In this issue of Blood, Acharya and colleagues provide evidence for the role of angiogenesis in the pathophysiology of hemophilic joint disease.1 Is this the linchpin that unravels this important clinical condition or merely a cog in a not so stepwise process?
Joint disease has always been the hallmark of severe hemophilia, but recent practice of regular infusions of intravenous factor VIII or IX (prophylaxis) from an early age has lessened its impact. Prophylaxis has now become the standard of care for children with hemophilia,2 but for those born in the preprophylaxis era or who demonstrate bleeding despite the regular infusion of factor concentrates, the treatment of established joint disease must include (alone or in combination) ongoing infusions of factor concentrate, radionuclide, or surgical synovectomy,3 or for those with end-stage arthropathy, chronic anti-inflammatory and pain management and/or joint replacement. Interestingly, it is well recognized that not all subjects with severe hemophilia will have joint bleeding and not all with joint bleeding demonstrate progressive joint disease. This paradox speaks to the role of factors other than the factor VIII/IX level and/or clinical bleeding as important to this process.
The hallmarks of hemophilic arthropathy involve joint bleeding, inflammation, synovial hypertrophy/villous formation, and cartilage/bony destruction; in essence, inflammation and cellular proliferation (angiogenesis). Iron deposition from joint bleeding, deep in synovial tissue, appears to be a key culprit. Iron has also correlated with increased c-myc expression,4 as well as overexpression of mdm2.5 The increased inflammatory response is a result of monocytes/macrophages recruited to the area along with accompanying inflammatory cytokines (interleukin-6 [IL-6], IL-1β, tumor necrosis factor α).6 The actual mediators of cellular proliferation and synovial hypertrophy are unknown, but evidence has partially implicated the previously mentioned cytokines and c-myc and mdm2 expression. In this issue of Blood, Acharya and colleagues provide evidence for the role of key angiogenic factors (vascular endothelial growth factor [VEGF], matrix metalloproteinase-9) in cellular proliferation.1
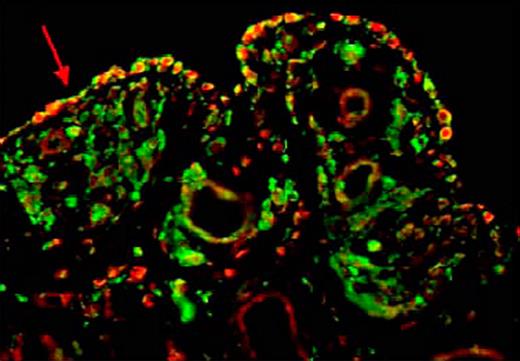
Several key experiments were performed to support the authors' hypothesis that angiogenesis plays a major role in the pathogenesis of hemophilic arthropathy. First, proangiogenic growth factors (previously mentioned) and monocytes/macrophages (CD68+, CD11b+) were both significantly elevated in synovium and peripheral blood of hemophilic patients with joint disease compared with controls. Second, sera and peripheral blood monocytes from subjects with hemophilia caused endothelial cell and synovial cell proliferation, and these proliferative responses were down-regulated by blocking the effect of VEGF. Finally, the potential role of hypoxia in this angiogenic process was suggested by expression of hypoxia-inducible factor 1α from human synovial cells when incubated with sera from subjects with hemophilic arthropathy. The figure is illustrative of the pivotal role of monocytes and key angiogenic factors in synovial hypertrophy (see figure). It demonstrates (red arrow) by immunofluorescent staining, CD68+ cells coexpressing VEGF in the synovium of a subject with hemophilic arthropathy.
It is not surprising that angiogenesis plays a key role in hemophilic arthropathy because neovascularization is crucial to tumor growth and rheumatoid arthritis progression. Could elevated levels of angiogenic markers such VEGF be a marker of hemophilic arthropathy? Nonetheless, despite the promise of this investigation, the exact mechanism underlying hemophilic joint disease remains unknown and although the contributions of iron deposition, inflammatory cell recruitment, and cytokine/growth factor production to hemophilic joint disease have individually been demonstrated, no linchpin for this process has been demonstrated. Collection of cytokine and growth factor samples from subjects on prophylaxis and with joint disease should be considered as a first step to more clearly define the etiology of hemophilic arthropathy. Until more data are obtained, VEGF and other angiogenic mediators remain a cog in this complicated pathway.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal