositol requiring protein 1 (IRE1), and the bZIP activating transcription factor 6.13,14 After proteasome inhibition, the accumulation of polyubiquitinated and misfolded proteins within the ER lumen leads to BiP/Grp78 dissociation from the luminal domains of these sensor proteins, leading to the initiation of the unfolded protein response (UPR): the cytosolic domain of activating transcription factor 6 is cleaved, enabling the protein to translocate and to activate a transcriptional program in the nucleus; IRE1 homodimerizes and cleaves 2 specific stem loop sequences in the mRNA sequence of X-box binding protein 1 (XBP-1), resulting in a larger, spliced, XBP-1 protein with transactivating properties; PKR-like ER kinase oligomerizes and phosphorylates the elongation initiation factor 2α to slow down protein synthesis.15,16 This coordinated cellular response initially promotes cell survival, but it will ultimately trigger apoptosis if cytoprotective mechanisms are overwhelmed. At its core, the accumulation of some Hsp proteins such as BiP/Grp78 and its co-chaperone Grp94 may promote cellular resistance to proteasome inhibitors.17,18
We demonstrate here a striking increase in BiP/Grp78 levels in MCL cells and MCL tumors with intrinsic or acquired resistance to bortezomib. This phenomenon is mediated by an increased chaperoning activity of Hsp90 that can be counteracted in vitro and in vivo by the Hsp90 inhibitor, IPI-504, allowing ER stress induction and restoration of MCL sensitivity to bortezomib. We therefore provide a rationale for the combination of bortezomib with Hsp90 inhibitors in clinical trials.
Methods
Isolation and culture of primary cells
Tumor cells from 10 patients with MCL according to the World Health Organization classification criteria,19 who had not received treatment for ≥ 3 months, were investigated. Approval for the study was obtained from the Ethics Committee at the Hospital Clínic in Barcelona, Spain, and informed consent was obtained from each patient according to the Declaration of Helsinki. The characteristics of these patients are listed in Table 1. For all samples, cyclin D1 overexpression was determined by immunohistochemistry or real-time polymerase chain reaction (PCR). Tumor cells were isolated from peripheral blood by Ficoll/Hypaque sedimentation (Seromed) and conserved within the hematopathology biobank of our institution (Biobanks from CDB-IDIBAPS-Hospital Clínic). Cells were cultured in X-vivo 10 Medium (Cambrex) at a density of 1-2 × 106 cells/mL, at 37°C, in a humidified atmosphere containing 5% carbon dioxide.
Characteristics of patients with MCL
| Patient . | Age, y/sex . | Disease status . | Tumor cells, %* . | P53 status† . | Bz LC50 at 24 h, nM . | IPI-504 GI50 at 72 h, μM . |
|---|---|---|---|---|---|---|
| MCL no.1 | 64/M | Relapse | 95 | del/mut | Not reached | 25.3 ± 0.1 |
| MCL no.2 | 56/M | Diagnosis | 80 | wt | 15.5 ± 0.2 | 2.9 ± 0.01 |
| MCL no.3 | 83/F | Diagnosis | 95 | del/mut | 263.1 ± 1.5 | 16.8 ± 0.2 |
| MCL no.4 | 79/M | Diagnosis | 95 | wt | 7.7 ± 0.7 | 0.7 ± 0.01 |
| MCL no.5 | 76/M | Diagnosis | 83 | wt | 236.2 ± 5.1 | 19.5 ± 0.04 |
| MCL no.6 | 85/M | Diagnosis | 85 | wt | 0.33 ± 0.1 | 1.7 ± 0.02 |
| MCL no.7 | 69/M | Relapse | 89 | del/mut | 34.1 ± 1.3 | 0.1 ± 0.01 |
| MCL no.8 | 78/F | Diagnosis | 97 | wt | 6.6 ± 0.5 | Not reached |
| MCL no.9 | 83/M | Relapse | 89 | wt | 17.8 ± 0.9 | 1.2 ± 0.05 |
| MCL no.10 | 37/M | Diagnosis | 86 | wt | 21.8 ± 1.2 | 0.1 ± 0.03 |
| Patient . | Age, y/sex . | Disease status . | Tumor cells, %* . | P53 status† . | Bz LC50 at 24 h, nM . | IPI-504 GI50 at 72 h, μM . |
|---|---|---|---|---|---|---|
| MCL no.1 | 64/M | Relapse | 95 | del/mut | Not reached | 25.3 ± 0.1 |
| MCL no.2 | 56/M | Diagnosis | 80 | wt | 15.5 ± 0.2 | 2.9 ± 0.01 |
| MCL no.3 | 83/F | Diagnosis | 95 | del/mut | 263.1 ± 1.5 | 16.8 ± 0.2 |
| MCL no.4 | 79/M | Diagnosis | 95 | wt | 7.7 ± 0.7 | 0.7 ± 0.01 |
| MCL no.5 | 76/M | Diagnosis | 83 | wt | 236.2 ± 5.1 | 19.5 ± 0.04 |
| MCL no.6 | 85/M | Diagnosis | 85 | wt | 0.33 ± 0.1 | 1.7 ± 0.02 |
| MCL no.7 | 69/M | Relapse | 89 | del/mut | 34.1 ± 1.3 | 0.1 ± 0.01 |
| MCL no.8 | 78/F | Diagnosis | 97 | wt | 6.6 ± 0.5 | Not reached |
| MCL no.9 | 83/M | Relapse | 89 | wt | 17.8 ± 0.9 | 1.2 ± 0.05 |
| MCL no.10 | 37/M | Diagnosis | 86 | wt | 21.8 ± 1.2 | 0.1 ± 0.03 |
Del indicates deleted; mut, mutated; wt, wild-type; and Bz, bortezomib.
CD19+ tumor cells quantified by flow cytometry.
Assessed by fluorescent in situ hybridization and direct sequencing.
Cell lines
The 9 MCL cell lines included in this study (Table 2) were cultured in RPMI 1640 or Dulbecco minimal essential medium, supplemented with 10%-20% fetal calf serum (FCS), 2mM glutamine (Gibco), 50 μg/mL penicillin-streptomycin, and 100 μg/mL normocin (Lonza). For the generation of bortezomib-resistant cell lines, Jeko-1 and Z-138 cells were initially treated for 96 hours with 10nM bortezomib and then were cultured in drug-free medium containing 20% FCS. After cell growth recovered, cells were treated with bortezomib for an additional 72 hours, and the selection cycle was repeated at the same concentration of bortezomib until cell growth recoveries were obtained within 2 weeks. At this point drug concentration was increased to the next step (12nM, 15nM, 20nM, and, finally, 30nM bortezomib). After repeated rounds of selection with the 30-nM dose of bortezomib over a period of 5 months, the established resistant cell lines were designated “JBR” and “ZBR.” At this point, these cell lines were removed from bortezomib, cryopreserved, and cultured under the same condition as the parental cell lines. Cultures were renewed every 6 weeks to avoid loss of bortezomib resistance during the culture process.
Characteristics of MCL cell lines
| Cell line . | P53 status* . | Cyclin D1 expression† . | Bz LC50 at 24 h, nM . | IPI-504 GI50 at 72 h, μM . |
|---|---|---|---|---|
| JVM-2 | wt | + | 7.6 ± 0.6 | 15.4 ± 0.04 |
| Z-138 | wt | +++ | 11.1 ± 0.3 | 0.4 ± 0.02 |
| HBL-2 | del/mut | ++ | 14.1 ± 1.2 | 8.9 ± 0.02 |
| Granta-519 | wt | +++ | 6.9 ± 0.7 | 0.9 ± 0.08 |
| Jeko-1 | del/mut | +++ | 11.9 ± 0.8 | 10.8 ± 0.09 |
| Rec-1 | wt | +++ | 21.6 ± 2.3 | 15.0 ± 0.04 |
| Maver | del/mut | + | 18.8 ± 2.7 | 4.8 ± 0.03 |
| UPN-1 | del/mut | +++ | 6.7 ± 0.3 | 0.9 ± 0.02 |
| Mino | del/mut | + | 150 ± 0.9 | 1.4 ± 0.01 |
| JBR | del/mut | +++ | 72.2 ± 1.2 | 9.2 ± 0.06 |
| ZBR | wt | +++ | 36.2 ± 0.9 | 0.5 ± 0.01 |
| Cell line . | P53 status* . | Cyclin D1 expression† . | Bz LC50 at 24 h, nM . | IPI-504 GI50 at 72 h, μM . |
|---|---|---|---|---|
| JVM-2 | wt | + | 7.6 ± 0.6 | 15.4 ± 0.04 |
| Z-138 | wt | +++ | 11.1 ± 0.3 | 0.4 ± 0.02 |
| HBL-2 | del/mut | ++ | 14.1 ± 1.2 | 8.9 ± 0.02 |
| Granta-519 | wt | +++ | 6.9 ± 0.7 | 0.9 ± 0.08 |
| Jeko-1 | del/mut | +++ | 11.9 ± 0.8 | 10.8 ± 0.09 |
| Rec-1 | wt | +++ | 21.6 ± 2.3 | 15.0 ± 0.04 |
| Maver | del/mut | + | 18.8 ± 2.7 | 4.8 ± 0.03 |
| UPN-1 | del/mut | +++ | 6.7 ± 0.3 | 0.9 ± 0.02 |
| Mino | del/mut | + | 150 ± 0.9 | 1.4 ± 0.01 |
| JBR | del/mut | +++ | 72.2 ± 1.2 | 9.2 ± 0.06 |
| ZBR | wt | +++ | 36.2 ± 0.9 | 0.5 ± 0.01 |
Bz indicates bortezomib; wt, wild-type; del, deleted; and mut, mutated.
Assessed by fluorescent in situ hybridization and direct sequencing.
Assessed by quantitative reverse transcription PCR.
Treatments and cytofluorimetric assessment of apoptosis
Cells were treated as indicated with bortezomib (Millennium Pharmaceuticals), IPI-504 (retaspimycin hydrochloride; Infinity Pharmaceuticals), 17-AAG (17-(allylamino)-17-demethoxygeldanamycin; Sigma), the selective IκBα kinase inhibitor BMS-345541 (Calbiochem) or the BH3-mimetic obatoclax (kindly provided by GeminX Biotechnologies). Phosphatidylserine exposure was quantified by double staining with Annexin V conjugated to fluorescein isothiocyanate (FITC) and propidium iodide (BenderMedsystems). Lethal concentration 50 (LC50) was defined as the concentration of drug required to reduce cell viability by 50%. Mitochondrial transmembrane potential was evaluated by staining cells with 20nM 3,3′-dihexyloxacarbocyanine iodide (Invitrogen). For the detection of activated caspase-3, cells were fixed with 4% paraformaldehyde (USB), permeabilized with saponin 0.1% (Sigma), and stained for 30 minutes with an antibody against active caspase-3 (BD Pharmingen), followed by an anti-rabbit-FITC antibody (Sigma). Ten thousand stained cells per sample were acquired and analyzed in a FACScan flow cytometer with the use of Cellquest and Paint-A-Gate softwares (Becton Dickinson). Combination indexes (CIs) were calculated with the use of the Calcusyn software Version 2.0 (Biosoft). The interaction between 2 drugs was considered synergistic with CI < 1.
Immunoprecipitation and Western blot assays
Five millions cells were lysed in Triton buffer [1% Triton X-100, 50mM Tris (tris hydroxymethyl-aminomethane)–HCl [pH 7.6], 150mM NaCl, 1mM EDTA (ethylene diamine tetraacetic acid), 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1mM phenylmethanesulfonyl fluoride, 5mM NaF, and 2mM Na3VO4)] and whole-cell lysates (50 μg/lane), were subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto phenylmethlsulfonyl fluoride membranes (Immobilon-P; Millipore). For Hsp90 and BiP/Grp78 immunoprecipitation, a modified Triton buffer was used (1% Triton X-100, 20mM Tris-HCl [pH 7.4], 2.5mM EDTA, 2.5mM ethylene glycol tetraacetic acid, 140mM NaCl, and inhibitors). Protein extracts were incubated overnight at 4°C with either anti-BiP (Cell Signaling Technologies) or anti-Hsp90 (clone 16F1; Assay Design) antibodies, followed by A- or G-protein beads (Amersham), respectively, and supernatant (nonimmunoprecipitated, unbound fraction) was recovered by centrifugation. A/G-protein beads (immunoprecipitated, bound fraction) were washed with lysis buffer and analyzed in 12% SDS-PAGE. Membranes were probed with the following monoclonal and polyclonal antibodies: anti-ubiquitinylated conjugates (clone FK2) and anti-NOXA (clone 114C307; Enzo Life Sciences), anti–phospho-IκBα (Ser32/Ser36), anti-BiP/Grp78 and anti-phospho-eIF2α (Ser 51; Cell Signaling Technologies), anti-p53 (Ab-2) and anti-p21 (Ab-1; Calbiochem), anti–MCL-1 (S-19) and anti–XBP-1 (M186; Santa Cruz Biotechnology), anti-Hsp90 (clone 16F1), anti-Hsp70 (clone C92F3A-5), anti-Hsp27 (clone G3.1) and anti-Grp94 (Assay Design), anti-IRE1 and anti-CHOP (Abcam), and anti–poly(ADP-ribose) polymerase (Roche). Membranes were then incubated with horseradish peroxidase–labeled anti-mouse (Sigma), anti-rabbit (Sigma and Cell Signaling Technologies) or anti-rat (Dako) secondary antibodies. Equal protein loading was confirmed by probing membranes with anti–β-actin or anti–α-tubulin antibodies (Sigma). Antibody binding was detected with the enhanced chemiluminescence (ECL) detection system (Amersham) and analyzed on a mini LAS4000 Fujifilm device with the Image Gauge software (Fujifilm).
20S proteasome assay
Cells were lysed by repeated freeze-thaw and 5mM EDTA (pH 8.0) for 15 minutes on ice and then centrifuged at 16 000g for 10 minutes at 4°C. Total proteins (20 μg) were mixed with substrate buffer containing the peptide Suc-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Millipore) and incubated at 37°C for 2 hours. Released dye was measured with the use of a 380/460 nm filter set. Percentage of inhibition was calculated by comparing the fluorescence of bortezomib-treated samples with untreated controls. Experiments were performed in duplicate.
RNA isolation and real-time PCR
RNA (1 μg) was isolated with the use of the TRIzol method (Invitrogen) and retrotranscribed to cDNA with the use of random primers and the Moloney murine leukemia virus reverse transcriptase (Invitrogen). mRNA expression was analyzed in duplicate by quantitative reverse transcription–PCR on the ABI Prism 7900HT sequence detection system with the use of predesigned assay-on-demand primers and probes (Applied Biosystems). mRNA quantification was done with the use of the comparative cycle threshold method. β-Glucuronidase was used as an endogenous control. Expression levels are given as arbitrary units, using control (untreated) cells as a calibrator.
RNA interference assay
JBR cells (7 × 106) were electroporated with a Nucleofector Device II (A23 program; Amaxa) in 100 μL of Ingenio Electroporation Solution (Mirus) containing 2.5μM of a mix of 2 small-interfering RNA (siRNA) double-stranded oligonucleotides targeting exons 8 and 6 of the human BIP gene (Applied Biosystems). The nontargeting (scramble) siRNA (5′-UUCUCCGAACGUGUCACGU-3′; QIAGEN) was used as a negative control.
Immunofluorescence staining
One million cells were fixed on poly-L-lysine–coated glass coverslips with 4% paraformaldehyde, permeabilized with a solution containing 0.1% saponin and 10% FCS, followed by incubation with an anti–calnexin antibody (H-70; Santa Cruz Biotechnology) and an anti–rabbit immunoglobulin G conjugated to cyanine 3 (Rockland Immunochemicals). Coverslips were washed 3 times with saponin 0.1% and incubated with anti-BiP/Grp78 (Assay Design) and anti–rabbit immunoglobulin G–FITC (Sigma) antibodies. Coverslips were mounted on glass slides with Vectashield-Hard Set medium (Vector Laboratories) and visualized on a Nikon H5505 microscope by means of a 100×/1.30 NA oil objective (Nikon) with the use of Isis Imaging System v5.3 software (MetaSystems GmbH).
3-(4,5-Dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide assay
MCL primary cells (3 × 105) and cell lines (5 × 104) were incubated for 72 hours with IPI-504, 17-AAG, or vehicle. The reagent 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide (Sigma) was added for 2-5 additional hours before spectrophotometric measurement. The growth inhibitory activity 50 (GI50) was calculated as the dose that produced 50% growth inhibition.
Xenograft mouse model and tumor phenotyping
CB17–severe combined immunodeficient mice (Charles River) were housed in the animal care facility, under a 12/12 hour light/dark cycle at 22°C, and they received a standard diet and acidified water ad libitum. With the use of a protocol approved by the animal testing ethical committee of the University of Barcelona, mice were inoculated subcutaneously into their lower dorsum with 107 JBR cells in Matrigel basement membrane matrix (Becton Dickinson). When tumors were palpable, mice were randomly assigned into cohorts of 6-9 mice each, receiving intraperitoneal injection twice a week with 0.15 mg/kg bortezomib, alone or combined with 50 mg/kg IPI-504, or an equal volume of vehicle. The shortest and longest diameters of the tumor were measured with external calipers every 3 days, and tumor volume (in mm3) was calculated with the use of the following standard formula: (the shortest diameter)2 × (the longest diameter) × 0.5. Animals were killed according to institutional guidelines, and tumor xenografts were extirpated. Tumor samples were snap-frozen in OCT medium (Sakura Tissue Tek) or formalin-fixed before paraffin embedding on silane-coated slides in a fully automated immunostainer (Bond Max; Vision Biosystems). Immunohistochemical staining studies were performed as previously described 20 with the use of anti–phospho-Histone H3 (1:1000; Epitomics), anti–caspase-3 cleaved (1:1000; 5A1E; Cell Signaling Technology) or anti BiP/Grp78 (1:1000; Cell Signaling Technology) primary antibodies. Preparations were evaluated with an Olympus DP70 microscope by means of a 20×/0.75 NA objective and DPManager software v2.1.1 (Olympus). Ten representative fields from each phospho-Histone H3 and activated caspase-3 tumor staining were recounted for the presence of positive cells.
To evaluate BiP/Grp78 levels, whole protein extracts were prepared from 3 representative tumors of each group with the use of T-PER lysis solution (Pierce). Briefly, 50 mg of tissue samples were resuspended in 400 μL of lysis buffer complemented with protease and phosphatase inhibitors and incubated for 1 hour on ice. Samples were centrifuged at 15 000g for 15 minutes, and 50 μg of supernatants were then analyzed by SDS-PAGE for BiP/Grp78 and β-actin levels.
Statistical analysis
Data are represented as mean ± SD of 3 independent experiments. All statistical analysis was done with the use of GraphPad Prism 3.0 software (GraphPad Software). Comparison between 2 groups of samples was evaluated by nonparametric Mann-Whitney test, and correlation coefficients were assessed with the Spearman test. Results were considered statistically significant when P < .05.
Results
Acquisition of bortezomib resistance in MCL cells involves the stabilization of the chaperone BiP/Grp78
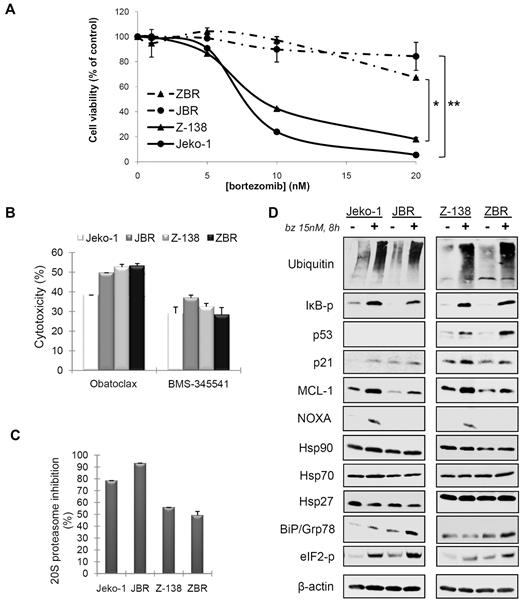
To study the mechanism of acquired resistance to bortezomib in MCL cells, we established 2 bortezomib-resistant cell lines designated JBR and ZBR derived from the parental Jeko-1 and Z-138, respectively, by repeated drug selection as described in “Methods.” Compared with their respective parental cell lines, JBR and ZBR presented approximately a 7-fold and a 3-fold increase in their bortezomib LC50 after 24 hours of treatment with the inhibitor (Table 2), with no significant differences in the growth curves between parental and derived cell lines (data not shown). JBR cells were almost completely resistant to bortezomib ≤ 20nM, a dose at which the parental Jeko-1 showed ∼ 95% induction of cell death at 24 hours (Figure 1A). A slight decrease (∼ 20%) of cell viability was detected in JBR cells on 48-hour incubation with 20nM bortezomib (data not shown). In parallel, ZBR cells were completely resistant to bortezomib at doses ≤ 10nM after 24 hours and showed a 35% cell viability decrease between 24 and 48 hours at this dose (data not shown). In these cells, the 20-nM dose was ∼ 50% less efficient compared with the parental cell line Z-138 at 24 hours of treatment (Figure 1A). Importantly, both JBR and ZBR remained equally sensitive to agents shown to activate the intrinsic apoptotic program in MCL cells,7,21 namely the BH3-mimetic Obatoclax and the nuclear factor-κB inhibitor BMS-345541 (Figure 1B). Accordingly, the expression of apoptotic transducers such as BAX, BCL-2 and caspase-9 remained unchanged between parental and resistant cell lines (data not shown).
Characterization of 2 derived MCL cell lines resistant to bortezomib. (A) Dose-response of Jeko-1 and Z-138 parental, and JBR and ZBR derived cell lines exposed for 24 hours to bortezomib (bz) (*P < .04; **P < .003). (B) Jeko-1, JBR, Z-138, and ZBR cells were treated for 24 hours with 5μM BMS-345541 and 2μM obatoclax, and cytotoxicity was assessed by Annexin V/propidium iodide staining. Cytotoxic values are compared with untreated cells as the means ± SDs of triplicate experiments. (C) Jeko-1, JBR, Z-138, and ZBR cells were incubated with 15nM bortezomib for 8 hours, and the inhibition of 20S proteasome activity was analyzed as described in “Methods.” (D) Modulation of proteasome-degraded proteins, apoptotic regulators, and molecular chaperone levels in parental and resistant cells exposed to bortezomib. Total protein extracts from Jeko-1, JBR, Z-138, and ZBR cell lines incubated for 8 hours with 15nM bortezomib were analyzed by Western blotting. Membranes were probed with suitable antibodies, using ubiquitin conjugates and β-actin to normalize misfolded protein accumulation and protein loading, respectively. eIF2-p represents elongation initiation factor 2α phosphorylated.
Characterization of 2 derived MCL cell lines resistant to bortezomib. (A) Dose-response of Jeko-1 and Z-138 parental, and JBR and ZBR derived cell lines exposed for 24 hours to bortezomib (bz) (*P < .04; **P < .003). (B) Jeko-1, JBR, Z-138, and ZBR cells were treated for 24 hours with 5μM BMS-345541 and 2μM obatoclax, and cytotoxicity was assessed by Annexin V/propidium iodide staining. Cytotoxic values are compared with untreated cells as the means ± SDs of triplicate experiments. (C) Jeko-1, JBR, Z-138, and ZBR cells were incubated with 15nM bortezomib for 8 hours, and the inhibition of 20S proteasome activity was analyzed as described in “Methods.” (D) Modulation of proteasome-degraded proteins, apoptotic regulators, and molecular chaperone levels in parental and resistant cells exposed to bortezomib. Total protein extracts from Jeko-1, JBR, Z-138, and ZBR cell lines incubated for 8 hours with 15nM bortezomib were analyzed by Western blotting. Membranes were probed with suitable antibodies, using ubiquitin conjugates and β-actin to normalize misfolded protein accumulation and protein loading, respectively. eIF2-p represents elongation initiation factor 2α phosphorylated.
It has been proposed that culture-induced resistance to bortezomib could lead to the selection of sublines presenting either an increased or a bortezomib-insensitive basal proteasome activity.22-24 To test this hypothesis in JBR and ZBR cells, both cell lines and their corresponding parental cells were exposed to bortezomib for 8 hours, and the 20S proteasome activity was analyzed in cytosolic protein extracts. As observed in Figure 1C, incubation of Jeko-1 and JBR cells with 15nM bortezomib caused, respectively, a 79% and a 92% reduction in proteasome activity, whereas a 56% and a 49% decrease was detected, respectively, in Z-138 and ZBR cells. Thus, bortezomib was equally effective at inhibiting proteasome activity in bortezomib-resistant and parental cell lines. Accordingly, the content of ubiquitinated residues after bortezomib exposure, as well as the accumulation or activation or both of traditional proteasome substrates involved in cell proliferation and cell death such as phospho-IκBα, p53, p21, and MCL-1, was similar between resistant and parental cell lines (Figure 1D). Similar to our previous results, apoptosis induction by bortezomib was tightly linked to the induction of NOXA,5 and its accumulation was observed in parental but not in resistant cells exposed to the inhibitor (Figure 1D).
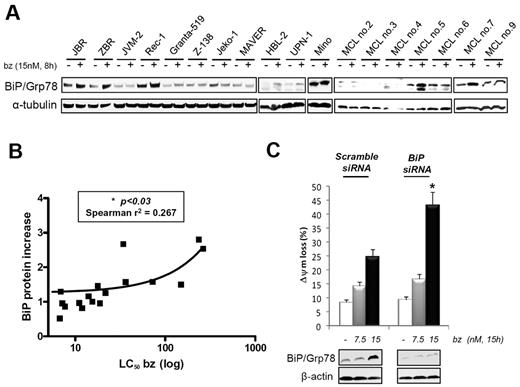
Recent data have suggested the importance of molecular chaperone activation and ER stress signaling in MCL response to bortezomib.25,26 Therefore, we analyzed the expression of Hsp90, its co-chaperones, Hsp70 and Hsp27, and the UPR components BiP/Grp78 and phospho-eIF2α in Jeko-1, JBR, Z-138, and ZBR cells. After bortezomib treatment, BiP/Grp78 was the only up-regulated chaperone in JBR and ZBR cells, compared with their parental cell lines (Figure 1D). Concordant with increased activation of the UPR pathway, the ER kinase eIF2α also presented higher phosphorylation levels in resistant cells treated with bortezomib (Figure 1D). To assess the relevance of these results, we analyzed basal and bortezomib-induced levels of BiP/Grp78 in 7 additional MCL cell lines and 7 MCL primary samples with distinct sensitivity to bortezomib (Tables 1 and 2). Rec-1 and Mino cell lines were used as primarily bortezomib-resistant lines with LC50 values of 21.6nM and 150nM, respectively. We observed a good correlation between the increase in BiP/Grp78 levels and a lower sensitivity to bortezomib, because BiP/Grp78 induction was more pronounced in resistant cell lines (JBR, ZBR, Rec-1, and Mino) and primary cultures (MCL cases no. 5 and no.7) (Figure 2A). Densitometric quantification of the BiP/α-tubulin ratio showed a significant correlation between BiP/Grp78 protein increase at 8 hours of exposure to bortezomib and the LC50 at 24 hours (P < .03) (Figure 2B; Tables 1 and 2).
Correlation between BiP/Grp78 levels and MCL sensitivity to bortezomib. (A) BiP/Grp78 protein accumulation after bortezomib treatment was analyzed by Western blot in a panel of 11 MCL cell lines and 7 MCL primary cultures. (B) Relative protein quantification of BiP/Grp78 levels in treated versus control extracts was performed with the use of Image Gauge Fujifilm software and α-tubulin levels for loading normalization. Values were plotted against the respective bortezomib LC50 of each MCL sample (Tables 1 and 2). (C) BiP/Grp78 siRNA and nonsilencing siRNA were transferred to JBR cells by electroporation, and transfected cells were treated with 7.5 or 15nM bortezomib for 15 hours. Viability was assessed by flow cytometry determination of mitochondrial transmembrane potential (ΔΨm) loss, and BiP/Grp78 protein levels were evaluated by Western blot. The results shown are the mean of 2 different experiments (*P < .05).
Correlation between BiP/Grp78 levels and MCL sensitivity to bortezomib. (A) BiP/Grp78 protein accumulation after bortezomib treatment was analyzed by Western blot in a panel of 11 MCL cell lines and 7 MCL primary cultures. (B) Relative protein quantification of BiP/Grp78 levels in treated versus control extracts was performed with the use of Image Gauge Fujifilm software and α-tubulin levels for loading normalization. Values were plotted against the respective bortezomib LC50 of each MCL sample (Tables 1 and 2). (C) BiP/Grp78 siRNA and nonsilencing siRNA were transferred to JBR cells by electroporation, and transfected cells were treated with 7.5 or 15nM bortezomib for 15 hours. Viability was assessed by flow cytometry determination of mitochondrial transmembrane potential (ΔΨm) loss, and BiP/Grp78 protein levels were evaluated by Western blot. The results shown are the mean of 2 different experiments (*P < .05).
To further characterize the putative protective role of BiP/Grp78 in bortezomib-mediated MCL cells apoptosis, we used a siRNA approach to selectively down-regulate BiP/Grp78 protein synthesis in JBR cells. A specific oligonucleotide mix targeting BIP RNA was able to abrogate basal BiP/Grp78 expression and to prevent the dose-dependent accumulation of the protein elicited by bortezomib in JBR cells, compared with cells transfected with a nontargeting siRNA (Figure 2C). BiP/Grp78 knockdown notably restored the ability of JBR cells to undergo bortezomib-mediated apoptosis, at similar rates to that observed in parental Jeko-1 cells exposed to equivalent bortezomib doses (Figure 1A). These results therefore suggest an inhibitory function of BiP/Grp78 accumulation in determining MCL sensitivity to bortezomib.
BiP/Grp78 accumulates within Hsp90 complexes after treatment of bortezomib-resistant cells
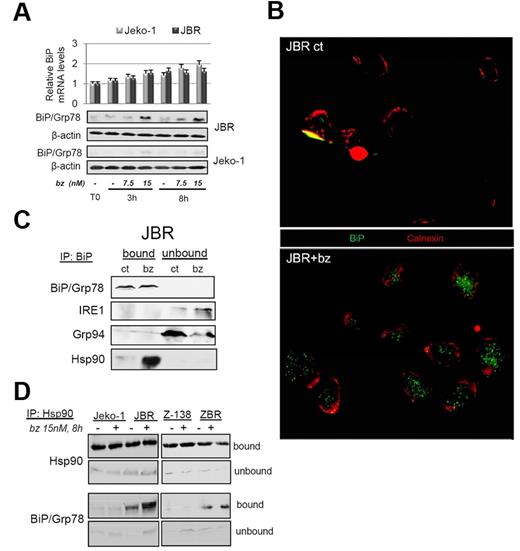
To characterize the mechanism underlying BiP/Grp78 protein increase in MCL cells treated with bortezomib, we analyzed BiP/Grp78 protein and mRNA levels in parental Jeko-1 cells and JBR-resistant cells exposed for 3 or 8 hours to 7.5 and 15nM bortezomib. As shown in Figure 3A, BIP transcript levels were unmodified in both cell lines after these short time exposures to bortezomib. In contrast, the time- and dose-dependent increase in BiP/Grp78 protein content was remarkable in JBR, while almost undetectable in Jeko-1 cells (Figure 3A), suggesting the existence of a posttranscriptional mechanism regulating BiP/Grp78 levels in cells resistant to bortezomib.
BiP/Grp78 stabilization on bortezomib treatment is ensured at a posttranscriptional level by increased chaperoning activity of Hsp90. (A) Jeko-1 and JBR cells were treated with 7.5 or 15nM bortezomib (bz). RNA and protein were isolated after 3 and 8 hours of incubation. BIP mRNA levels were quantified by reverse transcription–PCR. BiP/Grp78 protein levels were evaluated by Western blotting with the use of β-actin as equal loading control. (B) Microphotographs of BiP/Grp78 (green) and calnexin (red) labeling in JBR cells either untreated (ct) or treated for 8 hours with 15nM bortezomib. (C) JBR cells were treated as in panel B, and BiP immunoprecipitation was performed as described in “Methods.” Bound and unbound fractions were analyzed by Western blotting for the presence of BiP/Grp78, IRE-1, Grp94, and the cytosolic-activated Hsp90. (D) Jeko-1, JBR, Z-138, and ZBR cells were treated as indicated, and Hsp90/BiP complexes were analyzed as previously described by coimmunoprecipitation, followed by Western blot analysis of both factors.
BiP/Grp78 stabilization on bortezomib treatment is ensured at a posttranscriptional level by increased chaperoning activity of Hsp90. (A) Jeko-1 and JBR cells were treated with 7.5 or 15nM bortezomib (bz). RNA and protein were isolated after 3 and 8 hours of incubation. BIP mRNA levels were quantified by reverse transcription–PCR. BiP/Grp78 protein levels were evaluated by Western blotting with the use of β-actin as equal loading control. (B) Microphotographs of BiP/Grp78 (green) and calnexin (red) labeling in JBR cells either untreated (ct) or treated for 8 hours with 15nM bortezomib. (C) JBR cells were treated as in panel B, and BiP immunoprecipitation was performed as described in “Methods.” Bound and unbound fractions were analyzed by Western blotting for the presence of BiP/Grp78, IRE-1, Grp94, and the cytosolic-activated Hsp90. (D) Jeko-1, JBR, Z-138, and ZBR cells were treated as indicated, and Hsp90/BiP complexes were analyzed as previously described by coimmunoprecipitation, followed by Western blot analysis of both factors.
To analyze the changes in BiP/Grp78 intracellular distribution in JBR cells cultured with bortezomib, we performed a dual immunostaining of BiP/Grp78 and the ER resident protein calnexin. Figure 3B shows that BiP/Grp78 was almost undetectable in untreated JBR cells, whereas bortezomib-induced BiP/Grp78 displayed a diffused distribution throughout the cytoplasm that did not colocalize with calnexin in these cells. Accordingly, immunoprecipitation analysis of BiP/Grp78 complexes in JBR cells showed that bortezomib-induced BiP/Grp78 protein failed to interact with the ER factors IRE1 and Grp94 (Figure 3C). In contrast, analysis of the immunoprecipitated (bound) fraction of bortezomib-treated cells showed that substantial amounts of the cytosolic Hsp90 protein, unlike its ER homolog Grp94, were associated with BiP/Grp78 (Figure 3C). As a confirmation, the reverse immunoprecipitation of Hsp90 complexes showed that stabilization of Hsp90/BiP interaction after bortezomib exposure could be observed in JBR, as well as in ZBR cells (Figure 3D). Of note, some amounts of BiP/Grp78 were also present within Hsp90 complexes in bortezomib-treated Jeko-1 cells. Altogether, these results suggest that accumulation of BiP/Grp78 within MCL cells resistant to bortezomib is ensured by increased chaperoning activity of cytosolic Hsp90.
The Hsp90 inhibitor IPI-504 exerts cytostatic effect and restores bortezomib apoptogenicity in MCL cells mediated by BiP/Grp78 depletion and UPR inhibition
To assess the possible protective role of Hsp90 in MCL response to bortezomib, we first evaluated the antitumoral activity of 2 Hsp90 inhibitors of the ansamycin class, 17-AAG and IPI-504, in parental (Jeko-1 and Z-138) and bortezomib-resistant cells (JBR and ZBR). As shown in Figure 4A, both agents exerted a cytostatic effect on both parental and bortezomib-resistant MCL cell lines, at doses between 0.1 and 10 μM. Seven additional cell lines and 10 primary MCL samples were exposed for 72 hours to different doses of IPI-504, and the corresponding GI50 values were calculated (Tables 1 and 2). The GI50 values ranged from 0.1 to 25.3μM (mean, 6.8μM) and were independent of whether MCL cells contained complexed or free Hsp90 proteins (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The pharmacologic activity of IPI-504 was confirmed by the decrease in the Hsp90 client protein IκBα kinase-β and the accumulation of the Hsp90 co-chaperone Hsp70 in whole cell extracts and Hsp90 complexes purified from the sensitive cell line Z-138 (supplemental Figure 1B-C). We then checked whether IPI-504 pretreatment could be useful to enhance MCL response to bortezomib. A panel of 11 MCL cell lines and 9 MCL primary samples were pretreated for 24 hours with various IPI-504 doses, followed by a 16-hour exposure to doses of bortezomib ranging from 2.5 to 20nM, and the CI values were calculated. Figure 4B shows that IPI-504 0.5μM and bortezomib 10nM exerted a synergistic effect on almost all the samples analyzed, and the effect was tightly correlated to primary cell sensitivity to bortezomib. Indeed, the CI values of the bortezomib-resistant samples (mean, 0.36 ± 0.1) were significantly lower than the bortezomib sensitive samples (mean, 0.84 ± 0.07; **P = .001) and showed a notable synergistic interaction between both compounds in those samples that poorly respond to bortezomib alone. Importantly, this synergistic interaction was sequence dependent, because the effects were lost when cells were preincubated with bortezomib before adding IPI-504, or when both agents were added simultaneously (data not shown).
IPI-504 disrupts BiP/Hsp90 complexes, inhibits UPR activation, and synergistically induces apoptosis with bortezomib in resistant cells. (A) Jeko-1, JBR, Z-138, and ZBR cells were incubated for 72 hours with increasing concentrations of 17-AAG or IPI-504 and cytotoxicity was assessed by the 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide assay. The relative number of proliferating cells compared with control, untreated cells are presented as the mean ± SD of triplicate assays. (B) Relationship between sensitivity/resistance to bortezomib (bz) status and efficacy of IPI-504–bz combination in 20 MCL samples. Cells were pretreated for 24 hours with 0.01, 0.05, 0.1, or 0.5μM IPI-504, followed by an additional 16-hour exposure to 2.5, 5, 10, or 20nM bortezomib. Cytotoxicity was determined by Annexin V staining, and the CIs were calculated in both bortezomib-resistant (bz LC50 > 20nM) and bortezomib-sensitive (bz LC50 < 20nM) groups. Shown are the cytotoxicity and the mean CI values calculated on cell treatment with 10nM bortezomib and 0.5μM IPI-504. (C-D) JBR cells and cells from a representative bortezomib-resistant MCL sample were treated as above with 0.5μM IPI-504 and 20nM bortezomib. The levels of UPR proteins [BiP/Grp78, phospho–elongation initiation factor 2α (eIF2α), spliced (s) and unspliced (u) XBP-1, CHOP] and apoptosis hallmark proteins [NOXA, poly(ADP-ribose) polymerase (PARP) cleavage] were determined by Western blot. Mitochondrial transmembrane potential loss (ΔΨm), phosphatidylserine (PS) exposure, and caspase-3 cleavage were evaluated by flow cytometry. Percentages inside each chart refer to the cell population positive for the corresponding label.
IPI-504 disrupts BiP/Hsp90 complexes, inhibits UPR activation, and synergistically induces apoptosis with bortezomib in resistant cells. (A) Jeko-1, JBR, Z-138, and ZBR cells were incubated for 72 hours with increasing concentrations of 17-AAG or IPI-504 and cytotoxicity was assessed by the 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide assay. The relative number of proliferating cells compared with control, untreated cells are presented as the mean ± SD of triplicate assays. (B) Relationship between sensitivity/resistance to bortezomib (bz) status and efficacy of IPI-504–bz combination in 20 MCL samples. Cells were pretreated for 24 hours with 0.01, 0.05, 0.1, or 0.5μM IPI-504, followed by an additional 16-hour exposure to 2.5, 5, 10, or 20nM bortezomib. Cytotoxicity was determined by Annexin V staining, and the CIs were calculated in both bortezomib-resistant (bz LC50 > 20nM) and bortezomib-sensitive (bz LC50 < 20nM) groups. Shown are the cytotoxicity and the mean CI values calculated on cell treatment with 10nM bortezomib and 0.5μM IPI-504. (C-D) JBR cells and cells from a representative bortezomib-resistant MCL sample were treated as above with 0.5μM IPI-504 and 20nM bortezomib. The levels of UPR proteins [BiP/Grp78, phospho–elongation initiation factor 2α (eIF2α), spliced (s) and unspliced (u) XBP-1, CHOP] and apoptosis hallmark proteins [NOXA, poly(ADP-ribose) polymerase (PARP) cleavage] were determined by Western blot. Mitochondrial transmembrane potential loss (ΔΨm), phosphatidylserine (PS) exposure, and caspase-3 cleavage were evaluated by flow cytometry. Percentages inside each chart refer to the cell population positive for the corresponding label.
To check whether IPI-504 could facilitate bortezomib-induced apoptosis in resistant cells by modulating the Hsp90/BiP interaction, we performed an Hsp90 immunoprecipitation from JBR cell extracts treated as above with 20nM bortezomib or 0.5μM IPI-504. As shown in Figure 4C, IPI-504 substantially inhibited the basal interaction between Hsp90 and BiP/Grp78 in control cells and was able to prevent the aggregation of BiP/Grp78 after bortezomib addition. Accordingly, Western blot analysis of JBR cells and a representative bortezomib-resistant primary sample (MCL no. 7) showed that IPI-504 pretreatment could prevent the accumulation of BiP/Grp78 elicited by bortezomib and consequently interfere with the up-regulation of the UPR mediator transcription factor CHOP, the splicing of XBP-1, and the phosphorylation of eIF2α (Figure 4C). As a consequence, although IPI-504 or bortezomib alone slightly activated the mitochondrial apoptotic pathway in these cells, the combination of both compounds led to NOXA up-regulation and activation of mitochondrial cell death, characterized by enhanced phosphatidylserine exposure, loss of mitochondrial transmembrane potential, caspase-3 activation, and cleavage of the caspase substrate poly(ADP-ribose) polymerase (Figure 4C-D).
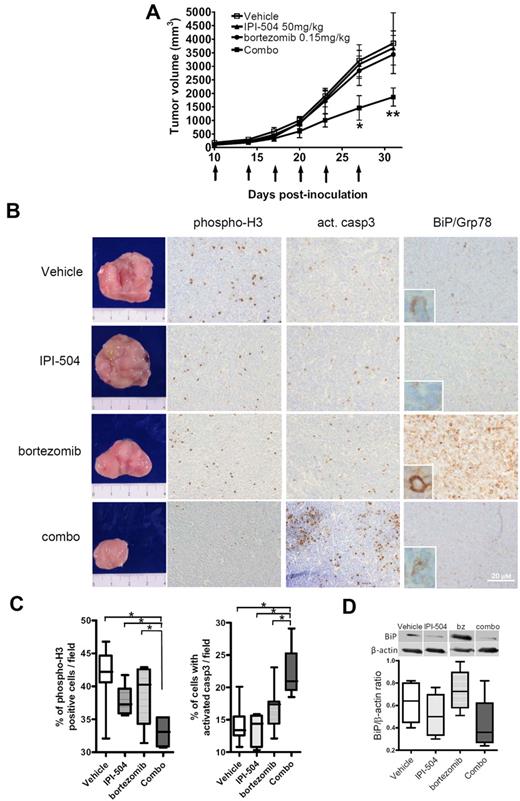
In vivo antitumoral activity of the IPI-504 and bortezomib combination involves UPR inhibition and apoptosis restoration
We next determined whether IPI-504 addition to bortezomib could restore the sensitivity of resistant tumors to the proteasome inhibitor in vivo. CB17–severe combined immunodeficient mice were inoculated with JBR cells to generate a bortezomib-resistant MCL xenograft animal model. Within 1 week of inoculation, animals developed tangible tumors that grew rapidly over the next 3 weeks (Figure 5A). On day 10, when the tumor size reached 5 mm in diameter, mice were randomly assigned into drug-treated [50 mg/kg IPI-504, 0.15 mg/kg bortezomib, IPI-504–bortezomib combination (combo)] and control (vehicle) groups, receiving the corresponding administration twice a week. Caliper measurements were performed every 3 days to calculate tumor volume. As shown in Figure 5A, administration of bortezomib or IPI-504 alone did not alter tumor growth compared with vehicle-treated tumors. In contrast, the IPI-504–bortezomib combination achieved significant MCL tumor regression, observable at day 27 (*P < .05) and increased at day 31 (**P < .01), compared with the other 3 groups (Figure 5A). Thirty-one days after inoculation, tumor size was reaching ∼ 10% of body weight in control mice, with no evidence of tumor-related toxicity and no differences in mean body weight between the different groups (data not shown). Animals were then killed according to animal care guidelines. Tumors isolated from control and drug-treated MCL-bearing mice showed a 40% reduction in tumor burden in the combo-receiving group (Figure 5B).
IPI-504 plus bortezomib synergistically inhibits the growth of bortezomib-resistant tumors in severe combined immunodeficient mice. (A) JBR cells (107 cells per mouse) were subcutaneously inoculated into the right flank of CB17–severe combined immunodeficient mice. Tumor-bearing mice received intraperitoneal injections of 50 mg/kg IPI-504 or 0.15 mg/kg bortezomib or both (n = 6), or equal volume of vehicle (n = 9), twice a week for ≤ 3 weeks. Arrows indicate the days of injection and tumor size recording (*P < .05, **P < .01). (B) Immunohistochemical staining of consecutive sections from tumor mass of representative specimen (magnification ×200). Specific anti–human antibodies were used to stain phospho-histone H3, activated caspase-3, and BiP/Grp78 in the whole tumor samples from each treated group. (C) Three representative fields from each phospho-Histone H3 and activated (act.) caspase-3 tumor staining were recounted for the presence of positive cells, and the mean percentages of positive cells in each treated group were compared (*P < .05). (D) BiP/Grp78 levels were evaluated by Western blot analysis of BiP/Grp78 expression and densitometric quantification of BiP/β-actin ratio in 3 representative specimens per tumor group. Shown are immunoblots from 4 representative tumor samples.
IPI-504 plus bortezomib synergistically inhibits the growth of bortezomib-resistant tumors in severe combined immunodeficient mice. (A) JBR cells (107 cells per mouse) were subcutaneously inoculated into the right flank of CB17–severe combined immunodeficient mice. Tumor-bearing mice received intraperitoneal injections of 50 mg/kg IPI-504 or 0.15 mg/kg bortezomib or both (n = 6), or equal volume of vehicle (n = 9), twice a week for ≤ 3 weeks. Arrows indicate the days of injection and tumor size recording (*P < .05, **P < .01). (B) Immunohistochemical staining of consecutive sections from tumor mass of representative specimen (magnification ×200). Specific anti–human antibodies were used to stain phospho-histone H3, activated caspase-3, and BiP/Grp78 in the whole tumor samples from each treated group. (C) Three representative fields from each phospho-Histone H3 and activated (act.) caspase-3 tumor staining were recounted for the presence of positive cells, and the mean percentages of positive cells in each treated group were compared (*P < .05). (D) BiP/Grp78 levels were evaluated by Western blot analysis of BiP/Grp78 expression and densitometric quantification of BiP/β-actin ratio in 3 representative specimens per tumor group. Shown are immunoblots from 4 representative tumor samples.
Histologic sections from whole tumors were analyzed by immunohistochemistry or Western blot, using phospho-histone H3, BiP/Grp78, and active caspase-3 antibodies to evaluate mitotic index, UPR activation, and apoptosis processing, respectively. Representative pictures and quantification of each labeling are shown in Figure 5B-D. In vehicle-treated tumors, the mitotic index was ∼ 42%, with a low apoptotic rate (13%) (Figure 5C) and low levels of BiP/Grp78 (Figure 5D). Bortezomib and IPI-504 monotherapies slightly decreased the tumor mitotic index, although not significantly and without increasing caspase-3 cleavage (Figures 5C). Despite this, and in accordance with our in vitro results, bortezomib treatment induced an 18% increase in BiP/Grp78 expression, as opposed to the down-regulation of the protein (∼ 17%) observed in IPI-504–treated tumors (Figure 5B,D). In contrast, the combo group showed a significant reduction in the mitotic index (−9%; P < .05) accompanied by an augmented processing of caspase-3 (9%; P < .05) and an impaired accumulation of BiP/Grp78 levels compared with the bortezomib-treated group (−40%). Taken together, these data confirm our in vitro observation that the IPI-504–bortezomib combination is able to overcome bortezomib resistance in MCL tumors, with its antitumor effect mediated by BiP/Grp78 down-regulation and apoptosis induction.
Discussion
MCL is an aggressive B-cell lymphoma, largely resistant to traditional chemotherapy and characterized by nondurable remissions. The molecular signature of MCL is complex and involves multiple signals that favor cell survival and growth.1,27 Therefore, new therapeutic options that take in account this peculiar signature are crucial to improve patient outcome. Bortezomib is a potent and reversible inhibitor of the proteasome that has shown therapeutic efficacy as a single agent in patients with relapsed and refractory MCL.28-30 Despite its approval as a second-line treatment, not all cases respond, and primary or acquired resistance to bortezomib occurs frequently.31
It has been proposed that after exposure to bortezomib, inhibition of the proteasome provokes an accumulation of misfolded or oxidized proteins within the ER-Golgi network. This primarily triggers UPR as a defense system and elicits the export of misfolded ER proteins from the ER into the cytosol to activate the ER-associated protein degradation system,32 a process recently shown to be involved in bortezomib-induced transcription of NOXA.11 Exactly how the misfolded proteins are shuttled to the proteasome remains unclear but may involve discrete structures known as aggresomes and molecular chaperones of the Hsp70 family.33,34 Accordingly, deregulated expression of several Hsp70 family members is associated with bortezomib resistance in a wide range of B-cell malignancies, including MCL,25 multiple myeloma (MM),35 and diffuse large B-cell lymphoma,36 and it may alter the response of cyclin D1–overexpressing B cells to chemotherapeutic agents.37
In the present work, we developed 2 MCL-derived cell lines with acquired resistance to bortezomib, showing no defect in proteasome activity or apoptosis machinery, but presenting altered levels of the ER Hsp70 homolog BiP/Grp78 after bortezomib treatment. In an extended set of samples, we found the accumulation of BiP/Grp78 to be significantly associated with a reduced sensitivity of MCL cells to the proteasome inhibitor. We also demonstrated that BiP/Grp78 levels can be used as a surrogate to determine MCL response to bortezomib, because its selective knockdown restored MCL sensitivity to the inhibitor. In line with a direct role of BiP/Grp78 protein stabilization in cell resistance to bortezomib, previous studies carried out in MM cells showed that transcriptional activation of BiP/Grp78 is a secondary event in cells treated with the inhibitor,35 pointing out that a reduced threshold for BiP/Grp78 accumulation partly regulates bortezomib activity38 and that decreased BiP/Grp78-mediated UPR signaling enhances the efficacy of the drug.39 All these results support the notion that BiP/Grp78, as a mediator of the UPR prosurvival arm, may play a fundamental role in reducing the load on the ER-Golgi network13 and also in facilitating tumor cell evasion from proteasome inhibitors and chemotherapeutics.40,41
We found BiP/Grp78 to accumulate in bortezomib-resistant cells at a posttranscriptional level and visibly in a non-ER compartment. In this context, a mitochondrial localization of BiP/Grp78, compatible with its function in UPR regulation, has been described in cells subjected to ER stress.42,43 Our immunoprecipitation studies further showed that BiP/Grp78 stabilization involved an increased interaction of the protein with Hsp90. Traditional Hsp90 client proteins are involved in the regulation of the cell cycle, cell growth, cell survival, apoptosis, and oncogenesis.44 Therefore, Hsp90 plays a key role in regulating cell response to environmental stress and in maintaining the malignant phenotype of tumor cells. The natural Hsp90 inhibitor and ansamycin antibiotic, geldanamycin, binds to the highly conserved 25 kDa N-terminal domain of the chaperone, shifting its conformation to the adenosine diphosphate–bound form associated with client protein degradation and conformational maturation. Animal toxicology studies of geldanamycin reported significant hepatotoxicity, which precluded its further development as a clinical therapeutic agent.44 Analog development led to the synthesis of 17-AAG, characterized by similar Hsp90-binding properties and a significantly improved toxicity profile, but with limited solubility and stability in solution.45 This has led to the development of IPI-504, a highly soluble hydroquinone ansamycin which has shown activity in several oncogenic models.45,46 We found MCL cells to be poorly sensitive to 17-AAG or IPI-504 monotherapy, possibly because of, as postulated in other models, the remarkable feedback induction of Hsp70.47 However, we demonstrated a meaningful synergistic effect of the IPI-504–bortezomib combination, especially in those cases with intrinsic or induced resistance to bortezomib, because of the capacity of IPI-504 to prevent BiP/Grp78 accumulation. By both in vitro and in vivo approaches, we also showed that impaired Hsp90/BiP interaction after IPI-504 treatment results in clear depletion of BiP/Grp78, leading to the blockade of bortezomib-elicited UPR signaling and to the restoration of bortezomib cytotoxicity in resistant cells and tumors. These observations thus confirm that targeting the mechanism involved in BiP/Grp78 stabilization is an efficient approach to counteract its protective activity. Consistent with these results, IPI-504 has been shown recently to reverse some processes typical of the UPR,48 whereas both IPI-504 and 17-AAG efficiently increased in vitro and in vivo bortezomib activity in MM.49,50
In summary, we demonstrate that the combination of bortezomib and IPI-504 is able to overcome intrinsic and acquired resistance to the proteasome inhibitor in MCL cultures and tumors, mediated by UPR modulation and enhanced apoptosis. These promising results offer a glimpse of the potential opportunities that this combinational strategy might hold for the treatment of patients with MCL refractory to bortezomib therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sandra Cabezas and Laura Jimenez for expert technical assistance. We gratefully acknowledge Dr Elisabeth Ametller for her help with in vivo experiments and Dr Antonio Martinez for fruitful discussion and help in immunochemistry (supported by grant PI080095).
This work was supported by grants from Ministerio de Ciencia y Innovación (SAF 06/8850 and 09/9503), Redes Temáticas de Investigacion Cooperativa de Cáncer from the Instituto de Salud Carlos III (RED 2006-20-014 and 039) (D.C. and E.C.), and Fondo de Investigación Sanitaria (CP07/00072 and PI09/00060) (G.R.). A.M. was partially supported by grant PI080095 from Fondo de Investigación Sanitaria. M.L.G. was the recipient of a FI predoctoral fellowship from Generalitat de Catalunya. S.X.T. and L.R. hold predoctoral fellowships from Ministerio de Ciencia e Innovación (FPU) and IDIBAPS, respectively.
Authorship
Contribution: G.R. contributed to the design and conception of the study, performed the research, analyzed and interpreted the data, and wrote the manuscript. P.P.-G., M.L.-G., S.X.-T., L.R., and I.S.-V. performed the research and analyzed and interpreted the data. A.M. realized the immunophenotyping of the tumors, analyzed data, and participated in writing the manuscript. E.N. provided essential compound and critically reviewed the manuscript. E.C. contributed to the revision of the manuscript critically. D.C. was the principal investigator and contributed to the design of the study, interpretation of data, and writing of the paper.
Conflict-of-interest disclosure: E.N. is an employee of Infinity Pharmaceuticals, Inc, with stock ownership. The remaining authors declare no competing financial interests.
Correspondence: Dolors Colomer, Hematopathology Unit, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain; e-mail: dcolomer@clinic.ub.es.

![Figure 4. IPI-504 disrupts BiP/Hsp90 complexes, inhibits UPR activation, and synergistically induces apoptosis with bortezomib in resistant cells. (A) Jeko-1, JBR, Z-138, and ZBR cells were incubated for 72 hours with increasing concentrations of 17-AAG or IPI-504 and cytotoxicity was assessed by the 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide assay. The relative number of proliferating cells compared with control, untreated cells are presented as the mean ± SD of triplicate assays. (B) Relationship between sensitivity/resistance to bortezomib (bz) status and efficacy of IPI-504–bz combination in 20 MCL samples. Cells were pretreated for 24 hours with 0.01, 0.05, 0.1, or 0.5μM IPI-504, followed by an additional 16-hour exposure to 2.5, 5, 10, or 20nM bortezomib. Cytotoxicity was determined by Annexin V staining, and the CIs were calculated in both bortezomib-resistant (bz LC50 > 20nM) and bortezomib-sensitive (bz LC50 < 20nM) groups. Shown are the cytotoxicity and the mean CI values calculated on cell treatment with 10nM bortezomib and 0.5μM IPI-504. (C-D) JBR cells and cells from a representative bortezomib-resistant MCL sample were treated as above with 0.5μM IPI-504 and 20nM bortezomib. The levels of UPR proteins [BiP/Grp78, phospho–elongation initiation factor 2α (eIF2α), spliced (s) and unspliced (u) XBP-1, CHOP] and apoptosis hallmark proteins [NOXA, poly(ADP-ribose) polymerase (PARP) cleavage] were determined by Western blot. Mitochondrial transmembrane potential loss (ΔΨm), phosphatidylserine (PS) exposure, and caspase-3 cleavage were evaluated by flow cytometry. Percentages inside each chart refer to the cell population positive for the corresponding label.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/4/10.1182_blood-2010-04-278853/4/m_zh89991065560004.jpeg?Expires=1767782290&Signature=fAmu6DoKjdOiFEF4I3ySp4jwuZNQQywbsKUGF7XH4EEgBTtqKzyJvclL8I3nUsNLpEE~8CuyOPwtiKU3kRjTAegrocRL2-n-VRpZ0RYDWM7g2llNA55VX8wVmvc~bNtZAs2wuQM4y6aBidvmfjGH6QrHjc-dB9d6T1y1y9K~5d90ZmkmqbHt8tML0dRB-cMaAYL7VO6D0-fbg-1VRQ8Iif4yiKNH1HzjSBIBLr8VU7YxLToFQN6UwgE1Q2iF4NBl4yKq4~aEV0Et9E0FengNPy7gouRh16BEwS6gLnbDYdCpxTcTkXNqqYafFtmxTtOo4MwAHjpr7qQUNyDP29Lwtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)