To the editor:
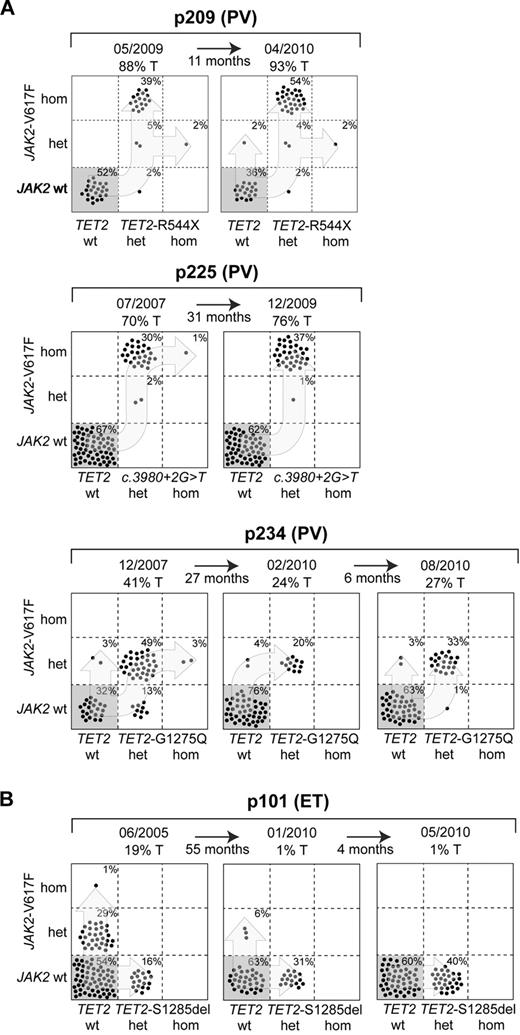
TET2 mutations are frequently found in hematologic malignancies including myeloproliferative neoplasms (MPNs).1,2 We previously reported on the clonal evolution of TET2 mutations in 8 JAK2-V617F–positive MPN patients by genotyping single BFU-E colonies and found there is no strict temporal order of occurrence between the TET2 and JAK2 mutations.3 The majority of patients with MPN display heterozygous TET2 mutations, but cases with homozygous TET2 mutations due to loss of heterozygosity (LOH) have also been described. Our clonal analysis revealed that 4/8 patients had a very small proportion (1%-3%) of colonies that were homozygous for the TET2 mutation through a mechanism of uniparental disomy or deletion in the TET2 locus. To determine whether cells carrying the homozygous TET2 mutation have a competitive advantage over heterozygous cells, we examined sequential follow up samples from 3 patients with homozygous mutations (Figure 1A). The homozygous TET2 mutation was present in the follow up sample of patient p209, but the proportion did not increase within the 11-month period between the 2 time points. In the other 2 patients (p225 and p234) we did not detect colonies homozygous for the TET2 mutation in the follow up analyses 31 months and 33 months later, respectively. These results argue against the hypothesis that the loss of both functional copies of TET2 provides an additional growth advantage over cells with the heterozygous mutation, as is frequently the case with tumor suppressor genes.
Analysis of single colonies for mutations in TET2 and JAK2. Mononuclear cells from peripheral blood were grown in methylcellulose. Single burst forming units erythroid (BFU-E) were picked and analyzed individually for the presence of TET2 and JAK2-V617F mutations by DNA sequencing and allele-specific polymerase chain reaction, respectively. Each colony is represented by a dot that is placed into one of 6 quadrangles representing the 6 possible genotypes: wild-type (wt), heterozygous (het) and homozygous (hom) for JAK2-V617F on the vertical axis, and for TET2 mutations on the horizontal axis. The unique patient numbers, the diagnoses (PMF indicates primary myelofibrosis; ET, essential thrombocythemia; and PV, polycythemia vera) and the allelic ratio of JAK2-V617F in purified granulocytes (%T) are shown above the corresponding boxes. Light blues arrows indicate the suggested order of mutation events. (A) Patterns from patients with homozygous colonies at the first time point, which did not expand. (B) Patterns compatible with a biclonal state of the disease with a gradual decrease in JAK2-V617F allelic burden.
Analysis of single colonies for mutations in TET2 and JAK2. Mononuclear cells from peripheral blood were grown in methylcellulose. Single burst forming units erythroid (BFU-E) were picked and analyzed individually for the presence of TET2 and JAK2-V617F mutations by DNA sequencing and allele-specific polymerase chain reaction, respectively. Each colony is represented by a dot that is placed into one of 6 quadrangles representing the 6 possible genotypes: wild-type (wt), heterozygous (het) and homozygous (hom) for JAK2-V617F on the vertical axis, and for TET2 mutations on the horizontal axis. The unique patient numbers, the diagnoses (PMF indicates primary myelofibrosis; ET, essential thrombocythemia; and PV, polycythemia vera) and the allelic ratio of JAK2-V617F in purified granulocytes (%T) are shown above the corresponding boxes. Light blues arrows indicate the suggested order of mutation events. (A) Patterns from patients with homozygous colonies at the first time point, which did not expand. (B) Patterns compatible with a biclonal state of the disease with a gradual decrease in JAK2-V617F allelic burden.
In our previous report, 2/8 patients showed a biclonal pattern, with mutations in JAK2 and TET2 representing separate clones. Our follow up analysis revealed that a third patient, p209, also acquired a second clone that carries JAK2-V617F without the TET2 mutation. We excluded the possibility that this pattern was acquired through uniparental disomy in the TET2 locus using the marker rs34402524, which remained heterozygous in this colony. Another patient (p101) with biclonal pattern of TET2 and JAK2-V617F surprisingly showed a reduction and disappearance of the JAK2-V617F single-positive colonies accompanied by decrease in the mutant allele burden in peripheral blood granulocytes (Figure 1B). The platelet counts decreased during this period (1467 × 109/L, 864 × 109/L and 810 × 109/L), but there were no signs of leukemic transformation. A similar decrease in the JAK2-V617F single-positive clone was reported in a biclonal TET2 positive MPN patient treated with pegylated interferon α.4 However, our patient was treated with 5-hydroxyurea only and did not receive interferon. The proportion of colonies single-positive for the heterozygous TET2 mutation increased from 16% to 40% in our patient, suggesting that the presence of the heterozygous TET2 mutation alone may provide a growth advantage.
TET2 has recently been shown to have hydroxylase activity for 5-methyl-cytosines.5 Our data are compatible with the hypothesis that a decrease in TET2 hydroxylase activity through haploinsufficiency causes alterations in the DNA-methylation patterns that provide a growth advantage. Loss of the TET2 hydroxylase activity in cells homozygous for TET2 mutations does not appear to increase their competitive advantage, suggesting that the TET2 hydroxylase activity is tightly regulated.
Authorship
Contribution: F.X.S. performed research, analyzed data and wrote the paper; R.L. and H.H.-S. performed research; T.L. and A.T. provided clinical data; and R.C.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek C. Skoda, MD, Department of Biomedicine, Experimental Hematology, University Hospital Basel, Hebelstr 20, 4031 Basel, Switzerland, radek.skoda@unibas.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal