Abstract
Platelets have evolved a highly specialized membrane skeleton that provides stability to the plasma membrane and facilitates adhesion under high shear stress. The cytoskeletal anchorage of glycoprotein (GP) Ibα plays an important role in regulating the membrane skeleton. However, its role in regulating membrane stability remains unknown. To investigate this role, we have developed a new mouse model that expresses wild-type human GPIbα (hGPIbαWT), or a mutant form of human GPIbα that has a selective defect in its ability to bind filamin A and anchor to the membrane skeleton (hGPIbαFW–Phe568Ala and Trp570Ala substitutions). Our study demonstrates that the link between platelet GPIb and the cytoskeleton does not alter the intrinsic ligand binding function of GPIbα or the ability of the receptor to stimulate integrin αIIbβ3-dependent spreading. However, exposure of hGPIbαFW platelets to pathologic shear rate levels (5000 to 40 000 s−1) leads to the development of unstable membrane tethers, defective platelet adhesion, and loss of membrane integrity, leading to complete disintegration of the platelet cell body. These outcomes suggest that the GPIbα–filamin A interaction not only regulates the architecture of the membrane skeleton, but also maintains the mechanical stability of the plasma membrane under conditions of high shear.
Introduction
The cytoskeleton of all eukaryotic cells plays a fundamental role in regulating the mechanical properties of the cells, influencing cell morphology, deformability, mechanotransduction, and migration. With the development of a high pressure closed circulatory system, blood cells, particularly erythrocytes and platelets, have developed highly specialized cytoskeletal networks linked to the plasma membrane to regulate membrane stability in the face of variable shear forces. The importance of interactions between erythrocyte integral membrane proteins and cytoskeletal structural proteins for erythrocyte membrane stability is well established. Diseases including hereditary spherocytosis and elliptocytosis result in hemolytic anemia as a consequence of genetic defects in erythrocyte membrane skeleton proteins including ankyrin, spectrin, and protein 4.1. These defects disrupt the anchorage of the cytoskeletal network to the plasma membrane resulting in decreased membrane deformability and increased membrane fragmentation. These results ultimately lead to loss of membrane surface area, abnormal cell morphology, and reduced cell survival because of sequestration and clearance in the spleen.1
In platelets, the actin-rich membrane skeleton is linked to the plasma membrane through the interaction between the cytoskeletal actin cross-linking protein, filamin A and the cytoplasmic tail of glycoprotein (GP) Ibα (GPIbα).2,3 Molecular defects within the GPIbα, GPIbβ, or GPIX genes result in the rare bleeding disorder Bernard-Soulier syndrome (BSS), which is characterized by giant platelets and thrombocytopenia. A recent study using GPIbα-null mice has demonstrated that expression of the cytoplasmic and transmembrane domains of human GPIbα (hGPIbα) are sufficient to partially rescue the giant platelet phenotype, thus providing some mechanistic insight into the potentially important role played by the cytoplasmic domain of GPIbα and its interaction with the membrane skeleton for regulating normal platelet morphology and cytoskeletal architecture.4 However, the importance of this interaction in regulating platelet membrane stability remains unknown. Interestingly, membrane deformability has also been demonstrated to be significantly increased in BSS platelets,5 raising the possibility that the GPIbα–filamin A interaction might be important in regulating platelet membrane stability.
In platelets, it has been difficult to provide direct evidence to demonstrate the role of receptor-cytoskeletal interactions in the regulation of platelet functions because it is not possible to use the molecular techniques applied to other nucleated cells. A previous in vitro study using local anesthetics suggested that the cytoskeleton may regulate the adhesive function of GPIb because brief exposure of platelets to dibucaine enhances von Willebrand factor (VWF)-dependent platelet aggregation,6 a finding that has been reproduced with other cytoskeletal disrupting agents.7 These anesthetics (including tetracaine, lidocaine, or dibucaine) induce proteolysis of cytoskeletal proteins and disrupt the physical linkage between GPIb and the membrane skeleton.8,9 However, analysis of the effects of these anesthetics on shear-dependent platelet adhesive function has been problematic because of their additional effects on the plasma membrane.10
Using GPIb/V/IX transfected cells, we previously identified 2 amino acids, Phe568 and Trp570, within a short hydrophobic motif (L567FLWV571) in the cytoplasmic tail of GPIbα that are essential for the association with filamin A.11 A crystallographic study subsequently confirmed that these residues form part of a hydrophobic filamin A binding site in the GPIbα cytoplasmic tail.12 In the current study, we developed a transgenic mouse expressing hGPIbα mutated at Phe568 and Trp570. We demonstrate that under shear conditions relevant to arterial thrombus formation, transgenic platelets devoid of the GPIbα/filamin/cytoskeleton axis exhibit a marked defect in membrane stability, leading to the development of unstable membrane tethers, membrane deposition on the VWF substrate, progressive reductions in the size of translocating platelets, and eventual disintegration of the platelet cell body. These findings suggest that the GPIb-filamin linkage not only plays an important role in regulating the cytoskeletal architecture of platelets, but also plays a major role in regulating the stability and integrity of the plasma membrane, particularly under conditions of high shear stress.
Methods
Materials
A detailed description of specific materials used in this study can be found in supplemental materials (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Generation of transgenic mice
A construct containing a 6-kb EcoRI fragment of the hGPIbα gene under the control of its endogenous promoter has been described previously.13 A second construct in which Phe568 and Trp570 were both replaced by alanine was also generated using a BsrGI-NsiI fragment digested from the pDX-FW-AA plasmid as previously described.11 Transgenic mice (C57BL/6 background) expressing either wild-type (WT) hGPIbα (hGPIbαWT) or GPIbα mutated at Phe568 and Trp570 (hGPIbαFW) were generated at Monash Mouseworks according to ethics committee approvals at the Alfred Medical Research & Education Precinct (AMREP) and School of Biomedical Sciences A (application E/0471/2006/M and SOBSA/MW/2008/14BC0). Platelet surface expression of hGPIbα was measured in hirudin anticoagulated blood samples (diluted 1:10) with CD42b-phycoerythrin (PE) monoclonal antibody (mAb) by flow cytometry on a FACSCalibur cytometer using CellQuest software (BD Biosciences) or FlowJo software (TreeStar). Platelet size was also assessed by flow cytometry using the forward scatter parameter.
Platelet preparation
Blood was collected from human volunteers who had given informed consent with the approval of the Monash University Human Ethics Committee in accordance with the Declaration of Helsinki. Blood was collected from mice according to AMREP animal ethics committee (SOP19–Collection of whole blood from mice). All experiments involving mice were approved under ethics applications E/0471/2006/M and E/0923/2010/M, AMREP animal ethics committee. Washed human and mouse platelets in Tyrode buffer were prepared as previously described.14 In experiments using human platelets treated with the BH3 mimetic compound ABT-73715 to induce caspase-dependent cleavage of cytoskeletal proteins, washed platelets were preincubated for 10 minutes with GM6001 (100μM) and ReoPro (20 μg/mL) to prevent metalloproteinase-dependent shedding of GPIbα16 and ligand binding to integrin αIIbβ3,17 respectively. To prevent caspase activation, platelets were preincubated with the broad spectrum caspase inhibitor Q-VD-OPh (50μM) for 10 minutes before ABT-737 treatment.
VWF binding assays
Human platelets were treated for 90 minutes with 0.001% dimethylsulfoxide (DMSO), ABT-737 (1μM), or ABT-737 after 10 minutes pretreatment with Q-VD-OPh (50μM) before incubation with human VWF. Transgenic mouse platelets were preincubated for 15 minutes with anti–mouse GPIbα blocking antibody, Xia.B2 (20 μg/mL), to prevent human VWF binding to endogenous mouse GPIbα. Washed platelets (50 μL, 2 × 107/mL) in Tyrode buffer were incubated with Alexa Fluor 488–VWF at 1 to 20 μg/mL in the presence or absence of the VWF-modulator botrocetin (10 μg/mL) for 30 minutes at room temperature, then diluted to 400 μL and analyzed by flow cytometry. In control studies, the anti–human GPIbα-specific mAb ALMA.12 (10 μg/mL) was used to block VWF binding to hGPIbα.
Immunoprecipitation of hGPIbα from human and transgenic mouse platelets
Washed human and transgenic mouse platelets (3 × 108/mL) in Tyrode buffer were lysed in 200 μL 1% Triton X-100 lysis buffer (1% Triton X-100, 20mM Tris, Complete protease inhibitor cocktail, 5mM ethylenediaminetetraacetic acid [pH 7.4]). Precleared supernatants were incubated with anti–human GPIbα mAb, ALMA.12, or isotype-matched control immunoglobulin G (20 μg/mL) for 1 hour, followed by 20 μL protein A/G Plus overnight at 4°C. Immunoprecipitated proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (7.5% for detection of filamin A or 12.5% for 14.3.3ζ), transferred to polyvinylidene difluoride and probed for hGPIbα with mAb, WM23 (0.8 μg/mL), filamin with polyclonal antibody (pAb), H-300 (400 μg/mL), or anti-14.3.3ζ pAb (1 μg/mL), followed by horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescence.
Static adhesion assays
Glass coverslips were derivatized with hexamethyldisilazane for 15 minutes, washed with water, and coated with 5 μg/mL human VWF in bovine serum albumin free Tyrode buffer overnight at 4°C. Coated coverslips were blocked with 2% (wt/vol) bovine serum albumin for 1 hour at room temperature. Washed transgenic mouse platelets (2 × 107/mL), were either untreated or preincubated with inhibitors of secondary mediators adenosine 5′-diphosphate (ADP) and thromboxane (either 1 U/mL apyrase or a combination of the ADP receptor antagonists MeSAMP [10μM] and MRS2179 [100μM] or 10μM MeSAMP/MRS2179 plus indomethacin), then allowed to adhere for 1 hour at 37°C in the presence of 1.5 mg/mL ristocetin to examine hGPIbα-specific adhesion. Platelets were fixed with 4% (wt/vol) paraformaldehyde in phosphate-buffered saline. C57BL/6 mouse platelets were used as controls. Platelet adhesion and spreading was imaged by differential interference contrast (DIC) microscopy (DMIRB, 63× or 100× Objective; Leica), and images captured using DVT tools (Pinnacle Systems) or Metamorph software (Molecular Devices). The number of adherent and spread platelets from duplicate coverslips was quantitated more than at least 3 random fields. In experiments to examine integrin αIIbβ3 activation, the remaining nonadherent platelet suspension was removed at 30 minutes and replaced with 20 μg/mL Oregon Green fibrinogen in Tyrode buffer for another 30 minutes. Samples were washed and fixed as initially described in this paragraph, and DIC and fluorescent images were captured and overlayed using Metamorph software.
Flow-based adhesion assays
Adhesion of human or transgenic mouse platelets was performed as previously described18 with some modifications. Washed human platelets (3 × 108/mL) in Tyrode buffer were adhered to human VWF in the presence of 1 mg/mL ristocetin in the absence of shear for 10 minutes before the initiation of flow at a shear rate of either 600 or 1800 s−1. Ristocetin was present to increase the affinity of the GPIbα–human VWF interaction thereby preventing translocation of platelets at the initiation of flow. Adherent platelets were visualized using DIC microscopy (63×, 1.2W Objective) and data recorded to DVD for offline analysis using Metamorph or ImageJ software (National Institutes of Health). Hirudin anticoagulated whole blood from transgenic mice was perfused more than glass microslides coated with 20 μg/mL human VWF at a rate of 1800 s−1 for 2 minutes. Shear rate was increased incrementally up to 20 000 s−1, and the number of adherent mouse platelets and their rolling velocity was quantitated as previously described.11 For analysis of membrane tether formation under flow conditions, washed transgenic mouse platelets were labeled with the lipophilic dye DiIC12 (1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine, 1μM, 530/560 Ex/Em), then reconstituted to 25% hematocrit with human red blood cells at a final platelet count of 3 × 107/mL and perfused over human VWF–coated coverslips prepared and assembled into microfluidic flow chambers according to the method of Nesbitt et al.19 Reconstituted transgenic mouse blood was perfused over human VWF at shear rates between 1800 and 10 000 s−1, with or without subsequent increases in shear to a maximum of 40 000 s−1. Adherent platelets were visualized on an Olympus IX 81 inverted microscope (UPlanSApo 100× Objective, 1.4NA) using a Sutter DG4 light source. DIC and fluorescent (tetramethylrhodamine isothiocyanate) time lapse stacks were captured using Metamorph software to visualize platelet adhesion, translocation, and membrane tether formation. Image analysis was performed using ImageJ software.
Statistical analyses
Results were analyzed using 1-way ANOVA, with Bonferroni posttest or Student t test using Prism software (Version 5.01; GraphPad).
Results
Cleavage of cytoskeletal proteins induces unstable platelet adhesion under shear conditions
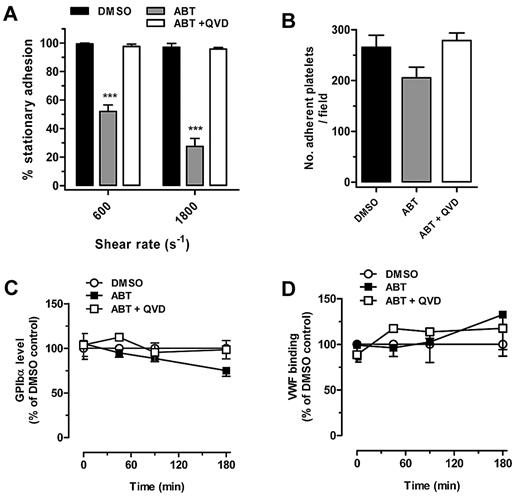
To examine the effect of disrupting cytoskeletal proteins on shear-dependent platelet adhesion, we used the BH3 mimetic compound ABT-737, which can induce caspase-dependent cleavage of cytoskeletal proteins, including filamin A and gelsolin, without causing severe disruption to the plasma membrane or platelet activation.20 In preliminary studies, we determined the optimal experimental conditions to induce filamin A proteolysis in ABT-737–treated human platelets without perturbing the surface expression of membrane receptors or inducing phosphatidylserine exposure. As demonstrated in Figure 1A, shear-dependent platelet adhesion to a VWF surface was significantly reduced after ABT-737 treatment of platelets. Whereas more than 95% of untreated control platelets maintained stationary adhesion at a rate of both 600 and 1800 s−1, only 52% ± 4.47% (P < .001, n = 5) maintained stationary adhesion at a rate of 600 s−1 and 22% ± 4.9% (P < .001, n = 5) at a rate of 1800 s−1. Significantly, this adhesive defect was completely prevented by preincubating platelets with the caspase inhibitor Q-VD-OPh, suggesting that the proteolysis of one or more cytoskeletal caspase substrates was responsible for the defect in human platelet adhesive function. This defect in adhesion was specific to shear conditions because adhesion of ABT-737–treated platelets to an immobilized VWF matrix under static conditions was not significantly different to vehicle-treated controls (Figure 1B). Control studies demonstrated that ABT-737 treatment had no effect on the surface expression of GPIbα (Figure 1C), and that ABT-737–treated human platelets bound comparable levels of soluble VWF (in the presence of botrocetin) to vehicle-treated control platelets (Figure 1D), confirming that the cleavage of cytoskeletal proteins does not diminish the ligand binding function of GPIbα in human platelets.
Cleavage of cytoskeletal proteins induces unstable platelet adhesion under shear conditions. (A) Washed human platelets prepared as described in ‘Methods’ were treated with either DMSO, 1μM ABT-737 (ABT), or 50μM Q-VD-OPh 10 minutes before ABT-737 treatment (ABT + QVD), then allowed to adhere to VWF for 10 minutes in the presence of 1 mg/mL ristocetin. Shear rates of 600 or 1800 s−1 were applied for up to 5 minutes, and the number of stationary platelets was analyzed from 5 independent experiments. ***P < .001). (B) Washed human platelets were allowed to adhere to VWF under static conditions in the presence of 1 mg/mL ristocetin for 60 minutes. The number of adherent platelets was analyzed as the mean (± SEM) from 3 experiments. (C) Surface expression of GPIbα was determined by flow cytometry using CD42b-PE anti–human GPIbα mAb as mean (± SEM) from 3 experiments. (D) VWF binding to DMSO, ABT-737, or Q-VD-OPh 10 minutes before ABT-737–treated human platelets was measured in the presence of 5 μg/mL botrocetin as mean (± SEM) from 3 experiments.
Cleavage of cytoskeletal proteins induces unstable platelet adhesion under shear conditions. (A) Washed human platelets prepared as described in ‘Methods’ were treated with either DMSO, 1μM ABT-737 (ABT), or 50μM Q-VD-OPh 10 minutes before ABT-737 treatment (ABT + QVD), then allowed to adhere to VWF for 10 minutes in the presence of 1 mg/mL ristocetin. Shear rates of 600 or 1800 s−1 were applied for up to 5 minutes, and the number of stationary platelets was analyzed from 5 independent experiments. ***P < .001). (B) Washed human platelets were allowed to adhere to VWF under static conditions in the presence of 1 mg/mL ristocetin for 60 minutes. The number of adherent platelets was analyzed as the mean (± SEM) from 3 experiments. (C) Surface expression of GPIbα was determined by flow cytometry using CD42b-PE anti–human GPIbα mAb as mean (± SEM) from 3 experiments. (D) VWF binding to DMSO, ABT-737, or Q-VD-OPh 10 minutes before ABT-737–treated human platelets was measured in the presence of 5 μg/mL botrocetin as mean (± SEM) from 3 experiments.
Caspase cleavage of cytoskeletal proteins induces a shear-specific defect in platelet membrane stability
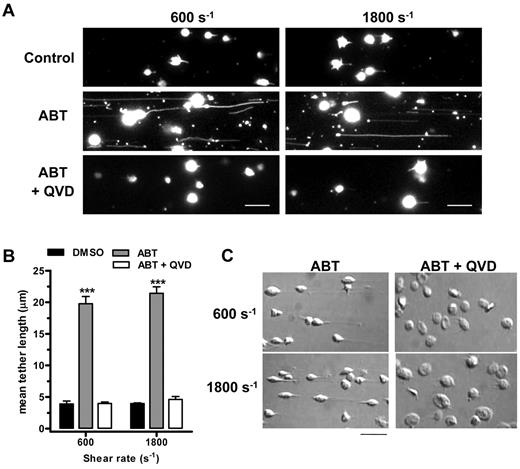
The shear-dependent adhesion of platelets to VWF is associated with the formation of filamentous membrane tethers, pulled from the surface of discoid platelets by hemodynamic drag forces.21 Membrane tethers are thought to facilitate platelet adhesion by decreasing the impact of tensile forces on VWF-GPIbα bonds.22 High magnification imaging of ABT-737–treated human platelets revealed that the principal defect in platelet adhesion was attributable to excessive elongation of membrane tethers (Figure 2A), with a mean tether length of 19.8 and 21.4 μm at a rate of 600 and 1800 s−1, respectively (Figure 2B). This resulted in platelet sliding and eventual detachment from the VWF surface, which was associated with tether deposition on the VWF surface (Figure 2A middle) These extended membrane tethers were never observed in control or Q-VD-OPh– treated platelets, both of which exhibited short membrane tethers, typically less than 5 μm in length (Figure 2B). These results suggest that the integrity of the membrane is likely to be weakened by ABT-737 induced proteolysis of cytoskeletal proteins. Consistent with this idea, morphologic analysis of adherent platelets by DIC microscopy (Figure 2C) revealed the presence of long membrane tethers and the loss of discoid platelet morphology in ABT-treated platelets compared with those pretreated with Q-VD-OPh, observations that are consistent with changes to the cytoskeleton. In addition to the loss of discoid morphology, the main platelet body also appeared to be reduced in size compared with Q-VD-OPh–treated platelets, an observation consistent with the notion of a shear-dependent loss of membrane material. The same experiments performed with C57BL/6 mouse platelets also demonstrated similar membrane tether formation, which was completely rescued by Q-VD-OPh (supplemental Figure 1). Overall, these findings in human platelets raise the possibility that caspase-dependent proteolysis of one or more cytoskeletal proteins, such as filamin A or gelsolin20 plays an important role in regulating membrane tether dynamics and platelet adhesion stability under shear, without significantly impacting on the ligand binding function of GPIb.
Caspase-dependent cleavage of cytoskeletal proteins induces a shear-specific defect in platelet membrane tether stability. (A) DiIC12-labeled washed human platelets in the presence of 1 mg/mL ristocetin were perfused over human VWF-coated microchannels and allowed to adhere for 10 minutes in the absence of shear. Shear was applied (at a rate of 600 or 1800 s−1), and platelet adhesive behavior was captured using Metamorph software. Single frame fluorescence images (after subjecting to a low pass filter of 3) show the formation of long membrane tethers from ABT-737–treated (1μM) platelets, which is prevented by pretreatment with Q-VD-OPh (50μM). Scale bar represents 10 μm. (B) Quantitation of mean tether length from 3 independent experiments demonstrates a significant increase in membrane tether length in ABT-737–treated platelets compared with control and QV-D-OPh–treated platelets. ***P < .001. (C) DIC images of platelets demonstrating membrane tethers and the loss of discoid platelet morphology after treatment with ABT-737 and prevention by Q-VD-OPh are shown under the same shear conditions as in panel A. Scale bar represents 10 μm.
Caspase-dependent cleavage of cytoskeletal proteins induces a shear-specific defect in platelet membrane tether stability. (A) DiIC12-labeled washed human platelets in the presence of 1 mg/mL ristocetin were perfused over human VWF-coated microchannels and allowed to adhere for 10 minutes in the absence of shear. Shear was applied (at a rate of 600 or 1800 s−1), and platelet adhesive behavior was captured using Metamorph software. Single frame fluorescence images (after subjecting to a low pass filter of 3) show the formation of long membrane tethers from ABT-737–treated (1μM) platelets, which is prevented by pretreatment with Q-VD-OPh (50μM). Scale bar represents 10 μm. (B) Quantitation of mean tether length from 3 independent experiments demonstrates a significant increase in membrane tether length in ABT-737–treated platelets compared with control and QV-D-OPh–treated platelets. ***P < .001. (C) DIC images of platelets demonstrating membrane tethers and the loss of discoid platelet morphology after treatment with ABT-737 and prevention by Q-VD-OPh are shown under the same shear conditions as in panel A. Scale bar represents 10 μm.
Development of a transgenic mouse model to investigate the role of the GPIbα-filamin A interaction in regulating shear-dependent platelet adhesion
To determine whether the defect in membrane stability and platelet adhesion may in part be because of defective GPIb anchorage to the membrane skeleton through the interaction with filamin A, we developed a transgenic mouse model expressing a mutant form of hGPIb that involved alanine substitution of 2 cytoplasmic residues, Phe568 and Trp570 (hGPIbαFW), both of which are important for GPIb binding to filamin A.11,12 The hGPIbαFW mice express GPIb receptors composed of hGPIbα complexed with murine GPIbβ and GPIX, as well as endogenous murine GPIb/V/IX. We maintained expression of murine GPIb to avoid the possibility that hGPIbαFW mice might exhibit macrothrombocytopenia because of a disruption of the GPIbα-filamin A interaction. A study shows the macrothrombocytopenia seen in GPIbα-null mice can be rescued by expression of the cytoplasmic and transmembrane domains of GPIbα.4 Transgenic mice expressing WT hGPIbα (hGPIbαWT) were generated as controls.
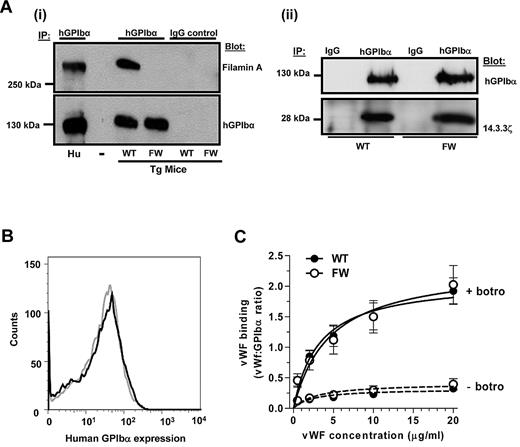
To determine whether the mutations in hGPIbα had successfully disrupted the GPIbα-filamin A interaction, hGPIbα was immunoprecipitated from transgenic mouse platelet lysates and samples probed for filamin A. As demonstrated in Figure 3Ai), whereas hGPIbαWT readily bound filamin A, there was no association with hGPIbαFW. This defect in filamin A binding had no negative impact on 14.3.3ζ binding to GPIb (Figure 3Aii) or on the surface expression of the human transgene on the platelet surface (Figure 3B). As expected, platelet size and morphology were unaffected by the mutation (supplemental Figure 2) because endogenous mouse GPIbα is still expressed at normal levels in these platelets (supplemental Figure 3).
Characterization of hGPIbα transgenic mice. (A) hGPIbα was immunoprecipitated from human platelets or hGPIbαWT (WT) and hGPIbαFW (FW) transgenic mouse platelet lysates using 10 μg/mL ALMA.12 mAb (hGPIbα) or an isotype-matched control mAb (immunoglobulin G control). The presence of filamin A was detected using pAb H-300 (Ai), and human GPIbα was immunoblotted with WM23 mAb (Ai-ii). Data shown are from 1 experiment representative of 3 performed. Substitution of Phe568 and Trp570 to alanine completely disrupts the interaction between GPIbα and filamin A but has no negative impact on 14.3.3ζ binding to GPIb (Aii). (B) Flow cytometric analysis of hGPIbα expression on the surface of hGPIbαWT (black line) and hGPIbαFW (gray line) platelets in whole blood samples (1:10 dilution) and stained with anti–human CD42b-PE mAb demonstrating that loss of filamin A binding has no effect on receptor surface expression. (C) Alexa Fluor 488-VWF binding to hGPIbα expressed on transgenic platelets was measured in the presence of 5 μg/mL botrocetin and 20 μg/mL Xia.B2 murine GPIbα blocking antibody. No differences were measured between hGPIbαWT and hGPIbαFW platelets over all concentrations of VWF (1-20 μg/mL).
Characterization of hGPIbα transgenic mice. (A) hGPIbα was immunoprecipitated from human platelets or hGPIbαWT (WT) and hGPIbαFW (FW) transgenic mouse platelet lysates using 10 μg/mL ALMA.12 mAb (hGPIbα) or an isotype-matched control mAb (immunoglobulin G control). The presence of filamin A was detected using pAb H-300 (Ai), and human GPIbα was immunoblotted with WM23 mAb (Ai-ii). Data shown are from 1 experiment representative of 3 performed. Substitution of Phe568 and Trp570 to alanine completely disrupts the interaction between GPIbα and filamin A but has no negative impact on 14.3.3ζ binding to GPIb (Aii). (B) Flow cytometric analysis of hGPIbα expression on the surface of hGPIbαWT (black line) and hGPIbαFW (gray line) platelets in whole blood samples (1:10 dilution) and stained with anti–human CD42b-PE mAb demonstrating that loss of filamin A binding has no effect on receptor surface expression. (C) Alexa Fluor 488-VWF binding to hGPIbα expressed on transgenic platelets was measured in the presence of 5 μg/mL botrocetin and 20 μg/mL Xia.B2 murine GPIbα blocking antibody. No differences were measured between hGPIbαWT and hGPIbαFW platelets over all concentrations of VWF (1-20 μg/mL).
To investigate the effect of disrupting the GPIbα-filamin A interaction on the VWF binding function of GPIb/V/IX, ligand binding studies were performed. Binding of Alexa Fluor 488–conjugated human VWF in the presence of botrocetin was measured in the presence of a mouse GPIbα function blocking antibody Xia.B2, to prevent human VWF binding to endogenous mouse GPIbα. Furthermore, all experiments were performed in the presence of ethylenediaminetetraacetic acid to prevent divalent cation-dependent ligand binding to integrin αIIbβ3. Figure 3C demonstrates that VWF binding to hGPIbαWT and hGPIbαFW transgenic mouse platelets is indistinguishable, indicating that loss of the filamin A interaction had no effect on the ligand binding function of hGPIbα expressed in mouse platelets. In studies with controls, botrocetin-dependent human VWF binding to nontransgenic C57BL/6 mouse platelets was completely abolished by blocking mouse GPIbα with Xia.B2 antibody, and human VWF binding to transgenic mouse platelets was completely inhibited by blocking hGPIbα with ALMA.12 mAb, demonstrating that VWF binding is specific to hGPIbα (data not shown).
Adhesion and spreading of transgenic mouse platelets to immobilized VWF under static conditions is unaffected by loss of the GPIb-filamin A interaction
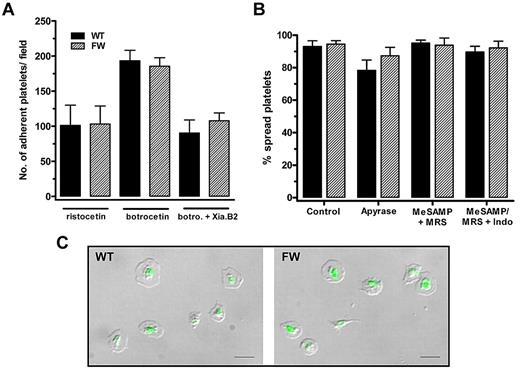
To examine the effect of disrupting the GPIbα-filamin A on VWF-dependent platelet adhesion and integrin activation, adhesion and spreading of transgenic mouse platelets under static conditions was analyzed. In studies with controls, we demonstrated that adhesion of transgenic mouse platelets to human VWF was dependent on exogenously added modulator (either botrocetin or ristocetin) consistent with a published report using normal mouse platelets.13 To examine hGPIbα-dependent adhesion and activation, transgenic mouse platelets were treated with 1.5 mg/mL ristocetin. This modulator discriminates between hGPIbα– and mouse GPIbα–mediated VWF binding and cannot support an interaction between human VWF and mouse GPIbα.13,23 Figure 4A demonstrates that there is no difference between the number of adherent hGPIbαWT and hGPIbαFW mouse platelets to VWF in the presence of ristocetin. This adhesion is mediated entirely through transgenic hGPIbα because control C57BL/6 mouse platelets are unable to adhere to VWF in the presence of ristocetin (supplemental Figure 4A) and adhesion of transgenic mouse platelets is completely inhibited in the presence of AK2, which binds hGPIbα not mouse GPIbα (supplemental Figure 4B). We also assessed the percentage of transgenic mouse platelets that became spread on the VWF surface as a measure of integrin αIIbβ3 activation. The hGPIbαFW platelets spread to the same extent as hGPIbαWT platelets (Figure 4B), suggesting that the interaction between GPIbα and filamin A is not required for integrin αIIbβ3 activation downstream of GPIbα-VWF. Transgenic mouse platelet spreading was also unaffected by the addition of inhibitors of the secondary mediators ADP and thromboxane, suggesting that integrin activation is occurring directly downstream of GPIb/V/IX (Figure 4B). Further evidence demonstrating GPIb-dependent integrin αIIbβ3 activation was normal in hGPIbαFW mouse platelets was that spread platelets on the human VWF substrate bound similar amounts of soluble Oregon Green fibrinogen as in the hGPIbαWT controls (Figure 4C).
Adhesion and spreading of transgenic mouse platelets to immobilized VWF under static conditions is unaffected by loss of filamin A interaction. (A) hGPIbαWT and hGPIbαFW platelets were allowed to adhere to human VWF (5 μg/mL) for 60 minutes in the presence of ristocetin (1.5 mg/mL), botrocetin (5 μg/mL), or botrocetin + Xia.B2 murine GPIbα blocking antibody (20 μg/mL). Results are mean (± SEM) from at least 4 independent experiments and demonstrate no significant difference in the total number of adherent platelets under all conditions tested. Ristocetin-dependent adhesion and botrocetin-dependent adhesion in the presence of Xia.B2 are identical and represent adhesion to human VWF mediated specifically by hGPIbα. (B) Platelet spreading on VWF (+ 1.5 mg/mL ristocetin) was analyzed from DIC images (at least 3 separate fields from 3 independent experiments) and demonstrated no significant difference between hGPIbαWT and hGPIbαFW platelets. Platelets treated with inhibitors of ADP and thromboxane (1 U/mL apyrase, a combination of 10μM MeSAMP and 100μM MRS2179, or MeSAMP/MRS2179 and 10μM indomethacin) demonstrated that integrin αIIbβ3 activation occurred directly downstream from GPIbα without a contribution from these secondary mediators. (C) Oregon Green fibrinogen binding to platelets adherent to human VWF (in the presence of 1.5 mg/mL ristocetin) was no different between hGPIbαWT and hGPIbαFW mice, indicating that integrin αIIbβ3 activation is unaffected by the loss of filamin A binding to GPIbα. DIC and fluorescent images were overlayed using Metamorph and are representative of 4 independent experiments. Scale bar represents 10 μm.
Adhesion and spreading of transgenic mouse platelets to immobilized VWF under static conditions is unaffected by loss of filamin A interaction. (A) hGPIbαWT and hGPIbαFW platelets were allowed to adhere to human VWF (5 μg/mL) for 60 minutes in the presence of ristocetin (1.5 mg/mL), botrocetin (5 μg/mL), or botrocetin + Xia.B2 murine GPIbα blocking antibody (20 μg/mL). Results are mean (± SEM) from at least 4 independent experiments and demonstrate no significant difference in the total number of adherent platelets under all conditions tested. Ristocetin-dependent adhesion and botrocetin-dependent adhesion in the presence of Xia.B2 are identical and represent adhesion to human VWF mediated specifically by hGPIbα. (B) Platelet spreading on VWF (+ 1.5 mg/mL ristocetin) was analyzed from DIC images (at least 3 separate fields from 3 independent experiments) and demonstrated no significant difference between hGPIbαWT and hGPIbαFW platelets. Platelets treated with inhibitors of ADP and thromboxane (1 U/mL apyrase, a combination of 10μM MeSAMP and 100μM MRS2179, or MeSAMP/MRS2179 and 10μM indomethacin) demonstrated that integrin αIIbβ3 activation occurred directly downstream from GPIbα without a contribution from these secondary mediators. (C) Oregon Green fibrinogen binding to platelets adherent to human VWF (in the presence of 1.5 mg/mL ristocetin) was no different between hGPIbαWT and hGPIbαFW mice, indicating that integrin αIIbβ3 activation is unaffected by the loss of filamin A binding to GPIbα. DIC and fluorescent images were overlayed using Metamorph and are representative of 4 independent experiments. Scale bar represents 10 μm.
Shear-dependent adhesion of hGPIbαFW transgenic mouse platelets to VWF is associated with enhanced membrane tether formation
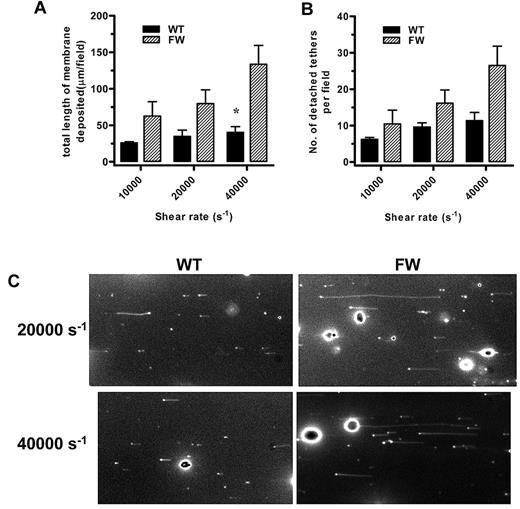
Examination of hGPIbαWT and hGPIbαFW platelet adhesion to human VWF at physiologic shear rates (1800 s−1) by DIC microscopy revealed no obvious differences in platelet morphology. However, when shear rates were increased, it became apparent that hGPIbαFW platelets generated numerous membrane tethers as they translocated over the VWF surface. The hGPIbαWT platelets were also observed to form membrane tethers, but these were less numerous and were generated only at higher shear rates. An analysis of both the number and total length of membrane tethers that were pulled from the main platelet body and which subsequently detached and deposited on the VWF surface, confirmed that hGPIbαFW platelets produced more tethers and deposited significantly more membrane tether material onto the VWF matrix than hGPIbαWT platelets (Figure 5A-B). Typical fluorescent images of tethers that were deposited from translocating platelets at shear rates of 20 000 and 40 000 s−1 are shown (Figure 5C) as well as images demonstrating how hGPIbαFW platelets pull long and numerous tethers compared with hGPIbαWT platelets (supplemental Video 1).
Shear-dependent membrane tether formation. (A-B) DiIC12-labeled transgenic platelets were perfused over human VWF at a rate of 1800 s−1 followed by an increase in shear rate to 10 000, 20 000, or 40 000 s−1. Metamorph stacks (500 frames) were acquired at approximately 3 fields per flow, and 5 fields per flow were analyzed for total length of membrane deposited and number of tethers deposited on the VWF matrix as mean (± SEM) for n = 4-5 independent experiments, *P < .05). (C) Typical fields are shown at a rate of 20 000 and 40 000 s−1 to demonstrate the significant difference between hGPIbαFW and hGPIbαWT (also shown in supplemental Video 1).
Shear-dependent membrane tether formation. (A-B) DiIC12-labeled transgenic platelets were perfused over human VWF at a rate of 1800 s−1 followed by an increase in shear rate to 10 000, 20 000, or 40 000 s−1. Metamorph stacks (500 frames) were acquired at approximately 3 fields per flow, and 5 fields per flow were analyzed for total length of membrane deposited and number of tethers deposited on the VWF matrix as mean (± SEM) for n = 4-5 independent experiments, *P < .05). (C) Typical fields are shown at a rate of 20 000 and 40 000 s−1 to demonstrate the significant difference between hGPIbαFW and hGPIbαWT (also shown in supplemental Video 1).
Transgenic hGPIbαFW mouse platelets have a shear-dependent defect in platelet adhesion
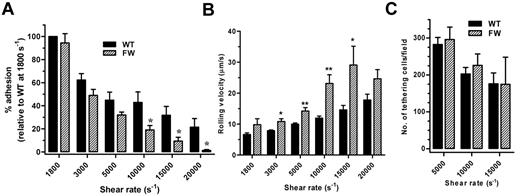
To examine whether the changes in membrane tether dynamics in hGPIbαFW platelets altered the stability of platelet adhesion under high shear, transgenic mouse blood was initially perfused over human VWF at a rate of 1800 s−1, and the shear rate was increased incrementally up to 20 000 s−1. In studies with control C57BL/6 mouse platelets, there was no tethering or rolling of platelets during perfusion of anticoagulated blood over human VWF at a rate of 1800 s−1, demonstrating that endogenous mouse GPIbα does not contribute to the adhesion of transgenic mouse platelets under these shear conditions (supplemental Video 2). Quantitation of the number of platelets remaining adherent at each shear rate revealed a significant difference at shear rates of 10 000 s−1 and above (Figure 6A). The hGPIbαFW platelets exhibited a shear-dependent defect in adhesion that became more profound as the shear rate increased (56% lower adhesion than hGPIbαWT at a shear rate of 10 000 s−1, 70.5% at 15 000 s−1, and 93.8% at 20 000 s−1), and in the majority of experiments, there were no hGPIbαFW platelets remaining adherent at 20 000 s−1. The rolling velocity of hGPIbαFW platelets was also significantly faster than hGPIbαWT at shear rates of 3000 s−1 and above (Figure 6B). To examine the ability of WT or mutant GPIbα to initiate platelet adhesion (tethering) in a high shear field, platelets were perfused over the VWF matrix at a rate of 5000, 10 000, and 15 000 s−1. Notably, there was no difference in the number of hGPIbαWT or hGPIbαFW platelets adhering to VWF from the bulk flow (Figure 6C). Taken together, these observations suggest that disrupting the filamin A–GPIbα interaction has no impact on the ability of GPIbα to initiate platelet adhesion on a VWF substrate, but results in a specific defect in their ability to maintain adhesion in a high shear field.
Adhesion of transgenic mouse platelets to VWF under high shear conditions. (A) Transgenic mouse blood was perfused over human VWF at a rate of 1800 s−1, followed by incremental increases in shear rate to a maximum of 20 000 s−1. Quantitation of the number of adherent platelets at each shear rate demonstrated a significant reduction in the number of hGPIbαFW platelets able to remain adherent at shear rates of 10 000 s−1 and above compared with hGPIbαWT as mean (± SEM) for n = 6 experiments. *P < .05. (B) Rolling velocity was analyzed from the same experiments as in panel A. At shear rates of 3000 s−1 and above, hGPIbαFW platelets rolled significantly faster that hGPIbαWT platelets for n = 6 experiments. *P < .05, **P < .01. Note that the rolling velocity at 20 000 s−1 was not significantly different because there were too few hGPIbαFW platelets remaining adherent to analyze. (C) Transgenic mouse blood was perfused over human VWF at 5000, 10 000, or 20 000 s−1 and the number of platelets adhering from the bulk flow was quantitated over at least 5 fields as the mean (± SEM) from 3 independent experiments. There was no significant difference between the number of adherent hGPIbαWT and hGPIbαFW platelets at any of the shear rates tested.
Adhesion of transgenic mouse platelets to VWF under high shear conditions. (A) Transgenic mouse blood was perfused over human VWF at a rate of 1800 s−1, followed by incremental increases in shear rate to a maximum of 20 000 s−1. Quantitation of the number of adherent platelets at each shear rate demonstrated a significant reduction in the number of hGPIbαFW platelets able to remain adherent at shear rates of 10 000 s−1 and above compared with hGPIbαWT as mean (± SEM) for n = 6 experiments. *P < .05. (B) Rolling velocity was analyzed from the same experiments as in panel A. At shear rates of 3000 s−1 and above, hGPIbαFW platelets rolled significantly faster that hGPIbαWT platelets for n = 6 experiments. *P < .05, **P < .01. Note that the rolling velocity at 20 000 s−1 was not significantly different because there were too few hGPIbαFW platelets remaining adherent to analyze. (C) Transgenic mouse blood was perfused over human VWF at 5000, 10 000, or 20 000 s−1 and the number of platelets adhering from the bulk flow was quantitated over at least 5 fields as the mean (± SEM) from 3 independent experiments. There was no significant difference between the number of adherent hGPIbαWT and hGPIbαFW platelets at any of the shear rates tested.
Decreased membrane stability in hGPIbαFW transgenic mouse platelets leads to loss of platelet membrane integrity under high shear
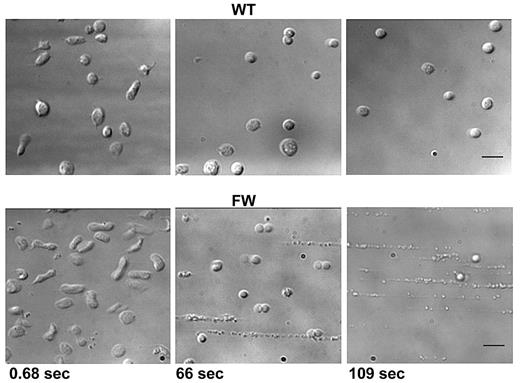
In addition to depositing increased amounts of membrane material on the VWF surface, it became apparent that with increasing time at rates of 20 000 s−1 and above, hGPIbαFW platelets became increasingly smaller, most likely as a consequence of depleting their membrane reserves. Representative images of hGPIbαWT and hGPIbαFW platelets after exposure to shear rates (with SEM) of 20 000 s−1 show that hGPIbαFW platelets were much smaller and of more spherical morphology than hGPIbαWT platelets (supplemental Figure 5). Finally, sustained exposure of transgenic mouse platelets to high shear revealed a profound structural instability of those hGPIbαFW platelets still adherent at these elevated shear rates. We observed that the main platelet body became highly vesiculated followed by a complete disintegration of the platelet into elongated string-like structures (Figure 7 bottom and supplemental Video 3). Some of these platelets were observed to detach from the matrix, whereas others remained adherent. The hGPIbαWT platelets also adopted a shear-induced spherical morphology as has been reported previously.18 However, in contrast to hGPIbαFW platelets, hGPIbαWT platelets continued to translocate across the VWF surface maintaining their structural integrity (Figure 7 top and supplemental Video 3). These observations suggest that under pathologic shear conditions, the link between GPIbα and the underlying platelet cytoskeleton, mediated through the filamin A interaction, is essential in maintaining the integrity of platelets, despite these platelets having a normal complement of endogenous mouse GPIb/V/IX receptor.
High shear–dependent platelet disintegration. Whole blood from transgenic mice was perfused into human VWF-coated microchannels at a rate of 1800 s−1. The shear rate was increased to 40 000 s−1, and platelet adhesion was monitored for up to 3 minutes. Single frame images were captured at the time points indicated. hGPIbαWT platelets progressively adopted a spherical morphology while maintaining rolling adhesion. In contrast, those hGPIbαFW platelets still able to maintain their adhesion were observed to progressively lose their normal structure, becoming highly vesiculated followed by complete disintegration (also shown in supplemental Video 3). Scale bar represents 5 μm.
High shear–dependent platelet disintegration. Whole blood from transgenic mice was perfused into human VWF-coated microchannels at a rate of 1800 s−1. The shear rate was increased to 40 000 s−1, and platelet adhesion was monitored for up to 3 minutes. Single frame images were captured at the time points indicated. hGPIbαWT platelets progressively adopted a spherical morphology while maintaining rolling adhesion. In contrast, those hGPIbαFW platelets still able to maintain their adhesion were observed to progressively lose their normal structure, becoming highly vesiculated followed by complete disintegration (also shown in supplemental Video 3). Scale bar represents 5 μm.
Discussion
Considerable evidence supports an important role for the GPIb-filamin A interaction in regulating the cytoskeletal organization of platelets.2,4,24 Most notably, expression of the transmembrane and cytoplasmic domain of GPIbα is sufficient to partially ameliorate the macrothrombocytopenia that is characteristic of BSS.4 Membrane deformability has also been noted to be increased in BSS platelets.5 However, whether this increase is because of defects in the GPIb-cytoskeletal linkage or secondary to gross changes in the cytoskeleton remains unknown. The results presented in this study suggest a critical role for the GPIb-filamin A linkage in regulating the stability of the platelet plasma membrane, particularly under conditions of high shear. Defective GPIb-cytoskeletal anchorage is associated with excessive membrane tether elongation, instability, and breakage leading to progressive reductions in the size of translocating platelets and eventual disintegration of the platelet cell body. These alterations in membrane stability lead to a specific decrease in platelet adhesion under pathologic shear conditions, raising the possibility that therapeutic targeting of the GPIb-filamin A interaction may represent a new approach to dampen platelet accumulation and thrombus growth under conditions of high shear stress.
An important finding in the current study was the observation that hGPIbαFW platelets have a greater propensity to form membrane tethers that detach from the main platelet body and become deposited on the VWF surface relative to hGPIbαWT controls. Human platelets translocating on VWF have been shown to generate membrane tethers in a shear-dependent manner,22,25 a process that facilitates the maintenance of platelet adhesion during the early stages of platelet aggregation26 and is a prerequisite step for membrane tether restructuring and the stabilization of discoid platelet aggregates.19 Our observations demonstrate an important role for the cytoskeletal anchorage of GPIb in limiting the excessive elongation of membrane tethers under conditions of elevated shear. Consistent with an important role for the cytoskeleton in regulating membrane stability, actin depolymerizing reagents such as the cytochalasins result in the formation of much longer tethers at a given shear force in platelets,22 neutrophils,27 and fibroblasts.28 It has also been suggested that the cytoskeleton is important in limiting the size of available membrane reservoir.29 This limiting affect is of particular relevance to platelets because they contain a significant pool of internal membrane reservoir in the form of the open canalicular system, which on platelet activation is required for filopodia and lamellipodia formation.30 In the absence of an intact GPIbα/filamin/cytoskeletal axis, it is possible that a translocating platelet under high shear conditions is unable to limit the amount of membrane reservoir that is extended and subsequently lost in the form of detaching membrane tethers. At the high shear rates examined in this study, hGPIbαFW platelets became highly unstable and were observed to completely disintegrate. This surprising observation occurs in a platelet in which the majority of the cytoskeleton should be normal because these platelets have normal levels of endogenous murine GPIbα, which is linked to the underlying membrane skeleton. Presumably, once hGPIbα is engaged with VWF, these bonds are exposed to force, resulting in tether formation followed by tether extraction. As more GPIbα-VWF bonds form, more tethers are pulled until the membrane reserve is exceeded and the platelet is no longer able to maintain membrane stability.
Importantly, this study also demonstrates that the GPIbα–filamin A interaction does not affect the intrinsic ligand binding function of GPIb/V/IX, even under high shear conditions. This finding is in contrast to several previous studies using GPIb-transfected cell models or indirect methods in platelets,31-34 which have demonstrated conflicting evidence that GPIb/V/IX function can be either negatively or positively regulated by disrupting its link to the cytoskeleton through filamin A. Resolution of this issue has been problematic because of the inability to genetically regulate the specific interaction between GPIb and filamin A in human platelets. The approach we have used in this study, to express mutant transgenic forms of hGPIbα in murine platelets while maintaining normal expression of endogenous mouse GPIb/V/IX, has enabled specific disruption of the GPIb-filamin interaction in transgenic mouse platelets without producing gross morphologic and cytoskeletal changes characteristic of the BSS phenotype. Our studies have clearly demonstrated that defective association of GPIbα with filamin does not lead to major changes in the ligand binding function of the GPIb receptor, or influence the ability of this receptor to mediate platelet tethering under high shear. In contrast, our studies suggest that the principal alteration in GPIb adhesive function relates to defects in membrane stability, leading to increased membrane tether formation and membrane deposition on the VWF surface, which are features likely to produce a progressive loss of platelet membrane reserves.
In erythrocytes, the importance of interactions between cytoskeletal and integral membrane proteins for normal morphology and deformability to ensure safe passage through the vasculature is well established. Although platelets have an equally specialized submembranous cytoskeletal network, the primary role for platelets is to mediate adhesion at sites of vascular injury to ensure efficient hemostasis. Because the link between adhesion receptors and the cytoskeleton plays an important role in regulating cell adhesion, spreading, and migration, it is likely that platelets have developed strategies to ensure appropriate regulation of their adhesion. Unlike other adhesion receptor-ligand interactions including the selectins and P-selectin glycoprotein ligand-1 that mediate capture and transient (rolling) adhesive interactions of neutrophils under low shear conditions, GPIb/V/IX is unique in its ability to initiate platelet adhesion at shear rates of 40 000 s−1 and above. The bond strengths required to maintain adhesion under these conditions are high, and combined with the hydrodynamic drag on the platelets, a balance will be reached between the ability of the bond to withstand the shear force and maintaining the stability of the membrane/membrane skeleton. Under the high shear conditions in which GPIbα becomes indispensable for platelet recruitment to the damaged vessel wall, combined with the high forces that these platelets would be subjected, the loss of this interaction could have a significant impact on platelet function. Moreover, human platelets are 10 times more resistant to deformation than erythrocytes,35 mediated primarily by the membrane skeleton and microtubule coil, rather than the intrinsic properties of the lipid bilayer. This resistance to deformation is likely to be important for the normal maintenance of platelet integrity under high shear forces.
Previous studies at shear rates of 10 000 s−1 and above have revealed an activation-independent mechanism of platelet aggregation that can prolong their adhesion to VWF for minutes without requiring integrin αIIbβ3 activation.36 This effect was suggested as a mechanism that might contribute to arterial thrombosis. Therefore, the consequences of destabilizing platelet integrity could be of significant importance at sites of severe stenosis (80%) in which shear rates have been estimated to reach 52 000 s−1 at the apex of the stenosis.37 Based on our observations, platelets with a defect in the GPIb-filamin linkage may be unable to maintain their adhesion contacts under such high shear forces, reducing the interaction lifetime of aggregating platelets and potentially undermining stable thrombus development. Future thrombosis studies with hGPIbαFW platelets, with and without endogenous mouse GPIb, will be required to address this finding. These studies may not be straightforward because in the absence of endogenous mouse GPIb, the FW-AA mutation may give rise to a macrothrombocytopenia characteristic of BSS. However, this additional mouse model will no doubt be informative in delineating the role of the GPIbα-cytoskeletal linkage in regulating normal megakaryocyte ultrastructure and internal membrane development during megakaryocyte maturation, which are features that are essential for normal platelet production.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Miriam Garcia, Tanya Templeton, and other Monash Mouseworks staff for generation, breeding, and maintenance of transgenic mice; Akiko Ono, Sharelle Sturgeon, Fu Jia, and Imala Alwis for their excellent technical assistance; Warwick Nesbitt and Erik Westein for helpful advice with imaging; and Joan Clark from Monash MicroImaging for assistance with scanning electron microscopy.
This work was supported by the National Health and Medical Research Council Australia.
Authorship
Contribution: S.L.C. provided intellectual input, designed and performed experiments, analyzed data, and cowrote the paper; K.J.A. performed experiments and analyzed data; Y.Y. performed experiments; M.C.B. and R.K.A. provided intellectual input; Z.M.R. provided intellectual input and DNA constructs; and S.P.J. provided intellectual input and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan L. Cranmer, PhD, Australian Centre for Blood Diseases, Monash University, Alfred Medical Research & Education Precinct, Commercial Rd, Melbourne, Victoria 3004, Australia; e-mail: sue.cranmer@monash.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal