Abstract
During a systemic inflammatory response endothelial-expressed surface molecules have been strongly implicated in orchestrating immune responses. Previous studies have shown enhanced extracellular nucleotide release during acute inflammatory conditions. Therefore, we hypothesized that endothelial nucleotide receptors could play a role in vascular inflammation. To address this hypothesis, we performed screening experiments and exposed human microvascular endothelia to inflammatory stimuli, followed by measurements of P2Y or P2X transcriptional responses. These studies showed a selective induction of the P2Y6 receptor (> 4-fold at 24 hours). Moreover, studies that used real-time reverse transcription–polymerase chain reaction, Western blot analysis, or immunofluorescence confirmed time- and dose-dependent induction of P2Y6 with tumor necrosis factor α or Lipopolysaccharide (LPS) stimulation in vitro and in vivo. Studies that used MRS 2578 as P2Y6 receptor antagonist showed attenuated nuclear factor κB reporter activity and proinflammatory gene expression in human microvascular endothelial cells in vitro. Moreover, pharmacologic or genetic in vivo studies showed attenuated inflammatory responses in P2Y6−/− mice or after P2Y6 antagonist treatment during LPS-induced vascular inflammation. These studies show an important contribution of P2Y6 signaling in enhancing vascular inflammation during systemic LPS challenge and implicate the P2Y6 receptor as a therapeutic target during systemic inflammatory responses.
Introduction
Vascular responses contribute significantly to inflammatory disorders such as systemic inflammatory response syndrome, sepsis, or acute lung injury.1-4 Because of its large surface area and its anatomic position at the interface between the blood stream and surrounding tissues, the vascular endothelium has an exquisite regulatory function in orchestrating acute inflammatory responses. In fact, inflammatory cells such as phagocytes or lymphocytes can emigrate from the bloodstream in response to molecular changes on the surface of blood vessels that signal injury or infection. For example, ∼ 70 million polymorphonuclear neutrophils exit the vasculature per minute. These inflammatory cells move into underlying tissue by initially passing between endothelial cells that line the inner surface of blood vessels. This process, referred to as transendothelial migration is particularly prevalent in inflamed tissues. Although several studies suggested that specific molecules may establish “bottlenecks” to the control of the inflammatory response of the vasculature, only limited information exists about the biochemical events that initialize and dynamically regulate vascular inflammation.
Under pathologic conditions such as acute inflammation, ischemia, or hypoxia extracellular nucleotides are released by multiple cell types.5,6 For example, vascular endothelia, platelets, erythrocytes, or inflammatory cells can release nucleotides. Moreover, activation of extracellular nucleotide receptors (P2X receptors, ligand-gated ion channels; P2Y receptors, G protein–coupled receptors) has been implicated in driving an inflammatory phenotype in different disease models.7 As such, studies in human and in mice implicate P2 receptor activation in pulmonary inflammation in the context of asthma8 or chronic lung injury.9 However, the role of nucleotide signaling during a systemic inflammatory response syndrome or sepsis remains largely unknown.
On the basis of these findings, we hypothesized that vascular inflammation could drive transcriptional alterations of P2 receptors involved in the dynamic regulation of vascular inflammation. Consistent with this hypothesis, we found a selective induction of endothelial-expressed P2Y6 receptors on tumor necrosis factor α (TNF-α), lipopolysaccharide (LPS), and interleukin-1α (IL-1α) exposure in vitro. Moreover, combination of pharmacologic and genetic studies showed a surprising role for P2Y6 signaling in enhancing vascular inflammatory responses during systemic LPS challenge.
Methods
An expanded section for the described methods can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell culture and inflammatory stimulation
The human microvascular endothelial cell line (HMEC-1) and human coronary artery endothelial cells (HCAECs) were cultured as previously described.10-14
Transcriptional analysis
Transcriptional analysis was performed as described previously.15-18 Primer sets and annealing temperatures are summarized in supplemental Table 1.
Microarray
Confluent HMEC-1 cells were exposed to 100 ng/mL recombinant human TNF-α (Promokine) for 4 and 24 hours. Three separate Petri dishes were pooled, and total RNA was isolated. Agilent Whole Human Genome Oligo Microarrays 4 × 44K were performed and analyzed by Miltenyi Biotec. Microarray data were accepted and published on Gene Expression Omnibus, National Center for Biotechnology Information, submission number 18102, and can be found at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18102.
Immunoblotting experiments
HMEC-1 cells were grown to confluence and were exposed at the indicated times to 10 ng/mL TNF-α (Promokine). Immunoblotting experiments were performed by a slight modification of methods as described previously.14
Evaluation of nuclear factor κB activity
We used reporter assays to assess nuclear factor κB (NF-κB) activity. To measure the transcriptional activity of NF-κB, endothelial cells were plated in 24-well plates at a density of 2.5 × 104 cells/well and were allowed to adhere overnight. The monolayers were then transfected with 0.25 μg of either NF-κB promoter reporter (Clontech) or control pGL3 vector for 4 hours with the use of GeneJuice Transfection Reagent (Novagen/Merck) according to the manufacturer's instructions. Cells were exposed to the P2Y6 receptor antagonist MRS 2578 (Tocris) or solvent (dimethyl sulfoxide [DMSO]) for 30 minutes. Subsequently, 10 ng/mL TNF-α were added for another 2 hours. At the end of the incubation period, cells were washed twice in ice-cold phosphate-buffered saline, and luciferase activity was measured with the use of the Luciferase Assay System (Promega). For normalization protein concentration was determined with the use of a BCA Protein Assay kit (Pierce Protein Research Products, Thermo Scientific).
Repression of inflammatory cytokine mRNA by MRS 2578
HMEC-1 cells were preincubated with 10μM MRS 2578 for 30 minutes. TNF-α (10 ng/mL) was added, and cells were lysed after indicated time points. mRNA levels of NF-κB–induced genes were determined with the use of the primer sets summarized in supplemental Table 1.
Immunofluorescence
HCAECs were seeded on glass coverslips and stimulated with 10 ng/mL TNF-α. After 24 hours of incubation, cells were fixed and permeabilized with Triton-X for 2 minutes. Cells were washed and blocked with 0.1% Tris (tris(hydroxymethyl)aminomethane)–buffered saline and Tween (TBST) and 3% skim milk for 1 hour. Anti–human P2Y6 receptor antibody (Santa Cruz Biotechnology) was administered for 2 hours. Cells were washed and incubated with Alexa Fluor 488 goat anti–rabbit immunoglobulin G antibody (Invitrogen) for 45 minutes. After that, cells were washed and mounted in 20 μL of Prolong Gold Antifade Reagent (Invitrogen).
Tissue slices of mouse aorta were obtained from samples set in paraffin. After deparaffinization, slides were boiled in citrate buffer in the microwave for 3 minutes. Probes were blocked with 3% bovine serum albumin in 0.5% TBST for 2 hours at room temperature. Slides were washed and then either incubated with Santa Cruz P2Y6 receptor goat anti–mouse antibody (concentration 4 μg/mL) or Santa Cruz–negative control normal goat immunoglobulin G (concentration 4 μg/mL) or TBST only for 2 hours at room temperature. Probes were washed and incubated with Alexa Fluor 488 donkey anti–goat antibody (Invitrogen) for 1 hour. After that, probes were washed and mounted in 20 μL of Prolong Gold Antifade Reagent (Invitrogen).
Murine endotoxinemia model
C57BL/6 mice (Charles River Laboratories) or previously described P2Y6−/− mice on C57BL/6 background19 or corresponding littermate controls matched in age, sex, and weight were used. Mice were anesthetized, and 300 μg of LPS (Escherichia coli O26:B6; Sigma) or vehicle were injected into the jugular vein. Where indicated, 100 μL of 10μM antagonist MRS 2578 were given before and 1 hour after the application of LPS. All animal studies were approved by the local animal ethics committee (Regierungspräsidium Tübingen or Freiburg) and performed according to the respective guidelines.
Data analysis
Statistical analysis was performed by Student t test with the use of GraphPad Prism 5. Values are expressed as mean ± SD from ≥ 3 separate experiments.
Results
The endothelial P2Y6 receptor is selectively induced after TNF-α exposure
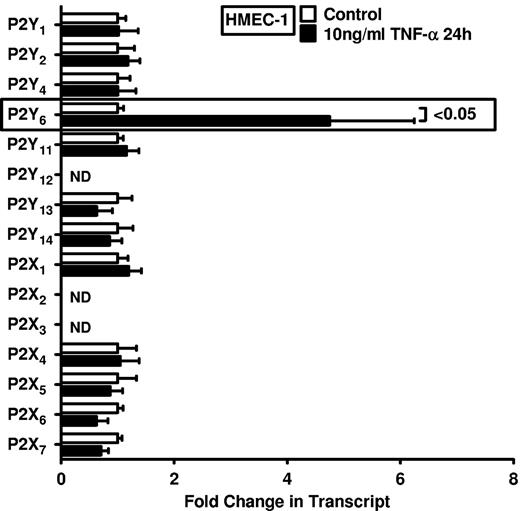
On the basis of studies showing enhanced nucleotide release during conditions of inflammation, ischemia, or hypoxia,5,6,11,20-22 and the known role of P2 receptors in driving inflammatory events,8,9,23-25 we pursued transcriptional responses of previously described nucleotide receptors in vascular endothelia. We gained first insight from a microarray study in HMEC-1 endothelia that were exposed to 100 ng/mL TNF-α. For the purpose of studying extracellular nucleotide signaling, we compiled all data for known nucleotide P2Y and P2X receptors (Table 1; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18102). Surprisingly, these studies showed a selective induction of the P2Y6 receptor transcript after TNF-α stimulation. As such, this screening experiment pointed us toward a potential role of P2Y6 as a candidate receptor for nucleotide-elicited alteration of vascular inflammation.
Microarray results of endothelial P2 receptor expression after TNF-α stimulation
| Gene . | TNF-α for 4 h (absolute fold change) . | TNF-α for 24 h (absolute fold change) . | Agilent array designation . |
|---|---|---|---|
| P2RY1 | 1.00 | 1.00 | A_23_P382835 |
| P2RY2 | 1.22 | 1.24 | A_23_P24903 |
| P2RY4 | 1.24 | 1.08 | A_23_P114307 |
| P2RY6 | 1.50 | 2.06* | A_23_P64611 |
| P2RY11 | 1.21 | 1.11 | A_23_P4696 |
| P2RY12 | 1.07 | 1.51 | A_23_P143902 |
| P2RY13 | 1.00 | 1.00 | A_23_P211948 |
| P2RY14 | 1.00 | 1.00 | A_24_P165864 |
| P2RX1 | 1.09 | 1.04 | A_23_P372848 |
| P2RX2 | 1.04 | 1.14 | A_23_P124003 |
| P2RX3 | 1.01 | 1.08 | A_23_P127721 |
| P2RX4 | 1.21 | 1.17 | A_23_P53623 |
| P2RX5 | 1.24 | 1.19 | A_23_P413760 |
| P2RX6 | 1.00 | 1.00 | A_23_P355980 |
| P2RX7 | 1.38 | 1.00 | A_24_P319113 |
| Gene . | TNF-α for 4 h (absolute fold change) . | TNF-α for 24 h (absolute fold change) . | Agilent array designation . |
|---|---|---|---|
| P2RY1 | 1.00 | 1.00 | A_23_P382835 |
| P2RY2 | 1.22 | 1.24 | A_23_P24903 |
| P2RY4 | 1.24 | 1.08 | A_23_P114307 |
| P2RY6 | 1.50 | 2.06* | A_23_P64611 |
| P2RY11 | 1.21 | 1.11 | A_23_P4696 |
| P2RY12 | 1.07 | 1.51 | A_23_P143902 |
| P2RY13 | 1.00 | 1.00 | A_23_P211948 |
| P2RY14 | 1.00 | 1.00 | A_24_P165864 |
| P2RX1 | 1.09 | 1.04 | A_23_P372848 |
| P2RX2 | 1.04 | 1.14 | A_23_P124003 |
| P2RX3 | 1.01 | 1.08 | A_23_P127721 |
| P2RX4 | 1.21 | 1.17 | A_23_P53623 |
| P2RX5 | 1.24 | 1.19 | A_23_P413760 |
| P2RX6 | 1.00 | 1.00 | A_23_P355980 |
| P2RX7 | 1.38 | 1.00 | A_24_P319113 |
Human microvascular endothelial cells (HMEC-1) were exposed to 100 ng/mL TNF-α for 24 hours. For each microarray, 3 different samples were pooled. RNA was isolated, and an Agilent Whole Genome Microarray was performed.
P of log ratio < .05.
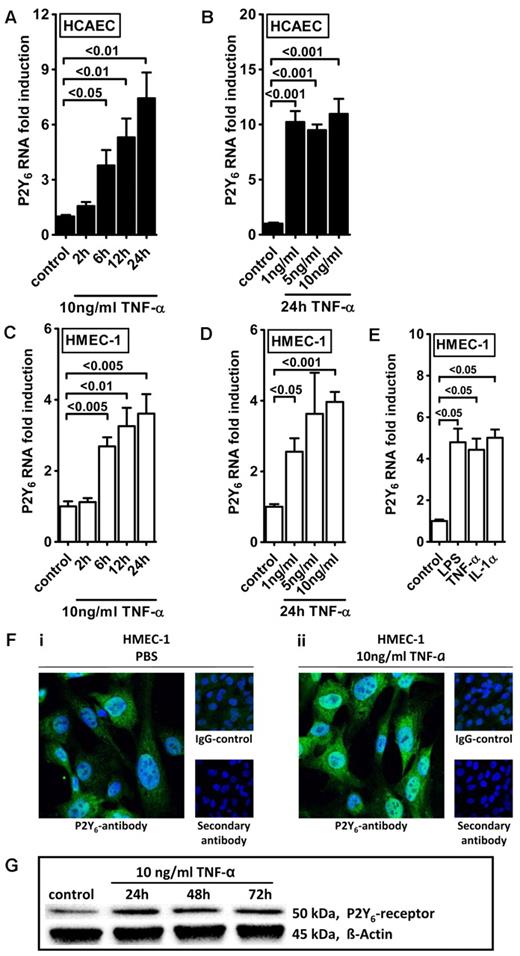
Intrigued by this observation, we used real-time reverse transcription–polymerase chain reaction (RT-PCR) to confirm our initial microarray findings. For this purpose, we stimulated HMEC-1 cells with TNF-α over 24 hours and assessed transcript levels of all known P2 receptors (Figure 1). Consistent with our microarray studies, we observed a selective and robust (4.2 ± 2; P < .05) induction of P2Y6 receptor transcript. To confirm these initial screening experiments, we exposed cultured endothelial cell lines (HMEC-1 and HCAEC) to different doses and time periods of TNF-α stimulation and assessed P2Y6 transcriptional responses. Here, we found time- and dose-dependent increases of P2Y6 transcript levels in HCAECs (Figure 2A-B) and in HMEC-1 cells (Figure 2C-D). Moreover, P2Y6 receptor induction was also observed on IL-1α or LPS exposure of HMEC-1 cells (Figure 2E). In addition, P2Y6 protein levels after inflammatory stimulation of vascular endothelia were elevated with the use of immunofluorescence (Figure 2F) or Western blotting (Figure 2G).
Endothelial P2 receptor expression after inflammatory stimulation. HMEC-1 cells were exposed to 10 ng/mL TNF-α for 24 hours. Receptor expression levels of the 15 known P2 receptors were determined in controls and TNF-α–stimulated cells by real-time RT-PCR. Data were calculated relative to the internal housekeeping gene β-actin and are expressed as mean ± SD fold change compared with the control (without TNF-α) (n = 3-4). Primers and PCR conditions are summarized in the supplemental data.
Endothelial P2 receptor expression after inflammatory stimulation. HMEC-1 cells were exposed to 10 ng/mL TNF-α for 24 hours. Receptor expression levels of the 15 known P2 receptors were determined in controls and TNF-α–stimulated cells by real-time RT-PCR. Data were calculated relative to the internal housekeeping gene β-actin and are expressed as mean ± SD fold change compared with the control (without TNF-α) (n = 3-4). Primers and PCR conditions are summarized in the supplemental data.
Endothelial P2Y6 receptor up-regulation after TNF-α stimulation. HCAECs (A-B) or HMEC-1 cells (C-D) were exposed to TNF-α for the indicated time periods or with indicated doses. P2Y6 receptor transcript was determined by real-time RT-PCR. Data were calculated relative to the internal housekeeping gene (β-actin) and are expressed as mean ± SD fold change compared with controls (without TNF-α) (n = 3-4). (E) HMEC-1 cells were exposed to LPS, IL-1α, and TNF-α for 24 hours. (F) HCAECs were grown to confluence on cover glasses and exposed to 10 ng/mL TNF-α for 24 hours. Cell layers were stained with antibodies specific for human P2Y6 receptor and Alexa Fluor 488–coupled secondary antibody (green) or isotype controls and Alexa Fluor 488–coupled secondary antibody or Alexa Fluor 488–coupled secondary antibody only. DAPI (4′,6-diamidino-2-phenylindole) was used as nuclear counterstain (blue). Slides were kept on ice before image acquisition. Probes were analyzed by confocal microscopy with the use of Zeiss Laser Scanning Microscope LSM 710 and the Plan-Apochromat 63×/1.40 Oil Dic M27 objective lens with oil immersion. Zen software was used for acquisition and image processing (γ adjustment) applied equally to all images. One representative image of 3 is displayed. (G) HMEC-1 cells were exposed to TNF-α for the indicated time periods, and P2Y6 receptor protein was determined by Western blotting. The same blot was stripped and reprobed for human β-actin as a control for protein loading. One of 3 representative Western blots is displayed.
Endothelial P2Y6 receptor up-regulation after TNF-α stimulation. HCAECs (A-B) or HMEC-1 cells (C-D) were exposed to TNF-α for the indicated time periods or with indicated doses. P2Y6 receptor transcript was determined by real-time RT-PCR. Data were calculated relative to the internal housekeeping gene (β-actin) and are expressed as mean ± SD fold change compared with controls (without TNF-α) (n = 3-4). (E) HMEC-1 cells were exposed to LPS, IL-1α, and TNF-α for 24 hours. (F) HCAECs were grown to confluence on cover glasses and exposed to 10 ng/mL TNF-α for 24 hours. Cell layers were stained with antibodies specific for human P2Y6 receptor and Alexa Fluor 488–coupled secondary antibody (green) or isotype controls and Alexa Fluor 488–coupled secondary antibody or Alexa Fluor 488–coupled secondary antibody only. DAPI (4′,6-diamidino-2-phenylindole) was used as nuclear counterstain (blue). Slides were kept on ice before image acquisition. Probes were analyzed by confocal microscopy with the use of Zeiss Laser Scanning Microscope LSM 710 and the Plan-Apochromat 63×/1.40 Oil Dic M27 objective lens with oil immersion. Zen software was used for acquisition and image processing (γ adjustment) applied equally to all images. One representative image of 3 is displayed. (G) HMEC-1 cells were exposed to TNF-α for the indicated time periods, and P2Y6 receptor protein was determined by Western blotting. The same blot was stripped and reprobed for human β-actin as a control for protein loading. One of 3 representative Western blots is displayed.
P2Y6 receptor antagonist MRS 2578 dampens mediator-induced inflammation of vascular endothelia in vitro
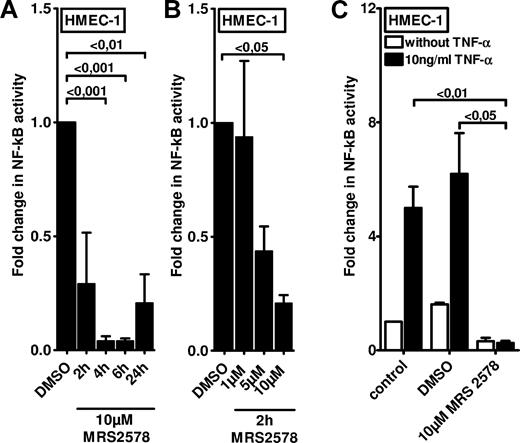
After having shown that P2Y6 receptor transcript and protein expressions are selectively increased on inflammatory stimulation, we next studied functional consequences of endothelial P2Y6 signaling in vitro. As in the in vitro model, we transfected HMEC-1 cells with a NF-κB reporter plasmid containing the binding sites for p50/p65. Treatment of HMEC-1 cells with the P2Y6 agonist uridine diphosphate only resulted in a modest increase in NF-κB activity (1.64-fold ± 0.45[fold; P < .05; supplemental Figure 1). However, and consistent with previous studies implicating P2Y6 signaling in NF-κB activity,26,27 we observed a profound inhibition of basal NF-κB activity in the presence of the P2Y6 antagonist MRS 2578. This reduction of NF-κB activity was time (Figure 3A) and dose (Figure 3B) dependent. As such, these studies indicate that downstream targets of P2Y6 activation are necessary but not sufficient for NF-κB activation and speak for an indirect effect in enhancing vascular inflammation.
Effect of P2Y6 receptor antagonist MRS 2578 on endothelial NF-κB activity. To measure the NF-κB activity, vascular endothelia (HMEC-1) were transfected with 0.25 μg of either NF-κB promoter reporter (Clontech) or control pGL3 vector. Cells were exposed to the P2Y6 receptor antagonist MRS 2578 either with or without TNF-α for the indicated time periods. As readout for NF-κB activity cells were lysed, and luciferase activity was determined relative to the total protein concentration. (A-B) Unstimulated NF-κB activity after exposure to indicated times or concentrations of MRS 2578. (C) NF-κB activity after stimulation with MRS 2578 (30 minutes) and after stimulation with TNF-α (10 ng/mL) for 2 hours. Results are displayed as mean ± SD (n = 3).
Effect of P2Y6 receptor antagonist MRS 2578 on endothelial NF-κB activity. To measure the NF-κB activity, vascular endothelia (HMEC-1) were transfected with 0.25 μg of either NF-κB promoter reporter (Clontech) or control pGL3 vector. Cells were exposed to the P2Y6 receptor antagonist MRS 2578 either with or without TNF-α for the indicated time periods. As readout for NF-κB activity cells were lysed, and luciferase activity was determined relative to the total protein concentration. (A-B) Unstimulated NF-κB activity after exposure to indicated times or concentrations of MRS 2578. (C) NF-κB activity after stimulation with MRS 2578 (30 minutes) and after stimulation with TNF-α (10 ng/mL) for 2 hours. Results are displayed as mean ± SD (n = 3).
Next, we examined if NF-κB activation by proinflammatory mediators (eg, TNF-α) was attenuated after pharmacologic inhibition of the P2Y6 receptor. For this purpose, we again used HMEC-1 endothelia that were transfected with a NF-κB reporter. Although treatment with 10 ng/mL TNF-α significantly increased NF-κB reporter activity in media alone or in vehicle-treated cells (DMSO), this response was completely abolished in HMEC-1 cells pretreated with the P2Y6 receptor antagonist MRS 2578 (Figure 3C). Together, these studies show a profound inhibition of TNF-α–induced NF-κB activation after pharmacologic blockade of the P2Y6 receptor.
After these studies, we examined the effect of treatment with the P2Y6 receptor antagonist on TNF-α–induced expression of proinflammatory cytokines and adhesion molecules. For this purpose we examined the transcript levels of IL-8 (Figure 4A), vascular cell adhesion molecule 1 (VCAM-1; Figure 4B), and intercellular adhesion molecule 1 (Figure 4C) after TNF-α exposure. In fact, these studies showed significant reduction of TNF-α–induced proinflammatory gene expression with pharmacologic inhibition of P2Y6. Taken together, these studies show that inhibition of P2Y6 dampens the inflammatory response of vascular endothelia in vitro.
Effect of P2Y6 receptor antagonist MRS 2578 on TNF-α–induced gene expression in vascular endothelia. Vascular endothelia (HMEC-1) were pretreated with vehicle (DMSO) or MRS 2578 (10μM) and after 30 minutes were exposed to TNF-α (10 ng/mL). Transcript levels of IL-8 (A), VCAM (B), or intercellular adhesion molecule (C) were determined by real-time RT-PCR relative to housekeeping gene β-actin 2 hours after TNF-α exposure (n = 4).
Effect of P2Y6 receptor antagonist MRS 2578 on TNF-α–induced gene expression in vascular endothelia. Vascular endothelia (HMEC-1) were pretreated with vehicle (DMSO) or MRS 2578 (10μM) and after 30 minutes were exposed to TNF-α (10 ng/mL). Transcript levels of IL-8 (A), VCAM (B), or intercellular adhesion molecule (C) were determined by real-time RT-PCR relative to housekeeping gene β-actin 2 hours after TNF-α exposure (n = 4).
P2Y6 receptor is induced in vivo after intravenous LPS treatment
After having shown a selective induction of the P2Y6 receptor in conjunction with a functional role in vascular inflammation in vitro, we next examined changes in P2Y6 receptor expression levels under vascular inflammatory conditions in vivo. As a murine model of vascular inflammation, we treated mice with an intravenous injection of 300 μg of LPS or vehicle given into the jugular vein and examined P2Y6 expression patterns at 24 hours after LPS treatment. Examination of P2Y6 transcript levels showed a robust induction of P2Y6 in vascular organs such as the kidney (Figure 5A) and the heart (Figure 5B). Moreover, immunofluorescence of the abdominal aorta (Figure 5C) showed vascular induction of the P2Y6 receptor after intravenous LPS treatment. Although these studies showed induction of the P2Y6 receptor in multiple cells (eg, vascular smooth muscle cells), this response was most prominent in the vascular endothelia.
P2Y6 receptor expression after intravenous LPS treatment and attenuated inflammatory responses in P2Y6−/− mice after intravenous LPS exposure. (A-B) C57BL/6 mice received an intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (phosphate-buffered saline [PBS]). Induction of P2Y6 mRNA was seen in kidney (A) and heart (B). (C) C57BL/6 mice received an intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS). Abdominal aorta was stained with antibodies specific for murine P2Y6 receptor and Alexa Fluor 488–coupled secondary antibody (green) or isotype controls and Alexa Fluor 488–coupled secondary antibody or Alexa Fluor 488–coupled secondary antibody only. DAPI (4′,6-diamidino-2-phenylindole) was used as nuclear counterstain (blue). Slides were kept on ice before image acquisition. Probes were analyzed by confocal microscopy with the use of Zeiss Laser Scanning Microscope LSM 710 and the Plan-Apochromat 20×/0.8 M27 objective lens with oil immersion. Zen software was used for acquisition and image processing (γ adjustment) applied equally to all images. White arrows indicate luminal side. One representative image of 3 is displayed. (D) Determination of plasma KC levels by enzyme-linked immunoabsorbent assay (ELISA) in C57Bl/6 wild-type mice treated with MRS 2578 and DMSO (solvent of MRS 2578) 2 hours after intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS) (wild-type control, n = 8; wild-type LPS, n = 8; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 6). (E) Determination of plasma KC levels by ELISA in C57Bl/6 wild-type versus P2Y6−/− mice 2 hours after intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS) (wild-type control, n = 10; wild-type LPS, n = 10; P2Y6−/− control, n = 8; P2Y6−/− LPS, n = 14). (F-G) Two hours after the LPS injection, mice were killed, and vascular organs (heart, kidney) were harvested. Transcript levels of VCAM were determined by real-time RT-PCR relative to the housekeeping gene β-actin in vascular organs (F) kidney (control, n = 3; LPS, n = 6) or (G) heart (control, n = 4; LPS, n = 6). All results are displayed as mean ± SD. (H-I) Determination of Evans blue concentration in kidney and heart tissue of C57Bl/6 wild-type versus P2Y6−/− mice 2 hours after intravenous challenge with 300 μg of LPS. (H) Kidney (wild-type control, n = 4; wild-type LPS, n = 10; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 12). (I) Heart (wild-type control, n = 4; wild-type LPS, n = 10; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 11).
P2Y6 receptor expression after intravenous LPS treatment and attenuated inflammatory responses in P2Y6−/− mice after intravenous LPS exposure. (A-B) C57BL/6 mice received an intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (phosphate-buffered saline [PBS]). Induction of P2Y6 mRNA was seen in kidney (A) and heart (B). (C) C57BL/6 mice received an intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS). Abdominal aorta was stained with antibodies specific for murine P2Y6 receptor and Alexa Fluor 488–coupled secondary antibody (green) or isotype controls and Alexa Fluor 488–coupled secondary antibody or Alexa Fluor 488–coupled secondary antibody only. DAPI (4′,6-diamidino-2-phenylindole) was used as nuclear counterstain (blue). Slides were kept on ice before image acquisition. Probes were analyzed by confocal microscopy with the use of Zeiss Laser Scanning Microscope LSM 710 and the Plan-Apochromat 20×/0.8 M27 objective lens with oil immersion. Zen software was used for acquisition and image processing (γ adjustment) applied equally to all images. White arrows indicate luminal side. One representative image of 3 is displayed. (D) Determination of plasma KC levels by enzyme-linked immunoabsorbent assay (ELISA) in C57Bl/6 wild-type mice treated with MRS 2578 and DMSO (solvent of MRS 2578) 2 hours after intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS) (wild-type control, n = 8; wild-type LPS, n = 8; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 6). (E) Determination of plasma KC levels by ELISA in C57Bl/6 wild-type versus P2Y6−/− mice 2 hours after intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS) (wild-type control, n = 10; wild-type LPS, n = 10; P2Y6−/− control, n = 8; P2Y6−/− LPS, n = 14). (F-G) Two hours after the LPS injection, mice were killed, and vascular organs (heart, kidney) were harvested. Transcript levels of VCAM were determined by real-time RT-PCR relative to the housekeeping gene β-actin in vascular organs (F) kidney (control, n = 3; LPS, n = 6) or (G) heart (control, n = 4; LPS, n = 6). All results are displayed as mean ± SD. (H-I) Determination of Evans blue concentration in kidney and heart tissue of C57Bl/6 wild-type versus P2Y6−/− mice 2 hours after intravenous challenge with 300 μg of LPS. (H) Kidney (wild-type control, n = 4; wild-type LPS, n = 10; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 12). (I) Heart (wild-type control, n = 4; wild-type LPS, n = 10; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 11).
Pharmacologic inhibition or genetic deletion of the P2Y6 receptor convey protection from LPS-induced inflammation
After having shown that murine P2Y6 receptor is induced after systemic LPS treatment, we next pursued pharmacologic and genetic studies to address a functional in vivo role of P2Y6 signaling. Here, we exposed wild-type mice treated with the P2Y6 receptor antagonist MRS 2578 or vehicle (DMSO) to intravenous LPS (300 μg) and compared corresponding serum keratinocyte derived chemokine levels by enzyme-linked immunoabsorbent assay. We observed that KC serum protein levels were dramatically increased in LPS-treated wild-type mice exposed to vehicle alone. In contrast, serum KC elevations were attenuated in mice treated with the P2Y6 receptor antagonist (Figure 5D).
Next, we examined previously described gene-targeted mice for P2Y619 or littermate control mice matched in weight, sex, and age. Two hours after administering 300 μg of intravenous LPS serum KC levels examined by enzyme-linked immunoabsorbent assay were significantly lower in P2Y6−/− mice than in the corresponding control mice (Figure 5E). Next, we examined transcript levels of VCAM-1 in vascular organs of LPS-treated P2Y6−/− mice or littermate controls matched in age, sex, and weight. In fact, these studies showed that renal (Figure 5F) and cardiac (Figure 5G) VCAM-1 levels were induced in LPS-treated control animals 2 hours after exposure to LPS. Importantly, however, increased VCAM-1 expression was almost completely abolished in P2Y6−/− mice. As additional readout for vascular inflammatory responses we used the albumin marker Evans blue. Here, we observed that LPS-induced increases in albumin leakage into vascular organs were significantly attenuated in P2Y6−/− mice (kidney, Figure 5H; heart, Figure 5F).Taken together, these studies provide genetic and pharmacologic in vivo evidence for a role of P2Y6 signaling in vascular inflammation after LPS treatment.
Discussion
Although research work over the past decades has clearly identified several groups of vascular inflammatory proteins that regulate inflammatory cell trafficking across the endothelial monolayer, biochemical processes that govern vascular inflammatory responses are less clearly defined. On the basis of the observation that extracellular nucleotide levels are dramatically increased after inflammatory stimulation, ischemia, or hypoxia, we pursued the hypothesis that extracellular nucleotide receptor signaling could play a role in vascular inflammation. Initial microarray studies pointed us toward a selective induction of the P2Y6 receptor after inflammatory stimulation. With the use of real-time RT-PCR and Western blotting, we were able to confirm a robust induction of P2Y6 transcript and protein levels on inflammatory stimulation of model endothelia. Moreover, functional in vitro and in vivo studies combining pharmacologic or genetic approaches have shown a role of P2Y6 signaling in enhancing vascular inflammation. Taken together, these studies provide evidence for a selective role of the P2Y6 receptor in enhancing vascular inflammatory responses and implicate the P2Y6 antagonist as a treatment form for conditions characterized by excessive vascular inflammation.
Although the present studies clearly indicate that endothelial P2Y6 receptors are transcriptionally induced on inflammatory stimulation, the transcriptional mechanism remains unknown. This could at least in part be related to the diversity of P2Y6 receptor variants. In fact, 4 variants of the P2Y6 receptor have been identified [(1) NM_176797.1, (2) NM_176798.1, (3) NM_176796.1, (4) NM_004154.3; http://www.ncbi.nlm.nih.gov/gene/5031; supplemental Figure 2A]. Studies that used different RT-PCR primers (supplemental Figure 2B) indicate the likelihood that all 4 variants are induced by inflammatory stimuli (supplemental Figure 2C). However, only P2Y6 receptor variant 1 and variant 3 share homology in their promoter region (supplemental Figure 3). In fact, transcription factor binding searches indicate binding sites for different transcription factors for the individual variants (eg, only the promoter of variant 2 contains a binding site for NF-κB), indicating that the transcriptional regulation of P2Y6 during inflammatory conditions is complex and could involve different transcription factors.
Consistent with the present results, a previous study addressed the role of nucleotide receptor signaling in epithelial inflammation.28 That study determined the effect of intestinal inflammation on P2Y6 receptor expression by PCR in the mouse, rat, and human. The investigators of that study found that epithelial inflammation induces epithelial P2Y2 and P2Y6 receptors in the mucosa of the colon of colitic mice. As such, that study was among the first to show a role of P2Y receptors in the inflammatory response of intestinal epithelia. Other studies have shown a role of P2Y6 receptor activation in enhancing NF-κB activity in osteoclasts and an associated increase in their survival.29 Further studies have shown that P2Y6 receptor activation mediates the release of proinflammatory cytokines and chemokines in monocytic cells.19,30 Taken together, these studies are consistent with the present in vitro and in vivo findings that implicate P2Y6 signaling in vascular inflammation. In fact, it is conceivable that enhanced inflammatory responses could also involve cross-talk pathways between epithelial cells and vascular endothelia.31 For instance, nucleotide release has been previously implicated in the cross talk between different cell types.5,11 Moreover, it is somewhat unclear whether the role of P2Y6 in inflammation and barrier function is direct or indirect. The fact that we observed only mild increases in inflammatory activation with P2Y6 agonist treatment of cultured endothelia (see supplemental Figure 1) argues for an indirect effect. As such, P2Y6 signaling could represent an additional mechanism for “fine-tuning” inflammatory pathways in the setting of a systemic stimulus (eg, LPS).
Although the present studies show that activation of the P2Y6 receptor can enhance vascular inflammation, extracellular nucleotides also represent the metabolic substrate for extracellular generation of adenosine and uridine. In fact, extracellular adenosine is known to be an important anti-inflammatory signaling molecule. This paradigm goes back to studies showing an anti-inflammatory effect of adenosine signaling on inflammatory cells.32,33 As such, adenosine triphosphate (ATP) or adenosine diphosphate (ADP) can be rapidly hydrolyzed to adenosine in a 2-step enzymatic process that involves the ectonucleoside triphosphate diphosphohydrolase 1 (CD39; conversion of ATP/ADP to adenosine monophosphate [AMP]) 11,18,21,22,34 and the ecto-5′-nucleotidase (CD73; conversion of AMP to adenosine).34-39 Several studies have implicated extracellular adenosine signaling in attenuating acute inflammatory responses during conditions such as hypoxia-induced vascular leakage and pulmonary edema,10,11,13,40 sepsis,41,42 or acute lung injury.15,16,42,43 As such, experimental approaches that will enhance extracellular nucleotide breakdown and their subsequent conversion to adenosine will simultaneously dampen activation of nucleotide receptors (such as the P2Y6) and increase extracellular adenosine signaling. Consistent with this concept, previous studies have shown that treatment with apyrase (enhances ATP/ADP phosphohydrolysis) or nucleotidase (enhances AMP phosphohydrolysis) are effective in the treatment of acute inflammatory disease such as acute lung injury,2,3,15,43,44 hypoxia-induced vascular permeability changes,39 or organ ischemia.21,22,36-38,45 Interestingly, also extracellular uridine (derived from the P2Y receptor agonist uridine triphosphate or uridine diphosphate) is implicated as an anti-inflammatory signaling molecule.46
Taken together, the present studies show selective induction of the vascular P2Y6 receptor after inflammatory stimulation in conjunction with enhanced systemic inflammation. These findings implicate pharmacologic strategies that dampen P2Y6 signaling (eg, apyrase treatment or selective P2Y6 receptor blockade) in the treatment of inflammatory disorders that involve a vascular phenotype, such as systemic inflammatory response syndrome, or sepsis. Future challenge will involve the definition of pharmacologic approaches, their translation from murine models toward the treatment of patients, and the identification of potentially unwanted side effects, such as alterations in coagulation or platelet function,47,48 heart rate, and blood pressure,49 or the consequences of chronic elevation of extracellular adenosine levels.3,50
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Alice Mager, Michaela Hoch-Gutbrod, Irene Vollmer and Christof Zanke for technical support.
This work was supported by the National Institutes of Health (grants R01-HL092188, R01-DK083385, and R01HL098294) (H.K.E.).
A.K.R. is a PhD candidate at the University of Tübingen, Germany, and this work is part of her fulfillment for the requirement of her PhD thesis.
National Institutes of Health
Authorship
Contribution: A.-K.R., S.Z., M.I., and P.R. performed experiments; A.-K.R. and M.I. analyzed results and provided the figures; B.R. and J.-M.B. provided P2Y6−/− mice; A.-K.R., M.F., P.R., M.I., and H.K.E. designed the research, and A.K.R. and H.K.E. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Holger K. Eltzschig, Mucosal Inflammation Program, Department of Anesthesiology, Research Complex II, Rm 7124, 12700 E 19th Ave, Aurora, CO 80045; e-mail: holger.eltzschig@ucdenver.edu; or Marco Idzko, Department of Pulmonary Medicine, University of Freiburg, Killianstr 5, 79106 Freiburg, Germany; e-mail: marco.idzko@uniklinik-freiburg.de.


![Figure 5. P2Y6 receptor expression after intravenous LPS treatment and attenuated inflammatory responses in P2Y6−/− mice after intravenous LPS exposure. (A-B) C57BL/6 mice received an intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (phosphate-buffered saline [PBS]). Induction of P2Y6 mRNA was seen in kidney (A) and heart (B). (C) C57BL/6 mice received an intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS). Abdominal aorta was stained with antibodies specific for murine P2Y6 receptor and Alexa Fluor 488–coupled secondary antibody (green) or isotype controls and Alexa Fluor 488–coupled secondary antibody or Alexa Fluor 488–coupled secondary antibody only. DAPI (4′,6-diamidino-2-phenylindole) was used as nuclear counterstain (blue). Slides were kept on ice before image acquisition. Probes were analyzed by confocal microscopy with the use of Zeiss Laser Scanning Microscope LSM 710 and the Plan-Apochromat 20×/0.8 M27 objective lens with oil immersion. Zen software was used for acquisition and image processing (γ adjustment) applied equally to all images. White arrows indicate luminal side. One representative image of 3 is displayed. (D) Determination of plasma KC levels by enzyme-linked immunoabsorbent assay (ELISA) in C57Bl/6 wild-type mice treated with MRS 2578 and DMSO (solvent of MRS 2578) 2 hours after intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS) (wild-type control, n = 8; wild-type LPS, n = 8; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 6). (E) Determination of plasma KC levels by ELISA in C57Bl/6 wild-type versus P2Y6−/− mice 2 hours after intravenous injection of LPS (300 μg; E coli O26:B6) or vehicle control (PBS) (wild-type control, n = 10; wild-type LPS, n = 10; P2Y6−/− control, n = 8; P2Y6−/− LPS, n = 14). (F-G) Two hours after the LPS injection, mice were killed, and vascular organs (heart, kidney) were harvested. Transcript levels of VCAM were determined by real-time RT-PCR relative to the housekeeping gene β-actin in vascular organs (F) kidney (control, n = 3; LPS, n = 6) or (G) heart (control, n = 4; LPS, n = 6). All results are displayed as mean ± SD. (H-I) Determination of Evans blue concentration in kidney and heart tissue of C57Bl/6 wild-type versus P2Y6−/− mice 2 hours after intravenous challenge with 300 μg of LPS. (H) Kidney (wild-type control, n = 4; wild-type LPS, n = 10; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 12). (I) Heart (wild-type control, n = 4; wild-type LPS, n = 10; P2Y6−/− control, n = 6; P2Y6−/− LPS, n = 11).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/8/10.1182_blood-2010-10-313957/5/m_zh89991166650005.jpeg?Expires=1769121001&Signature=HiJdqtSalRq3T89SU7m-roHHu7CigL4jm1BhWPp~FLMdX9BnNX8zVFUXf8UnfDiY-RrjO2uh04Ut1rx-JsRNp1hVC5rvqZfuS74LVeFmSS1We8LyMKQEzrJGp0wzW2JhqmHHC06nWGOK9LwmCIDN8NyEC-tgL2I5edcnXsEWTbZhxo01Azi9P--H3HRVmLdQoUxzxI3wBMAQIvu1iJ2ROH7RTQuk4zB5VJlBqevdMzJRbT259Eg6tg1OuhDuWuXRzoLHjCvgmyOWhybfHrHA7NF2Ci8DhQ3yWUFxeKI1egm-bmr38d2oIHJqyCccB7Aw7ebgS0dnWijfF~KFDZ3Wyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)