In this issue of Blood, Trotta et al define a novel mechanism of human granzyme B and perforin regulation and identify 2 new signaling players involved in modulating NK cytotoxicity.1
Natural killer (NK) cells play an important role in viral and antitumor innate immunity, commonly through 2 primary functions: cytotoxicity and cytokine production.2 Of the several mechanisms available to kill target cells, NK cells primarily kill virus-infected or tumor targets using the granule exocytosis pathway.3 After appropriate triggering events occur via cell-surface receptor interactions, an NK cell forms a cytotoxic synapse with a target cell, releasing granzymes (a family of serine proteases) that enter the target with the aid of perforin, and initiate an apoptotic-like cell death. Of the granzymes, granzyme B is one of the best studied, and plays a nonredundant role in NK-mediated cytotoxicity. More recently, additional functions have been attributed to granzyme B, including direct antiviral effects. Simply put, granzyme B and perforin proteins comprise the ammunition for NK cell–mediated killing. Thus, how an NK cell controls the transcription, translation, and activity of granzyme B and perforin (and other effector molecules) is an important topic in NK-cell biology.
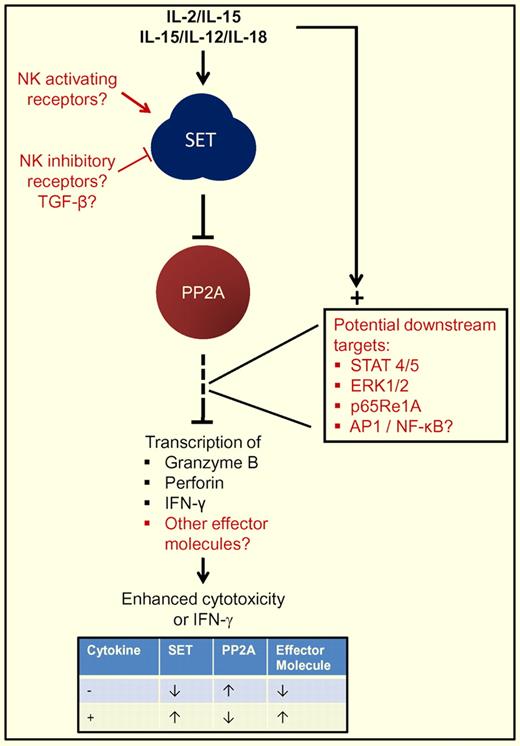
Simplified pathway of SET-PPT2A regulation of key NK-cell effector molecules. Cytokine stimulation results in both activating signal transduction pathways and increased SET expression. SET in turn down-regulates PP2A, an indirect negative regulator of IFN-γ, granzyme B, and perforin resulting in their enhanced expression. Potential contributors or participants in the pathways are annotated in blue text. The inset table summarizes the simplified regulatory circuit with (+) and without (−) cytokine activation.
Simplified pathway of SET-PPT2A regulation of key NK-cell effector molecules. Cytokine stimulation results in both activating signal transduction pathways and increased SET expression. SET in turn down-regulates PP2A, an indirect negative regulator of IFN-γ, granzyme B, and perforin resulting in their enhanced expression. Potential contributors or participants in the pathways are annotated in blue text. The inset table summarizes the simplified regulatory circuit with (+) and without (−) cytokine activation.
The regulation of granzyme B in NK cells is incompletely understood, and species differences may exist. In specific pathogen free (SPF) laboratory mice, granzyme B and perforin mRNA are constitutively present in resting NK cells, but only minimal protein is detectable. After cytokine activation, there is rapid translation of granzyme B and perforin protein, resulting in an armed or primed NK cell with enhanced cytotoxic capacity.4,5 Similar mRNA and protein patterns have been observed for interferon-γ6 in resting and cytokine-activated NK cells, suggesting that this may be a general regulatory approach for murine NK cells. In this setting, transcription of key effector molecules appears to be regulated during development, and a final arming/priming event results in fully functional NK cells. In contrast, most mature human NK cells in peripheral blood constitutively express granzyme B and perforin protein and have basal cytotoxicity against NK-sensitive target cells, indicating differences in human and murine NK cells. Hypotheses explaining these species differences include inherent differences in mouse and human biology and differing innate immune stimulatory environments for SPF mice versus people.7 For human NK cells, cytokine exposure with interleukin-2 (IL-2) or IL-15 is known to increase baseline human NK-cell granzyme B and perforin protein abundance and cytotoxic activity, and also converts basal NK cytotoxicity to lymphokine-activated killing. However, the molecular mechanisms responsible for transducing this signal have remained unclear and are the focus of the current study.
In this issue, Trotta and colleagues demonstrate that modulation of SET (I2PPA2A, IGAAD, TAF1b) or its target protein phosphatase type 2A (PP2A) can affect granzyme B mRNA and protein levels in primary human NK cells.1 SET and PP2A are signal transduction proteins that participate in normal cellular functions; however, neither has been implicated in regulating cytotoxicity. Overexpression of SET or knockdown of PP2A in primary human NK cells and human NK-cell lines enhanced granzyme B and perforin expression, as well as killing of NK-sensitive tumor cells. Furthermore, this study demonstrates that the cytokines IL-2 and IL-15 directly induce SET, leading to PP2A inhibition and subsequent granzyme B production (see figure). In previous work, this group also demonstrated that interferon-γ, a prototype cytokine produced by activated NK cells, was also controlled by SET-PP2A.8 Collectively, these data suggest that SET-PP2A may have a shared downstream signaling pathway culminating in the transcription of multiple, key genes expressed in cytokine-activated NK cells.
Many new questions in NK-cell biology arise from these observations. What are the downstream targets of PP2A that lead to granzyme B and perforin control? Do activating or inhibitory NK receptor-based signals lead to modulation of SET and PP2A? Does SET-PP2A regulate other granzymes or additional effector molecules in NK cells? What physiologic or disease conditions modulate SET expression in NK cells, and may thereby alter NK-cell responsiveness? How does SET-PP2A fit into the differences observed between human and murine granzyme B and perforin expression? Answers to these and other questions arising from this work will continue to shed new light on the molecular mechanisms orchestrating NK-cell function.
One intriguing issue stemming from this work is how SET-PP2A participates in the activation of different human NK subsets. Two functionally and developmentally distinct types of human NK cells exist in the peripheral blood, defined by CD56 expression. CD56dimNK cells (“stage 5” developing human NK cells) express high levels of granzyme B and perforin at rest and kill NK cell–sensitive targets by granule exocytosis. In contrast, CD56bright NK cells (“stage 4” developing human NK cells) possess minimal granzyme B or perforin protein, and are poorly cytotoxic in the resting state.9 SET has been shown to be increased in resting CD56bright, compared with CD56dim NK cells,8 suggesting that additional mechanisms controlling granzyme B and perforin are likely operative in human NK cells. Cytokine activation with IL-15/IL-12/IL-18 for CD56bright or IL-2/IL-15 for CD56dim NK cells increase SET expression in both subsets, thereby amplifying downstream effects on the relevant effector molecule. Thus, the end result of stimulating the SET-PP2A pathway may depend on the cellular context of expression and interplay with other cooperating regulatory pathways.
In summary, Trotta et al have identified an important new link in human NK cells between IL-2/IL-15 receptor signaling, SET-PP2A, and effector molecules of cytotoxicity including granzyme B and perforin. This study enhances our understanding of human granzyme B and perforin regulation, and leads to an exciting new series of hypotheses in NK-cell biology that await evaluation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal