Abstract
The mechanisms by which megakaryocytes (MKs) differentiate and release platelets into the circulation are not well understood. However, growing evidence indicates that a complex regulatory mechanism involving MK-matrix interactions may contribute to the quiescent or permissive microenvironment related to platelet release within bone marrow. To address this hypothesis, in this study we demonstrate that human MKs express and synthesize cellular fibronectin (cFN) and transglutaminase factor XIII-A (FXIII-A). We proposed that these 2 molecules are involved in a new regulatory mechanism of MK-type I collagen interaction in the osteoblastic niche. In particular, we demonstrate that MK adhesion to type I collagen promotes MK spreading and inhibits pro-platelet formation through the release and relocation to the plasma membrane of cFN. This regulatory mechanism is dependent on the engagement of FN receptors at the MK plasma membrane and on transglutaminase FXIII-A activity. Consistently, the same mechanism regulated the assembly of plasma FN (pFN) by adherent MKs to type I collagen. In conclusion, our data extend the knowledge of the mechanisms that regulate MK-matrix interactions within the bone marrow environment and could serve as an important step for inquiring into the origins of diseases such as myelofibrosis and congenital thrombocytopenias that are still poorly understood.
Introduction
Hemopoietic stem cells reside in bone marrow–specialized niches that dictate how they differentiate, proliferate, mature, and enter the peripheral circulation.1-4 Megakaryocyte (MK) maturation and platelet generation are consequent to MK migration from the osteoblastic to the vascular niche, where MKs extend pro-platelets and newly generated platelets are released into the bloodstream.5,6
The characteristics of the microenvironment surrounding MKs play an important role in the regulation of platelet production within the bone marrow.3,7 In particular, the interaction of MKs with different extracellular matrices (ECMs) that fill the bone marrow spaces seems to orchestrate their maturation in specific sites.8 It has been demonstrated that interactions of primary human MKs with matrices thought to fill the vascular niche, such as fibrinogen or von Willebrand factor, are able to sustain MK maturation and pro-platelets, whereas type I collagen totally suppresses these events and prevents premature platelet release in the osteoblastic niche.7,9 The negative regulation of pro-platelets by type I collagen is mediated by the interaction with the integrin α2β1 and involves the Rho/ROCK pathway.10,11 However, the exact sequence of events that determines the interaction of MKs with the ECM, and therefore their regulation, is not completely understood.12 Recent studies13 have demonstrated that the encounter between a cell and an adhesive substrate involves an initial passive interaction characterized by cell adhesion and spreading, followed by an active stage that involves actin polymerization and myosin contraction triggered by the activation of a complex cascade of signaling events. Interestingly, nothing is known about the mechanisms that regulate the dynamics of the MK-ECM interaction within the bone marrow environment.
Recent studies have demonstrated that fibronectin (FN) may represent a new regulator of MK maturation and platelet release.14-16 FNs are produced from a single gene, but alternative splicing of their pre-mRNA leads to the creation of several isoforms.17,18 The amino acid sequence variations are the consequence of the alternative processing of a single primary transcript at 3 conserved sites: extra domain B (EDB) or extra type III homology B (also called EIII-B), extra domain A (EDA or EIII-A), and a type III homology connecting segment (IIICS; the V region in the rat). EDA and EDB are single exons coding for single type III repeats that are included or excluded from the FN mRNA by exon skipping.19 The EDB FN isoform protein has been detected in guinea pig20 and human21 MKs, but its function remains poorly understood.
FN and type I collagen are cross-linked at a cellular site by activated transglutaminases (TGs). The TG family consists of 9 known members, of which at least 3 are expressed in the vascular system: type 1 TG, type 2 TG, and factor XIII.22 Recent data suggest that TGs play a role in several other processes such as cell differentiation,23 adhesion,24 and migration.25 In platelets, TG activity depends on the presence of FXIII-A, and a significant reduction of soluble fibrinogen binding in response to thrombin receptor agonist peptide was observed in FXIII-deficient platelets.26 In contrast, platelet FXIII-A has been proposed to negatively regulate αIIbβ3 integrin function in collagen-adherent platelets.27
In this study, we show for the first time that human MKs express and synthesize cellular FN (cFN). Therefore, we propose that MK adhesion to type I collagen prevents pro-platelets and sustains MK spreading through a mechanism that involves FN, membrane receptors, and FXIII-A TG activity. This mechanism seems to be mediated by the exposure of cFN to the cell membrane and to be maintained by FN polymerization catalyzed by FXIII-A. These data address a new role for FN that, upon specific activation, could be released and thereby modulate MK interaction with ECMs. In this context, FXIII-A catalyzed FN cross-linking at cellular sites stabilizes FN assembly and promotes the organization of ECMs. These experiments open new insight into an understanding of the role of force development and cell spreading on the MK-ECM interaction, which are still poorly understood.
Methods
Reagents and antibodies
Type I collagen and human plasma FN (pFN) were purified as described previously.28,45 Human plasma fibrinogen was from Calbiochem, human factor XIII was from Haematologic Technologies, and biotin-cadaverine was from Anaspec. Iodoacetamide (IAA), cystamine (CYS), monodansylcadaverine (MDC), thrombin from human plasma, blebbistatin, and fluorescein isothiocyanate (FITC) were from Sigma-Aldrich. The following antibodies were used: rabbit polyclonal anti–plasmatic and cFN (a kind gift of L.V.28 ); mouse monoclonal anti–FN (clone IST-4), mouse anti–EDA+ FN (clone FN-3E2), phalloidin-TRITC, mouse anti–β-actin (clone AC-15), mouse anti–α-tubulin (clone DM1A), and FITC-anti–mouse immunoglobulin M (IgM; Sigma-Aldrich); mouse monoclonal anti–factor XIIIA Ab-1 (clone AC-1A1; LabVision); mouse monoclonal anti–CD49b (clone HAS-4; Abcam); mouse monoclonal anti–CD49e (clone SAM-1), anti–CD49d (clone P1H4) were from Chemicon/Millipore, anti–CD49e (clone IIA1; BD Bioscience); mouse monoclonal anti–CD61 (clone SZ21; Immunotech); Alexa Fluor–conjugated antibodies (Invitrogen); and colloidal gold–conjugated goat antibody to rabbit IgG with a 40-nm particle size (EY Laboratories).
Human cord blood–derived MKs
Human cord blood was collected following normal pregnancies and deliveries upon informed consent of the parents, in accordance with the ethical committee of the IRCCS Policlinico San Matteo Foundation and the principles of the Declaration of Helsinki. CD34+ cells were separated and cultured as described previously.7,29
Evaluation of pro-platelets and cell spreading in adhesion to different matrices
To analyze pro-platelets onto different adhesive substrates, 12-mm glass coverslips were coated with fibrinogen or type I collagen as described previously.7 Cells at day 13 of culture were harvested and allowed to adhere for 3 or 16 hours (1 × 105 cells/well) at 37°C and 5% CO2. Cell spreading was determined as described previously.7 In some experiments, MKs were harvested and pre-incubated with specific inhibitors or antibodies for 30 minutes at 37°C used at the following final concentrations: 10μM IAA, 10μM CYS, 0.1mM MDC, 10 μg/mL anti–CD49d, 10 μg/mL anti–CD49e (clone IIA1), 1mM RGDS, and 50μM blebbistatin.
Immunofluorescence and confocal microscopy analysis
Preparation of samples for the analysis of subcellular localization of cFN in activated MKs was performed as described previously.29 MKs were stained with rabbit polyclonal anti–FN antibody (10 μg/mL) and goat anti–rabbit secondary antibody, while the distributions of CD49b and CD49e were analyzed with anti–CD49b (clone HAS-4) and anti–CD49e (clone SAM-1) both diluted 1:100. FXIII-A was stained in nonpermeabilized cells using antibody clone AC-1A1 diluted 1:100. MKs extending pro-platelets were stained with a mouse monoclonal anti–α-tubulin diluted 1:700.
Analysis by conventional fluorescence and confocal microscopy were performed with an Olympus BX51 microscope using a 63×/1.25 or a 100×/1.30 UplanF1 oil-immersion objective and a TCS SPII confocal laser scanning microscopy system (Leica) equipped with a DM IRBE inverted microscope (Leica), as described previously.7,29
RT-PCR and quantitative RT-PCR
CD61+ MKs at day 13 of culture were separated using the immunomagnetic beads technique (Miltenyi Biotec), and total cellular RNA was extracted using the Mammalian GeneElute Total RNA Kit (Sigma-Aldrich) and reverse transcribed to cDNA using MuLV reverse transcriptase (Applera) following the manufacturer's instructions.
For reverse-transcription polymerase chain reaction (RT-PCR) detection of FN and its splice variants EDA+ and EDB+ FN, one-third of the resulting cDNA was amplified using the following primers: 5′ GGAGAGAGTCAGCCTCTGGTTCAG 3′ and 5′ TGTCCACTGGGCGCTCAGGCTTGTG 3′, which are located in the exons III-11 and III-12 flanking the EDA region and amplify both EDA+ FN and EDA− FN mRNA; 5′ CGGCCTGGAGTACAATGTCAGTGT 3′ and 5′ CAGGTGACACGCATGGTGTCTGGA 3′, which are located in the exons III-7 and III-8 flanking the EDB region and amplify both EDB+ FN and EDB− FN mRNA; 5′ GCCTGGTACAGAATATGTAGTG 3′ and 5′ ATCCCAGCTGATCAGTAGGCTGGTG 3′, which amplify a FN region (III-9) that does not undergo alternative splicing and is common to all FN mRNA.30 The amplification reaction was performed in 25 μL using an Applied Biosystems GeneAmp 9700 thermocycler, and 20-μL PCR products were electrophoresed in a 2% agarose gel stained with ethidium bromide.
For TG expression, 1/10 of the resulting cDNA was amplified using the following set of primers: TGM1 (NM_000359) 5′ CCATGATTCTGTCTGGAACT 3′ and 5′ CACAGAGACCATCAGATCCT 3′, TGM2 (NM_004613.2) 5′ ATGAGAAATACCGTGACTGC 3′ and 5′ GGCATATTTTGCTCACTAGC 3′, TGM3 (NM_003245.3) 5′ GGATAGCGTCTTTATGGGTA 3′ and 5′ GAGATTTCGGTCTGTGTCAT 3′, FXIIIA1 (NM_001994) 5′ AAGGTGTCATGAAATCAAGG 3′ and 5′ TGGTCTTGTTACTCCAGGAC 3′, FN1 (NM_212482.1) 5′ GAACTATGATGCCGACCAGAA 3′ and 5′ CTGATCTCCAATGCGGTACAT 3′, β-actin (NM_001101) 5′ TCCTGTGGCATCCACGAAACT 3′ and 5′GAAGCATTTGCGGTGGACGAT 3′. Twenty microliters of the PCR products were loaded on ethidium bromide–stained 1% agarose gel.
For quantitative expression analysis of TGs, real-time PCR was performed using the Rotorgene 6000 (Eurogentec), and 1/20 of the resulting cDNA was amplified in triplicate using the MESA GREEN qPCR MasterMix Plus for SYBR assay no ROX sample (Eurogentec). Rotorgene 6000 series software Version 1.7 was used for the comparative concentration analysis.
Western immunoblotting
Cell-culture supernatants were collected and centrifuged for 10 minutes at 17 800g to pellet-floating cells. Adherent MKs were washed with phosphate-buffered saline (PBS) and lysed with radioimmunoprecipitation assay buffer 1× (PBS with Nonidet P-40 2%, sodium deoxycholate 1%, sodium dodecyl sulfate [SDS] 0.2%, and protease inhibitors). Protein concentrations of both the supernatant and cellular fractions were determined using the bicinchoninic acid assay (Pierce). Equal amounts of proteins were then used for SDS-polyacrylamide gel electrophoresis (SDS-PAGE): secreted FN was assessed in the suspension medium by immunoprecipitation and visualized in a 7.5% reduced gel with the antibody anti–FN (clone IST-4), diluted 1:1000, and detected by a horseradish peroxidase–labeled secondary antibody using enhanced chemiluminescence (Pierce). Proteins from adherent MKs were separated by SDS-PAGE and immunostained with the antibody anti–β actin diluted 1:10 000.
Flow cytometry
For DNA content analysis, low-ploidy MKs at day 9 of culture were split and incubated with 25 μg/mL type I collagen, fibrinogen, or PBS as a control and reseeded for another 4 days in fresh medium. At the end of culture, cells were harvested and fixed with cold ethanol 70% and frozen overnight at −20°C. Subsequently, cells were stained with an FITC-conjugated antibody against human CD41 (clone HIP8; BioLegend) for 30 minutes on ice in the dark with 50 μg/mL propidium iodide (Sigma) supplemented with 100 μg/mL RNAse (Sigma) for 30 minutes at 37°C. The ploidy distribution in the CD41+ population was determined by 2-color flow cytometry (FACSCalibur flow cytometer; BD Biosciences). A minimum of 20 000 events were collected in the CD41+ gate. Offline data analysis was performed using FCS Express Version 3.0 software (DeNovo Software).
Pull-down assay of GTP-loaded Rho
RhoA activity assay was performed using a Rho activation assay kit (Upstate Biotechnology) following the manufacturer's instructions. In parallel, the total amount of Rho was determined by immunoblotting.
Assembly of FN by adherent MKs under static conditions
MKs were incubated for 30 minutes at 37°C with 25 μg/mL FITC-labeled human pFN, seeded for an additional 16 hours on matrix-coated coverslips, and immunofluorescence microscopy was performed as described in “Immunofluorescence and confocal microscopy analysis.”
Deoxycholic acid extraction of fibrillar deposition of FN
MKs were plated on different matrix-coated well plates for 16 hours in the presence of 25 μg/mL human pFN (pFN) at 37°C. Conditioned medium was then removed and the matrix was solubilized using 200 μL of deoxycholic acid (DOC) lysis buffer (PBS, DOC 0.1%, EDTA [ethylenediaminetetraacetic acid] 2mM). After centrifugation, the DOC-insoluble pellet was solubilized in 25 μL of 2% SDS, 20mM Tris-HCl, pH 8.8, 2mM phenylmethanesulfonyl fluoride, 2mM IAA, 2mM N-ethylmaleimide, and 2 mM EDTA. Equal volumes of DOC-insoluble samples were analyzed by SDS-PAGE using 5% polyacrylamide gels. Samples were immunoblotted and incubated with IST-4 anti–FN monoclonal antibody. To ensure the same number of cells in the experiments, in a parallel sample, cells were scraped and proteins separated by SDS-PAGE and then incubated with anti–β actin antibody. In some experiments, the DOC-insoluble matrix was visualized by immunofluorescence using the rabbit polyclonal anti–fibronectin antibody followed by an appropriate secondary antibody.
TG activity associated with the MK cell surface
TG activity was measured by the incorporation of biotinylated cadaverine into fibronectin. For this assay, 96-well plates were precoated with human pFN (5 μg/well) and cells were then plated at a density of 2 × 105 cells/mL in the presence of 0.1mM biotinylated cadaverine. Cells were allowed to adhere for 16 hours at 37°C. As a negative control, pFN-coated 96-well plates were incubated with medium containing 0.1mM biotinylated cadaverine alone. In parallel samples, cells were pretreated with TG-activity inhibitors such as IAA and CYS 10μM for 15 minutes. The detergent solution consisting of 0.1% DOC in PBS was then added to each well and incubated for 20 minutes. The supernatant was discarded, and the remaining FN layer washed 3 times with Tris-HCl. The reaction was blocked with 3% bovine serum albumin in Tris-HCl, and the incorporated biotinylated cadaverine was revealed with a 1:5000 dilution of peroxidase conjugate avidin after 1 hour of incubation at 37°C. Then, 200 μL of substrate solution (a mixture of hydrogen peroxide and tetramethylbenzidine) was added for 20 minutes at room temperature. Color development was stopped by adding 50 μL of stop solution, and optical density was measured with an enzyme-linked immunosorbent assay plate reader (Bio-Rad) set at 450 nm.
Intracellular TG activity in human MKs
TG activity was measured using the method of Jayo et al.26 Briefly, 1 × 106 MKs were lysed on ice for 20 minutes in 10mM Tris-HCl (pH 7.4) containing 0.1% Triton X-100, 140mM NaCl, 0.4mM phenylmethanesulfonyl fluoride, and 1mM dithiothreitol. After clearing by centrifugation, 10 μg of proteins were incubated with 400μM biotinylated cadaverine with or without 5mM EDTA and dimethylsulfoxide as negative controls. After 1 hour at 37°C, the reaction was stopped by adding loading buffer, then resolved in 10% SDS-PAGE under reducing conditions and transferred to polyvinylidene fluoride membranes. The membranes were incubated with peroxidase-conjugated avidin (1:5000 dilution) and visualized using the enhanced chemiluminescence system. To analyze the effect of thrombopoietin (TPO) on TG activity, cells were seeded in the presence of different concentrations of TPO (0, 50, 100 ng/mL) for 2 hours prior to TG activity analysis.
Scanning electron microscopy and immunogold staining
Samples were fixed with 2.5% glutaraldehyde solution in 0.1M sodium-cacodylate buffer for 1 hour at 4°C. Immunogold staining of FN was performed by incubating the cells for 2 hours with a polyclonal anti–FN antibody, followed by 1 hour of incubation with a goat anti–rabbit secondary antibody conjugated with 40-nm gold beads, and then dehydrated at room temperature in a gradient ethanol series up to 100%. Negative controls were performed by omitting the primary antibody. The specimens were mounted on aluminum stubs, sputter-coated with gold, and observed with a Cambridge Stereoscan 440 microscope (Leica Microsystems) at 17.5 kV and a magnification of 5000×.
Statistics
The t test was used to analyze data, with a significant difference set at P < .05. Data are presented as means ± SD of at least 3 different experiments.
Results
Human MKs express and synthesize cFN
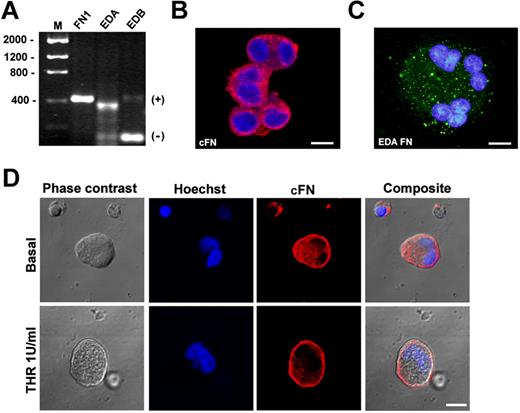
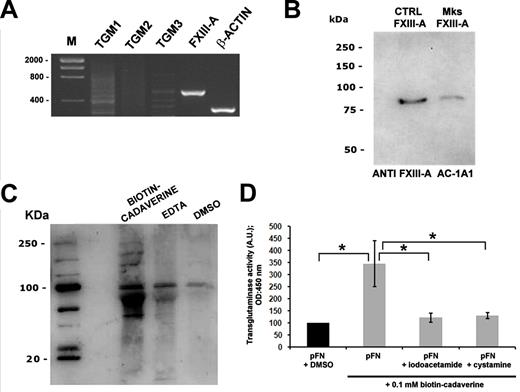
MKs were derived from human umbilical cord blood progenitor cells, and the expressions of cFN and the 2 isoforms, EDA and EDB, were analyzed by RT-PCR. As shown in Figure 1A, EDA FN RT-PCR revealed 374- and 104-bp amplication products corresponding, respectively, to EDA+ FN and the EDA-spliced FN mRNA sequence. EDB RT-PCR revealed 413- and 143-bp products corresponding to the EDB+ FN and spliced EDB isoform, and RT-PCR for total FN (FN1) revealed a 419-bp product.30 Further, cFN was also detected in MK cytoplasm using a polyclonal antibody created in our laboratory28 (Figure 1B) and a specific antibody against the EDA isoform (clone 3E2; Figure 1C). Finally, cFN relocated to the MK membrane upon thrombin stimulation (Figure 1D). Overall, these results demonstrated that cFN was expressed by human MKs with a prevalence of the EDA+ isoform and was actively involved in MK activation.
Human MKs express cFN isoforms. CD61+ MKs at 13 days of culture were separated using the immunomagnetic beads technique and total cellular RNA was extracted. (A) RT-PCR demonstrated expression of cFN (FN1 gene), the EDA+ FN and EDA− spliced FN mRNA sequence, and the EDB+ FN and spliced EDB− isoform. (B) MKs were permeabilized and stained in immunofluorescent dye with a polyclonal antibody against cFN (red) and (C) a monoclonal antibody against the EDA isoform (green). Scale bars = 20 μm and 10 μm, respectively. (D) cFN relocated to the MK plasma membrane upon activation with thrombin (1 U/mL; top panels) or omitting thrombin (bottom panels), as revealed by immunofluorescence with polyclonal antibody against cFN (red). Nuclei were stained with Hoechst 33288 (blue). Scale bar = 10 μm.
Human MKs express cFN isoforms. CD61+ MKs at 13 days of culture were separated using the immunomagnetic beads technique and total cellular RNA was extracted. (A) RT-PCR demonstrated expression of cFN (FN1 gene), the EDA+ FN and EDA− spliced FN mRNA sequence, and the EDB+ FN and spliced EDB− isoform. (B) MKs were permeabilized and stained in immunofluorescent dye with a polyclonal antibody against cFN (red) and (C) a monoclonal antibody against the EDA isoform (green). Scale bars = 20 μm and 10 μm, respectively. (D) cFN relocated to the MK plasma membrane upon activation with thrombin (1 U/mL; top panels) or omitting thrombin (bottom panels), as revealed by immunofluorescence with polyclonal antibody against cFN (red). Nuclei were stained with Hoechst 33288 (blue). Scale bar = 10 μm.
cFN differently modulates MK adhesion to bone marrow ECMs
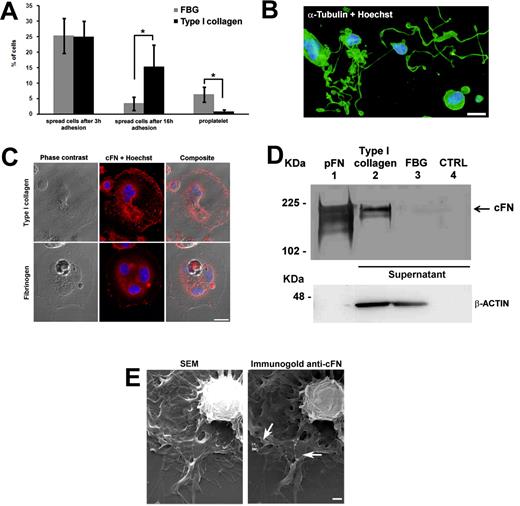
To analyze the role of cFN in regulating the MK-ECM interaction, mature MKs were plated on 2 different bone marrow ECMs: type I collagen, known to inhibit MK maturation and pro-platelets11 through the Rho-ROCK pathway, or fibrinogen, known to support MK maturation and pro-platelets.3 After 3 hours of incubation, approximately 25% of MKs were spread on both ECMs (Figure 2A); however, after prolonging incubation to 16 hours, MKs on fibrinogen returned round and started to extend pro-platelets (Figure 2B), while MKs on type I collagen remained spread and did not proceed on maturation (Figure 2A). To evaluate the effect of these ECMs on MK differentiation, low-ploidy MKs at day 9 of culture were also cultured with type I collagen or fibrinogen for 4 days, but no significant variation in ploidy level was observed (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To confirm the involvement of the α2β1 in type I collagen–dependent spreading, pull-down experiments were performed to evaluate the quantity of GTP-loaded Rho in MKs adherent to type I collagen using the GST-RBD (a fusion protein containing Rho-binding domain of Rhotekin), which selectively binds to the GTP-bound form of RhoA. Levels of GTP-bound RhoA increased upon adhesion of MKs to type I collagen with respect to MKs maintained in suspension (supplemental Figure 2).
cFN modulates MK adhesion on collagen type I, but not on fibrinogen. Mature MKs were differentiated from human cord blood CD34+ cells as described in “Methods.” Mature MKs were then incubated on type I collagen or fibrinogen-coated coverslips for 16 hours at 37°C in an atmosphere of 5% CO2 in the presence of TPO. (A) After 3 and 16 hours of incubation, coverslips were fixed and MK spreading and pro-platelets were evaluated as described in “Methods.” (B) Representative image of MK forming pro-platelets upon fibrinogen adhesion. Cells were stained with an anti–α-tubulin antibody (green) and Hoechst 33288 for nuclear staining (blue). Scale bar = 20 μm. (C) After 3 hours of incubation, coverslips were fixed, permeabilized, and stained with a polyclonal antibody against cFN. Subcellular localization of cFN (red) was analyzed by confocal microscopy. Scale bar = 10 μm. (D) Immunoprecipitation of released FN in supernatants harvested after 3 hours of MK adhesion to type I collagen (lane 2) or fibrinogen (lane 3). FN from human plasma was used as a positive control (lane 1), while unconditioned StemSpan medium was used as a negative control (lane 4). Actin was revealed by Western blotting in adherent cells on both matrices to ensure the same number of cells. (E) Scanning electron microscopy of MKs spread on type I collagen after 3 hours of incubation. Right panel, immunogold staining with polyclonal FN antibody. Arrows indicate FN exposure on the MK membrane. Scale bar = 1 μm. *P < .05
cFN modulates MK adhesion on collagen type I, but not on fibrinogen. Mature MKs were differentiated from human cord blood CD34+ cells as described in “Methods.” Mature MKs were then incubated on type I collagen or fibrinogen-coated coverslips for 16 hours at 37°C in an atmosphere of 5% CO2 in the presence of TPO. (A) After 3 and 16 hours of incubation, coverslips were fixed and MK spreading and pro-platelets were evaluated as described in “Methods.” (B) Representative image of MK forming pro-platelets upon fibrinogen adhesion. Cells were stained with an anti–α-tubulin antibody (green) and Hoechst 33288 for nuclear staining (blue). Scale bar = 20 μm. (C) After 3 hours of incubation, coverslips were fixed, permeabilized, and stained with a polyclonal antibody against cFN. Subcellular localization of cFN (red) was analyzed by confocal microscopy. Scale bar = 10 μm. (D) Immunoprecipitation of released FN in supernatants harvested after 3 hours of MK adhesion to type I collagen (lane 2) or fibrinogen (lane 3). FN from human plasma was used as a positive control (lane 1), while unconditioned StemSpan medium was used as a negative control (lane 4). Actin was revealed by Western blotting in adherent cells on both matrices to ensure the same number of cells. (E) Scanning electron microscopy of MKs spread on type I collagen after 3 hours of incubation. Right panel, immunogold staining with polyclonal FN antibody. Arrows indicate FN exposure on the MK membrane. Scale bar = 1 μm. *P < .05
Interestingly, upon adhesion to type I collagen, and not fibrinogen, relocation of cFN to the plasma membrane was observed after 3 hours of incubation and was maintained thereafter (Figure 2C). Moreover, upon MK adhesion to type I collagen, and not fibrinogen, cFN was also released in the supernatant, as revealed by Western blotting performed upon immunoprecipitation (Figure 2D). Finally, scanning electron microscopy analysis confirmed that after 3 hours of incubation, MKs on type I collagen started to accumulate cFN on the plasma membrane, as revealed by immunogold staining (Figure 2E).
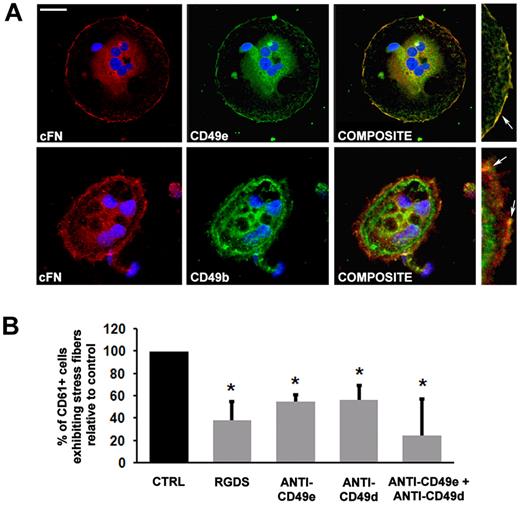
Codistribution analysis of the α5 (CD49e) and α2 (CD49b) subunits with cFN demonstrated a strict association between the α5 subunit with membranous cFN and, to a lesser extent, with the α2 subunit in MKs spread on type I collagen (Figure 3A). Further, MK spreading was evaluated on type I collagen upon incubation with the monoclonal antibodies anti–CD49e (clone SAM-1) or anti–CD49d (α4 subunit) (clone P1H4) or their combination. Both antibodies determined a significant decrease in MK spreading of approximately 50% with respect to control MKs incubated with a nonrelated IgG antibody, and this effect was amplified by the combination of antibodies (Figure 3B). Consistently, MK spreading was also decreased upon incubation with the RGDS peptide (Figure 3B).
Integrin engagement in MK adhesion to type I collagen. (A) Subcellular localization of cFN (red), CD49e (green, top panel), CD49b (green, bottom panel) in MK spread on type I collagen. Colocalization between cFN and the receptor components was analyzed by merging images obtained in green and red channels in each confocal optical section (composite). In the right panels, image magnification shows protein colocalization on the MK plasma membrane (yellow staining, arrows). All slides were also incubated with Hoechst 33288 for nuclear staining (blue). Scale bar = 10 μm. (B) Effect of RGDS, CD49e, and CD49d antibodies and their combination on MK spreading upon incubation for 16 hours. Results are reported as a percentage of inhibitor-treated MKs adherent to collagen type I compared with MKs treated with PBS alone, and are the means ± SD of 3 different experiments. *P < .05.
Integrin engagement in MK adhesion to type I collagen. (A) Subcellular localization of cFN (red), CD49e (green, top panel), CD49b (green, bottom panel) in MK spread on type I collagen. Colocalization between cFN and the receptor components was analyzed by merging images obtained in green and red channels in each confocal optical section (composite). In the right panels, image magnification shows protein colocalization on the MK plasma membrane (yellow staining, arrows). All slides were also incubated with Hoechst 33288 for nuclear staining (blue). Scale bar = 10 μm. (B) Effect of RGDS, CD49e, and CD49d antibodies and their combination on MK spreading upon incubation for 16 hours. Results are reported as a percentage of inhibitor-treated MKs adherent to collagen type I compared with MKs treated with PBS alone, and are the means ± SD of 3 different experiments. *P < .05.
Fibronectin assembly is dependent on MK interaction with type I collagen
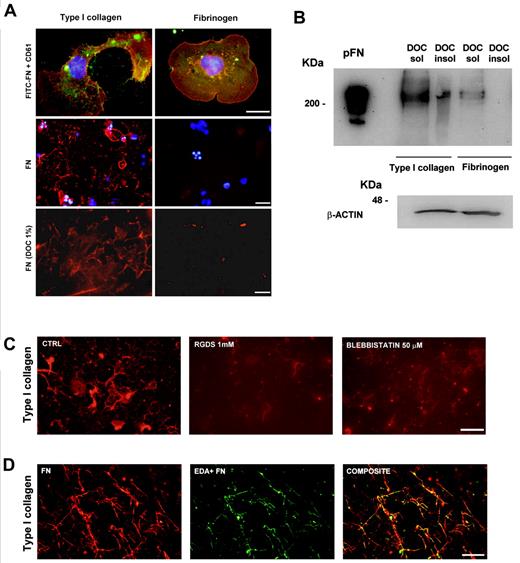
Because relocation on the plasma membrane and secretion of cFN were dependent on the interaction of MKs with type I collagen, the ability of MKs to organize pFN in an insoluble matrix was also explored. Mature MKs were plated either on type I collagen or fibrinogen and allowed to adhere for 16 hours before incubation with FITC-labeled FN. As observed for cFN, only MKs plated on type I collagen, and not on fibrinogen, assembled FITC-FN, as revealed by immunofluorescence analysis (Figure 4A). Moreover, analysis of pFN assembly after DOC treatment revealed the presence of fibrillar pFN only when MKs were plated on type I collagen (Figure 4A). Consistently, immunoblot analysis revealed the presence of fibrillar pFN in the DOC-insoluble fraction in MK-type I collagen, and not in MK-fibrinogen cocultures, while pFN was present in the soluble fractions of both cocultures (Figure 4B).
FN assembly by MKs adherent to type I collagen. The ability of MKs to assembly pFN was evaluated on type I collagen and fibrinogen, as described in “Methods.” (A) Assembly of FITC-labeled FN (green) by CD61+ MKs (red) adherent on type I collagen and fibrinogen. Scale bars = 10 μm (top), 50 μm (middle) and 30 μm (bottom). In parallel experiments, staining of fibrillar pFN with a polyclonal antibody in MKs plated on type I collagen and fibrinogen. MKs adherent on type I collagen and fibrinogen were removed after DOC treatment, and DOC-insoluble pFN fibrils were stained with a polyclonal antibody anti–FN (red). Nuclei were always counterstained with Hoechst 33288 (blue). (B) The presence of DOC-insoluble assembly of pFN was also revealed by immunoblot analysis using a monoclonal anti–FN, clone IST-4. In a parallel sample, cells were scraped and proteins separated by SDS-PAGE and then stained with anti–β actin to ensure the same number of cells in the experiment. (C) Evaluation of cytoskeleton and integrin roles in FN matrix assembly by MKs in samples treated with RGDS or blebbistatin with respect to controls. FN matrix after DOC treatment was stained with the polyclonal antibody anti–FN and visualized in immunofluorescent dye. (D) The presence of endogenous EDA+ FN during pFN deposition and assembly was evaluated with immunofluorescent dye, and staining was performed using both the polyclonal antibody against FN and monoclonal anti–EDA FN. Scale bars = 20 μm (C-D).
FN assembly by MKs adherent to type I collagen. The ability of MKs to assembly pFN was evaluated on type I collagen and fibrinogen, as described in “Methods.” (A) Assembly of FITC-labeled FN (green) by CD61+ MKs (red) adherent on type I collagen and fibrinogen. Scale bars = 10 μm (top), 50 μm (middle) and 30 μm (bottom). In parallel experiments, staining of fibrillar pFN with a polyclonal antibody in MKs plated on type I collagen and fibrinogen. MKs adherent on type I collagen and fibrinogen were removed after DOC treatment, and DOC-insoluble pFN fibrils were stained with a polyclonal antibody anti–FN (red). Nuclei were always counterstained with Hoechst 33288 (blue). (B) The presence of DOC-insoluble assembly of pFN was also revealed by immunoblot analysis using a monoclonal anti–FN, clone IST-4. In a parallel sample, cells were scraped and proteins separated by SDS-PAGE and then stained with anti–β actin to ensure the same number of cells in the experiment. (C) Evaluation of cytoskeleton and integrin roles in FN matrix assembly by MKs in samples treated with RGDS or blebbistatin with respect to controls. FN matrix after DOC treatment was stained with the polyclonal antibody anti–FN and visualized in immunofluorescent dye. (D) The presence of endogenous EDA+ FN during pFN deposition and assembly was evaluated with immunofluorescent dye, and staining was performed using both the polyclonal antibody against FN and monoclonal anti–EDA FN. Scale bars = 20 μm (C-D).
To demonstrate that FN assembly was strictly dependent on integrin binding and cell contractility, in some experiments, pFN was added to MK cultures on type I collagen, as described previously, upon either incubation with RGDS, a synthetic peptide containing the RGD sequence that inhibits integrin-related functions, or treatment with 50μM blebbistatin, a selective antagonist of myosin IIA ATPase activity. Figure 4C shows the decrease of matrix pFN assembly in the presence of both RGDS and blebbistatin with respect to controls.
Finally, to verify the incorporation of endogenous EDA+ FN during pFN assembly, DOC insoluble matrix was stained with both the antibody against the EDA isoform and the polyclonal antibody against FN. Figure 4D shows that the pFN and cFN EDA+ isoforms colocalized in the FN matrix assembly.
Expression and activity of TGs in human MKs
To search for possible mechanisms underlying matrix FN assembly by MKs, TG expression and localization were analyzed. Of the most well-characterized members of the TG family, only FXIII-A resulted in highly expressed by MKs, as verified by RT-PCR (Figure 5A) and quantitative RT-PCR. Quantification of different TG mRNA levels by quantitative RT-PCR demonstrated that type 1 TG (TG1) and type 2 TG (TG2) expressions were barely detected, type 3 TG (TG3) expression was not detected, and FXIII-A was easily amplified (data not shown). Consistently, FXIII-A was also detected at the protein level by Western blot analysis using FXIII-A from human plasma as a control (Figure 5B). Intracellular TG activity of FXIII-A in MKs was demonstrated by the incorporation of biotinylated cadaverine, a small-molecule amine-donor TG substrate, into different proteins in MK lysate using the method of Jayo et al.26 This activity was completely reverted upon calcium sequestering with EDTA and completely absent in the control dimethylsulfoxide (Figure 5C). Interestingly, the TPO concentration was inversely associated with FXIII-A activity, because a reduction of biotinylated proteins was observed when MKs were incubated for 2 hours with increased TPO concentrations (supplemental Figure 3).
MKs express and synthesize FXIII-A. (A) RT-PCR analysis of different TGs by human MKs. The amplification products were resolved on agarose gel and visualized by ethidium bromide staining. Actin amplification was used as a control. (B) Western blot analysis of FXIII-A in human MKs. FXIII from human plasma was used as a positive control. (C) Analysis of intracellular TG activity of FXIII in human MKs. MK lysates were incubated with biotinylated cadaverine in the absence or presence of EDTA or Ca2+, resolved by SDS-PAGE, and blotted with peroxidase-conjugated avidin. TG activity was completely reverted upon calcium sequestering with EDTA. (D) Extracellular TG activity was demonstrated by ELISA assay of cadaverine incorporation into pFN upon adhesion of MKs for 16 hours and DOC treatment. TG activity was significantly reduced by incubation with TG inhibitors such as IAA or CYS (10μM each). *P < .05.
MKs express and synthesize FXIII-A. (A) RT-PCR analysis of different TGs by human MKs. The amplification products were resolved on agarose gel and visualized by ethidium bromide staining. Actin amplification was used as a control. (B) Western blot analysis of FXIII-A in human MKs. FXIII from human plasma was used as a positive control. (C) Analysis of intracellular TG activity of FXIII in human MKs. MK lysates were incubated with biotinylated cadaverine in the absence or presence of EDTA or Ca2+, resolved by SDS-PAGE, and blotted with peroxidase-conjugated avidin. TG activity was completely reverted upon calcium sequestering with EDTA. (D) Extracellular TG activity was demonstrated by ELISA assay of cadaverine incorporation into pFN upon adhesion of MKs for 16 hours and DOC treatment. TG activity was significantly reduced by incubation with TG inhibitors such as IAA or CYS (10μM each). *P < .05.
Further, cell surface–associated TG activity, presumably due to FXIII-A, was shown by the incorporation of biotinylated cadaverine into a layer of FN and detected by ELISA assay upon adhesion of MKs for 16 hours on FN (Figure 5D). Decreased FXIII-A activity was shown upon incubation with TG inhibitors such as IAA and CYS (Figure 5D).
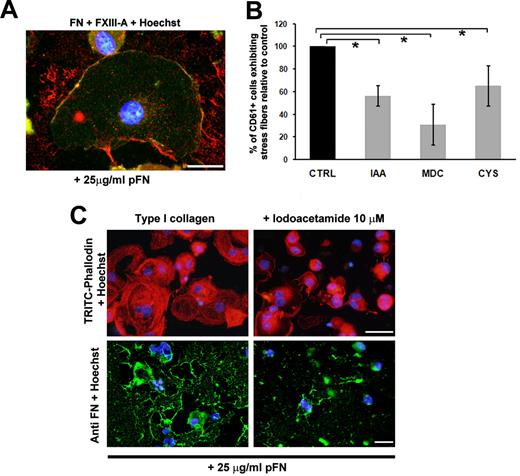
Function and localization of FXIII-A in human MKs
To demonstrate the FN-FXIII-A interaction during the assembly of exogenous FN, codistribution of pFN and FXIII-A was analyzed. As shown in Figure 6A, pFN-FXIII-A colocalized on the plasma membrane of spread MKs on type I collagen after incubation for 16 hours (Figure 6A). To better understand the role of FXIII-A in regulating MK function, MK spreading on type I collagen was evaluated upon 16 hours of incubation with TG inhibitors such as IAA, CYS, and MDC. As shown in Figure 6B, TG inhibitors revealed a significant decrease in MK spreading on type I collagen, demonstrating the fundamental role of FXIII-A in stabilizing the MK cytoskeleton and in moderating the MK-type I collagen interaction. Consistently, in the presence of IAA, MKs presented an evident reduction in actin stress fiber formation and lost their ability to assemble pFN with respect to the controls, as revealed by immunofluorescence analysis (Figure 6C).
FXIIIa modulates MK spreading and FN assembly on type I collagen. (A) Colocalization during FN fibrillogenesis of FXIII-A (green) and pFN (red) at the membrane level of MKs adherent on type I collagen. Scale bar = 10 μm. (B) MK spreading on type I collagen was modulated by TG activity: analysis of MK CD61+ exhibiting stress fibers in the presence of TG inhibitors such as IAA, MDC, and CYS. Results are reported as a percentage of inhibitor-treated MKs adherent to collagen type I compared with MKs treated with PBS alone, and are the means ± SD of 3 different experiments. (C) Effects of IAA treatment on MK spreading and FITC-labeled FN assembly on type I collagen. Cells were stained with tetramethyl rhodamine isothiocyanate–phalloidin and incubated with 25 μg/mL FITC-FN and immunofluorescent dye as described in “Immunofluorescence and confocal microscopy analysis.” Nuclei were counterstained with Hoechst 33288 (blue). Scale bar = 50 μm. *P < .05
FXIIIa modulates MK spreading and FN assembly on type I collagen. (A) Colocalization during FN fibrillogenesis of FXIII-A (green) and pFN (red) at the membrane level of MKs adherent on type I collagen. Scale bar = 10 μm. (B) MK spreading on type I collagen was modulated by TG activity: analysis of MK CD61+ exhibiting stress fibers in the presence of TG inhibitors such as IAA, MDC, and CYS. Results are reported as a percentage of inhibitor-treated MKs adherent to collagen type I compared with MKs treated with PBS alone, and are the means ± SD of 3 different experiments. (C) Effects of IAA treatment on MK spreading and FITC-labeled FN assembly on type I collagen. Cells were stained with tetramethyl rhodamine isothiocyanate–phalloidin and incubated with 25 μg/mL FITC-FN and immunofluorescent dye as described in “Immunofluorescence and confocal microscopy analysis.” Nuclei were counterstained with Hoechst 33288 (blue). Scale bar = 50 μm. *P < .05
Discussion
The characteristics of the bone marrow environment play an important role in the regulation of MK development and pro-platelet formation. Type I collagen is known to inhibit pro-platelet extension through the engagement of the integrin α2β19,12 and activation of the Rho/ROCK signaling cascade with the assistance of myosin IIa.10,11 Despite advances in knowledge about the biochemical niche, little is known about the complex and dynamic regulation of MK interactions with the bone marrow–matrix environment.31 Demonstrating the expression of cFN and FXIII-A by human MKs, we propose a new, more complex mechanism underlying the MK-type I collagen interaction in the osteoblastic niche (supplemental Figure 4).
In a previous study, Schick et al20 showed that guinea pig MKs synthesize an EDB FN isoform protein that is relocated to the MK membrane in response to thrombin. Therefore, the investigators assumed that the redistribution of EDB FN on the MK surface may modulate the MK-matrix interaction. In the present study, we extend these observations by focusing on the expression and function of the cFN isoforms in human MKs. We demonstrate for the first time that human MKs express cFN with a prevalence of the EDA isoform. Further, exposure on the MK plasma membrane of cFN was shown to occur only upon MK adhesion on type I collagen and not on fibrinogen. Moreover, relocation of cFN on the MK plasma membrane seemed to be related to a specific MK effect on type I collagen; in fact, cFN was detected only on the plasma membrane of MKs spread on type I collagen. Interestingly, MK spreading on fibrinogen was not accompanied by cFN exposure on the plasma membrane and resulted in a shortened process that was replaced by pro-platelets in 16 hours. Consistent with this, a recent study13 has described cell spreading as a dynamic process that leads to the generation of forces regulated by a complex cascade of signaling events. Therefore, these results may account for the mechanism by which cFN promotes the anchoring of MKs to type I collagen, which results in the activation of biochemical signaling and the generation of contractile force that maintains MK spreading over time. Further confirmation of these data was achieved by the evidence that only MKs on type I collagen were able to release cFN that bound to its integrin receptors on spread MK plasma membrane. Finally, the integrins α5β1 and α4β1 played a fundamental role as regulators of MK function on type I collagen, because their inhibition with specific antibodies or incubation with the RGDS peptide led to a significant reduction of MK spreading. These results suggest a new role for cFN as a modulator of biochemical and mechanical signaling underlying the MK-type I collagen interaction.
In addition to the cellular form, FN exists in a soluble form in the blood plasma (pFN) that is known to enhance platelet thrombus formation on type I collagen.32 Further, several studies have demonstrated that fibroblasts, endothelial cells, and vascular smooth muscle cells secrete, bind, and assemble FN into fibrils in the ECM.33 Interestingly, FN fibrillogenesis is initiated by the binding of the integrin α5β1 to FN on the plasma membrane,34,35 while FN fibril elongation is dependent on interactions with type I collagen.36 Further, FN fibrillogenesis is dependent on cell cytoskeleton reorganization triggered by the activation of Rho/ROCK signaling through myosin IIA.37 In this context, the present work shows that MKs can also promote the assembly of exogenous FN in a substrate-dependent manner. FN-matrix assembly was detected only around spread MKs on type I collagen, as revealed after DOC treatment by Western blotting and immunofluorescence techniques. Moreover, FN assembly was strictly dependent on forces generated by the contractility of the MK cytoskeleton, because this was prevented by inhibition of the actin-myosin reorganization with blebbistatin, a selective antagonist of myosin IIA ATPase activity.37,38 Finally, FN-matrix assembly was also prevented by the presence of RGDS, demonstrating that integrin activation plays a fundamental role in FN-matrix assembly by human MKs.39 These results indicate that MKs can control FN assembly, with integrins and MK cytoskeleton playing a central role in this regulatory mechanism.40 Further, specificity of the matrix substrate is fundamental for this process, because MKs can support FN assembly only upon adhesion on collagen type I and not on fibrinogen.
Interestingly, FN is a major component of bone marrow ECM and may modulate homing of hemopoietic progenitor cells41 and the organization and composition of bone marrow ECMs and cell-matrix adhesion sites.40 The present work suggests a new role for MKs as promoters of matrix deposition and remodeling within the bone marrow environment, with particular emphasis on the type I collagen–rich osteoblastic niche.
TGs are a widely distributed group of enzymes that catalyze the posttranslational modification of proteins by the formation of isopeptide bonds. TGs have been shown to bind and cross-link several extracellular proteins, in particular FN, for which they have a high binding affinity.42 The physiologic implications related to matrix protein cross-linking indicate that its function is not only to stabilize these proteins (ie, increasing their proteolytic, chemical, and mechanical resistance), but also to facilitate cell adhesion and cell motility.25 Interestingly, a recent study by Jayo et al reported that FXIII-A represents the only detectable source of TG activity by protein analysis in platelets.26 In the present study, we demonstrate that, of all of the TGs, FXIII-A is the most expressed by human MKs at both the mRNA and protein levels. Moreover, FXIII-A was constitutively active in MKs, because cross-linking activity was detected by biotinylated cadaverine incorporation in different intracellular substrates. In addition, TGase activity was shown to be associated with the MK plasma membrane, as revealed by analysis of cross-linked biotinylated cadaverine to the adsorbed FN matrix in an ELISA assay.
These results support the idea that active FXIII-A may represent an important biochemical modulator of different MK functions related to its enzymatic activity at both intracellular and membrane sites. In particular, we analyzed how FXIII-A activity affects MK spreading and FN matrix assembly on type I collagen. FXIII-A was shown to regulate MK spreading and stress fibers formation on type I collagen, because a significant decrease of these processes was observed upon incubation with TG inhibitors. Consistently, FXIII-A colocalized with FN on the plasma membrane of spread MKs, and FN matrix assembly by MKs on type I collagen was inhibited upon incubation with the TG inhibitor IAA. These results confirmed an important role of FXIII-A in stabilizing the MK-type I collagen interaction through FN fibrillogenesis.
The bone marrow microenvironment has been shown to undergo continual remodeling with a balance between ECM deposition and destruction. Interestingly, our data represent a sort of mirror image of previous studies43,44 demonstrating that local secretion of proteases induces hemopoietic stem cell mobilization from the bone marrow niche by altering the hemopoietic stem cell-stromal cell interaction and that the production of MMP-9 by MKs is one of the mechanisms by which mature MKs free themselves from the bone marrow matrix and travel to the vascular niche. This study provides important new elements in the understanding of the regulatory pathways for MK-matrix interactions within the bone marrow environment. In particular, our results demonstrate that FNs and FXIII-A modulate MK spreading on type I collagen by promoting matrix assembly.
In conclusion, this work opens new doors in the study of diseases such as primary myelofibrosis or MYH9-related thrombocytopenia, which are characterized by a defect of MK-matrix interactions within the bone marrow environment and whose origin is still a matter of debate.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. Vaghi (Centro Grandi Strumenti, University of Pavia) and D. Picenoni (Politecnico of Milan) for their technical assistance in confocal and scanning electron microscopy studies; G. Viarengo for technical assistance in flow cytometry studies; C. Di Buduo, V. Abbonante, and S. Badalucco (Department of Biochemistry, University of Pavia) for assistance in performing experiments; and V. Rosti for critical review of the paper.
This paper was supported by the Cariplo Foundation (2006.0596/10.8485 to A.B.), by FAR (Fondi per le Agevolazioni alla Ricerca; 2008 to L.V.), by the Banca del Monte di Lombardia Foundation (to L.V. and A.B.), and by PRIN (Progretti di Ricerca di Interesse Nazionale; 2007TWL3MX_003 to M.E.T.) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contributions: A.M., C.G., P.R., and L.V. performed the research; A.M., C.B., M.E.T., and A.B. designed research and interpreted and analyzed the data; C.P. provided essential reagents; R.M. analyzed data; and A.M. and A.B. wrote the paper.
Conflict-of-interest declaration: The authors declare no competing financial interests.
Correspondence: Alessandra Balduini, Biotechnology Research Laboratories, Department of Biochemistry, University of Pavia, San Matteo Foundation, via Bassi 21, 27100 Pavia, Italy; e-mail: alessandra.balduini@unipv.it; or Department of Biomedical Engineering, Tufts University, 4 Colby St, Medford, MA 02155; e-mail: alessandra.balduini@tufts.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal