Abstract
Rituximab is a chimeric anti-CD20 monoclonal B cell–depleting antibody indicated for certain hematologic malignancies and active rheumatoid arthritis with inadequate response to tumor necrosis factor antagonists. Despite counseling to avoid pregnancy, women may inadvertently become pregnant during or after rituximab treatment. Using the rituximab global drug safety database, we identified 231 pregnancies associated with maternal rituximab exposure. Maternal indications included lymphoma, autoimmune cytopenias, and other autoimmune diseases. Most cases were confounded by concomitant use of potentially teratogenic medications and severe underlying disease. Of 153 pregnancies with known outcomes, 90 resulted in live births. Twenty-two infants were born prematurely; with one neonatal death at 6 weeks. Eleven neonates had hematologic abnormalities; none had corresponding infections. Four neonatal infections were reported (fever, bronchiolitis, cytomegalovirus hepatitis, and chorioamnionitis). Two congenital malformations were identified: clubfoot in one twin, and cardiac malformation in a singleton birth. One maternal death from pre-existing autoimmune thrombocytopenia occurred. Although few congenital malformations or neonatal infections were seen among exposed neonates, women should continue to be counseled to avoid pregnancy for ≤ 12 months after rituximab exposure; however, inadvertent pregnancy does occasionally occur. Practitioners are encouraged to report complete information to regulatory authorities for all pregnancies with suspected or known exposure to rituximab.
Introduction
Rituximab is a chimeric (mouse/human) monoclonal antibody directed against B-cell surface antigen CD20. Administration results in rapid and sustained depletion of peripherally circulating CD20+ B cells. Treatment regimens usually involve 4 weekly infusions of 375 mg/m2 (malignancy) or 2 infusions 14 days apart of 1000 mg (rheumatoid arthritis [RA]). Discovered by Biogen Idec, rituximab received approval in November 1997 for the treatment of relapsed or refractory, low-grade or follicular, CD20+, B-cell non-Hodgkin lymphoma (NHL) as a single agent. It is also approved for previously untreated follicular NHL in combination with cyclophosphamide, vincristine, prednisone chemotherapy, for nonprogressing low-grade NHL as a single agent after cyclophosphamide, vincristine, prednisone, and for diffuse large B-cell NHL in combination with CHOP or other anthracycline-based chemotherapy regimens. More recently, rituximab was approved for CD20+ chronic lymphocytic leukemia in combination with fludarabine and cyclophosphamide.1
Given a greater understanding of the role of B cells in several autoimmune diseases, rituximab received approval in 2006 in combination with methotrexate for the treatment of adults with moderately to severely active RA with an inadequate response to tumor necrosis factor antagonists. It has additionally been studied in randomized controlled trials, but not approved, in other autoimmune diseases, including systemic lupus erythematosus (SLE), idiopathic thrombocytopenia purpura (ITP), thrombotic thrombocytopenia (TTP), and multiple sclerosis (MS).
Like other monoclonal antibodies, rituximab contains an immunoglobulin G1κ (IgG1κ) construct and can therefore cross the placenta. Human IgG is selectively transported across the placenta into the fetal circulation in a time-dependent fashion, most probably by Fc receptors in the placenta.2 Fetal concentrations of IgG1 exceed those of other IgG subclasses at all time points. Very little IgG is seen in fetal circulation during the first trimester of pregnancy. Levels slowly rise during the second trimester and reach maternal serum concentrations by ∼ 26 weeks of gestation. Maximum IgG transfer across the maternal-fetal interface occurs during the last 4 weeks of gestation, and fetal concentration often exceeds maternal concentration at term delivery.2 Because transfer of rituximab from maternal into fetal circulation depends on gestational age, timing of exposure may be important when assessing outcomes.
Rituximab carries a Food and Drug Administration Pregnancy Category C: “Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.”3 Extensive reproductive toxicity studies have been performed on cynomolgus monkeys.1 Rituximab does cross the monkey placenta. Administration to pregnant cynomolgus monkeys during organogenesis resulted in a dose-dependent decrease in lymphoid B cells. Peripheral B-cell depletion and immunosuppression was seen in offspring at birth but returned to normal levels by 6 months of age. No teratogenic effects were observed.
Several reports have been published of rituximab administration during an established pregnancy, often in conjunction with combination chemotherapy, to treat malignancies or severe nonmalignant hematologic abnormalities that were discovered or recurred during the pregnancy. These reports have generally found reassuring neonatal outcomes.4-12 In these situations, risks of undertreating life-threatening maternal disease outweighed potential risks of antenatal exposure.
The estimated median terminal elimination half-life of rituximab is 18-22 days. Active drug has been detected in peripheral blood beyond 24 weeks after the last infusion in some patients.13 Peripheral B cells remain depleted for ∼ 6 months after infusion; however, B-cell reconstitution is highly heterogeneous and, in a small percent of patients, may not occur after years of follow-up.14,15 Much less is known about the effects of drug half-life or B-cell depletion in primary lymphoid organs.
Except in instances of potentially life-threatening disease occurring during an established pregnancy, all persons of childbearing potential are strongly advised to avoid pregnancy for ≥ 12 months after therapy. All Roche/Genentech/Biogen/Idec-sponsored clinical trials that use rituximab contain strong language in informed consent documents requiring women of childbearing potential and nonsterile men to use effective methods of contraception throughout study participation and for ≥ 12 months after the last administration of the study drug.
Despite best efforts, women may occasionally become pregnant during or after exposure to rituximab. Nearly one-half of all pregnancies in the United States are unplanned,16 including those in women with underlying medical conditions. Particularly in women with malignant or chronic autoimmune diseases, pregnancies, even unplanned, are often desired but fraught with uncertainty about risks posed by underlying maternal disease as well as risks related to inadvertent medication exposure. With long-term use of rituximab for maintenance therapy, an increasing number of women and men will have prolonged exposure during periods of relative disease quiescence. This may lead to increasing numbers of pregnancies occurring with antenatal exposure in the future.17
Given that the likelihood of randomized controlled studies to look at effects of antenatal rituximab exposure on pregnancy and neonatal outcomes is negligible, observational data are critical to better understanding of the risks of therapy to the developing fetus. Although not replacing counseling to avoid pregnancy, data about pregnancy outcomes are important when counseling women who may discover pregnancy after rituximab exposure.
Methods
Acquisition of data
Since the initial studies of rituximab, Biogen Idec/Genentech/Roche have undertaken diligent pharmacovigilance to monitor experiences of patients and infants exposed before or during pregnancy. All attempts were made to obtain complete information about the timing of pregnancy in relation to conception, concomitant medications, and pregnancy outcomes. Pregnancy case information was derived from the Biogen Idec/Genentech/Roche rituximab global drug safety database, which contains all reported pregnancies/pregnancy-related events from clinical trials and events spontaneously reported through regulatory health authorities, direct reporting of exposure by consumers and health care providers, and review of the literature for published case reports. Follow-up for case details from health care providers was conducted.
Analysis
Timing of exposure and onset of pregnancy were estimated from best available information provided. Reports that did not disclose the outcome of pregnancy or those for which the temporal association between rituximab exposure and onset of pregnancy could not be determined were excluded from analyses. Pregnancy outcomes of interest included spontaneous abortion, premature delivery, congenital malformations, and neonatal hematologic abnormalities or infections.
Concomitant medications and treatments were not consistently provided in spontaneous reports compared with clinical trials; therefore, medication exposure was only evaluated in the context of clinical trial reports and was limited to data provided by individual investigators. Data about important confounders, including disease severity or activity, smoking or alcohol use, length of treatment with rituximab, and complete ascertainment of concomitant medications, were not provided; however, patients were required to have moderate-to-severe underlying disease to be eligible for enrollment in clinical trials, and many trials mandated concomitant use of potentially teratogenic or abortifacient chemotherapeutic or immunosuppressive medications. Given the nature and variability of reported data, only summary and descriptive analyses were performed.
Results
Pregnancy exposures and outcomes
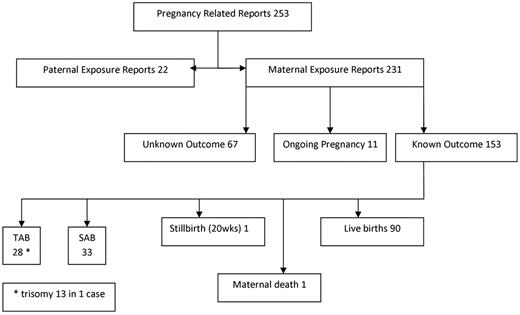
A total of 253 pregnancy-related reports were identified worldwide as of November 30, 2009 (Figure 1), most of which were reports of maternal exposure. Sixty-nine reports (29%) did not describe pregnancy outcomes, and pregnancies were ongoing in 11 reports (5%). In 4 clinical trial reports, patients were unblinded to placebo immediately after pregnancy discovery; 3 pregnancies were terminated despite unblinding. The fourth pregnancy resulted in a healthy full-term infant. Therefore, 153 reported pregnancies had known outcomes. Nearly 60% (n = 90) resulted in live births. First-trimester miscarriages were reported in 33 pregnancies (21%). One fetal loss at 20 weeks of gestation was reported; cause of death was fetal hypoxemia from an umbilical cord knot. No congenital abnormalities were found at postmortem examination. One maternal death during pregnancy was reported from cerebral hemorrhage in a patient with underlying ITP. Twenty-eight pregnancies (18%) were electively terminated; however, reasons for termination were not described.
Flow diagram of all pregnancy related reports in therituximab global drug safety database. TAB indicates therapeutic abortion/elective termination; and SAB, spontaneous abortion/miscarriage.
Flow diagram of all pregnancy related reports in therituximab global drug safety database. TAB indicates therapeutic abortion/elective termination; and SAB, spontaneous abortion/miscarriage.
Seventy pregnancies occurred during or after rituximab administration in clinical trials (supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Fifty-three reports (76%) occurred in industry sponsored or cosponsored trials. Indications for rituximab included RA (41 pregnancies), SLE (12 pregnancies), lymphoma (8 pregnancies), ITP/TTP (5 pregnancies), MS (2 pregnancies), and Castleman disease in patients positive for HIV (2 pregnancies). Improved, but not complete, reporting of concomitant medications was available. Concomitant exposure to potentially teratogenic medications was reported in more than one-half of pregnancies, most commonly methotrexate, but exposures were also documented with mycophenolate mofetil, oral contraceptives, and combination chemotherapy. Nine pregnancies resulted in preterm deliveries and 22 were full-term. Twenty-one ended with first-trimester spontaneous abortions, ≥ 60% of which were exposed to concomitant teratogenic medications. The reported fetal loss occurred in a clinical trial subject. Seventeen pregnancies were electively terminated.
Outcomes of live births
Of the 90 live births after maternal exposure, 68 (76%) resulted in full-term deliveries (Table 1). Indication for rituximab among exposed mothers included RA (29 pregnancies), NHL (24 pregnancies), SLE (11 pregnancies), ITP (11 pregnancies), autoimmune hemolytic anemia (3 pregnancies), MS (3 pregnancies), TTP (3 pregnancies), unreported indication (3 pregnancies), and one each to women with Castleman disease, mixed connective tissue disease, and renal transplantation. Twenty-two infants (24%) were born prematurely (before37 weeks of gestation); however, none had extreme prematurity (< 30 weeks of gestation). One neonate died at 6 weeks of life from an unknown cause. The pregnancy occurred 14 months after the last dose of rituximab and was complicated by underlying SLE, gestational diabetes, and exposure to mycophenolate mofetil, warfarin sodium, and prednisone. A 2255-g male was delivered at 33 weeks of gestation secondary to nonreassuring fetal status and was healthy at routine 3-week pediatrician visit. Three weeks later, he developed sudden onset diarrhea and respiratory failure. Cardiopulmonary resuscitation was unsuccessful. Postmortem examination identified the cause of death as multisystem organ failure of unknown cause. No congenital malformations or infections were identified.
Outcomes of live births
| Event . | All pregnancies . | Clinical trials . | Administration during established pregnancies . |
|---|---|---|---|
| Total no. of live births | 91 | 31 | 20 |
| Full-term infants, n | 68 | 22 | 11 |
| Twins, no. of sets | 2 | 0 | 0 |
| Premature delivery (< 37 wk), n | 16*† | 9* | 9 |
| Hematologic abnormalities, n | 11 | 1 | 7 |
| B-cell depletion, n | 4 | 0 | 3 |
| Lymphopenia, n | 3 | 0 | 1 |
| Neutropenia and anemia, n | 1 | 0 | 1 |
| Thrombocytopenia, n | 3 | 1 | 2 |
| Low birth weight, n | 1 | 0 | 0 |
| Congenital abnormalities, n | 3 | 1 | 0 |
| Club foot, n | 1 of twin pair | 0 | 0 |
| VSD/PFO/PDA, n | 1 | 1 | 0 |
| Turner syndrome, n | 1‡ | 0 | 1 |
| Neonatal infections, n | 4 | 2 | 1 |
| Fever at 3 weeks of age, n | 1 | 1 | 0 |
| Neonatal bronchiolitis, n | 1 | 0 | 0 |
| CMV hepatitis, n | 1 | 0 | 1 |
| Acute chorioamnionitis, n | 1 | 1 | 0 |
| Neonatal death (unknown cause), n | 1 | 1 | 0 |
| Event . | All pregnancies . | Clinical trials . | Administration during established pregnancies . |
|---|---|---|---|
| Total no. of live births | 91 | 31 | 20 |
| Full-term infants, n | 68 | 22 | 11 |
| Twins, no. of sets | 2 | 0 | 0 |
| Premature delivery (< 37 wk), n | 16*† | 9* | 9 |
| Hematologic abnormalities, n | 11 | 1 | 7 |
| B-cell depletion, n | 4 | 0 | 3 |
| Lymphopenia, n | 3 | 0 | 1 |
| Neutropenia and anemia, n | 1 | 0 | 1 |
| Thrombocytopenia, n | 3 | 1 | 2 |
| Low birth weight, n | 1 | 0 | 0 |
| Congenital abnormalities, n | 3 | 1 | 0 |
| Club foot, n | 1 of twin pair | 0 | 0 |
| VSD/PFO/PDA, n | 1 | 1 | 0 |
| Turner syndrome, n | 1‡ | 0 | 1 |
| Neonatal infections, n | 4 | 2 | 1 |
| Fever at 3 weeks of age, n | 1 | 1 | 0 |
| Neonatal bronchiolitis, n | 1 | 0 | 0 |
| CMV hepatitis, n | 1 | 0 | 1 |
| Acute chorioamnionitis, n | 1 | 1 | 0 |
| Neonatal death (unknown cause), n | 1 | 1 | 0 |
VSD indicates ventricular septal defect; PFO, patent foramen ovale; PDA, patent ductus arteriosus; and CMV, cytomegalovirus.
One resulted in neonatal death.
One set of twins was born at 36 weeks of gestation.
Diagnosis was made before rituximab administration.
Occasionally life-threatening illnesses are diagnosed or relapse during an established pregnancy. Table 2 describes all known reports of rituximab administered to women with established pregnancies. Treatment was indicated for malignant disease or profound hematologic disturbances. In all but 2 cases, rituximab was administered during the second or third trimester of pregnancy, well beyond the period of organogenesis. Six of 21 pregnancies were exposed to multiagent chemotherapy in addition to rituximab for lymphoma. All pregnancies, except for one that was still ongoing, resulted in live births. No maternal deaths, neonatal deaths, or congenital malformations were reported. The majority of infants were full term (11 of 20); however, 7 of 11 reports of neonatal cytopenias occurred when rituximab was administered during an established pregnancy
Outcomes of established pregnancies treated with antepartum rituximab for severe maternal disease
| Country . | Age, y . | Indication . | Narrative . | Pregnancy outcome . |
|---|---|---|---|---|
| Germany | 35 | Burkitt lymphoma | Treatment with rituximab began after week 16 | Healthy female |
| Germany4 | 29 | NHL | Rituximab, vincristine, doxorubicin, and prednisone administered during the second trimester | Healthy female, 35 wk |
| France | 20 | NHL | Rituximab was administered at 28 wk of pregnancy | Female, 32 wk; leukopenia and anemia |
| France | 34 | Hodgkin disease | Rituximab administered at week 33 | Healthy, 39 wk |
| France | 41 | Autoimmune hemolytic anemia | Rituximab administered between weeks 7 and 10 | Healthy female, 38 wk |
| France | 45 | ITP | Rituximab administered at week 33 for profound thrombocytopenia | Male, 35 wk; lymphopenia* |
| Australia | B-cell lymphoma | Rituximab, cyclophosphamide, doxorubicin, and vincristine administered during the third trimester | Premature male, 35 wk | |
| France | 28 | NHL | Rituximab and CHOP were administered at 18 wk | Healthy baby |
| United States | 26 | TTP | Rituximab, prednisone, and labetalol administered during the second trimester | Premature infant, 31 wk |
| France | 19 | TTP | RItuximab administered during the second trimester | Premature male, 36 wk; CMV hepatitis |
| Switzerland | 35 | Burkitt lymphoma | Rituximab and CHOP administered at week 16 | Healthy female, 41 wk; B-cell depletion with Rituxan in cord blood |
| United Kingdom | 21 | TTP | Rituximab administered during the third trimester | Healthy male |
| United States | 28 | NHL | Rituximab + CHOP administered at week 21 | Healthy female, 33 wk; preeclampsia |
| United States | 19 | Burkitt lymphoma | Rituximab and multiagent chemotherapy were administered at 13 wk | Healthy female, 39 wk |
| United Kingdom | 28 | ITP | Rituximab administered in seventh month | Female, 39 wk; neonatal thrombocytopenia with cerebral hemorrhage |
| United States | 27 | ITP | Rituximab administered during week 34 | Baby |
| Sweden | 38 | NHL | Rituximab administered in third trimester | Healthy female |
| Netherlands | 36 | ITP | Rituximab administered weeks 30-34 | Healthy female, 38 wk; Rituxan in cord blood |
| Canada | 35 | ITP | Rituximab administered in sixth month (after amniocentesis diagnosed Turner syndrome) | Delivery at 36 wk; absent B cells in neonate |
| Switzerland | 31 | NHL | Rituximab + CHOP administered at 15 wk of gestation | Female, 33 wk; low B cells at birth |
| France | 30 | ITP | Rituximab administered during the fifth month | Ongoing |
| Country . | Age, y . | Indication . | Narrative . | Pregnancy outcome . |
|---|---|---|---|---|
| Germany | 35 | Burkitt lymphoma | Treatment with rituximab began after week 16 | Healthy female |
| Germany4 | 29 | NHL | Rituximab, vincristine, doxorubicin, and prednisone administered during the second trimester | Healthy female, 35 wk |
| France | 20 | NHL | Rituximab was administered at 28 wk of pregnancy | Female, 32 wk; leukopenia and anemia |
| France | 34 | Hodgkin disease | Rituximab administered at week 33 | Healthy, 39 wk |
| France | 41 | Autoimmune hemolytic anemia | Rituximab administered between weeks 7 and 10 | Healthy female, 38 wk |
| France | 45 | ITP | Rituximab administered at week 33 for profound thrombocytopenia | Male, 35 wk; lymphopenia* |
| Australia | B-cell lymphoma | Rituximab, cyclophosphamide, doxorubicin, and vincristine administered during the third trimester | Premature male, 35 wk | |
| France | 28 | NHL | Rituximab and CHOP were administered at 18 wk | Healthy baby |
| United States | 26 | TTP | Rituximab, prednisone, and labetalol administered during the second trimester | Premature infant, 31 wk |
| France | 19 | TTP | RItuximab administered during the second trimester | Premature male, 36 wk; CMV hepatitis |
| Switzerland | 35 | Burkitt lymphoma | Rituximab and CHOP administered at week 16 | Healthy female, 41 wk; B-cell depletion with Rituxan in cord blood |
| United Kingdom | 21 | TTP | Rituximab administered during the third trimester | Healthy male |
| United States | 28 | NHL | Rituximab + CHOP administered at week 21 | Healthy female, 33 wk; preeclampsia |
| United States | 19 | Burkitt lymphoma | Rituximab and multiagent chemotherapy were administered at 13 wk | Healthy female, 39 wk |
| United Kingdom | 28 | ITP | Rituximab administered in seventh month | Female, 39 wk; neonatal thrombocytopenia with cerebral hemorrhage |
| United States | 27 | ITP | Rituximab administered during week 34 | Baby |
| Sweden | 38 | NHL | Rituximab administered in third trimester | Healthy female |
| Netherlands | 36 | ITP | Rituximab administered weeks 30-34 | Healthy female, 38 wk; Rituxan in cord blood |
| Canada | 35 | ITP | Rituximab administered in sixth month (after amniocentesis diagnosed Turner syndrome) | Delivery at 36 wk; absent B cells in neonate |
| Switzerland | 31 | NHL | Rituximab + CHOP administered at 15 wk of gestation | Female, 33 wk; low B cells at birth |
| France | 30 | ITP | Rituximab administered during the fifth month | Ongoing |
NHL indicates non-Hodgkin lymphoma; ITP, idiopathic thrombocytopenia purpura; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; TTP, thrombotic thrombocytopenia; and CMV, cytomegalovirus.
Congenital abnormalities
Two infants (2.2%) were born with congenital malformations: one infant in a set of twins had club foot,17 and another full-term singleton's condition was diagnosed as ventral septal defect, patent foramen ovale, and patent ductus arteriosus. Turner syndrome was diagnosed in one pregnancy before rituximab administration at 6 months of gestation.
Neonatal hematologic complications
Table 3 summarizes reported data on pregnancy complicated by neonatal hematologic or infectious complications. Eleven infants were reported to have hematologic abnormalities at birth (peripheral B-cell depletion, neutropenia, lymphopenia, thrombocytopenia and anemia); in 3 cases, rituximab was administered during the second trimester of pregnancy for lymphoma and relapsed TTP during pregnancy.6,7,10 In 2 reports, rituximab was inadvertently administered around the time of conception before the pregnancy was discovered.4,11 Neonatal thrombocytopenia was reported in 3 cases: 2 for maternal ITP, and 1 for RA. One infant had a cerebral hemorrhage due to thrombocytopenia of unreported severity (mother was treated at 7 months of gestation for ITP). In most cases, cytopenias were transient and had recovered spontaneously within weeks to months.6,7,10,11 Three reports included data on rituximab levels in cord or infant blood; rituximab was detected in cord or infant blood at birth with corresponding undetectable peripheral B-cell counts. Maternal rituximab was administered < 12 weeks before delivery in each case, and serum rituximab concentrations declined postnatally at a rate consistent with the drug's half-life. Infant rituximab concentrations were highest in the 2 cases in which maternal rituximab was administered within 5 weeks of delivery. No infectious complications were reported among any of the affected neonates.4,6,7,10,11
Pregnancies complicated by neonatal hematologic abnormalities or infections
| Country . | Age, y . | Indication . | Narrative . | Rituximab eose . | Pregnancy outcome . | Hematologic abnormality . | Follow-up . | Infection . |
|---|---|---|---|---|---|---|---|---|
| France10 | 41 | AIHA | Rituximab inadvertently administered between weeks 7 and 10 of pregnancy | 375 mg/m2 weekly × 4 | Female, 40 wk | Low WBC count | None reported | None |
| United States | NR | Transplant rejection | Kidney/pancreas transplant rejection prevention treatment | Not reported | Baby | B-cell depleted | None reported | None |
| United Kingdom | 28 | ITP | Rituximab administered during seventh month | Unreported dose weekly × 4 | Female, 39 wk; cerebral hemorrhage | Thrombocytopenia | Normal platelets at 4 mo | None |
| Canada | 35 | ITP | Rituximab administered in sixth month; amniocentesis previously diagnosed Turner mosaic | Unreported dose over a 4-wk period | Female, 36 wk; Turner mosaic syndrome | Absent B cells at 1 wk | None reported | None |
| Sweden3 | 37 | NHL | Pregnancy occurred within 1 mo of rituximab | 375 mg/m2 weekly × 4 | Female, 40 wk | Low granulocyte count | Normalized by 18 mo; normal response to vaccinations | None |
| France | 20 | NHL | Rituximab administered at 28 wk of gestation | Unreported dose weekly × 4 | Female, 33 wk | Neutropenia, anemia | Normalized in 3 d | None |
| France | 45 | ITP | Rituximab administered at 33 wk gestation | 375 mg/m2 at week 33 and 35 | Male, 35 wk | Lymphopenia | None reported | None |
| United States | 22 | RA | Pregnancy occurred 4 mo after last rituximab administration | 1 g every 14 d × 2 | Female, 41 wk, respiratory distress | Mild thrombocytopenia | None reported | None |
| Switzerland6 | 35 | NHL | Rituximab + CHOP administered at 16 wk of gestation | 375 mg/m2 weekly × 4 | Female, 41 wk | Rituximab in cord blood, B-cell depletion | Recovered by 12 wk | None |
| Switzerland5 | 31 | NHL | Rituximab + CHOP administered at 15 weeks gestation | 375mg/m2 every 14 days × 6 | female, 33 weeks | Low b cells at birth. Immunoglobulins normal | Normalized by week 12 | None |
| France | 19 | ITP | Rituximab administered during seventh month; mother had CMV infection during pregnancy | 375 mg/m2 weekly × 2 | Male, 36 wk | Thrombocytopenia | None reported | CMV hepatitis, vertical transmission |
| Australia | 33 | NHL | Patient was treated with rituximab + CHOP before pregnancy | 375 mg/m2 every 14 d | Male, 35 wk | None | None reported | Bronchiolitis |
| United States | 30s | SLE | 18 mo after rituximab | 1 g every 14 d × 2, repeat at 6 mo | Premature infant, 36 wk | None | None reported | Acute chorioamnionitis diagnosed on placental pathology |
| Norway | 32 | ITP | 9 mo after rituximab | Not reported | Male, 41 wk | None | None reported | Fever at 3 wk old, presumed to be viral |
| Country . | Age, y . | Indication . | Narrative . | Rituximab eose . | Pregnancy outcome . | Hematologic abnormality . | Follow-up . | Infection . |
|---|---|---|---|---|---|---|---|---|
| France10 | 41 | AIHA | Rituximab inadvertently administered between weeks 7 and 10 of pregnancy | 375 mg/m2 weekly × 4 | Female, 40 wk | Low WBC count | None reported | None |
| United States | NR | Transplant rejection | Kidney/pancreas transplant rejection prevention treatment | Not reported | Baby | B-cell depleted | None reported | None |
| United Kingdom | 28 | ITP | Rituximab administered during seventh month | Unreported dose weekly × 4 | Female, 39 wk; cerebral hemorrhage | Thrombocytopenia | Normal platelets at 4 mo | None |
| Canada | 35 | ITP | Rituximab administered in sixth month; amniocentesis previously diagnosed Turner mosaic | Unreported dose over a 4-wk period | Female, 36 wk; Turner mosaic syndrome | Absent B cells at 1 wk | None reported | None |
| Sweden3 | 37 | NHL | Pregnancy occurred within 1 mo of rituximab | 375 mg/m2 weekly × 4 | Female, 40 wk | Low granulocyte count | Normalized by 18 mo; normal response to vaccinations | None |
| France | 20 | NHL | Rituximab administered at 28 wk of gestation | Unreported dose weekly × 4 | Female, 33 wk | Neutropenia, anemia | Normalized in 3 d | None |
| France | 45 | ITP | Rituximab administered at 33 wk gestation | 375 mg/m2 at week 33 and 35 | Male, 35 wk | Lymphopenia | None reported | None |
| United States | 22 | RA | Pregnancy occurred 4 mo after last rituximab administration | 1 g every 14 d × 2 | Female, 41 wk, respiratory distress | Mild thrombocytopenia | None reported | None |
| Switzerland6 | 35 | NHL | Rituximab + CHOP administered at 16 wk of gestation | 375 mg/m2 weekly × 4 | Female, 41 wk | Rituximab in cord blood, B-cell depletion | Recovered by 12 wk | None |
| Switzerland5 | 31 | NHL | Rituximab + CHOP administered at 15 weeks gestation | 375mg/m2 every 14 days × 6 | female, 33 weeks | Low b cells at birth. Immunoglobulins normal | Normalized by week 12 | None |
| France | 19 | ITP | Rituximab administered during seventh month; mother had CMV infection during pregnancy | 375 mg/m2 weekly × 2 | Male, 36 wk | Thrombocytopenia | None reported | CMV hepatitis, vertical transmission |
| Australia | 33 | NHL | Patient was treated with rituximab + CHOP before pregnancy | 375 mg/m2 every 14 d | Male, 35 wk | None | None reported | Bronchiolitis |
| United States | 30s | SLE | 18 mo after rituximab | 1 g every 14 d × 2, repeat at 6 mo | Premature infant, 36 wk | None | None reported | Acute chorioamnionitis diagnosed on placental pathology |
| Norway | 32 | ITP | 9 mo after rituximab | Not reported | Male, 41 wk | None | None reported | Fever at 3 wk old, presumed to be viral |
AIHA indicates autoimmune hemolytic anemia; WBC, white blood cell; NR, not reported; ITP, idiopathic thrombocytopenia purpura; NHL, non-Hodgkin lymphoma; RA, rheumatoid arthritis; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CMV, cytomegalovirus; and SLE, systemic lupus erythematosus.
Perinatal infections
Four neonatal infections were reported. One infant was admitted at 3 weeks of age for observation for a fever suspected to be viral in origin. In another case, rituximab was administered during the second trimester of pregnancy for severe ITP; during the pregnancy, the mother's condition was diagnosed as acute cytomegalovirus infection. The infant, born at 36 weeks of gestation, had cytomegalovirus hepatitis. No additional information about maternal or infant outcomes was provided. The third case involved a woman with NHL treated with rituximab and cyclophosphamide, doxorubicin, vincristine, prednisone initiated during the third trimester of pregnancy. Delivery was induced at 35 weeks for poor fetal growth. At birth, the normal-appearing premature male weighed 2364 g with excellent APGAR scores. On unknown date, the baby presented with bronchiolitis, leading to a 5- to 6-week hospitalization. Further details were not provided. A fourth pregnancy was complicated by acute chorioamnionitis. The pregnancy, occurring 18 months after exposure, was induced at 36 weeks of gestation for oligohydramnios. The infant was born without complications, but placental biopsy showed chorioamnionitis.
Paternal exposure
Of 22 reported pregnancies, 11 had enough data for evaluation (Table 4). Two pregnancies miscarried; 7 resulted in live births without complications, and 2 were ongoing. No information was provided about paternal dose of rituximab, maternal health, or paternal or maternal concomitant medications in exposed pregnancies.
Outcomes of pregnancies with paternal exposure to rituximab
| Country . | Indication . | Report type . | Narrative . | Pregnancy Outcome . |
|---|---|---|---|---|
| United Kingdom | TTP | Study | No information provided | SAB |
| United States | RA | Study | No information provided | Healthy female, 33 wk |
| United States | Unknown | Spontaneous | No information provided | SAB |
| United States | Unknown | Spontaneous | No information Provided | SAB |
| United States | Unknown | Spontaneous | No information Provided | SAB |
| United States | MS | Study | Patient's partner became pregnant 4 mo after patient received rituximab | SAB |
| Israel | RA | Study | Patient's partner was in the first trimester of pregnancy when patient received rituximab | SAB |
| Brazil | Lymphoma | Spontaneous | Patient's partner became pregnant 2 mo after patient received rituximab | Healthy female |
| Venezuela | Lymphoma | Study | Patient's partner became pregnant 1 mo after patient received rituximab | Healthy twins, 37 wk |
| Australia | TTP | Spontaneous | Patient's partner became pregnant 7 mo after patient received rituximab | Healthy male |
| United Kingdom | Lymphoma | Spontaneous | Patient's partner became pregnant 7 mo after patient received rituximab | Healthy male |
| Canada | RA | Study | Patient's partner became pregnant 5 mo after patient received rituximab | Healthy female, 39 wk |
| Netherlands | TTP | Study | Patient's partner became pregnant within 1 y after patient received rituximab | Healthy male |
| France | RA | Spontaneous | Patient's partner became pregnant within 1 y after patient received rituximab | Healthy male, 37 wk |
| Australia | Lymphoma | Study | Patient's partner became pregnant 3 months after patient received rituximab | Ongoing |
| United States | malignancy | Spontaneous | Patient's partner became pregnant 2 wk after patient received rituximab | Ongoing |
| Country . | Indication . | Report type . | Narrative . | Pregnancy Outcome . |
|---|---|---|---|---|
| United Kingdom | TTP | Study | No information provided | SAB |
| United States | RA | Study | No information provided | Healthy female, 33 wk |
| United States | Unknown | Spontaneous | No information provided | SAB |
| United States | Unknown | Spontaneous | No information Provided | SAB |
| United States | Unknown | Spontaneous | No information Provided | SAB |
| United States | MS | Study | Patient's partner became pregnant 4 mo after patient received rituximab | SAB |
| Israel | RA | Study | Patient's partner was in the first trimester of pregnancy when patient received rituximab | SAB |
| Brazil | Lymphoma | Spontaneous | Patient's partner became pregnant 2 mo after patient received rituximab | Healthy female |
| Venezuela | Lymphoma | Study | Patient's partner became pregnant 1 mo after patient received rituximab | Healthy twins, 37 wk |
| Australia | TTP | Spontaneous | Patient's partner became pregnant 7 mo after patient received rituximab | Healthy male |
| United Kingdom | Lymphoma | Spontaneous | Patient's partner became pregnant 7 mo after patient received rituximab | Healthy male |
| Canada | RA | Study | Patient's partner became pregnant 5 mo after patient received rituximab | Healthy female, 39 wk |
| Netherlands | TTP | Study | Patient's partner became pregnant within 1 y after patient received rituximab | Healthy male |
| France | RA | Spontaneous | Patient's partner became pregnant within 1 y after patient received rituximab | Healthy male, 37 wk |
| Australia | Lymphoma | Study | Patient's partner became pregnant 3 months after patient received rituximab | Ongoing |
| United States | malignancy | Spontaneous | Patient's partner became pregnant 2 wk after patient received rituximab | Ongoing |
Discussion
Rituximab is widely used for the treatment of CD20+ lymphoid malignancies, as well as hematologic and autoimmune diseases. A single course of rituximab can result in prolonged peripheral B-cell depletion. For this reason, counseling about contraception must include the potential for delayed B-cell reconstitution for months and occasionally years after administration. We report the outcomes of all reported pregnancies with maternal or paternal rituximab exposure. Of 231 pregnancies with preconceptional or antepartum exposure to rituximab, most resulted in uncomplicated live births. The preterm delivery rate in this cohort (19% of live births) was found to be higher than the general population (10%-12%)18 but may be similar to rates reported in women with certain chronic medical conditions.19,20 Two congenital malformations (2.2%) were reported, a rate consistent with what is seen in the general obstetric population.21 No pattern of congenital anomalies was identified to be associated with rituximab exposure. First-trimester pregnancy loss occurred in 21% of pregnancies with known outcomes. This rate is somewhat higher than the 10%-15% rate that has been published in the general population; however, established incidences of early miscarriage are unknown for women with autoimmune or malignant disease. In addition, because of frequent pregnancy testing performed during clinical trials, some early pregnancies may have been identified that would not have otherwise been clinically detected. Although there does not appear to be a pattern of teratogenicity or embryotoxicity, because of the limited cohort size, this finding cannot be interpreted to equal a conclusion of safety for rituximab use in the preconception period. Therefore, counseling strategies should still emphasize the importance of effective contraception throughout the duration of therapy and for ≤ 12 months after administration. Twenty-two reports concerned paternal exposure to rituximab, 11 of which provided data on timing of rituximab exposure to pregnancy. Given the small number of complete reports and the absence of confounding data about maternal and paternal health, specific recommendations about appropriate time periods for pregnancy avoidance after paternal rituximab exposure are difficult to make at this time.
When interpreting data on antenatal medication exposure, it is important to keep in mind the confounding factors that may have a significant affect on pregnancy outcomes. Rituximab is indicated for severe underlying conditions, CD20+ malignancies and refractory RA, and is studied and occasionally used for severe manifestations of other autoimmune conditions. These diseases are commonly managed with multiagent chemotherapeutic or immunosuppressive agents that may themselves be associated with increased risks of teratogenicity and neonatal immunosuppression.22-24 Similarly, maternal autoimmune, hematologic, and malignant disease may have independent effects on pregnancy outcome.19,20,25-28 There is little published data about outcomes of pregnancies occurring shortly after diagnosis or under therapy for lymphoma or other malignancies. In several diseases, increased maternal disease activity is independently associated with increased risk of adverse pregnancy outcomes.20,25,29 Limited data were available in this dataset to assess disease severity or concomitant medication use, precluding adjustment for these important confounders. Disentangling separate effects from the medication under study from concomitant medications or underlying disease can be nearly impossible.
Most of available data about outcomes of pregnancies exposed to medications remains limited to voluntary reporting, either by registries, reporting of occurrences during clinical trials, description of isolated events as case series or case reports, or voluntary reporting to sponsors by patients or providers. The number of reported pregnancies probably represents an underestimation of the actual number of exposed pregnancies. This makes interpretations of rates of pregnancy outcomes difficult because the denominator of exposed pregnancies is unknown and difficult to estimate. In addition, there is no way to standardize data elements for reports, yielding great heterogeneity in the extent and quality of data, as well as the length of follow-up of outcomes. These data include only immediate pregnancy outcomes, without assessment of longer term developmental follow-up of exposed children. Health care providers and patients need access to the best available data when making decisions should a pregnancy be discovered after exposure to rituximab, or when rituximab is indicated for the treatment of serious maternal conditions during an established pregnancy.
Given that the known mechanism of action of rituximab is CD20+ B-cell depletion, perinatal and neonatal immunosuppression and subsequent infection remain a serious concern. Of the 11 hematologic abnormalities in exposed newborns, none were associated with infections. No pattern about the timing of rituximab exposure and the 4 infections was apparent; exposures ranged from 18 months before conception to treatment initiation during the third trimester of pregnancy. There was not enough data to evaluate effects of various doses of rituximab or the use as induction or maintenance therapy.
Given the limitations of these data and probable confounding by severe maternal disease and potentially teratogenic concomitant medications, women who receive rituximab for routine clinical care or in clinical trials should continue to be counseled to avoid pregnancy for the duration of treatment and for ≤ 12 months after the last dose of rituximab. In cases when a pregnancy is discovered after exposure, potential risks about exposure to rituximab; other potentially teratogenic medications, including cyclophosphamide, methotrexate, mycophenolate mofetil, or warfarin sodium; and the severity and activity of underlying maternal disease should be reviewed in detail. The 3 pregnancy terminations occurring despite unblinding to placebo further highlight the multifactoral medical and social issues involved in decisions about unplanned pregnancies. When rituximab is indicated during an established pregnancy to treat life-threatening maternal malignant or hematologic disease, current data suggest that the risks of treatment may be outweighed by the benefits to the mother.
Although relatively few infections were reported in exposed pregnancies to date, continued vigilance is essential for early detection of potential infections complicating the pregnancy or the neonate. Maternal fetal medicine specialists should be considered for antepartum monitoring, particularly given the complexities of management of pregnancy in women with underlying medical conditions requiring multiple medications for control of disease activity. Given the mechanism of action of rituximab, particular attention should focus on early signs of infection during the pregnancy or in the neonate. Because reported cases of leucopenia were asymptomatic, transient, and without infectious complications, serial monitoring of peripheral leukocytes in exposed neonates may not be warranted. However, a neonatal complete blood count at birth may be considered to assess for clinically significant cytopenias, particularly in cases when maternal exposure occurred shortly before or during gestation.
Practitioners caring for women with either preconceptional or antepartum exposure to rituximab are encouraged to report the event, irrespective of outcome, to regulatory agencies with as complete information as possible to best understand the relationship between medication exposure and pregnancy outcome.30
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.F.C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. E.F.C. participated in conception and design, analysis and interpretation of data, statistical analysis, and drafting of the manuscript; E.R.M., A.K., and P.F. were involved in conception and design, acquisition of data, interpretation of data, and critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: E.R.M., A.K., and P.F. are paid employees of Genentech Inc. E.F.C. has been a site Principal Investigator of several Genentech/Roche sponsored multicenter randomized controlled clinical trials and has served in the past as a paid consultant for Genentech.
Correspondence: Eliza F. Chakravarty, Division of Immunology and Rheumatology, Stanford University School of Medicine, 1000 Welch Rd, Ste 203, Palo Alto, CA 94304; e-mail: echakravarty@stanford.edu.