Abstract
Dentin matrix protein 1 (DMP1) is a member of the small integrin–binding ligand N-linked glycoprotein (SIBLING) family, a group of proteins initially described as mineralized extracellular matrices components. More recently, SIBLINGs have been implicated in several key steps of cancer progression, including angiogenesis. Although proangiogenic activities have been demonstrated for 2 SIBLINGs, the role of DMP1 in angiogenesis has not yet been addressed. We demonstrate that this extracellular matrix protein induced the expression of vascular endothelial cadherin (VE-cadherin), a key regulator of intercellular junctions and contact inhibition of growth of endothelial cells that is also known to modulate vascular endothelial growth factor receptor 2 (VEGFR-2) activity, the major high-affinity receptor for VEGF. DMP1 induced VE-cadherin and p27Kip1 expression followed by cell-cycle arrest in human umbilical vein endothelial cells (HUVECs) in a CD44-dependent manner. VEGF-induced proliferation, migration, and tubulogenesis responses were specifically blocked on DMP1 pretreatment of HUVECs. Indeed, after VE-cadherin induction, DMP1 inhibited VEGFR-2 phosphorylation and Src-mediated signaling. However, DMP1 did not interfere with basic fibroblast growth factor–induced angiogenesis. In vivo, DMP1 significantly reduced laser-induced choroidal neovascularization lesions and tumor-associated angiogenesis. These data enable us to put DMP1 on the angiogenic chessboard for the first time and to identify this protein as a new specific inhibitor of VEGF-induced angiogenesis.
Introduction
Dentin matrix protein 1 (DMP1) is a member of the small integrin–binding ligand N-linked glycoprotein (SIBLING) gene family, which also includes osteopontin (OPN), bone sialoprotein (BSP), and dentin sialophosphoprotein (DSPP).1,2 DMP1 was originally considered to be dentin-specific,3 but later its expression was also detected in bone and in nonmineralized tissues such as salivary glands and kidney.4,5 Whereas its precise biologic activities have not been identified, this glycoprotein has been associated mainly with the regulation of extracellular matrix mineralization.6 We and others have demonstrated the expression of SIBLINGs in human tumors, with OPN and BSP being the 2 most studied in relation to cancer progression and metastasis development.1 Using cancer-profiling arrays, Fisher et al demonstrated the presence of SIBLING mRNAs in different cancers and found that DMP1 was significantly overexpressed in human breast, uterine, colon, and lung cancer tissue compared with normal tissue.7 More recently, immunohistochemical studies allowed us to demonstrate the up-regulation of DMP1 in human lung and breast tumors8,9 and of DSPP in prostate cancer.10
Angiogenesis is a multistep process in which activated endothelial cells of existing vessels migrate and proliferate in the perivascular stroma to form capillary sprouts. These sprouting endothelial cells stop proliferating, align, form tubes, and deposit a basement membrane to finally yield operational new vessels.11 Vascular endothelial cadherin (VE-cadherin), the principal cell-cell junction molecule in endothelial cells,12 is required for normal vasculature development in the mouse embryo and for new vessel formation in the adult.13 VE-cadherin engagement is associated with the cessation of proliferation commonly known as contact inhibition of growth,14 in which endothelial cells show a reduced proliferative response to specific factors such as vascular endothelial growth factor (VEGF). Binding of VEGF to VEGF receptor 2 (VEGFR-2) is the principal extracellular signal triggering an angiogenic response and leads to VEGFR-2 dimerization and autophosphorylation. One of the mechanisms of the contact-inhibitory activity of VE-cadherin occurs through the modulation of VEGFR-2 signaling.14 Src family kinases (SFKs) are involved in VEGFR-2 signaling and in the regulation of angiogenesis.15 VE-cadherin interacts with C-terminal Src kinase (Csk), an inhibitor of SFKs in vascular endothelial cells. On VEGF stimulation, the VE-cadherin-Csk complex is disrupted, allowing the activation of Src kinase and its downstream signaling.16
Endothelial cell matrix interactions mediated by integrins play a critical role in vascular development and angiogenesis.17 One characteristic feature of the SIBLINGs is the presence of a highly conserved RGD motif in their primary structure2 that is recognized by endothelial integrins. Indeed, DMP1 promotes αVβ3-mediated cell attachment and migration.18 DMP1 has been also shown to interact with the CD44 cell-surface receptor,19 which is detectable both on vascular endothelium in situ and on cultured human endothelial cells and is involved in tumor angiogenesis.20 Whereas OPN21 and BSP22 display proangiogenic properties through their interaction with endothelial integrins, the possibility of a potential influence of DMP1 on the behavior of endothelial cells has not yet been addressed.
In the present study, we investigated whether DMP1 is involved in the multistep process required for the formation of new blood vessels by studying the effects of recombinant human DMP1 on human umbilical vein endothelial cells (HUVECs). Our data are the first demonstration of a CD44-dependent function of DMP1 in endothelial morphogenesis through VE-cadherin induction. We show that the DMP1-mediated VE-cadherin increase is accompanied by an arrest of proliferation in sparse HUVECs, mimicking the contact inhibition of growth that occurs in endothelial cells cultured at high cell density. Because VE-cadherin expression regulates VEGFR-2 signaling, we investigated further the role of DMP1 on VEGF-induced proliferation, migration, and tubulogenesis responses. We demonstrate that DMP1 interferes with each of these essential events of the angiogenic process, most notably by inhibiting VEGFR-2 phosphorylation and by modulating Src activity. Finally, we were able to demonstrate that DMP1 interferes with 2 in vivo angiogenesis models, one involving wound healing and the other tumor development.
Methods
Cell culture and recombinant proteins
HUVECs were isolated and maintained in culture as described previously.23 Cells were cultured in MCDB131 medium (GIBCO) supplemented with 20% fetal bovine serum (FBS), 2mM l-glutamine, 50 μg/mL of heparin, and 50 μg/mL of endothelium cell growth supplement (ECGS) referred to as complete medium hereafter. Recombinant human DMP1, VEGF165, and basic fibroblast growth factor (bFGF) were purchased from R&D Systems.
Antibodies
Anti-p27Kip1 and anti-pRb were from BD Pharmingen. Anti–phospho-p27Kip1(Ser10) was from Zymed Laboratories. Anti–Hsc 70, anti-p21, anti–VEGFR-2, anti-phospho–VEGFR-2, and anti-phospho–VEGFR-1 were from Santa Cruz Biotechnology. Anti–VEGFR-1 and anti–β-actin were from Sigma-Aldrich. Anti–VE-cadherin, anti-phospho–VE-cadherin (Y685), anti-Csk, anti-poly(ADP-ribose) polymerase, and anti–αV-integrin were from BD Biosciences. Anti–platelet endothelial cell adhesion molecule 1 (anti–PECAM-1) was from Dako. Anti-phospho–VE-cadherin (Y731)/(Y658) was from Invitrogen. Anti–zonula occludens 1 (anti–ZO-1), anti-phospho–VEGFR-2 and anti–phospho-Src (Y416)/(Y527) were from Cell Signaling Technology. Anti-DMP1 was from Takara Bio. For blocking experiments, HUVECs were incubated with RGD peptide (GRGDS; Calbiochem), anti-αVβ3 (LM609), anti-αVβ5 (P1F6) (Chemicon), anti-CD44 (BU75; Ancell), and mouse-purified immunoglobulin G (IgG; Serotec) before DMP1 treatment.
Western blotting
Equal amounts of proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted, and membranes were first probed with the indicated antibodies and reprobed with β-actin or Hsc 70 antibodies. Scanned bands were quantified using ImageJ software Version 1.43 (National Institutes of Health, http://rsb.info.nih.gov/ij/).
RNA interference
HUVECs (15 × 104, 6-well plates) were grown overnight in complete medium. Cells were transfected with 100 nmol/L of small interfering RNAs (siRNAs) using the calcium phosphate precipitation method, and cultured for 48 hours or treated for the last 24 hours of transfection in complete medium without ECGS. VE-cadherin siRNA and control nontargeting siRNA SMARTpool reagents were purchased from Dharmacon RNAi Technologies.
Adhesion assay
Bacteriologic 96-well plates (Greiner Bio-One) were coated with DMP1 or vitronectin (Dako), and HUVECs were seeded at 2 × 104 cells per well. After 2 hours, attached cells were stained with crystal violet and the incorporated dye was measured by reading the absorbance at 560 nm.
Migration assay
To assess the migration toward DMP1, HUVECs (12 × 104) were tested in modified Boyden chambers (Neuroprobe) as described previously.22 For the other migration assays, HUVECs (105) in serum-free Dulbecco modified Eagle medium containing 0.1% bovine serum albumin (BSA) were seeded into the upper part of a Transwell filter (Becton Dickinson), and the lower compartment was filled with Dulbecco modified Eagle medium containing 1% BSA, 1% FBS, and VEGF (2 ng/mL) where indicated. After overnight incubation, migrating cells were counted in 3 random fields per well and expressed as the average number of cells per field. Three wells per condition were counted.
Tubulogenesis assay
HUVECs (3 × 104, 24-well plates) were seeded on Matrigel (Chemicon) in 2% serum complete medium without ECGS. For VEGF- or bFGF-induced tubulogenesis, HUVECs (3 × 103 cells per well) were seeded on Matrigel in 15-well μ-Slide angiogenesis plates (Integrated BioDiagnostics) in 0.2% serum complete medium without ECGS and treated with the mentioned growth factor in the presence of DMP1 where indicated. Tubulogenesis was determined by counting vessels in 2 random fields per well and is expressed as the average number of vessels per field.
Proliferation assay
HUVECs (2 × 104, 24-well plates) were treated with DMP1 in complete medium without ECGS and grown up to 48 hours. For VEGF- or bFGF-induced proliferation, sparse (2 × 104, 24-well plates) and confluent cells (4 × 105, 24-well plates) were treated with DMP1 in complete medium without ECGS for 24 hours and then treated with growth factor in presence of DMP1 for another 24 hours in 2% serum complete medium without ECGS. Fluorimetric DNA titration was performed with a SPECTRAmax GEMINI-XS using SoftMax Pro software Version 3.1.1.
Cell-cycle analysis
To have better control in the study of the cell-cycle arrest, all experiments were performed with synchronized cultures of HUVECs arrested in the G0/G1 phase by serum starvation. Briefly, cells (15 × 104, 6-well plates) were grown overnight in complete medium and were then serum starved for 48 hours in 2% serum complete medium without ECGS. For the last 4 hours of serum starvation, cells were treated with DMP1 or mimosine (Sigma-Aldrich). Cells were serum-released for 20 hours either with complete medium without ECGS or with VEGF where indicated. The relative percentage of cells in each stage of the cell cycle was analyzed using the Cycle TEST Plus DNA reagent kit (Becton Dickinson).
Immunocytochemistry
For double-labeling experiments, sparse HUVECs (3 × 104, 24-well plates) were grown on glass coverslips in complete medium. After overnight incubation, cells were treated with DMP1 in complete medium without ECGS for 3 and 24 hours. Cells were incubated with anti–VE-cadherin followed by Alexa-488–conjugated antibody and TO-PRO-3 iodide (Molecular Probes). Images were obtained under a TCS SP laser-scanning confocal microscope (Leica). For bromodeoxyuridine (BrdU) incorporation, sparse or confluent HUVECs prepared as above were treated with DMP1. At28 hours from the beginning of DMP1 treatment, BrdU (20μM; Sigma-Aldrich) was added, and the incubation was continued for another 20 hours. After incubation with anti–VE-cadherin and its secondary antibody, anti-phalloidin–Alexa-633 (Molecular Probes) was added. Cells were fixed and subjected to DNA denaturation followed by incubation with an anti-BrdU–Alexa-543 (Sigma-Aldrich). Images were obtained under a laser scanning confocal microscope (Olympus).
CNV
Two-month-old C57bl6 mice (5 per condition) were maintained in a 12-hour light-dark cycle with free access to food and water. All animal experiments were performed in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Choroidal neovascularization (CNV) was induced by laser burns as described previously.24 Eyes were locally anesthetized and subjected to intravitreal injection with DMP1 (500 nmol/L) or PBS used as a vehicle. At day 7 after laser burn, mice were intravenously injected with fluorescein isothiocyanate–dextran (2000 kDa; Sigma) before being killed. Eyes were fixed in paraformaldehyde at room temperature. Retinas were discarded and the choroids were flat-mounted in Vectashield medium (Vector Laboratories) for epifluorescence microscopy analysis. Quantitation was realized by measuring total vessel fluorescence surface (ImageJ software) as we described previously.25
Experimental glioma model
Human glioma cells (U87-MG) were maintained in Mimimum Essential Medium with 10% FBS, 2mM l-glutamine, 1% nonessential amino acid, and 1mM sodium pyruvate. Cells (45 × 105 in Petri dishes) were grown overnight and transfected with 1 μg/mL of plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions for 24 hours before implantation on chick embryo chorioallantoic membrane (CAM). DMP1 expression plasmid (pDMP1) and its control empty plasmid (pEmpty) were purchased from GeneCopoeia. Transfected U87-MG cells (5 × 106) were deposited on the CAM of fertilized day-10 eggs, and tumors developed after 5 days were harvested as we described previously.26 Tumor volumes were calculated as described previously.27 Tumors were fixed with 4% paraformaldehyde and processed for cryo-sectioning. Tissue sections were stained with hematoxylin and eosin (H&E). The vessels were stained using fluorescein-coupled Sambucus nigra lectin SNA-1 (FL-1301; Vector Laboratories). For determination of DMP1 protein expression by CAM tumors, control and DMP1 tumors at day 5 (pool of 3-5 tumors/condition) were homogenized in lysis buffer (Sigma-Aldrich) and extracts were submitted to Western blot analysis.
Statistical analysis
The Student t test was used to compare differences between experimental conditions. A P value less than .05 was considered statistically significant. The analyses were carried out using STATISTICA software Version 8.1 (StatSoft).
Results
DMP1 induces HUVEC adhesion, migration, and differentiation but inhibits proliferation
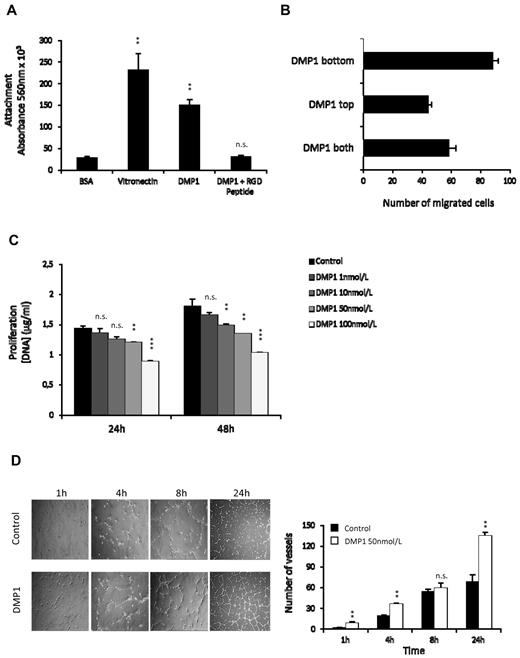
HUVECs adhered to DMP1 to a similar extent as the other RGD-containing protein, vitronectin, but they did not adhere to control BSA. The addition of an RGD peptide completely inhibited adhesion to DMP1, suggesting that DMP1 mediates HUVEC attachment through molecular interaction with integrins (Figure 1A). When placed in the lower chamber of a modified Boyden chamber, DMP1 stimulated HUVEC migration (Figure 1B). To determine the importance of a concentration gradient for the observed migratory response, cell migration was evaluated when DMP1 was placed either in the top chamber only or in both chambers. DMP1 has chemotactic properties, because placing this molecule in both chambers at the same concentration reduced maximal migration by 34%. However, because the absence of a concentration gradient did not totally abolish cell migration, we can also consider that DMP1 exhibits chemokinetic ability toward endothelial cells. We next showed that DMP1-treated HUVECs were less proliferative than control cells (Figure 1C). Results of an annexin V assay (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and a Western blot to poly(ADP-ribose) polymerase (supplemental Figure 1B) demonstrated that DMP1-treated cells were not apoptotic.
DMP1 induces the adhesion, migration, and differentiation of HUVECs in vitro but decreases their proliferation. (A) Cells were plated onto DMP1 (50 nmol/L) or incubated for 1 hour in the presence of 50 nmol/L of RGD peptide and then plated onto DMP1 (50 nmol/L). Vitronectin (50 nmol/L) and BSA 1% were used as positive and negative controls, respectively. Cells were allowed to adhere for 2 hours at 37°C and were quantified as described in “Adhesion assay.” Error bars represent the means ± SD of 6 replicates of a representative experiment (n = 2). **P ≤ .005 vs control; n.s., not significant. (B) Modified Boyden chamber chemotaxis assays were performed on HUVECs with DMP1 (100 nmol/L) placed in the bottom chamber (DMP1 bottom), in the top chamber with the cells (DMP1 top), or in both the top and bottom chambers (DMP1 both). Each bar represents the mean ± SD of the total number of migrated cells within 4 replicates (n = 2). (C) HUVECs were treated with DMP1 (1-100 nmol/L) and proliferation was followed for 24 and 48 hours and scattered as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .001, ***P ≤ .0001 vs control; n.s., not significant. (D) Capillary tube–like assay using HUVECs treated with DMP1 (50 nmol/L) for 24 hours and then cultured on Matrigel for 1, 4, 8 (100×), and 24 hours (40×). Phase-contrast microscopy photomicrographs were taken for each culture time. The quantification of the assay is shown and was realized by counting the number of vessels from 2 representative fields from 2 replicates (n = 3). **P ≤ .005 vs control; n.s., not significant.
DMP1 induces the adhesion, migration, and differentiation of HUVECs in vitro but decreases their proliferation. (A) Cells were plated onto DMP1 (50 nmol/L) or incubated for 1 hour in the presence of 50 nmol/L of RGD peptide and then plated onto DMP1 (50 nmol/L). Vitronectin (50 nmol/L) and BSA 1% were used as positive and negative controls, respectively. Cells were allowed to adhere for 2 hours at 37°C and were quantified as described in “Adhesion assay.” Error bars represent the means ± SD of 6 replicates of a representative experiment (n = 2). **P ≤ .005 vs control; n.s., not significant. (B) Modified Boyden chamber chemotaxis assays were performed on HUVECs with DMP1 (100 nmol/L) placed in the bottom chamber (DMP1 bottom), in the top chamber with the cells (DMP1 top), or in both the top and bottom chambers (DMP1 both). Each bar represents the mean ± SD of the total number of migrated cells within 4 replicates (n = 2). (C) HUVECs were treated with DMP1 (1-100 nmol/L) and proliferation was followed for 24 and 48 hours and scattered as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .001, ***P ≤ .0001 vs control; n.s., not significant. (D) Capillary tube–like assay using HUVECs treated with DMP1 (50 nmol/L) for 24 hours and then cultured on Matrigel for 1, 4, 8 (100×), and 24 hours (40×). Phase-contrast microscopy photomicrographs were taken for each culture time. The quantification of the assay is shown and was realized by counting the number of vessels from 2 representative fields from 2 replicates (n = 3). **P ≤ .005 vs control; n.s., not significant.
We tested the impact of DMP1 on tubulogenesis in a Matrigel in vitro assay. As early as 1 hour after seeding, we observed that DMP1-treated endothelial cells spread and attached better on Matrigel than control cells. Vessel counting after 4 hours demonstrated that DMP1-treated HUVECs rapidly formed a tubular network that was maintained after 24 hours (Figure 1D). These data indicate that DMP1 could act as a pro-differentiating factor for HUVECs and contribute to the organization and/or stability of developing endothelial tubular networks.
DMP1 blocks the cell cycle in G1, modulates the expression of cell cycle–related proteins, and induces p27Kip1 through CD44 ligation
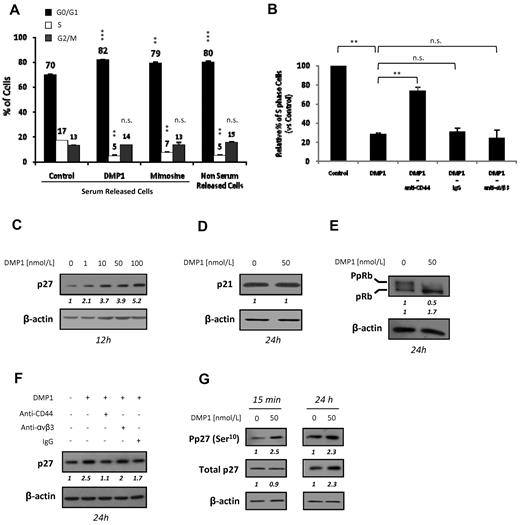
We next investigated whether the decrease of HUVEC proliferation was because of an arrest of the cell cycle. Synchronized HUVECs were released with medium containing 20% serum in presence of DMP1 or mimosine, an inhibitor of DNA replication that leads to mammalian cell-cycle arrest in the G1 phase,28 which was used as a control. DMP1 treatment significantly impaired cell-cycle progression after serum release, with an accumulation of HUVECs in the G1 phase from 70% to 82% and a decrease of the S-phase cell population from 17% to 5% (Figure 2A).
DMP1 blocks the cell cycle in G1, modulates the expression of cell cycle–related proteins, and induces p27Kip1 through CD44 ligation. (A) Cell-cycle analysis of serum-starved HUVECs treated with DMP1 (50 nmol/L) and mimosine (200μM). Mimosine and non-serum–released cells were used to assess for G1 arrest. Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .001 and ***P ≤ .0005 vs control serum-released cells; n.s., not significant. (B) S-phase cell-cycle analysis of serum-starved HUVECs incubated with blocking antibodies to CD44 and αVβ3, with IgG used as a control, followed by treatment with DMP1 (50 nmol/L), as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 2). **P ≤ .001 vs control or DMP1 condition; n.s., not significant. (C) Western blot analysis with an antibody to p27Kip1 using total lysates from HUVECs treated with increasing concentrations of DMP1. (D) Western blot analysis with antibodies to p21Cip1 using total lysates from DMP1-treated cells. (E) Western blot analysis with antibodies to pRb using total lysates from DMP1-treated cells. (F) Western blot analysis with an antibody to p27Kip1 using total lysates from DMP1-treated cells. Before DMP1 treatment (50 nmol/L) for 24 hours, cells were incubated for 1 hour in the presence of 10 μg/mL of anti-CD44 and anti-αVβ3 blocking antibodies or IgG as a control. (G) Western blot analysis with antibodies to p27Kip1 and P-p27Kip1(Ser10) using total lysates from DMP1-treated HUVECs. All Western blotting results were evaluated by densitometric scanning, corrected with respect to β-actin expression, and expressed relative to the value obtained with the corresponding control (arbitrarily set as 1). These relative protein level values are shown in italics below the lanes. Western blots were performed at least 2 times with similar results. Equal protein loading was assessed by anti–β-actin immunoblotting.
DMP1 blocks the cell cycle in G1, modulates the expression of cell cycle–related proteins, and induces p27Kip1 through CD44 ligation. (A) Cell-cycle analysis of serum-starved HUVECs treated with DMP1 (50 nmol/L) and mimosine (200μM). Mimosine and non-serum–released cells were used to assess for G1 arrest. Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .001 and ***P ≤ .0005 vs control serum-released cells; n.s., not significant. (B) S-phase cell-cycle analysis of serum-starved HUVECs incubated with blocking antibodies to CD44 and αVβ3, with IgG used as a control, followed by treatment with DMP1 (50 nmol/L), as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 2). **P ≤ .001 vs control or DMP1 condition; n.s., not significant. (C) Western blot analysis with an antibody to p27Kip1 using total lysates from HUVECs treated with increasing concentrations of DMP1. (D) Western blot analysis with antibodies to p21Cip1 using total lysates from DMP1-treated cells. (E) Western blot analysis with antibodies to pRb using total lysates from DMP1-treated cells. (F) Western blot analysis with an antibody to p27Kip1 using total lysates from DMP1-treated cells. Before DMP1 treatment (50 nmol/L) for 24 hours, cells were incubated for 1 hour in the presence of 10 μg/mL of anti-CD44 and anti-αVβ3 blocking antibodies or IgG as a control. (G) Western blot analysis with antibodies to p27Kip1 and P-p27Kip1(Ser10) using total lysates from DMP1-treated HUVECs. All Western blotting results were evaluated by densitometric scanning, corrected with respect to β-actin expression, and expressed relative to the value obtained with the corresponding control (arbitrarily set as 1). These relative protein level values are shown in italics below the lanes. Western blots were performed at least 2 times with similar results. Equal protein loading was assessed by anti–β-actin immunoblotting.
To investigate whether the DMP1 effect on the cell cycle was mediated through an interaction with αVβ3 or CD44, we used specific blocking antibodies. In DMP1-treated cells, the S-phase population was decreased to 28% compared with the control S-phase cell population arbitrarily set as 100%. However, cells pretreated with anti-CD44 proved to be able to enter the S phase, with the population of S-phase cells being significantly increased to 74%. No significant changes were observed with cells pretreated with anti-αVβ3 or control IgG (Figure 2B). Thus, the inhibitory effect of DMP1 on the cell cycle is mediated, at least in part, by the CD44 receptor and not by αVβ3.
The cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1 can bind and inhibit the kinase activities of several cyclin–cyclin-dependent kinase inhibitor complexes and arrest cell growth at the G1/S boundary. The expression of p27Kip1 was induced by DMP1 in a dose-dependent manner (Figure 2C), whereas the expression of p21Cip1 was unaffected (Figure 2D). Consistent with the cell-cycle arrest observed in DMP1-treated HUVECs, these cells showed a significant decrease of phospho-pRb (Figure 2E). The importance of p27Kip1 in the DMP1-induced effect on HUVEC growth was further demonstrated by the use of specific siRNAs. We found that DMP1 treatment failed to inhibit the proliferation of p27Kip1-silenced HUVECs compared with either mock or irrelevant siRNA–transfected cells (supplemental Figure 2).
We next demonstrated that p27Kip1 induction was abolished when cells were treated with anti-CD44 before DMP1 treatment, whereas significant p27Kip1 induction was still observed in cells pretreated with anti-αVβ3 or control IgG (Figure 2F). Our data are consistent with CD44 being the key receptor involved in mediating the observed effects of DMP1 on cell-cycle arrest and on the increase of p27Kip1 expression in HUVECs. It has been shown that the phosphorylation on serine 10 (Ser10) increases the stability of p27Kip1.29 DMP1 treatment of HUVECs rapidly induced a significant increase of phospho-p27Kip1 (Ser10), whereas the total p27Kip1 level was unchanged (Figure 2G). After 24 hours, the total p27Kip1 level also increased, suggesting its accumulation over time.
DMP1 induces CD44-dependent VE-cadherin expression and mediates inhibition of growth in sparse HUVECs
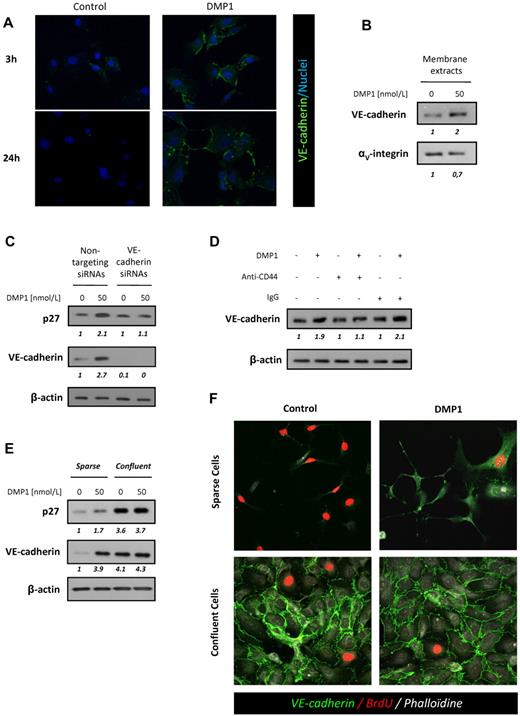
VE-cadherin is a major homophilic cell-to-cell adhesion molecule involved in the control of blood vessel formation and contact inhibition of endothelial cell growth. Using immunofluorescence, we demonstrated that DMP1 treatment significantly induced the expression of VE-cadherin at the cell membrane after 3 hours (Figure 3A). Western blot analysis performed on membrane extracts showed a 2-fold increase of VE-cadherin expression in DMP1-treated HUVECs (Figure 3B). Fluorescence-activated cell sorting analysis confirmed this increase, whereas DMP1 did not affect the surface expression of other endothelial cell-cell junction proteins such as ZO-1 and PECAM-1 (supplemental Figure 3).
DMP1 induces CD44-dependent VE-cadherin expression and mediates inhibition of growth in sparse HUVECs. (A) Immunofluorescence microscopy of sparse HUVECs treated with DMP1 (50 nmol/L) for 3 and 24 hours. Nuclei appear blue after TO-PRO-3 staining. Representative confocal fields of one experiment (n = 3) are shown (magnification, 40×). (B) Western blot analysis with antibodies to VE-cadherin and αV-integrin using membrane lysates from DMP1-treated HUVECs. (C) Western blot analysis with antibodies to p27Kip1 and VE-cadherin using total lysates from HUVECs transfected for 48 hours with VE-cadherin or nontargeting siRNAs and treated with DMP1 (50 nmol/L) for the last 24 hours of transfection. (D) Western blot analysis with an antibody to VE-cadherin using total lysates from HUVECs incubated for 1 hour in the presence of 10 μg/mL of anti-CD44 blocking antibody or IgG before DMP1 treatment (50 nmol/L) for 24 hours. (E) Western blot analysis with antibodies to VE-cadherin and p27Kip1 using total lysates from sparse and confluent DPM1-treated HUVECs, as described in “Methods.” All Western blotting results were evaluated by densitometric scanning. The relative protein level values are shown in italics below the lanes. Western blots were performed 3 times with similar results. Equal protein loading was assessed by anti–β-actin immunoblotting. (F) Immunofluorescence of sparse and confluent DMP1-treated HUVECs incubated with BrdU for 20 hours to evaluate the S-phase population, as described in “Methods.” VE-cadherin, BrdU, and phalloidin are shown in green, red, and gray, respectively. As expected, control sparse cells presented with S-phase–positive and VE-cadherin–negative staining compared with control confluent cells. DMP1-treated sparse cells showed strong positive VE-cadherin staining and less BrdU incorporation than control cells, similar to that of control or DMP1-treated confluent cells. Confluent cells did not show any modulation of VE-cadherin staining intensity or BrdU incorporation after DMP1 treatment. Representative confocal fields of one experiment (n = 3) are shown (magnification, 40×).
DMP1 induces CD44-dependent VE-cadherin expression and mediates inhibition of growth in sparse HUVECs. (A) Immunofluorescence microscopy of sparse HUVECs treated with DMP1 (50 nmol/L) for 3 and 24 hours. Nuclei appear blue after TO-PRO-3 staining. Representative confocal fields of one experiment (n = 3) are shown (magnification, 40×). (B) Western blot analysis with antibodies to VE-cadherin and αV-integrin using membrane lysates from DMP1-treated HUVECs. (C) Western blot analysis with antibodies to p27Kip1 and VE-cadherin using total lysates from HUVECs transfected for 48 hours with VE-cadherin or nontargeting siRNAs and treated with DMP1 (50 nmol/L) for the last 24 hours of transfection. (D) Western blot analysis with an antibody to VE-cadherin using total lysates from HUVECs incubated for 1 hour in the presence of 10 μg/mL of anti-CD44 blocking antibody or IgG before DMP1 treatment (50 nmol/L) for 24 hours. (E) Western blot analysis with antibodies to VE-cadherin and p27Kip1 using total lysates from sparse and confluent DPM1-treated HUVECs, as described in “Methods.” All Western blotting results were evaluated by densitometric scanning. The relative protein level values are shown in italics below the lanes. Western blots were performed 3 times with similar results. Equal protein loading was assessed by anti–β-actin immunoblotting. (F) Immunofluorescence of sparse and confluent DMP1-treated HUVECs incubated with BrdU for 20 hours to evaluate the S-phase population, as described in “Methods.” VE-cadherin, BrdU, and phalloidin are shown in green, red, and gray, respectively. As expected, control sparse cells presented with S-phase–positive and VE-cadherin–negative staining compared with control confluent cells. DMP1-treated sparse cells showed strong positive VE-cadherin staining and less BrdU incorporation than control cells, similar to that of control or DMP1-treated confluent cells. Confluent cells did not show any modulation of VE-cadherin staining intensity or BrdU incorporation after DMP1 treatment. Representative confocal fields of one experiment (n = 3) are shown (magnification, 40×).
It has been previously reported that N-cadherin–30 and E-cadherin31 –mediated signaling is involved in contact inhibition of growth by inducing p27Kip1 expression and cell-cycle arrest at the G1 phase. However, no direct evidence of an effect of VE-cadherin on p27Kip1 expression in endothelial cells has been reported to date. To explore this possibility, we transfected VE-cadherin siRNAs in HUVECs before treatment with DMP1. VE-cadherin–silenced cells did not show an increased p27Kip1 expression after DMP1 treatment compared with cells transfected with nontargeting siRNAs (Figure 3C), indicating that VE-cadherin expression is essential to DMP1-mediated p27Kip1 up-regulation and subsequent growth control of HUVECs. We also evaluated the involvement of CD44 on the observed DMP1-induced VE-cadherin up-regulation. As shown in Figure 3D, VE-cadherin induction was abolished when HUVECs were treated with anti-CD44 before DMP1 treatment, but not with control IgG. Our data pointed to CD44 as a major actor in the increase in VE-cadherin expression, p27Kip1 induction, and cell-cycle arrest induced by DMP1 in HUVECs.
VE-cadherin induction has been shown to control contact inhibition of growth in endothelial cells.14 Using sparse and confluent HUVECs, we addressed the question of a contact inhibition of growth signal mimicry occurring after DMP1 treatment. We showed that DMP1 induced the expression of VE-cadherin and p27Kip1 in sparse cells, whereas, as expected, high-density cells did not show any modulation of expression of these proteins (Figure 3E). We then evaluated VE-cadherin staining by immunofluorescence in parallel with BrdU incorporation in sparse and confluent HUVECs. A large proportion of sparse HUVECs were BrdU positive compared with confluent cells (Figure 3F). DMP1-treated sparse cells showed positive VE-cadherin staining and less BrdU incorporation than control sparse cells, a pattern similar to that of control or DMP1-treated confluent cells. Because confluent HUVECs established VE-cadherin–dependent junctions and underwent growth arrest, they did not show any modulation of their VE-cadherin staining intensity, and the proportion of BrdU-positive cells was approximately the same in both control and DMP1-treated conditions. These observations indicate that DMP1 induced contact inhibition of growth signal mimicry on sparse endothelial cells.
DMP1 counteracts VEGF-induced angiogenesis
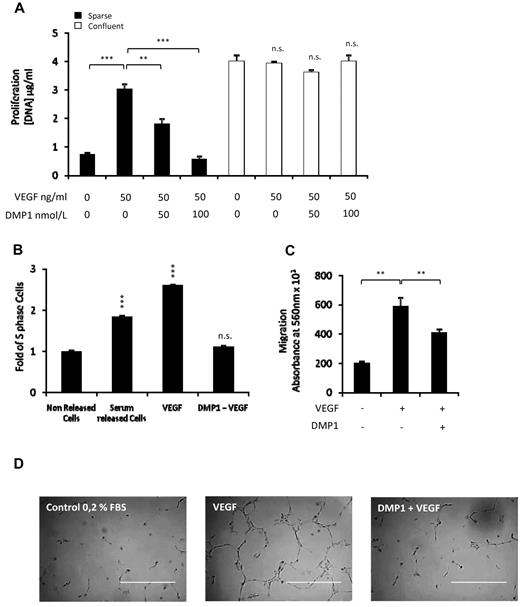
Knowing that VEGF is essential for angiogenic processes in both normal and pathologic conditions32 and based on our previous observations, we decided to investigate the effects of DMP1 on VEGF-induced proliferation, migration, and tubulogenesis. Sparse and confluent cells were treated with DMP1 for 24 hours and then with VEGF for another 24 hours. We observed that DMP1 pretreatment impaired VEGF-induced sparse cell proliferation in a dose-dependent manner compared with VEGF-treated sparse cells (Figure 4A), whereas it did not affect confluent cells. We next studied the impact of DMP1 on VEGF-induced cell-cycle progression. Synchronized HUVECs responded to VEGF release by a significant entry into the S phase compared with control cells released with serum. However, the S-phase cell population corresponded to that of nonreleased cells after DMP1 treatment (Figure 4B). The migration of endothelial cells is a critical step during vessel formation. We showed that DMP1 impaired the migration of cells toward VEGF compared with control cells (Figure 4C).
DMP1 counteracts VEGF-induced angiogenesis. (A) Proliferation was assessed in sparse and confluent cells treated with DMP1 for 48 hours. For the last 24 hours of DMP1 treatment, cells were treated with VEGF (50 ng/mL), as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .005 and ***P ≤ .0005 vs control or vs VEGF; n.s., not significant. (B) S-phase cell-cycle analysis of serum-starved HUVECs treated with DMP1 (50 nmol/L) and released with either serum or VEGF (50 ng/mL). Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). ***P ≤ .0005 vs control. (C) Assessment of HUVEC migration toward VEGF (2 ng/mL) of cells treated with DMP1 (50 nmol/L) for 24 hours and then seeded into the upper compartment of fibronectin coated inserts, as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .005 vs VEGF condition. (D) Capillary tube-like assay of HUVECs treated with DMP1 (100 nmol/L) for 24 hours and then cultured on Matrigel and treated with VEGF (25 ng/mL). Phase-contrast microscopy photomicrographs were taken after 4 hours, and representative fields from one replicate of 2 from one experiment are shown (n = 2). Scale bar = 400 μm.
DMP1 counteracts VEGF-induced angiogenesis. (A) Proliferation was assessed in sparse and confluent cells treated with DMP1 for 48 hours. For the last 24 hours of DMP1 treatment, cells were treated with VEGF (50 ng/mL), as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .005 and ***P ≤ .0005 vs control or vs VEGF; n.s., not significant. (B) S-phase cell-cycle analysis of serum-starved HUVECs treated with DMP1 (50 nmol/L) and released with either serum or VEGF (50 ng/mL). Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). ***P ≤ .0005 vs control. (C) Assessment of HUVEC migration toward VEGF (2 ng/mL) of cells treated with DMP1 (50 nmol/L) for 24 hours and then seeded into the upper compartment of fibronectin coated inserts, as described in “Methods.” Error bars represent the means ± SD of 3 replicates of a representative experiment (n = 3). **P ≤ .005 vs VEGF condition. (D) Capillary tube-like assay of HUVECs treated with DMP1 (100 nmol/L) for 24 hours and then cultured on Matrigel and treated with VEGF (25 ng/mL). Phase-contrast microscopy photomicrographs were taken after 4 hours, and representative fields from one replicate of 2 from one experiment are shown (n = 2). Scale bar = 400 μm.
To finally assess the effect of DMP1 on VEGF-induced angiogenesis, we tested the impact of DMP1 on VEGF-induced tubulogenesis. After 6 hours, we observed that DMP1 treatment impaired VEGF-induced tubular network formation on Matrigel (Figure 4D). Interestingly, these DMP1 effects appeared to be specific to VEGF-induced angiogenesis, because DMP1 did not interfere with bFGF-induced proliferation (supplemental Figure 4A) or tubulogenesis (supplemental Figure 4B).
DMP1 inhibits VEGFR-2 phosphorylation through the induction of VE-cadherin expression
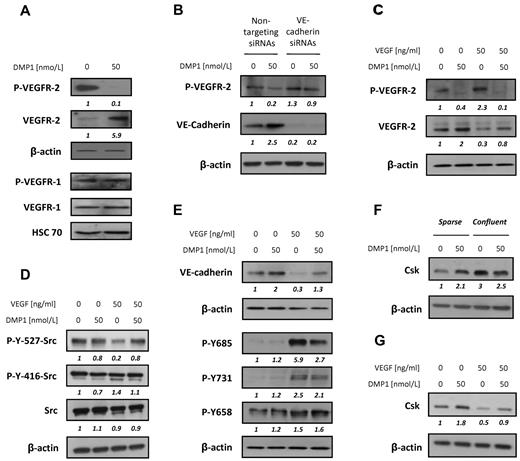
At the surface of endothelial cells, VEGFR-2 has been identified as the major mediator of VEGF-dependent signaling and cellular activities.33 VEGFR-2 expression was significantly increased and its phosphorylation was completely inhibited after DMP1 treatment. Under the same conditions, DMP1 did not show any significant effect on VEGFR-1 expression or phosphorylation (Figure 5A).
DMP1 affects VEGFR-2, not VEGFR-1, phosphorylation, induces Src inactivation, and inhibits VEGF-mediated VE-cadherin down-regulation and phosphorylation. (A) Western blot analysis with antibodies to VEGFR-2 and VEGFR-1 and their phosphorylated forms using total lysates from HUVECs treated with DMP1 for 24 hours. (B) Western blot analysis with antibodies to P-VEGFR-2 and VE-cadherin using total lysates from HUVECs transfected for 48 hours with VE-cadherin or nontargeting siRNAs and treated with DMP1 (50 nmol/L) for the last 24 hours of transfection. (C) Western blot analysis with antibodies to VEGFR-2 and P-VEGFR-2 using total lysates from HUVECs treated with DMP1 for 24 hours and then pulsed with VEGF (50 ng/mL) for a further 10 minutes. (D) Western blot analysis with specific antibodies to Src, P-Src Tyr416, and P-Src Tyr527 using total lysates from HUVECs treated with DMP1 and VEGF as in panel C. (E) Western blot analysis with antibodies to VE-cadherin and P-VE-cadherin Tyr658, Tyr731, and Tyr685 using total lysates from HUVECs treated with DMP1 and VEGF as in panel C. (F) Western blot analysis with an antibody to Csk using total lysates from sparse and confluent HUVECs treated with DMP1 for 24 hours. (G) Western blot analysis with an antibody to Csk using total lysates from HUVECs treated with DMP1 and VEGF as in panel C. All Western blots were evaluated by densitometric scanning (in italics below the lanes) and were performed 3 times with similar results. Equal protein loading was assessed by anti–β-actin or anti–Hsc 70 immunoblotting.
DMP1 affects VEGFR-2, not VEGFR-1, phosphorylation, induces Src inactivation, and inhibits VEGF-mediated VE-cadherin down-regulation and phosphorylation. (A) Western blot analysis with antibodies to VEGFR-2 and VEGFR-1 and their phosphorylated forms using total lysates from HUVECs treated with DMP1 for 24 hours. (B) Western blot analysis with antibodies to P-VEGFR-2 and VE-cadherin using total lysates from HUVECs transfected for 48 hours with VE-cadherin or nontargeting siRNAs and treated with DMP1 (50 nmol/L) for the last 24 hours of transfection. (C) Western blot analysis with antibodies to VEGFR-2 and P-VEGFR-2 using total lysates from HUVECs treated with DMP1 for 24 hours and then pulsed with VEGF (50 ng/mL) for a further 10 minutes. (D) Western blot analysis with specific antibodies to Src, P-Src Tyr416, and P-Src Tyr527 using total lysates from HUVECs treated with DMP1 and VEGF as in panel C. (E) Western blot analysis with antibodies to VE-cadherin and P-VE-cadherin Tyr658, Tyr731, and Tyr685 using total lysates from HUVECs treated with DMP1 and VEGF as in panel C. (F) Western blot analysis with an antibody to Csk using total lysates from sparse and confluent HUVECs treated with DMP1 for 24 hours. (G) Western blot analysis with an antibody to Csk using total lysates from HUVECs treated with DMP1 and VEGF as in panel C. All Western blots were evaluated by densitometric scanning (in italics below the lanes) and were performed 3 times with similar results. Equal protein loading was assessed by anti–β-actin or anti–Hsc 70 immunoblotting.
VE-cadherin has been shown to control contact inhibition of endothelial cell growth by inhibiting VEGFR-2 phosphorylation, notably through the recruitment of specific phosphatases.14 Therefore, we postulated that the observed decrease of VEGFR-2 phosphorylation could be subsequent to the induction of VE-cadherin expression after DMP1 treatment. To test this hypothesis, we inhibited VE-cadherin expression in HUVECs before the addition of DMP1. VE-cadherin–silenced cells did not show any significant decrease of VEGFR-2 phosphorylation after DMP1 treatment compared with cells transfected with nontargeting siRNAs (Figure 5B), indicating that VE-cadherin expression is indispensable in DMP1-mediated VEGFR-2 phosphorylation inhibiton.
DMP1 blocks VEGF-induced VEGFR-2 phosphorylation and inhibits Src activation
VEGFR-2 is expressed and phosphorylated on activation by its ligands. Therefore, we next evaluated whether DMP1 could impair VEGF influence on VEGFR-2. HUVECs were treated with DMP1 for 24 hours and challenged with VEGF (50 ng/mL) for 10 minutes. DMP1 pretreatment not only induced VEGFR-2 expression, but also completely impaired its phosphorylation in the presence of VEGF (Figure 5C).
SFKs are involved in VEGFR-2 signaling in the context of angiogenesis. Src possesses 2 sites of tyrosine (Tyr) phosphorylation that are critical to the regulation of its kinase activity. Autophosphorylation of Tyr416 (chicken c-Src numbering) increases its catalytic activity, whereas phosphorylation of the C-terminal Tyr527 inhibits it.34 To exert its pro-angiogenic activities, VEGF activates Src through phosphorylation of Tyr416 and dephosphorylation of Tyr527. On DMP1 addition, the phosphorylation of Src Tyr416 was reduced almost to the basal level and that of Tyr527 was increased (Figure 5D), indicating that DMP1 counteracts VEGF-triggered Src activation.
DMP1 inhibits VEGF-mediated VE-cadherin down-regulation and phosphorylation
It is known that VE-cadherin undergoes both a decrease of expression and an inactivation through tyrosine phosphorylation by VEGF.35 DMP1 impaired the VEGF-mediated decrease of VE-cadherin expression (Figure 5E). As expected, VEGF induced the phosphorylation of VE-cadherin on Tyr731, Tyr658, and Tyr685, whereas DMP1 alone did not affect its phosphorylation status. Interestingly, DMP1 treatment specifically reversed VEGF-induced VE-cadherin phosphorylation on Tyr685 (Figure 5E).
It has been shown that the Src phospho-Tyr527 level is dictated by the activities of Csk,36 which is recruited at the membrane by VE-cadherin. Accordingly, we found that DMP1 induced the expression of Csk in HUVEC sparse cultures and mimicked the induction of Csk that occurs in confluent cells (Figure 5F). Finally, we observed that, in good accordance with its Src kinase–promoting activity, VEGF induced a significant decrease of Csk whereas DMP1 treatment proved to be able to counteract this effect (Figure 5G). The ability of DMP1 to block VEGF-triggered Src activation through the induction of Csk expression is consistent with its potent antagonistic role on VEGF-induced angiogenesis.
DMP1 inhibits angiogenesis in the CNV model in mice
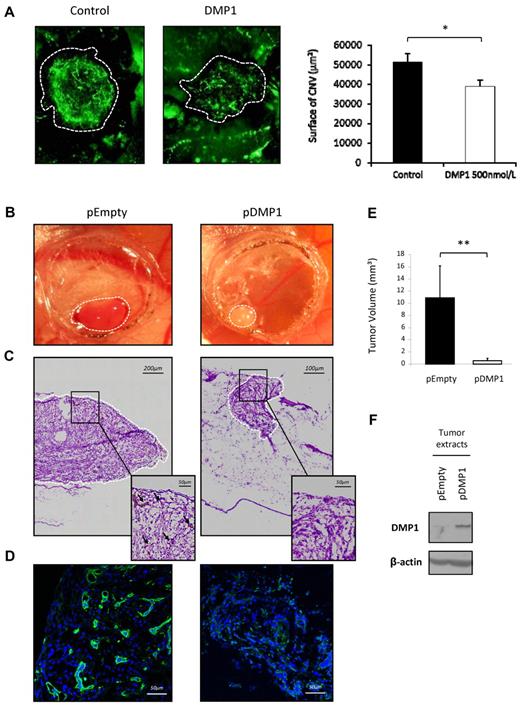
To further investigate the antiangiogenic effect of DMP1, we took advantage of the laser-induced CNV model in which VEGF has been shown to be a major stimulator of subretinal angiogenesis.37 In this experiment, mice were injected intravitreally with DMP1 (500 nmol/L) on the day of the laser injuries. After 7 days, the whole-mount choroids were stained to reveal neoformed blood vessels. As shown in Figure 6A, DMP1 significantly limited the size of neovascularized ocular lesions compared with control lesions. Quantification of the lesions showed a 30% decrease after DMP1 treatment (Figure 6A).
DMP1 impairs in vivo angiogenesis in the CNV model and its overexpression decreases glioma tumor growth and tumor angiogenesis in vivo. (A) Mice were injured by laser shots onto the retina (areas within the dotted lines) and were then subjected to intravitreous injection of DMP1 (500 nmol/L). Eyes were removed after 7 days and the angiogenic response was measured, as described in “Methods.” The quantification represents the measure of total vessel fluorescence surface for each impact. Error bars represent the means ± SEM of 20 impacts of a representative experiment (n = 2). *P ≤ .05 vs control vehicle. (B) The growth of both DMP1-overexpressing (pDMP1) and control (pEmpty) tumors on CAM was documented by biomicroscopy at day 5 after U87-MG cell grafting. A representative tumor for each condition is shown. DMP1 tumors appeared completely white compared with control, in which blood vessels can be seen under the surface. (C) H&E photos of the tumors shown in panel B were taken right under the surface of the tumor (magnification, 40×). Numerous irregular and dilated capillaries (black arrows in the enlarged insert) were visible in control pEmpty tumors but not in DMP1-overexpressing tumors. (D) Specific fluorescein isothiocyanate–lectin staining was used for visualization of blood vessels in the tumors shown in panel B, which confirmed the avascular phenotype of DMP1-overexpressing tumors compared with control pEmpty tumors. Nuclei appear blue after TO-PRO-3 staining (magnification, 40×). (E) Tumor volume was calculated 5 days after grafting for each condition, as described in “Methods.” DMP1 overexpression induced a significant decrease of tumor volume compared with the controls. Results are expressed as the means ± SD of 6 replicates of a representative experiment (n = 3). **P ≤ .005 vs pEmpty. (F) Western blot analysis with an antibody to DMP1 on tumor lysates showing that pDMP1 tumors expressed significantly more DMP1 than control pEmpty tumors.
DMP1 impairs in vivo angiogenesis in the CNV model and its overexpression decreases glioma tumor growth and tumor angiogenesis in vivo. (A) Mice were injured by laser shots onto the retina (areas within the dotted lines) and were then subjected to intravitreous injection of DMP1 (500 nmol/L). Eyes were removed after 7 days and the angiogenic response was measured, as described in “Methods.” The quantification represents the measure of total vessel fluorescence surface for each impact. Error bars represent the means ± SEM of 20 impacts of a representative experiment (n = 2). *P ≤ .05 vs control vehicle. (B) The growth of both DMP1-overexpressing (pDMP1) and control (pEmpty) tumors on CAM was documented by biomicroscopy at day 5 after U87-MG cell grafting. A representative tumor for each condition is shown. DMP1 tumors appeared completely white compared with control, in which blood vessels can be seen under the surface. (C) H&E photos of the tumors shown in panel B were taken right under the surface of the tumor (magnification, 40×). Numerous irregular and dilated capillaries (black arrows in the enlarged insert) were visible in control pEmpty tumors but not in DMP1-overexpressing tumors. (D) Specific fluorescein isothiocyanate–lectin staining was used for visualization of blood vessels in the tumors shown in panel B, which confirmed the avascular phenotype of DMP1-overexpressing tumors compared with control pEmpty tumors. Nuclei appear blue after TO-PRO-3 staining (magnification, 40×). (E) Tumor volume was calculated 5 days after grafting for each condition, as described in “Methods.” DMP1 overexpression induced a significant decrease of tumor volume compared with the controls. Results are expressed as the means ± SD of 6 replicates of a representative experiment (n = 3). **P ≤ .005 vs pEmpty. (F) Western blot analysis with an antibody to DMP1 on tumor lysates showing that pDMP1 tumors expressed significantly more DMP1 than control pEmpty tumors.
Overexpression of DMP1 in glioma tumor cells decreases tumor growth and tumor angiogenesis in vivo
Hagedorn et al27 have established a robust and highly reproducible in vivo human tumor model that allows fast and precise analysis of the main steps of tumor progression and tumor-associated angiogenesis. In this model, the human glioma cells U87-MG grafted onto the vascularized chicken CAM developed into a tumor within a short period of time. DMP1-overexpressing U87-MG cells developed into tumors that were smaller and less vascularized, as they appeared much whiter than fully vascularized control tumors (Figure 6B). H&E staining and specific vasculature immunostaining confirmed that DMP1-overexpressing tumors were significantly less angiogenic than control tumors (Figures 6C-D, respectively). The estimation of tumor volume demonstrated that the experimental gliomas were significantly smaller on DMP1 overexpression (Figure 6E). DMP1 Western blot on glioma tumor extracts validated the overexpression of DMP1 in vivo (Figure 6F).
Discussion
Angiogenesis is the process by which new blood vessels are formed from preexisting vasculature. The present study reports for the first time the specific functional responses elicited by DMP1 on human endothelial cells, and demonstrates a novel biologic role for this SIBLING protein during the angiogenic process. Both receptors known to bind DMP1 are expressed on HUVECs and have been implicated in critical endothelial cell functions. In the present study, we show that CD44 ligation is responsible for DMP1-induced cell-cycle blockade. The mechanism by which DMP1 inhibits endothelial cell growth implicates, at least in part, a CD44-dependent up-regulation of p27Kip1. The ligation of CD44, by either a specific monoclonal antibody or its preferential ligand hyaluronan, has previously been associated with cell-cycle control via p27Kip1 regulation in leukemic cells.38 It is noteworthy that DMP1-mediated up-regulation of p27Kip1 in HUVECs is subsequent to specific VE-cadherin induction. This new finding is in accordance with previous reports showing that E- and N-cadherin mediate antiproliferative responses in the context of contact-induced inhibition of cell growth through p27Kip1 up-regulation.30,31,39 DMP1 increases VE-cadherin surface expression in sparse HUVECs, thereby inducing a mimicry of contact inhibition of the growth mechanism exemplified further by the entry of the cells into the G1 phase of the cell cycle.
In addition to the control of contact-induced inhibition, VE-cadherin is also an important player in capillary tube formation, a specialized endothelial cell function.40 Indeed, cells lacking VE-cadherin are unable to initiate in vitro morphogenesis, defined here as the process whereby endothelial cells assemble into cell cords in a 2D culture (Matrigel).41 DMP1-treated HUVECs demonstrate a precocious and sustained morphogenesis that corresponds well with the reduced cell division and the enhanced attachment and migratory responses observed in the presence of DMP1.
Our exploration of endothelial cell functional responses induced by DMP1 in the presence of VEGF includes cell migration and proliferation, which are essential for more complex processes such as the formation of the endothelial tube network during angiogenesis in vitro and in vivo. Pretreatment of HUVECs with DMP1 significantly blocked all of these responses and let us envisage DMP1 as a new specific inhibitor of VEGF-induced angiogenesis, because DMP1 does not interfere with bFGF-induced proliferation or tubulogenesis.
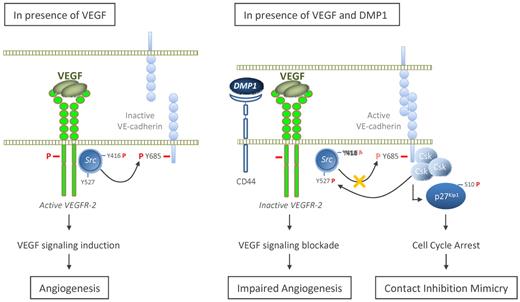
The other major finding of this study was the specific inhibition of VEGFR-2 activity by DMP1. In the presence of DMP1 (Figure 7 right panel), HUVECs no longer responded to the VEGF stimulus. Even though VEGFR-2 expression is increased as a consequence of VE-cadherin induction,42 its phosphorylation no longer occurs and VEGF-induced Src kinase activation is counteracted in DMP1-treated cells. The role for VE-cadherin in modulating downstream signaling of VEGF has been largely recognized,13,15,43 and VEGF-activated Src kinase phosphorylates VE-cadherin and makes it inactive in the control of endothelial functions35 (Figure 7 left panel). DMP1 specifically impairs VEGF-induced Tyr685 phosphorylation through Src activity, which has been exclusively associated with the phosphorylation of this tyrosine.44 Supporting this, DMP1 does not affect VEGF-induced VE-cadherin phosphorylation on other tyrosines such as Tyr658 and Tyr731. The repression of Src is known to be dependent on the activity of Csk when the latter is bound to VE-cadherin Tyr685.16 It is noteworthy that DMP1: (1) partially reverses VEGF-induced Tyr685 phosphorylation, which still allows Csk binding to VE-cadherin, and (2) induces Csk expression, which inactivates Src despite the presence of VEGF. We found that Csk, which has also been involved in cadherin-driven proliferation arrest at high density,16 is expressed at a high level in both confluent and DMP1-treated HUVECs. This observation adds weight to DMP1-induced mimicry of contact inhibition of growth as discussed in the first paragraph of this section.
Model for a role of DMP1 in VEGF-induced signaling. In the presence of VEGF, VEGFR-2 is activated through phosphorylation (bold red “P”). Src is subsequently phosphorylated on Tyr416, whereas it is dephosphorylated on Tyr527, which results in its activation. Active Src thereby inactivates VE-cadherin function through phosphorylation of its intracytoplasmic domain tyrosines, particularly on Tyr685. In the presence of VEGF and on DMP1 binding to CD44, VE-cadherin expression level is increased. On the one hand, this VE-cadherin up-regulation induces p27Kip1 expression and cell-cycle arrest, thus mimicking contact inhibition of growth; on the other hand, active VE-cadherin sequestrates VEGFR-2 and therefore impedes its activation through the inhibition of its phosphorylation. DMP1 pretreatment partially impairs (light red “P”) VE-cadherin phosphorylation on its Tyr685, which still allows the recruitment of Csk. In turn, DMP1 inactivated-VEGFR-2 is not able to phosphorylate further Src on Tyr416, whereas it is phosphorylated on Tyr527 by Csk, the expression of which is induced in the presence of DMP1.
Model for a role of DMP1 in VEGF-induced signaling. In the presence of VEGF, VEGFR-2 is activated through phosphorylation (bold red “P”). Src is subsequently phosphorylated on Tyr416, whereas it is dephosphorylated on Tyr527, which results in its activation. Active Src thereby inactivates VE-cadherin function through phosphorylation of its intracytoplasmic domain tyrosines, particularly on Tyr685. In the presence of VEGF and on DMP1 binding to CD44, VE-cadherin expression level is increased. On the one hand, this VE-cadherin up-regulation induces p27Kip1 expression and cell-cycle arrest, thus mimicking contact inhibition of growth; on the other hand, active VE-cadherin sequestrates VEGFR-2 and therefore impedes its activation through the inhibition of its phosphorylation. DMP1 pretreatment partially impairs (light red “P”) VE-cadherin phosphorylation on its Tyr685, which still allows the recruitment of Csk. In turn, DMP1 inactivated-VEGFR-2 is not able to phosphorylate further Src on Tyr416, whereas it is phosphorylated on Tyr527 by Csk, the expression of which is induced in the presence of DMP1.
In a previous study, we investigated DMP1 expression by immunohistochemistry in human breast cancer tumors.9 SIBLING protein expression is generally associated with bad prognosis and poor survival for cancer patients,1 so we have been a little puzzled by the observation that patients with tumors expressing high levels of DMP1 presented with a better survival than patients with low-DMP1–expressing tumors. In this study, we observed a striking impact of DMP1 on tumor growth and tumor-related angiogenesis in vivo. In fact, the inhibitory effect of DMP1 on both processes was comparable with that of siRNAs directed against VEGF, which was assessed using the same in vivo model in a previous study.26 In light of these new data, it is tempting to speculate that high-DMP1–expressing tumors may be associated with limited neovessel formation, because tumor-secreted DMP1 could favor endothelial cell differentiation at the expense of their proliferation. Arguing for this possibility is the observation that high-DMP1–expressing human tumors are consistently small in size.9
In conclusion, we demonstrate for the first time that DMP1 is implicated in endothelial cell morphogenesis in vitro, indicating that secreted extracellular matrix proteins are endowed with specific functions that influence the dynamic balance controlling vessel growth. The other outcome of this study is that DMP1 induces the up-regulation of VE-cadherin and VEGFR-2 inactivation, specifically leading to impaired VEGF-mediated wound healing and tumor-associated angiogenesis in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. Heneaux, V. Hennequière, and N. Maloujahmoum for their expert technical assistance.
S.P. is a Télévie Research Fellow, S.O. is a logistic research worker, D.M. is a research associate, and A.B. is a senior research associate, all with the National Fund for Scientific Research (FNRS). This work was supported by grants from FNRS, Télévie, Center Anti-Cancéreux, Fonds Léon Fredericq, Direction Générale des Technologies, de la Recherche et de l'Énergie and SPW-DG06 Convention no. 0616476 “NéoAngio” from the “Région Wallonne,” the European Union Framework Program FP7-projects, and the University of Liège.
Authorship
Contribution: S.P. designed and performed the research, analyzed the data, and wrote the manuscript; V. Lamour designed and performed the CAM model, analyzed the data, and reviewed the manuscript; V. Lambert and M.-L.A.G. designed and performed the CNV model, analyzed the data, and reviewed the manuscript; S.O. provided miscroscopy expertise and reviewed the manuscript; A.N. designed the CNV model and critically reviewed the manuscript; D.M. analyzed the data and critically reviewed the manuscript; V.C. designed the research, analyzed the data, and critically reviewed the manuscript; and A.B. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akeila Bellahcène, Metastasis Research Laboratory, GIGA-Cancer, Pathology Tour, B23, level +4, 4000 Liège, Belgium; e-mail: A.Bellahcene@ulg.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal