Abstract
The contribution of specific cancer therapies, comorbid medical conditions, and host factors to mortality risk after pediatric Hodgkin lymphoma (HL) is unclear. We assessed leading morbidities, overall and cause-specific mortality, and mortality risks among 2742 survivors of HL in the Childhood Cancer Survivor Study, a multi-institutional retrospective cohort study of survivors diagnosed from 1970 to 1986. Excess absolute risk for leading causes of death and cumulative incidence and standardized incidence ratios of key medical morbidities were calculated. Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of risks for overall and cause-specific mortality. Substantial excess absolute risk of mortality per 10 000 person-years was identified: overall 95.5; death due to HL 38.3, second malignant neoplasms 23.9, and cardiovascular disease 13.1. Risks for overall mortality included radiation dose ≥ 3000 rad ( ≥ 30 Gy; supra-diaphragm: HR, 3.8; 95% CI, 1.1-12.6; infradiaphragm + supradiaphragm: HR, 7.8; 95% CI, 2.4-25.1), exposure to anthracycline (HR, 2.6; 95% CI, 1.6-4.3) or alkylating agents (HR, 1.7; 95% CI, 1.2-2.5), non–breast second malignant neoplasm (HR, 2.6; 95% CI 1.4-5.1), or a serious cardiovascular condition (HR, 4.4; 95% CI 2.7-7.3). Excess mortality from second neoplasms and cardiovascular disease vary by sex and persist > 20 years of follow-up in childhood HL survivors.
Introduction
Current 5-year survival rates for people treated for Hodgkin lymphoma (HL) in childhood exceed 90%.1,2 Approximately 31 500 childhood HL survivors live in the United States, and this population increases annually.3 Long-term HL survivors commonly experience treatment-related morbidity that impairs thyroid, pulmonary, gonadal, cerebrovascular, and cardiovascular (CV) functions.4-13 In addition, their curative therapy has been linked to an excess risk of developing second malignant neoplasms (SMNs).14-21 These late treatment sequelae can negatively affect survivor's health and predispose to premature death.22-25
Despite extensive characterization of mortality in HL survivors, the literature contains some important limitations. Prior study populations have mixed survivors of adult and childhood HL.25,26 In addition, studies have included multiple childhood cancer diagnoses27 or reported on few mortality events10,23 and had median follow-up time < 20 years from diagnosis. Finally, studies have lacked detailed cancer treatment data to establish risk models for mortality.10,23,25,26 Specifically, neither the Childhood Cancer Survivor Study (CCSS) nor any other study of childhood HL survivors have evaluated the association between mortality, patient characteristics, treatment, and treatment-mediated comorbid medical conditions.4,6,13,27,28
Elucidation of factors influencing mortality risk is important to inform clinical care and future interventions aimed at preventing premature death in HL survivors. To address these gaps in knowledge, we sought to (1) delineate cause-specific mortality in a cohort of pediatric HL survivors, (2) characterize the incidence and latency of key morbidities underlying mortality in survivors of childhood HL, and (3) investigate predictors of overall and leading cause-specific mortality.
Methods
Characteristics of study participants
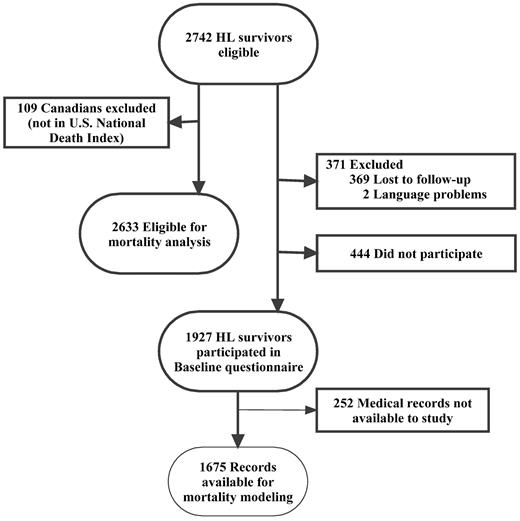
The CCSS is an ongoing multi-institutional study of persons who survived > 5 years after treatment of childhood cancer. Subjects for this analysis met the following eligibility criteria: (1) diagnosis of HL; (2) diagnosis and initial treatment at 1 of 26 collaborating CCSS institutions; (3) diagnosis date between January 1, 1970, and December 31, 1986; (d) age < 21 years at diagnosis; and (e) survival ≥ 5 years from diagnosis. A detailed description of the methods and cohort characteristics has been reported previously.29 The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution. Among the 2742 eligible subjects with HL, 1927 (70.3%) enrolled survivors represent 13% of the CCSS respondent cohort. Figure 1 summarizes HL survivors eligible for mortality analysis, versus those enrolled in the cohort, and with available treatment data.
Data sources and definitions
The data sources for this CCSS analysis include the U.S. National Death Index (NDI); self-report questionnaires administered to the survivors or parent proxy; and treatment data for the initial HL, abstracted by the survivor's treating institution.
Mortality was evaluated among non-Canadian HL subjects eligible for CCSS as described previously.27 The vital status and cause of death of all subjects eligible for CCSS was ascertained as of December 31, 2002, linking to the NDI and followed up with a death certificate request from the state where the death occurred.27,30
Eligible subjects (or a legal proxy for subjects who had died or were ≤ 18 years of age) who agreed to participate completed a baseline questionnaire at entry into the cohort. For traceable living subjects, follow-up questionnaires were collected at 3 subsequent times. Copies of all questionnaires and the treatment abstraction form are available for review at http://ccss.stjude.org. Questionnaire items specific to this analysis included demographic information; CV, pulmonary, or thyroid conditions diagnosed by a physician; cancer recurrence; and development of a benign neoplasm, SMN, or non–melanoma skin cancer (NMSC). For medical conditions, subjects were asked to provide an age at first occurrence of the condition. Self-report of all SMNs was collected at each questionnaire and validated by pathology review of the report as previously described.19,21 Histology for invasive SMNs was coded and classified with the use of the International Classification Diseases for Oncology. Recurrences are captured by participant self-report and verified by review of medical records.31
Chronic condition data were collected and scored on the basis of baseline questionnaire response. Scoring of severity of chronic conditions was based on the Common Terminology Criteria for Adverse Events (Version 3) system developed through the National Cancer Institute (http://ctep.cancer.gov). Conditions are graded as mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening or disabling (grade 4), or fatal (grade 5) as previously described.4 The current analysis used grade 3-5 CV conditions, including congestive heart failure requiring medication; myocardial infarction; angina or coronary heart disease on anti–angina medication or requiring cardiac catheterization, angioplasty, or coronary artery bypass graft surgery; heart transplantation; or cerebrovascular accident. Grade 3-5 pulmonary conditions included: emphysema on medication, pulmonary fibrosis on oxygen, or pulmonary embolism and infarction.4,32 Thyroid chronic conditions included thyroid nodules, hypothyroidism, or hyperthyroidism.6,33
For participants with HL who signed a medical record release, a detailed summary of cancer treatment was abstracted by CCSS trained staff at the treating institution as previously described.4,29 Cumulative anthracycline or alkylating agent chemotherapy exposure was expressed as a total score (0-3) on the basis of the tertile of cumulative doses received, according to methods reported previously.34 Radiation therapy (RT) records were submitted to CCSS by the treating institution and were centrally abstracted with calculation of absorbed dose coordinated through the Department of Radiation Physics, the University of Texas M,D. Anderson Cancer Center, Houston.29 RT exposure was divided into 5 categories by treatment field in reference to the diaphragm (supradiaphragmatic versus infradiaphragmatic or infradiaphragmatic plus supradiaphragmatic) and dose (< 3000 rad [30 Gy] or ≥ 3000 rad [30 Gy]).
Statistical analyses
The primary outcomes for this analysis were overall and cause-specific mortality. Secondary outcomes included key chronic conditions, including SMNs. Descriptive statistics are presented by sex. To compare demographic and cancer treatment variables between sexes, Pearson chi-square tests were performed.
Follow-up time was defined as the interval from entry into the CCSS cohort (5 years after original cancer diagnosis) to the date of death from any cause, or censored at date of NDI search (December 31, 2002). Standardized mortality ratios (SMRs) were calculated for overall and cause-specific death in US residents in the HL cohort (Figure 1) and compared with the US resident cohort. SMR was computed as the number of observed deaths divided by the expected number of deaths in an age-, sex-, and calendar year–matched general population, based on US mortality rates from the National Center for Health Statistics. A 95% confidence interval (CI) of each SMR was calculated on the basis of Poisson probability models, and differences between groups were evaluated with likelihood ratio tests from these models.35
Because the SMR only reflects the relative increase in risk, excess adverse risks (EARs) were also calculated to show the absolute increase in risk for a young population that has low expected mortality rates. EAR per 10 000 person-years was determined by subtracting the expected number of events from the observed number, dividing the difference by total person-years of follow-up, and multiplying by 10 000. Cause of death was missing for 44 persons; these were not included in cause-specific calculations. Estimated probabilities of overall survival were calculated by the method of Kaplan-Meier with associated 95% CI based on the Greenwood formula. Cumulative incidence and 95% CI were estimated for each cause-specific mortality, treating other causes of death as competing risks and conditioned on survival of 5, 10, 15, and 20 years since the original diagnosis.36
The remaining analyses use questionnaire and treatment data from the respondent HL cohort (Figure 1). Cumulative incidence and 95% CI were estimated for each of the following events: first recurrence of HL, histologically confirmed SMN, NMSC, grade 3-5 CV condition, grade 3-5 pulmonary condition, and thyroid chronic conditions. Death was treated as a competing risk event, and participants were censored at date of last questionnaire at which the outcome was ascertained. Events dated before cohort entry were included as prevalent at 5 years from diagnosis. All SMNs and recurrences reported up to and including the follow-up 2005 questionnaire were included in this analysis. Excess risk of SMN (exclusive of NMSC) was evaluated with standardized incidence ratio (SIR) and EAR. SIR was calculated in a similar manner to that described earlier for SMR, using US Surveillance Epidemiology and End Results (SEER) age-, sex-, and race-specific rates to calculate the expected number of cases.19,37 All multiple SMNs were counted in the numerator of SIRs. EAR was determined as described earlier for mortality.
Cox proportional hazards regression, with age as the time scale, was used to assess the effects of demographics, cancer treatment, and medical conditions of interest (recurrence, SMN, CV event, NMSC, thyroid disease, pulmonary condition) on overall or cause-specific mortality risk.38 Regression diagnostics to assess the proportional hazards assumption for key variables (such as sex) were assessed and appeared valid. Initial regression models were constructed to screen treatment and comorbidity explanatory variables of interest, testing each factor individually while controlling for age at diagnosis, race, sex, household income, and education, which were a priori included in all models. Risk factors significant at α = .1 level in initial adjusted models were eligible for evaluation in the final multivariable model. The ultimate set of factors was determined from step-down procedures, using P value < .05 as inclusion criteria. Recurrence of HL, occurrence of a SMN, report of a grade 3-5 cardiac condition (inclusive of cerebrovascular events), an NMSC, grade 3-5 pulmonary condition, and thyroid chronic condition were each examined as time-dependent covariates for the all-cause mortality model. Of these, recurrence of HL, thyroid chronic condition, and NMSC occurrence were also evaluated as time-dependent covariates for the SMN mortality model. Interactions between sex and treatment variable, between sex and comorbidity variables, and between anthracycline exposure and RT field were also tested in all-mortality and SMN-mortality models. An interaction was found between sex and SMN in the overall mortality model; hence, the final Cox model was stratified on sex to evaluate separate sex-specific effects of SMN. Because of the smaller number of cardiac deaths, a more parsimonious model (including sex, household income, and anthracycline score) was fit for CV mortality, and a log-rank test of RT was performed. Hazard ratios (HRs), 95% CIs, and P values are reported.
Data were analyzed with SAS Version 9.0 (SAS Institute) and Tibco-Spotfire S+ Version 8.0 (Tibco Software Inc), using 2-tailed statistical tests at the α = .05 level.
Results
Characteristics of childhood HL survivors
Figure 1 delineates the HL survivors included in mortality analysis, those enrolled in CCSS, and those with treatment data used for models to predict mortality. Among all 2633 US HL survivors eligible for the analysis, 57% were male and 58% were treated from 1970 to 1979 (Table 1). Of the 1927 HL survivors participating in CCSS, the median age at diagnosis was 14.0 years (range, 2.0-20.0 years). Most participants were white. The median follow-up for these participants is 23.8 years (range, 16.0-33.0 years) and 16.1 years (range, 5.0-31.5 years) from diagnosis for those alive and deceased, respectively. Participants treated in the early treatment era versus the later treatment era did not differ significantly by sex (P = .30) or race/ethnicity (P = .20) but differed by age at diagnosis (P ≤ .001).
Consort diagram of 5-year survivors of childhood Hodgkin lymphoma in the Childhood Cancer Survivor Study.
Consort diagram of 5-year survivors of childhood Hodgkin lymphoma in the Childhood Cancer Survivor Study.
Characteristics of 5-year survivors of childhood Hodgkin lymphoma in the CCSS
| . | HL cohort, n (%)* . | Male, n (%)* . | Female, n (%) . | P† . |
|---|---|---|---|---|
| All eligible CCSS survivors‡ | 2633 | 1507 (57) | 1126 (43) | |
| Treatment era | .63 | |||
| 1970-1979 | 1546 (59) | 879 (58) | 667 (59) | |
| 1980-1986 | 1087 (41) | 628 (42) | 459 (41) | |
| Age at diagnosis | < .001 | |||
| 0-9 y | 476 (18) | 379 (25) | 100 (90) | |
| 10-14 y | 884 (34) | 490 (33) | 394 (35) | |
| 15-21 y | 1273 (48) | 641 (43) | 632 (56) | |
| Participants, n§ | 1927 | 1049 | 878 | |
| Treatment era | .30 | |||
| 1970-1979 | 1097 (57) | 586 (56) | 511 (58) | |
| 1980-1986 | 830 (43) | 463 (44) | 367 (42) | |
| Age at diagnosis | < .001 | |||
| 0-9 y | 329 (17) | 244 (23) | 85 (10) | |
| 10-14 y | 663 (34) | 350 (33) | 313 (36) | |
| 15-21 y | 935 (49) | 455 (43) | 480 (55) | |
| Race/ethnicity‖ | .20 | |||
| White, non-Hispanic | 1653 (86) | 892 (85) | 761 (87) | |
| Other | 268 (14) | 156 (15) | 112 (13) | |
| Missing, n | 6 | 1 | 5 | |
| Household income‖ | .12 | |||
| $0-19 999 | 288 (17) | 167 (18) | 121 (15) | |
| ≥ $20 000 | 1423 (83) | 753 (82) | 670 (85) | |
| Missing, n | 216 | 129 | 87 | |
| Education‖ | < .001 | |||
| Through high school | 503 (27) | 323 (32) | 180 (22) | |
| After High School | 1339 (73) | 691 (68) | 648 (78) | |
| Missing, n | 85 | 35 | 50 | |
| Treatment group¶ | .77 | |||
| Radiation only | 548 (33) | 285 (32) | 263 (33) | |
| Chemotherapy + radiation | 1024 (61) | 552 (62) | 472 (60) | |
| Chemotherapy only | 98 (6) | 52 (6) | 46 (6) | |
| Missing | 257 | 160 | 97 | |
| Splenectomy (yes) | 1441 (76) | 760 (74) | 681 (79) | .02 |
| Chemotherapy categories# | .10 | |||
| Radiation only | 548 (33) | 285 (32) | 263 (34) | |
| Chemotherapy, no anthracycline | 689 (41) | 355 (40) | 334 (43) | |
| Chemotherapy, including anthracycline | 428 (26) | 247 (28) | 181 (23) | |
| Missing | 262 | 162 | 100 | |
| Anthracycline score# | .21 | |||
| 0 | 1237 (76) | 640 (74) | 597 (78) | |
| 1 | 174 (11) | 98 (11) | 79 (10) | |
| 2 | 166 (10) | 99 (12) | 67 (9) | |
| 3 | 47 (3) | 25 (3) | 22 (3) | |
| Missing, n | 303 | 187 | 116 | |
| Alkylating agents score# | .02 | |||
| 0 | 606 (44) | 317 (43) | 289 (44) | |
| 1 | 118 (9) | 58 (8) | 60 (9) | |
| 2 | 108 (8) | 45 (6) | 63 (10) | |
| 3 | 555 (40) | 316 (43) | 239 (37) | |
| Missing, n | 540 | 313 | 227 | |
| RT field by dose | < .01 | |||
| Chemotherapy only | 98 (6) | 52 (6) | 46 (6) | |
| Supradiaphragmatic, < 30 Gy** | 156 (10) | 104 (12) | 52 (7) | |
| Supradiaphragmatic, ≥ 30 Gy** | 406 (25) | 201 (24) | 205 (27) | |
| Infradiaphragmatic + supradiaphragmatic, < 30 Gy**†† | 147 (9) | 82 (10) | 65 (9) | |
| Infradiaphragmatic + supradiaphragmatic, ≥ 30 Gy**†† | 790 (49) | 409 (48) | 381 (51) | |
| Missing, n | 330 | 201 | 129 |
| . | HL cohort, n (%)* . | Male, n (%)* . | Female, n (%) . | P† . |
|---|---|---|---|---|
| All eligible CCSS survivors‡ | 2633 | 1507 (57) | 1126 (43) | |
| Treatment era | .63 | |||
| 1970-1979 | 1546 (59) | 879 (58) | 667 (59) | |
| 1980-1986 | 1087 (41) | 628 (42) | 459 (41) | |
| Age at diagnosis | < .001 | |||
| 0-9 y | 476 (18) | 379 (25) | 100 (90) | |
| 10-14 y | 884 (34) | 490 (33) | 394 (35) | |
| 15-21 y | 1273 (48) | 641 (43) | 632 (56) | |
| Participants, n§ | 1927 | 1049 | 878 | |
| Treatment era | .30 | |||
| 1970-1979 | 1097 (57) | 586 (56) | 511 (58) | |
| 1980-1986 | 830 (43) | 463 (44) | 367 (42) | |
| Age at diagnosis | < .001 | |||
| 0-9 y | 329 (17) | 244 (23) | 85 (10) | |
| 10-14 y | 663 (34) | 350 (33) | 313 (36) | |
| 15-21 y | 935 (49) | 455 (43) | 480 (55) | |
| Race/ethnicity‖ | .20 | |||
| White, non-Hispanic | 1653 (86) | 892 (85) | 761 (87) | |
| Other | 268 (14) | 156 (15) | 112 (13) | |
| Missing, n | 6 | 1 | 5 | |
| Household income‖ | .12 | |||
| $0-19 999 | 288 (17) | 167 (18) | 121 (15) | |
| ≥ $20 000 | 1423 (83) | 753 (82) | 670 (85) | |
| Missing, n | 216 | 129 | 87 | |
| Education‖ | < .001 | |||
| Through high school | 503 (27) | 323 (32) | 180 (22) | |
| After High School | 1339 (73) | 691 (68) | 648 (78) | |
| Missing, n | 85 | 35 | 50 | |
| Treatment group¶ | .77 | |||
| Radiation only | 548 (33) | 285 (32) | 263 (33) | |
| Chemotherapy + radiation | 1024 (61) | 552 (62) | 472 (60) | |
| Chemotherapy only | 98 (6) | 52 (6) | 46 (6) | |
| Missing | 257 | 160 | 97 | |
| Splenectomy (yes) | 1441 (76) | 760 (74) | 681 (79) | .02 |
| Chemotherapy categories# | .10 | |||
| Radiation only | 548 (33) | 285 (32) | 263 (34) | |
| Chemotherapy, no anthracycline | 689 (41) | 355 (40) | 334 (43) | |
| Chemotherapy, including anthracycline | 428 (26) | 247 (28) | 181 (23) | |
| Missing | 262 | 162 | 100 | |
| Anthracycline score# | .21 | |||
| 0 | 1237 (76) | 640 (74) | 597 (78) | |
| 1 | 174 (11) | 98 (11) | 79 (10) | |
| 2 | 166 (10) | 99 (12) | 67 (9) | |
| 3 | 47 (3) | 25 (3) | 22 (3) | |
| Missing, n | 303 | 187 | 116 | |
| Alkylating agents score# | .02 | |||
| 0 | 606 (44) | 317 (43) | 289 (44) | |
| 1 | 118 (9) | 58 (8) | 60 (9) | |
| 2 | 108 (8) | 45 (6) | 63 (10) | |
| 3 | 555 (40) | 316 (43) | 239 (37) | |
| Missing, n | 540 | 313 | 227 | |
| RT field by dose | < .01 | |||
| Chemotherapy only | 98 (6) | 52 (6) | 46 (6) | |
| Supradiaphragmatic, < 30 Gy** | 156 (10) | 104 (12) | 52 (7) | |
| Supradiaphragmatic, ≥ 30 Gy** | 406 (25) | 201 (24) | 205 (27) | |
| Infradiaphragmatic + supradiaphragmatic, < 30 Gy**†† | 147 (9) | 82 (10) | 65 (9) | |
| Infradiaphragmatic + supradiaphragmatic, ≥ 30 Gy**†† | 790 (49) | 409 (48) | 381 (51) | |
| Missing, n | 330 | 201 | 129 |
Percentages are calculated on the basis of the number of values not missing for each factor as the denominator.
Comparisons are based on 2-tail chi-square for categorical variables.
All US survivors with HL who met eligibility criteria for CCSS study and were included in national death index search (Figure 1).
HL survivors who participated in the study (at baseline).
Race, income, and education were not reported at baseline by some participants.
Summary data on treatment data for first diagnosis of HL available in 1675 respondents. Other specific treatment factors have various numbers of missing values as indicated.
Chemotherapy categories are mutually exclusive. Exposure to anthracycline and alkylating agents was expressed as a total score on the basis of the tertiles of various alkylating agents received, according to methods reported previously. A score of 0 indicates no exposure to the agent.34
Multiply by 100 to convert gray to rad.
Includes 49 subjects with field limited to infradiaphragmatic sites only.
Treatment records available for 1675 HL participants show 6% received chemotherapy alone for their initial HL treatment, whereas the rest received RT alone (33%) or combined with chemotherapy (61%). Chemotherapy included anthracyclines in 24% and alkylating agents in 56% of participants.
Mortality
Among 2633 US members of the eligible cohort, the 500 observed deaths are due to HL (n = 175; 35%), SMN (n = 116; 23%), benign neoplasms (n = 4, < 1%), cerebrovascular and heart disease (CV disease; n = 70; 14%), nonmalignant respiratory disease (n = 22; 4%), external cause (n = 33; 7%), other (n = 40; 8%), and unknown causes (n = 44; 9%) (Table 2).
EAR and SMR for leading causes of death by time since diagnosis in 5-year survivors of Hodgkin lymphoma (n = 2633)
| Cause of death . | Years after diagnosis . | Overall . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-9 y . | 10-19 y . | ≥ 20 y . | |||||||
| n . | EAR/10 000 PY (95% CI) . | n . | EAR/10 000 PY (95% CI) . | n . | EAR/10 000 PY (95%CI) . | n . | EAR/10 000 PY (95% CI) . | SMR/1000 PY (95% CI) . | |
| All deaths* | 148 | 10.5 (8.7-12.6) | 205 | 7.6 (6.8-9.0) | 147 | 12.6 (10.3-15.1) | 500 | 95.5 (86.1-105.5) | 7.8 (7.2-8.5) |
| Hodgkin lymphoma | 89 | 69.6 (55.9-85.7) | 64 | 28.1 (21.6-35.8) | 22 | 21.7 (13.6-32.9) | 175 | 38.3 (32.4-44.4) | 779.2 (668.0-903.5) |
| All other malignant neoplasms | 21 | 15.8 (9.6-24.5) | 48 | 19.9 (14.4-26.8) | 47 | 43.1 (30.7-58.4) | 116 | 23.9 (19.5-29.0) | 16.8 (13.9-20.1) |
| Leukemia | 7 | 5.3 (2.0-11.1) | 4 | 1.6 (0.3-4.3) | 0 | 11 | 2.3 (1.0-4.2) | 15.5 (7.7-27.7) | |
| All other hematopoietic | 3 | 2.3 (0.4-6.8) | 8 | 3.4 (1.4-6.8) | 10 | 9.7 (4.5-18.0) | 21 | 4.5 (2.7-6.9) | 42.5 (26.3-64.97) |
| Solid tumors | 11 | 8.2 (3.9-15.0 | 36 | 14.9 (10.2-21.0) | 37 | 33.6 (22.7-47.4) | 84 | 17.2 (13.4-21.5) | 14.9 (11.9-18.4) |
| Breast | 1 | 0.8 (0.0-4.3) | 9 | 3.8 (1.7-7.4) | 11 | 10.3 (4.9-18.9) | 21 | 4.4 (2.6-6.8) | 22.3 (13.8-34.1) |
| Buccal cavity/pharynx | 0 | 2 | 0.9 (0.1-3.2) | 0 | 2 | 0.4 (0.0-1.6) | 17.9 (2.01-64.5) | ||
| Lung/bronchus/trachea | 1 | 0.8 (0-4.3) | 4 | 1.7 (0.4-4.4) | 10 | 9.3 (4.2-17.6) | 15 | 3.1 (1.7-5.2) | 19.4 (10.9-32.0) |
| Digestive organs/peritoneum | 2 | 1.5 (0.1-5.6) | 10 | 4.2 (1.9-7.9) | 9 | 8.2 (3.4-16.2) | 21 | 4.4 (2.6-6.8) | 18.7 (11.6-28.6) |
| CNS | 2 | 1.5 (0.1-5.6) | 4 | 1.6 (0.4-4.4) | 1 | 0.8 (−0.2 to 5.3) | 7 | 1.4 (0.5-3.0) | 12.0 (4.89-24.6) |
| Kidney | 0 | 1 | 0.4 (0.0-2.4) | 0 | 1 | 0.2 (0.0-1.2) | 8.4 (0.1-46.8) | ||
| Melanoma | 1 | 0.8 (0-4.3) | 0 | 1 | 0.9 (−0.1 to 5.4) | 2 | 0.4 (0.0-1.5) | 6.3 (0.7-22.9) | |
| Others | 4 | 3.1 (0.8-7.9) | 6 | 2.5 (0.8-5.6) | 5 | 4.6 (1.3-11.2) | 15 | 3.1 (1.7-5.3) | 21.4 (12.0-35.3) |
| Benign neoplasms | 2 | 1.5 (0.1-5.6) | 1 | 0.4 (0-2.4) | 1 | 0.9 (0.0-5.5) | 4 | 0.8 (0.2-2.2) | 23.7 (6.4-60.6) |
| Cerebrovascular disease | 2 | 0.7 (0.1-3.0) | 3 | 2.4 (0.1-8.1) | 5 | 0.9 (0.1-2.3) | 4.6 (1.5-10.7) | ||
| All heart disease | 7 | 5.1 (1.8-10.9) | 30 | 12.3 (8.0-18.0) | 28 | 25.0 (15.7-37.3) | 65 | 13.1 (9.9-17.0) | 12.7 (9.8-16.2) |
| Ischemic heart disease | 4 | 3.1 (0.8-7.9) | 20 | 8.5 (5.1-13.3) | 13 | 11.4 (5.4-20.5) | 37 | 7.6 (5.2-10.7) | 16.5 (11.6-22.8) |
| Chronic endocardial, other myocardial insufficiency | 2 | 1.5 (0.2-5.6) | 5 | 2.1 (0.7-5.1) | 8 | 7.8 (3.3-15.5) | 15 | 3.2 (1.8-5.4) | 62.4 (34.9-102.92) |
| Nonmalignant respiratory disease | 5 | 3.7 (1.1-8.9) | 12 | 5.0 (2.4-8.9) | 5 | 4.4 (1.0-10.9) | 22 | 4.5 (2.7-7.0) | 14.2 (8.9-21.5) |
| Cause of death . | Years after diagnosis . | Overall . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-9 y . | 10-19 y . | ≥ 20 y . | |||||||
| n . | EAR/10 000 PY (95% CI) . | n . | EAR/10 000 PY (95% CI) . | n . | EAR/10 000 PY (95%CI) . | n . | EAR/10 000 PY (95% CI) . | SMR/1000 PY (95% CI) . | |
| All deaths* | 148 | 10.5 (8.7-12.6) | 205 | 7.6 (6.8-9.0) | 147 | 12.6 (10.3-15.1) | 500 | 95.5 (86.1-105.5) | 7.8 (7.2-8.5) |
| Hodgkin lymphoma | 89 | 69.6 (55.9-85.7) | 64 | 28.1 (21.6-35.8) | 22 | 21.7 (13.6-32.9) | 175 | 38.3 (32.4-44.4) | 779.2 (668.0-903.5) |
| All other malignant neoplasms | 21 | 15.8 (9.6-24.5) | 48 | 19.9 (14.4-26.8) | 47 | 43.1 (30.7-58.4) | 116 | 23.9 (19.5-29.0) | 16.8 (13.9-20.1) |
| Leukemia | 7 | 5.3 (2.0-11.1) | 4 | 1.6 (0.3-4.3) | 0 | 11 | 2.3 (1.0-4.2) | 15.5 (7.7-27.7) | |
| All other hematopoietic | 3 | 2.3 (0.4-6.8) | 8 | 3.4 (1.4-6.8) | 10 | 9.7 (4.5-18.0) | 21 | 4.5 (2.7-6.9) | 42.5 (26.3-64.97) |
| Solid tumors | 11 | 8.2 (3.9-15.0 | 36 | 14.9 (10.2-21.0) | 37 | 33.6 (22.7-47.4) | 84 | 17.2 (13.4-21.5) | 14.9 (11.9-18.4) |
| Breast | 1 | 0.8 (0.0-4.3) | 9 | 3.8 (1.7-7.4) | 11 | 10.3 (4.9-18.9) | 21 | 4.4 (2.6-6.8) | 22.3 (13.8-34.1) |
| Buccal cavity/pharynx | 0 | 2 | 0.9 (0.1-3.2) | 0 | 2 | 0.4 (0.0-1.6) | 17.9 (2.01-64.5) | ||
| Lung/bronchus/trachea | 1 | 0.8 (0-4.3) | 4 | 1.7 (0.4-4.4) | 10 | 9.3 (4.2-17.6) | 15 | 3.1 (1.7-5.2) | 19.4 (10.9-32.0) |
| Digestive organs/peritoneum | 2 | 1.5 (0.1-5.6) | 10 | 4.2 (1.9-7.9) | 9 | 8.2 (3.4-16.2) | 21 | 4.4 (2.6-6.8) | 18.7 (11.6-28.6) |
| CNS | 2 | 1.5 (0.1-5.6) | 4 | 1.6 (0.4-4.4) | 1 | 0.8 (−0.2 to 5.3) | 7 | 1.4 (0.5-3.0) | 12.0 (4.89-24.6) |
| Kidney | 0 | 1 | 0.4 (0.0-2.4) | 0 | 1 | 0.2 (0.0-1.2) | 8.4 (0.1-46.8) | ||
| Melanoma | 1 | 0.8 (0-4.3) | 0 | 1 | 0.9 (−0.1 to 5.4) | 2 | 0.4 (0.0-1.5) | 6.3 (0.7-22.9) | |
| Others | 4 | 3.1 (0.8-7.9) | 6 | 2.5 (0.8-5.6) | 5 | 4.6 (1.3-11.2) | 15 | 3.1 (1.7-5.3) | 21.4 (12.0-35.3) |
| Benign neoplasms | 2 | 1.5 (0.1-5.6) | 1 | 0.4 (0-2.4) | 1 | 0.9 (0.0-5.5) | 4 | 0.8 (0.2-2.2) | 23.7 (6.4-60.6) |
| Cerebrovascular disease | 2 | 0.7 (0.1-3.0) | 3 | 2.4 (0.1-8.1) | 5 | 0.9 (0.1-2.3) | 4.6 (1.5-10.7) | ||
| All heart disease | 7 | 5.1 (1.8-10.9) | 30 | 12.3 (8.0-18.0) | 28 | 25.0 (15.7-37.3) | 65 | 13.1 (9.9-17.0) | 12.7 (9.8-16.2) |
| Ischemic heart disease | 4 | 3.1 (0.8-7.9) | 20 | 8.5 (5.1-13.3) | 13 | 11.4 (5.4-20.5) | 37 | 7.6 (5.2-10.7) | 16.5 (11.6-22.8) |
| Chronic endocardial, other myocardial insufficiency | 2 | 1.5 (0.2-5.6) | 5 | 2.1 (0.7-5.1) | 8 | 7.8 (3.3-15.5) | 15 | 3.2 (1.8-5.4) | 62.4 (34.9-102.92) |
| Nonmalignant respiratory disease | 5 | 3.7 (1.1-8.9) | 12 | 5.0 (2.4-8.9) | 5 | 4.4 (1.0-10.9) | 22 | 4.5 (2.7-7.0) | 14.2 (8.9-21.5) |
Cause of death not detailed in the table include external causes (n = 33), other (n = 40), and unknown causes (n = 44).
EAR indicates, excess absolute risk; SMR, standardized mortality ratio; PY indicates person-year; CI, confidence interval; and CNS, central nervous system.
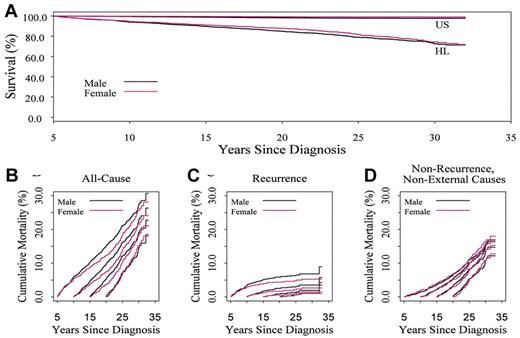
Overall survival at 30 years after diagnosis is 74.1% (95% CI, 71.8-76.6), and differs by sex (P = .007, Cox regression model results not shown; Figure 2A). The SMR differs by sex (P < .001) and is 6.3 (95% CI, 5.6-7.1) for males and 12.0 (95% CI, 10.4-13.8) for females. The EAR for overall death is 95.5 per 10 000 person-years (95% CI, 86.1-105.5). The EAR for the leading causes of death was 38.3 for HL, 23.9 for non-HL SMN, and 13.1 for cardiovascular disease (all per 10 000 person-years).
Overall survival in 5-year survivors of childhood Hodgkin lymphoma by sex. (A) Expected on the basis of US population mortality; (B) 30-year cumulative incidence of mortality varies when conditioned on time since diagnosis: 26.7% (95% CI, 24.2-29.2) in 5-year survivors, 22.3% (95% CI, 19.7-24.9) in 10-year survivors, and 14.9% (95% CI, 12.3-17.5) in 20-year survivors. Pattern of mortality differs with recurrent disease in the early years (C) and nonrecurrence causes in later years (D).
Overall survival in 5-year survivors of childhood Hodgkin lymphoma by sex. (A) Expected on the basis of US population mortality; (B) 30-year cumulative incidence of mortality varies when conditioned on time since diagnosis: 26.7% (95% CI, 24.2-29.2) in 5-year survivors, 22.3% (95% CI, 19.7-24.9) in 10-year survivors, and 14.9% (95% CI, 12.3-17.5) in 20-year survivors. Pattern of mortality differs with recurrent disease in the early years (C) and nonrecurrence causes in later years (D).
Estimates of cause-specific mortality and EAR for cause-specific mortality change when conditioned on time from initial diagnosis (Figure 2B; Table 2). Notably, the EAR of death from relapse decreases over the follow-up period, whereas the EAR from solid tumors, cerebrovascular disease, and all heart disease increases over time.
Subsequent neoplasms
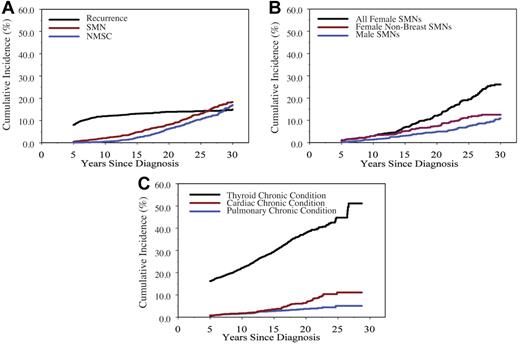
Recurrent HL, SMN, and noninvasive second neoplasms indicative of morbidity in 5-year HL survivors were delineated. The prevalence of recurrent HL was 8.1% (95% CI, 6.8%-9.3%) at cohort entry, with an increase to 11.9% (95% CI, 10.5%-13.4%) at 10 years and 15.0% (95% CI, 13.2%-16.7%) at 30 years from diagnosis (Figure 3A).
Cumulative incidence of leading chronic medical conditions in 5-year survivors of childhood Hodgkin lymphoma. (A) all neoplasms; (B) invasive second malignant neoplasms (SMNs) by sex (log-rank for women with no breast SMN vs male SMN, P = .05); (C) other nonneoplastic conditions.
Cumulative incidence of leading chronic medical conditions in 5-year survivors of childhood Hodgkin lymphoma. (A) all neoplasms; (B) invasive second malignant neoplasms (SMNs) by sex (log-rank for women with no breast SMN vs male SMN, P = .05); (C) other nonneoplastic conditions.
The incidence of invasive SMN surpasses the risk of relapse by 20 years from diagnosis (Figure 3A). There have been 277 invasive SMNs among 257 HL 5-year survivors (Table 3). The median time to first SMN is 18.7 years from diagnosis (range, 5.0-33 years). The sex difference in 30-year cumulative incidence of any SMN of 10.9% (95% CI, 8.3%-13.4%) among males and 26.1% (95% CI, 22.4%-29.8%) in females (log-rank test, P < .001) is driven by the incidence of invasive breast cancer (Figure 3B). Compared with the general US population, SIRs are highest for solid epithelial cancers: bone cancer 22.3 (95% CI, 10.0-49.6), thyroid cancer 17.6 (95% CI, 13.0-24.0), breast cancer 17.0 (95% CI, 14.0-21.7) per 10 000 person-years, respectively (Table 3). Cumulative incidence of invasive breast SMN at 30 years after diagnosis is 18.3% (95% CI, 16.0%-20.6%), with no apparent plateau in incidence within the cohort at this time (Figure 3B). The median time to occurrence of invasive breast SMN is 21.0 years (range, 6.7-33.5 years) from diagnosis.
SIR and EAR of invasive SMN among 5-year survivors of childhood Hodgkin lymphoma
| Second cancer diagnosis . | Observed . | Expected . | SIR . | 95% CI . | EAR/10 000 PY . | 95% CI . |
|---|---|---|---|---|---|---|
| All SMN | 277 | 31.9 | 8.7 | 7.7-9.8 | 69.2 | 60.3-79.3 |
| Males | 82 | 13.0 | 6.3 | 5.0-8.0 | 37.0 | 27.9-48.4 |
| Females | 195 | 18.9 | 10.3 | 8.9-11.8 | 105.1 | 89.8-122.7 |
| Hematopoietic | ||||||
| Leukemia | 13 | 1.0 | 12.7 | 7.4-21.9 | 3.4 | 1.8-6.0 |
| Non-Hodgkin lymphoma | 15 | 1.9 | 8.1 | 4.6-14.3 | 3.7 | 1.9-7.0 |
| Solid tumors | ||||||
| Breast | 109 | 6.4 | 17.0 | 14.0-21.7 | 29.0 | 23.5-35.7 |
| Thyroid | 42 | 2.4 | 17.6 | 13.0-24.0 | 11.2 | 8.1-15.5 |
| Head and neck | 10 | 0.9 | 11.7 | 6.3-21.7 | 2.6 | 1.3-5.0 |
| Lung/bronchus | 7 | 1.1 | 6.3 | 3.0-13.2 | 1.7 | 0.6-3.8 |
| Small intestine/colorectal | 11 | 1.7 | 6.6 | 3.7-12.0 | 2.6 | 1.3-5.1 |
| Bone cancer | 6 | 0.3 | 22.3 | 10.0-49.6 | 1.6 | 0.7-3.7 |
| Soft tissue sarcomas | 22 | 2.0 | 10.9 | 7.2-16.3 | 5.6 | 3.5-8.9 |
| CNS | 7 | 1.2 | 6.1 | 2.9-12.7 | 1.7 | 0.6-3.8 |
| Kidney | 5 | 0.7 | 7.2 | 3.0-17.3 | 1.2 | 0.4-3.2 |
| Melanoma | 13 | 3.3 | 4.0 | 2.3-6.8 | 2.8 | 1.2-5.4 |
| Female genital | 4 | 3.0 | 1.3 | 0.5-3.54 | 0.3 | −0.4-2.2 |
| Others | 12 | 4.5 | 2.7 | 1.5-4.7 | 2.1 | 0.6-4.7 |
| Second cancer diagnosis . | Observed . | Expected . | SIR . | 95% CI . | EAR/10 000 PY . | 95% CI . |
|---|---|---|---|---|---|---|
| All SMN | 277 | 31.9 | 8.7 | 7.7-9.8 | 69.2 | 60.3-79.3 |
| Males | 82 | 13.0 | 6.3 | 5.0-8.0 | 37.0 | 27.9-48.4 |
| Females | 195 | 18.9 | 10.3 | 8.9-11.8 | 105.1 | 89.8-122.7 |
| Hematopoietic | ||||||
| Leukemia | 13 | 1.0 | 12.7 | 7.4-21.9 | 3.4 | 1.8-6.0 |
| Non-Hodgkin lymphoma | 15 | 1.9 | 8.1 | 4.6-14.3 | 3.7 | 1.9-7.0 |
| Solid tumors | ||||||
| Breast | 109 | 6.4 | 17.0 | 14.0-21.7 | 29.0 | 23.5-35.7 |
| Thyroid | 42 | 2.4 | 17.6 | 13.0-24.0 | 11.2 | 8.1-15.5 |
| Head and neck | 10 | 0.9 | 11.7 | 6.3-21.7 | 2.6 | 1.3-5.0 |
| Lung/bronchus | 7 | 1.1 | 6.3 | 3.0-13.2 | 1.7 | 0.6-3.8 |
| Small intestine/colorectal | 11 | 1.7 | 6.6 | 3.7-12.0 | 2.6 | 1.3-5.1 |
| Bone cancer | 6 | 0.3 | 22.3 | 10.0-49.6 | 1.6 | 0.7-3.7 |
| Soft tissue sarcomas | 22 | 2.0 | 10.9 | 7.2-16.3 | 5.6 | 3.5-8.9 |
| CNS | 7 | 1.2 | 6.1 | 2.9-12.7 | 1.7 | 0.6-3.8 |
| Kidney | 5 | 0.7 | 7.2 | 3.0-17.3 | 1.2 | 0.4-3.2 |
| Melanoma | 13 | 3.3 | 4.0 | 2.3-6.8 | 2.8 | 1.2-5.4 |
| Female genital | 4 | 3.0 | 1.3 | 0.5-3.54 | 0.3 | −0.4-2.2 |
| Others | 12 | 4.5 | 2.7 | 1.5-4.7 | 2.1 | 0.6-4.7 |
SIR indicates standardized incidence ratio; EAR, excess absolute risk; SMN, second malignant neoplasm; CI, confident interval; PY, person-year; and CNS, central nervous system.
Noninvasive neoplasms (NMSC; other benign neoplasms) also confer morbidity. Cumulative incidence of NMSCs at 30 years after diagnosis is 16.7% (95% CI, 14.5%-19.4%; Figure 3A). Eighty-two additional noninvasive neoplasms were reported, including non–infiltrating intraductal carcinoma of the breast (n = 41), other breast disorders (n = 12), noninvasive meningioma (n = 2), neurilemmoma (n = 3), noninvasive female genitourinary cancer (n = 8), and other histologically unspecified neoplasms (n = 16).
Chronic medical conditions
At least one chronic condition (grade 1-4) was reported by 70% (1348 of 1927) of survivors by baseline questionnaire response. Serious chronic conditions (grade 3-4) were enumerated in 27% (516 of 1927). Of these, 34% (174 of 516) were present at cohort entry, and 63% (327 of 516) were incident events after 5 years from diagnosis. Multiple morbidities were reported by HL survivors, with 44% having > 2 and 28% reporting > 3 chronic health conditions. The 30-year cumulative incidence of grade 3-5 CV conditions reported by the cohort is 11.1% (95% CI, 8.5%-13.8%; Figure 3C). There is no sex difference in incidence of reported CV conditions (log-rank test, P = .20). Specific CV conditions of concern among the 1927 participants included coronary artery disease requiring medication (2%; n = 39), myocardial infarction (1.2%; n = 24), congestive heart failure (1.5%; n = 28), heart transplantation for cardiomyopathy (0.1%; n = 2), cerebrovascular accident (0.78%; n = 15), and other (0.57%; n = 11).
The 30-year cumulative incidence of grade 3-5 pulmonary conditions or any thyroid conditions was 5.1% (95% CI, 3.3%-6.9%) and 51.1% (95% CI, 44.6%-57.7%), respectively (Figure 3C). Among these, 72% (38 of 53) of grade 3-5 pulmonary conditions and 52% (334 of 639) of thyroid conditions occurred after cohort entry.
Risks for overall and cause-specific mortality
Adjusting for demographics and key medical conditions, overall mortality was independently associated with initial cancer treatment (Table 4). Infradiaphragmatic and supradiaphragmatic extended field RT together increased risk at any dose (< 3000 rad [< 30 Gy]: HR, 3.9; 95% CI, 1.1-13.8]; ≥ 3000 rad [≥ 30 Gy]: HR, 7.8; 95% CI, 2.4-25.1), as did supradiaphragmatic RT at ≥ 3000 rad (≥ 30 Gy; HR 3.8; 95% CI, 1.1-12.6). Overall mortality also was associated with any alkylating agent exposure (HR, 1.7; 95% CI, 1.2-2.5) and risk increased with increasing exposure to anthracycline chemotherapy (highest cumulative dose score; HR, 4.4; 95% CI, 1.9-10.1). There were no interactions between sex and treatment variables.
Multivariable model of predictors of mortality from any cause in 5-year survivors of childhood Hodgkin lymphoma*
| Variable . | HR of death from any cause . | 95% CI . | P . |
|---|---|---|---|
| Age at diagnosis | |||
| < 10 y (reference) | 1.0 | ||
| 10-14 y | 0.9 | 0.5-1.6 | .68 |
| 15-21 y | 1.1 | 0.6-2.0 | .82 |
| Race | |||
| Other (reference) | 1.0 | ||
| White not Hispanic | 1.3 | 0.8-2.3 | .26 |
| Household Income | |||
| ≥ $20 000 (reference) | 1.0 | ||
| < $20 000 | 2.4 | 1.6-3.5 | < .01 |
| Education | |||
| After high school (reference) | 1.0 | ||
| <High school | 1.6 | 1.1-2.2 | .01 |
| Radiation field + dose | |||
| No radiation (reference) | 1.0 | ||
| Supradiaphragm, < 30 Gy† | 0.9 | 0.2-4.6 | .92 |
| Supradiaphragm, ≥ 30 Gy† | 3.8 | 1.1-12.6 | .03 |
| Infradiaphragm or Infra diaphragm + supradiaphragm, < 30 Gy† | 3.9 | 1.1-13.9 | .04 |
| Infradiaphragm or Infradiaphragm + supradiaphragm, ≥ 30 Gy | 7.8 | 2.4-25.1 | < .01 |
| Chemotherapy | |||
| Anthracycline score | |||
| 0 (reference) | 1.0 | ||
| 1 | 1.9 | 1.1-3.2 | .02 |
| 2 | 2.6 | 1.6-4.3 | < .01 |
| 3 | 4.4 | 1.9-10.1 | < .01 |
| Alkylating agent | |||
| No (reference) | 1.0 | ||
| Yes | 1.7 | 1.2-2.5 | < .01 |
| Grade 3-5 CV condition | |||
| No (reference) | 1.0 | ||
| Yes | 4.4 | 2.7-7.3 | < .01 |
| NMSC | |||
| No (reference) | 1.0 | ||
| Yes | 0.1 | 0.04-0.40 | < .01 |
| SMN within sex stratum | |||
| Female no SMN (female reference) | 1.0 | ||
| Female with breast cancer | 0.7 | 0.3-1.6 | .38 |
| Female with non breast cancer SMN | 2.6 | 1.4-5.1 | < .01 |
| Male, no SMN (male reference) | 1.0 | ||
| Male with SMN | 2.3 | 1.4-3.9 | < .01 |
| Variable . | HR of death from any cause . | 95% CI . | P . |
|---|---|---|---|
| Age at diagnosis | |||
| < 10 y (reference) | 1.0 | ||
| 10-14 y | 0.9 | 0.5-1.6 | .68 |
| 15-21 y | 1.1 | 0.6-2.0 | .82 |
| Race | |||
| Other (reference) | 1.0 | ||
| White not Hispanic | 1.3 | 0.8-2.3 | .26 |
| Household Income | |||
| ≥ $20 000 (reference) | 1.0 | ||
| < $20 000 | 2.4 | 1.6-3.5 | < .01 |
| Education | |||
| After high school (reference) | 1.0 | ||
| <High school | 1.6 | 1.1-2.2 | .01 |
| Radiation field + dose | |||
| No radiation (reference) | 1.0 | ||
| Supradiaphragm, < 30 Gy† | 0.9 | 0.2-4.6 | .92 |
| Supradiaphragm, ≥ 30 Gy† | 3.8 | 1.1-12.6 | .03 |
| Infradiaphragm or Infra diaphragm + supradiaphragm, < 30 Gy† | 3.9 | 1.1-13.9 | .04 |
| Infradiaphragm or Infradiaphragm + supradiaphragm, ≥ 30 Gy | 7.8 | 2.4-25.1 | < .01 |
| Chemotherapy | |||
| Anthracycline score | |||
| 0 (reference) | 1.0 | ||
| 1 | 1.9 | 1.1-3.2 | .02 |
| 2 | 2.6 | 1.6-4.3 | < .01 |
| 3 | 4.4 | 1.9-10.1 | < .01 |
| Alkylating agent | |||
| No (reference) | 1.0 | ||
| Yes | 1.7 | 1.2-2.5 | < .01 |
| Grade 3-5 CV condition | |||
| No (reference) | 1.0 | ||
| Yes | 4.4 | 2.7-7.3 | < .01 |
| NMSC | |||
| No (reference) | 1.0 | ||
| Yes | 0.1 | 0.04-0.40 | < .01 |
| SMN within sex stratum | |||
| Female no SMN (female reference) | 1.0 | ||
| Female with breast cancer | 0.7 | 0.3-1.6 | .38 |
| Female with non breast cancer SMN | 2.6 | 1.4-5.1 | < .01 |
| Male, no SMN (male reference) | 1.0 | ||
| Male with SMN | 2.3 | 1.4-3.9 | < .01 |
HR indicates hazard ratio; and CI, confidence interval.
Chronic cardiovascular (CV) conditions, nonmelanoma skin cancer (NMSC), and second malignant neoplasms (SMNs) are adjusted as time-dependent covariates. No significant effect of pulmonary or thyroid condition or recurrent HL was observed on overall mortality in univariate or multivariable modeling; hence, they were not included in the final model. SMN effects differed within sex stratum, with differences primarily because of lower risks due to breast cancer. Effects are presented within sex stratum to show this phenomenon. No interaction was observed between sex and chronic cardiac condition. Exposure to anthracycline was expressed as a total score, based on the tertile of cumulative dose received; a score of 0 indicates no exposure to the agent.34
Multiply by 100 to convert gray to rad.
In evaluating leading morbidities driving overall mortality risk, there was a statistically significant interaction of sex with the type of SMN. Separation of effects of SMN type showed overall mortality was not associated with female breast cancer but rather was associated with other SMNs in both males and females (Table 4). Finally, report of a grade 3-5 cardiac condition was also an independent risk factor for mortality (HR, 4.4; 95% CI, 2.7-7.3). The occurrence of recurrent HL was evaluated in mortality models and was neither significant nor a confounder on treatment or key medical condition effect; hence, recurrent HL was not included in final models.
A similar model for risk of death from SMN also showed independent associations of treatment with death (Table 5), including RT (3000 rad [≥ 30 Gy]; HR, 7.4; 95% CI, 1.8-30.3) to any site, alkylating agent exposure (HR, 2.3; 95% CI, 1.4-3.9), or any anthracycline dose (highest cumulative dose score: HR, 6.6; 95% CI, 2.6-16.2).
Multivariable model of predictors of mortality from a SMN in 5-year survivors of childhood Hodgkin lymphoma
| Variable . | HR for fatal SMN . | 95% CI . | P . |
|---|---|---|---|
| Age at diagnosis | |||
| <10 y (reference) | 1.0 | ||
| 10-14 y | 0.9 | 0.4-1.9 | .68 |
| 15-21 y | 1.6 | 0.7-3.6 | .28 |
| Sex | |||
| Female (reference) | 1.0 | ||
| Male | 1.3 | 0.9-2.0 | .15 |
| Race | |||
| Other (reference) | 1.0 | ||
| White not Hispanic | 1.8 | 0.9-3.7 | .10 |
| Household income | |||
| ≥ $20 000 (reference) | 1.0 | ||
| < $20 000 | 2.4 | 1.5-3.9 | < .01 |
| Education | |||
| After high school (reference) | 1.0 | ||
| < High school | 1.5 | 1.0-2.3 | .05 |
| Radiation dose | |||
| No radiation (reference) | 1.0 | ||
| < 30 Gy* | 1.9 | 0.4-8.7 | .43 |
| ≥ 30 Gy | 7.4 | 1.8-30.3 | < .01 |
| Chemotherapy | |||
| Anthracycline score | |||
| 0 (reference) | 1.0 | ||
| 1 | 2.3 | 1.2-4.2 | .01 |
| 2 | 5.0 | 2.9-8.7 | < .01 |
| 3 | 6.6 | 2.6-16.2 | < .01 |
| Alkylating agent | |||
| No (reference) | 1.0 | ||
| Yes | 2.3 | 1.4-3.9 | < .01 |
| NMSC | |||
| No (reference) | 1.0 | ||
| Yes | 0.3 | 0.1-1.0 | .04 |
| Variable . | HR for fatal SMN . | 95% CI . | P . |
|---|---|---|---|
| Age at diagnosis | |||
| <10 y (reference) | 1.0 | ||
| 10-14 y | 0.9 | 0.4-1.9 | .68 |
| 15-21 y | 1.6 | 0.7-3.6 | .28 |
| Sex | |||
| Female (reference) | 1.0 | ||
| Male | 1.3 | 0.9-2.0 | .15 |
| Race | |||
| Other (reference) | 1.0 | ||
| White not Hispanic | 1.8 | 0.9-3.7 | .10 |
| Household income | |||
| ≥ $20 000 (reference) | 1.0 | ||
| < $20 000 | 2.4 | 1.5-3.9 | < .01 |
| Education | |||
| After high school (reference) | 1.0 | ||
| < High school | 1.5 | 1.0-2.3 | .05 |
| Radiation dose | |||
| No radiation (reference) | 1.0 | ||
| < 30 Gy* | 1.9 | 0.4-8.7 | .43 |
| ≥ 30 Gy | 7.4 | 1.8-30.3 | < .01 |
| Chemotherapy | |||
| Anthracycline score | |||
| 0 (reference) | 1.0 | ||
| 1 | 2.3 | 1.2-4.2 | .01 |
| 2 | 5.0 | 2.9-8.7 | < .01 |
| 3 | 6.6 | 2.6-16.2 | < .01 |
| Alkylating agent | |||
| No (reference) | 1.0 | ||
| Yes | 2.3 | 1.4-3.9 | < .01 |
| NMSC | |||
| No (reference) | 1.0 | ||
| Yes | 0.3 | 0.1-1.0 | .04 |
Neither recurrence of Hodgkin lymphoma nor the occurrence of a chronic cardiac condition was significant in models adjusted for treatment effects and was therefore not included in final model. Occurrence of NMSC was included as a time-dependent covariate. Exposure to anthracycline was expressed as a total score, based on the tertile of cumulative dose received; a score of 0 indicates no exposure to the agent.34
SMN indicates second malignant neoplasm; HR, hazard ratio; CI, confidence interval; and NMSC, nonmelanoma skin cancer.
Multiply by 100 to convert gray to rad.
Males had a higher risk of cardiac-specific death (HR, 3.2; 95% CI, 1.4-7.7) compared with females (adjusted Cox regression model; data not shown). There was no significant effect of anthracycline or alkylating exposure on cardiac mortality. The RT effect was not estimable, because all cardiac deaths occurred in those who received [≥ 3000 rad (≥ 30 Gy) to any field. However, because of the small number of cardiac deaths, in a log-rank test, RT was not significant (P = .81).
Discussion
Despite excellent 5-year survival rates for HL treated in childhood, the overall EAR of death after 5 years is significantly elevated at 95.5/10 000 person-years in a cohort treated between 1970 and 1986. Underlying this excess are SMNs and CV disease, both of which are elevated above population norms. Consistent with previous studies, death in the first 10 years after diagnosis is largely attributable to HL.10,23,25,26 With extended follow-up beyond 10 years, treatment complications, predominantly SMNs and CV disease, emerge as leading causes of excess death and exhibit novel sex relationships in this young cohort. Notably, despite the 17-fold elevated incidence of breast cancer in this cohort and with the current length of follow-up, women with breast cancer do not appear to have an excess risk of overall mortality. Longer follow-up will be important to know if this observation persists. Yet, non–breast SMNs in both men and women confer an excess independent risk of mortality after adjustment for treatment effects. Overall and SMN-specific mortality are associated with both RT and chemotherapy.
Importantly, 20 years after initial HL treatment, the excess death risk from CV disease rivals that from solid-tumor SMN. The EAR for a CV mortality of 13.1 per 10 000 person-years is comparable to the estimate of 9.5 and 17.7 per 10 000 person-years in those treated at < 20 years age in the Dutch and Stanford cohorts, respectively,25,39 and to the myocardial infarction-specific mortality of 12.6 per 10 000 person-years in a British cohort of both pediatric and adult HL survivors.9 We noted an elevated incidence of congestive heart failure, coronary artery disease, and cerebrovascular events in the cohort. The rates of fatal SMNs in our cohort differ by site/histology from previous literature. Breast cancer mortality is similar to that in the Dutch experience, but we found lower EAR of fatal respiratory or gastrointestinal SMNs at > 20 years of follow-up.25
Our regression model, adjusted for attained age, indicates no effect of age at treatment on overall, SMN-, or CV-specific mortality within a cohort treated during childhood. This does not negate prior findings in cohorts of combined pediatric and adult HL that noted increased mortality risk from SMN or CV disease in patients treated at < 21 years of age compared with those older at diagnosis.25,40 After adjustment for key chronic medical conditions, initial cancer treatments (including RT ≥ 3000 rad [≥ 30 Gy], any anthracycline, or alkylating agent chemotherapy exposure) remain significant independent risks of overall and SMN-specific mortality. The lack of significance for anthracycline dose as a independent predictor of CV-specific mortality in our cohort must be interpreted in the context of 94% of the CCSS cohort having received RT as initial therapy.9 The effects of anthracycline and other chemotherapy (such as alkylating agents) will need to be distinguished from that of RT in childhood HL cohorts treated with contemporary approaches that minimize or avoid RT.
Sex affects multiple dimensions of health after childhood cancer therapy.41 We show that male survivors of HL have a higher overall mortality than females, echoing previous single-institution and cohort studies with smaller event numbers among those treated in childhood.23,25,39 This finding parallels mortality excess for males in the general population of North America, where CV events are the leading cause of death. Although Ng et al26 noted no sex effect in their multivariable model of survival among patients with low-stage HL, unadjusted for treatment or comorbidities, Dutch investigators reported similar findings to ours in a cohort of adult and childhood patients with HL. They found male sex conferred a HR of 2.4 for CV-specific mortality, unadjusted for treatment factors.25 Conversely, some studies among survivors who received anthracyclines find females to have an increased risk of cardiotoxicity compared with males.41 Importantly, the reported incidence of grade 3-5 CV conditions in our cohort was not significantly higher in male survivors, yet their risk of mortality from CV disease was 3-fold than that of their female counterparts. Possible explanations for our findings are that male survivors are twice as likely to receive no medical care.42 An alternate explanation is that perception of CV risk or the symptom complex of CV events may differ between the sexes.
The steep rise in the cumulative incidence of serious CV conditions begins at 10 years after diagnosis, highlighting the temporal window for modifiable injury regardless of sex.12,39 Hence, investigation for biomarkers of subclinical CV injury and related interventions to preserve CV health should target this critical period. This is germane to HL that often presents in adolescence and necessitates transition from pediatric oncology to community primary medical care providers who may not be familiar with cancer-related health risks.
Our EAR estimates for breast cancer are consistent with the well-described elevated risk of breast cancer and its association with RT in female HL survivors.16-18,20,28,40 We now show that despite the high rates of noninvasive and invasive breast neoplasms, HL survivors with breast malignancies in the CCSS cohort have a comparable mortality risk to females with no history of SMN. Continued follow-up of this cohort will be important to evaluate this mortality effect over time. Although one could postulate that lower mortality is due to better surveillance and treatment options for breast cancer compared with the other second tumor types noted in the cohort, previous investigations have shown suboptimal adherence to mammography screening recommendations in the CCSS cohort.43 Alternately, an index invasive or noninvasive breast neoplasm may lead to better overall health surveillance for these women. Our data support the current practice guidelines for heightened breast cancer surveillance and the potential benefit associated with early detection in female childhood cancer survivors treated with chest RT.44-46 Although organ-targeted cancer screening programs (analogous to breast screening in women) may not be feasible in male survivors, regular health surveillance may allow increased awareness and modification of health behaviors to modify risk of RT-associated malignancies.
The rates of NMSCs and noninvasive breast neoplasms in HL survivors underscore the morbidity of these “low-grade” entities, which often require multiple surgical procedures for surveillance and management.47 Although development of an NMSC within the RT field is a recognized risk, it portends increased tissue sensitivity to RT. Therefore, the decreased risk of overall mortality in cohort members who developed an NMSC was unexpected. Our finding could be explained by the fact that the occurrence of a NMSC serves as a gateway diagnosis for better overall health surveillance. Conversely, this population, who already has regular medical care, may be more likely to have an NMSC diagnosed. It is also possible that the mechanisms leading to RT-associated invasive or life-threatening SMNs are independent from those for development of an NMSC.
Although the CCSS cohort of 5-year survivors of HL is rich in treatment and comorbidity data, results must be interpreted in light of limitations of its unique retrospective-prospective design. The current analysis used a mixture of well-validated variables (death, SMN, treatment, RT dose and field) and variables based on self-report, which have not been fully adjudicated (pulmonary, CV condition). In addition, multivariable models constructed on information from the 87% of the cohort with full available treatment data may reflect participants with more serious illness. However, the Nordic cohort, comparable to the CCSS treatment era, with 94% follow-up of all survivors and outcomes verified by physician examination, reported chronic illness rates similar to that of the CCSS.48 Finally, risks derived primarily from rare events that occur late in follow-up may be inaccurate predictors of the outcome of more contemporary therapy.
Regardless, the CCSS remains the largest available cohort with the most extended follow-up, making it a rich resource for understanding long-term outcomes of childhood cancer survivors. The expanded CCSS cohort, currently being assembled will have the ability to evaluate HL survivors treated with contemporary chemotherapy only and combined modality approaches, because it encompasses children treated from 1987 to 1999. Although it is anticipated that more targeted contemporary approaches (ie, risk-and response-based chemotherapy, immunotherapy; anti–angiogenic agents, involved and targeted nodal RT) will minimize the incidence of SMN and CV disease in the next generation of survivors, decades of follow-up will be required to document the magnitude of effect of such approaches delivered to the growing child. The strong association of RT with SMN and CV morbidity and mortality substantiates current and future cooperative group strategies to minimize and obviate the use of RT while maintaining disease-free survival in the treatment of childhood HL. However, given the radiosensitivity of HL, it remains that some proportion of patients will ultimately require RT to optimize disease control.
In summary, HL survivors continue to be at risk of treatment-related mortality for decades beyond their initial disease. SMN and CV conditions represent the leading morbidities underlying the risk of premature death; sex and specific treatment modality influence this risk. HL survivors and clinicians supervising their medical care should be vigilant for these sex- and therapy-specific health risks. Research is needed to evaluate whether health surveillance programs that facilitate early diagnosis of cancer-related morbidity can optimize long-term health outcomes in childhood cancer survivors.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in part as oral presentation at the meeting of the American Society of Clinical Oncology, Chicago, IL, June 2, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the participants and the institutional investigators in the Childhood Cancer Survivor Study.
This work was supported by the National Institutes of Health (grant U01 CA55727, L.L.R; grant R25 CA122061, S.M.C) and by the American Lebanese-Syrian Associated Charities (ALSAC; L.L.R).
National Institutes of Health
Authorship
Contribution: S.M.C. designed and performed analyses and wrote the manuscript; W.M.L., J.A.T., and P.G. performed and oversaw all analyses; A.M.G., A.C.M., and M.M.H contributed to the interpretation of data; M.M.H, A.M.G., and L.L.R. edited the paper; M.S. performed radiation dosimetry analyses; P.G. prepared figures; A.C.M collected data; and L.L.R. is the principal investigator of the CCSS project and designed and assembled the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of CCSS participants, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Sharon M. Castellino, Department of Pediatrics, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157; e-mail: scastell@wfubmc.edu.