Abstract
In healthy adults, the major peripheral blood γδ T-cell subset expresses the Vγ9Vδ2 TCR and displays pleiotropic features. Here we report that coculture of naive Vγ9Vδ2 T cells with phosphoantigens and a cocktail of cytokines (IL-1-β, TGF-β, IL-6, and IL-23), leads to selective expression of the transcription factor RORγt and polarization toward IL-17 production. IL-17+ Vγ9Vδ2 T cells express the chemokine receptor CCR6 and produce IL-17 but neither IL-22 nor IFN-γ; they have a predominant terminally differentiated (CD27−CD45RA+) phenotype and express granzyme B, TRAIL, FasL, and CD161. On antigen activation, IL-17+ Vγ9Vδ2 T cells rapidly induce CXCL8-mediated migration and phagocytosis of neutrophils and IL-17–dependent production of β-defensin by epithelial cells, indicating that they may be involved in host immune responses against infectious microorganisms. Accordingly, an increased percentage of IL-17+ Vγ9Vδ2 lymphocytes is detected in the peripheral blood and at the site of disease in children with bacterial meningitis, and this pattern was reversed after successful antibacterial therapy. Most notably, the phenotype of IL-17+ Vγ9Vδ2 T cells in children with meningitis matches that of in vitro differentiated IL-17+ Vγ9Vδ2 T cells. Our findings delineate a previously unknown subset of human IL-17+ Vγ9Vδ2 T lymphocytes implicated in the pathophysiology of inflammatory responses during bacterial infections.
Introduction
IL-17 is a cytokine that induces mobilization and activation of neutrophils and triggers the production of proinflammatory cytokines and chemokines by a broad range of cellular targets.1 It is predominantly produced by αβ T cells, but also by natural killer (NK) T cells,2 γδ T cells,3 and other non-T cells, such as macrophages and neutrophils.4,5 Differentiation of CD4 T cells producing IL-17 (Th17) is initiated in naive Th cells by antigen-specific stimulation in the presence of the polarizing cytokines IL-1β, TGF-β, and IL-6 (and autocrine IL-21), which induce the expression of IL-23 receptor (IL-23R), the chemokine receptor CCR6, and the Th17-specifying transcription factor RORγt, which is necessary and sufficient for induction of IL-17.1,6 In mice, γδ T cells represent an innate source of IL-17 and precede the development of the adaptive Th17-cell response. For instance, during Mycobacterium tuberculosis and Escherichia coli infection, γδ T cells are the primary source of IL-177,8 and their depletion causes decreased IL-17 production and neutrophil infiltration into the peritoneal cavity.8 In Listeria monocytogenes infection, γδ T cells producing IL-17 enhance the antibacterial activity of nonphagocytic cells, which correlates with the induction of β-defensin gene expression.9 These results indicate a novel IL-17–dependent protective mechanism of γδ T cells that acts against intracellular bacterial infections in the mouse. The authors of several recent studies have provided data on the differentiation, phenotype, and functions of murine γδ T cells producing IL-1710-15 and have demonstrated that signals through the γδ TCR are not required for IL-17 production; instead, this process seems to be controlled by innate cytokines produced by accessory cells such as macrophages or dendritic cells (DCs).11,15,16 Conversely, few groups have investigated IL-17 production by human γδ T cells.
Most human peripheral blood γδ T cells express a TCR consisting of the Vγ9 and the Vδ2 chains (here and thereafter referred to as Vγ9Vδ2 T cells) and recognize nonpeptidic phosphorylated metabolites of isoprenoid biosynthesis produced by microorganisms and stressed cells.17-19 On activation, Vγ9Vδ2 T cells promote DC maturation,20 B-cell activation,21 and polarize adaptive immunity toward a Th1 immune response.10 Such a broad plasticity emphasizes the capacity of Vγ9Vδ2 T cells to influence the nature of immune response to different challenges. Human γδ T cells producing IL-17 have been detected in the peripheral blood of patients with tuberculosis22 or HIV infection,23 but in neither of these studies did the authors characterize the IL-17– and IL-22–producing γδ T cells or examine the cytokine requirements for IL-17 production.
The authors of a very recent study have demonstrated that IL-17A– and IL-22–producing Vγ9Vδ2 T cells exist at low but significant frequencies in human and nonhuman primates,24 and have suggested that Vγ9Vδ2 T cells can be polarized into Th17 (producing only IL-17), Th1/17 (producing both IFN-γ and IL-17), and Th22 (producing only IL-22) populations, with distinct cytokine requirements for their initial polarization and later maintenance. Finally, Maggi et al25 have found that circulating γδ T lymphocytes that produce IL-17 express the distinctive marker CD161 and that CD161 expression identifies umbilical cord blood γδ T cells that express RORC and IL-23R mRNA and can be induced to differentiate into IL-17–producing cells by IL-1β and IL-23.25
We show here that in human naive Vγ9Vδ2 T cells, RORC expression and polarization toward IL-17 production are efficiently induced by coordinated TCR triggering and a combination of polarizing cytokines, IL-1β, IL-6, TGF-β, and IL-23. Moreover, we provide detailed phenotypic and functional analysis of IL-17+ Vγ9Vδ2 T cells, as well as in vivo evidence of their involvement in the pathogenesis and inflammatory response during bacterial infections.
Methods
Study population
Buffy coats of healthy volunteers were obtained from the Blood Bank of the University Hospital, Palermo. Paired samples of PBMCs and cerebrospinal fluid (CSF) samples were obtained by 12 children with bacterial meningitis (9 boys, 3 girls, 7.8 ± 4.9 years of age; range, 3-14 years) from the Children Hospital G. Di Cristina, Palermo. The diagnosis of bacterial meningitis was established by the presence of clinical symptoms, clinical history, the results of computed tomography scanning, CSF examination, and positive CSF cultures. Eight children (4 boys, 4 girls, 9.2 ± 3.4 years of age; range, 5-14 years) affected by other noninflammatory neurologic disease and who underwent lumbar puncture for diagnostic purposes also were recruited at the Children Hospital G. Di Cristina, Palermo, to serve as control patients. None of the recruited patients and control children had any evidence of HIV infection, nor were any of the patients being treated with steroids or other drugs at the time of first sampling. Informed consent was obtained for each patient and control subject by their parents in accordance with the Declaration of Helsinki.
Cell purification and culture
Peripheral blood CD14+ monocytes and Vγ9Vδ2 T cells were isolated by positive selection with CD14- and Vδ2-specific microbeads, respectively (Miltenyi Biotec). DCs were obtained from sorted CD14+ monocytes after culture for 5-6 days in the presence of 25 ng/mL GM-CSF and 1000 U/mL IL-4 (both from Euroclone).26 Subsets of Vγ9Vδ2 T cells were isolated to > 99% purity after staining with PE-conjugated anti-CD27 (BD Biosciences) and allophycocyanin (APC)–conjugated anti-CD45RA mAbs (BD Biosciences), followed by cell sorting with a FACSAria (BD Biosciences). Cells were cultured in IMDM medium or RPMI-1640 (Euroclone) supplemented with 2mM l-glutamine, 20nM HEPES buffer, 10 μg/mL gentamicin, 100 U/mL penicillin/streptomycin (Sigma-Aldrich), and containing 10% pooled human AB+ serum (kindly provided by the Blood Bank of the University Hospital, Palermo).
Sorted Vγ9Vδ2 T-cell subsets (5 × 104) were cultured in U-bottom 96-well plates, with an equal number of irradiated (30 Gy from a cesium source) DCs and isopentenyl pyrophosphate (IPP; Sigma-Aldrich; 10−5M final concentration) supplemented with recombinant TGF-β (10 ng/mL final concentration; R&D Systems), IL-1β (10 ng/mL final concentration; R&D Systems), IL-6 (50 ng/mL final concentration; BD Biosciences), and IL-23 (10 ng/mL final concentration; R&D Systems) added in all possible combinations. On day 6, one-half of the medium was removed and replaced with fresh medium containing recombinant IL-2 (20 IU/mL final concentration; Novartis Pharma), and the cells were maintained in culture for additional 6 days. In some experiments (Figure 3), cultures were performed as described previously, but combinations of priming cytokines were added 6 hours after initial stimulation with IPP.
FACS analysis
Expression of surface markers was determined by flow cytometry (FACS) analysis. The following unconjugated or FITC-, PE-, PE-Cy5-, or APC-conjugated mAbs were used: anti-TCRVδ2 (B6; BD Biosciences), anti-CD16 (3G8; BD Biosciences), anti-CD56 (B159; BD Biosciences), anti-CD161 (DX12; BD Biosciences), antigranzyme B (GB11; eBioscience), anti-Fas Ligand (FasL, 2C101; Alexis through Vinci Biochem), anti-TRAIL (RIK-2; eBioscience), anti-NKG2D (1D11; eBioscience), antiperforin (δG2; Vinci Biochem), anti-CCR3 (61828.111; R&D Systems), anti-CCR4 (1G1; BD Biosciences), anti-CCR5 (2D7; BD Biosciences), anti-CCR6 (11A9; BD Biosciences), anti-CXCR3 (1C6/CXCR3; BD Biosciences), anti-CXCR5 (51 505; R&D Systems), and isotype control mAbs. Vγ9Vδ2 cells were incubated in U-bottom 96-well plates with labeled mAbs in PBS containing 1% FCS for 30 minutes at 4°C according to manufacturers' recommendations, washed, and analyzed by flow cytometry on an FACSCalibur or FACSCanto (BD Biosciences) and analyzed with FlowJo software (TreeStar). Viable cells were gated by forward and side scatter, and the analysis was performed on 100 000 acquired events for each sample.
ELISA and intracellular cytokine analysis
The cytokine-producing capacity of primed Vγ9Vδ2 T cells was assessed by stimulation of cells (105/mL) for 24 hours with IPP (10−5M final concentration). Cytokines in culture supernatants were measured by ELISA according to the manufacturer's instructions (R&D Systems). Intracellular staining for IFN-γ, IL-17, IL-4, IL-10, and IL-22 was performed on Vγ9Vδ2 T cells stimulated for 6 hours with IPP (10−5M final concentration) in the presence of GolgiStop (BD Biosciences) for the final 3 hours of culture. Cells were fixed and made permeable with BD Cytofix/Cytoperm Plus (BD Biosciences) according to the manufacturer's instructions. Cells were incubated with FITC-labeled anti-IFN-γ mAb (B27; BD Biosciences), PE-labeled anti-IL-4 mAb (8D4-8; BD Biosciences), PE-labeled anti-IL-22 mAb (142928; R&D Systems), PE-labeled anti-IL-10 mAb (JES5-16E3; BD Biosciences), and APC-labeled anti-IL-17 mAb (eBIO64-DEC17; eBioscience) or isotype-control mAbs. Cells were washed, and data were acquired on a FACSCalibur or FACSCanto (BD Biosciences) and analyzed with FlowJo software.
Neutrophil migration and phagocytosis assays
Human neutrophils were isolated from the citrate-anticoagulated peripheral blood of healthy volunteers by the Polymorphoprep (Nycomed Pharma) centrifugation techniques.27 The purity of human neutrophils was > 95%, as estimated by the Wright-Giemsa stain. Neutrophil migration was performed as described previously.28 Cells (105/mL) were placed in the top well of a Boyden Chamber (Neuro Probes) with a 3-μm porous membrane. Vγ9Vδ2 T cells (105/mL) were placed in the lower chamber in the presence or absence of IPP (10−5M final concentration). As a negative control, IPP or medium alone was added in the lower chamber. In some experiments, neutralizing mAb to CXCL8 (6217; R&D Systems) or isotype control mAbs were added to the lower chamber.
After 3 hours' incubation at 37°C, migrated neutrophils that adhered to the lower side of the membrane were stained by the use of a modified Giemsa staining (DiffQuik). All cells in 3 or 4 randomly chosen fields (×400 magnification) were counted. For each experiment, 3 replicates were performed. Chemotaxis of neutrophils was quantified as percentage of migrated cells among input. To study phagocytic activity, neutrophils separated as described previously were incubated with PE-fluorescent beads (BD Biosciences) in the presence of Vγ9Vδ2 T cells (105/mL) and IPP (10−5M final concentration). In some experiments, neutralizing mAbs to CXCL8 (6217; R&D Systems) or isotype control mAbs were added to cultures. After 2 hours, the percentage of PE-positive neutrophils was determined by FACS.
ELISA assay for β-defensins
The tumor epithelial cell line HT29 was used as a source of β-defensins. HT29 cells (105/mL) were incubated with Vγ9Vδ2 T cells (105/mL) in the presence or absence of IPP (10−5M final concentration). As a negative control, IPP or medium alone was placed in the cultures. In some experiments, we added neutralizing mAbs to IL-17 (eBIO64-DEC17, eBioscience) or isotype control mAbs to the cultures. After 24 hours, supernatants were collected, and β-defensins were quantitated, according to the manufacturer's instructions, with a commercially available ELISA (Alpco Diagnostics).
Real-time quantitative RT-PCR
Total RNA was extracted with the ABI PRISM 6100 Nucleic Acid PrepStation (Perkin-Elmer Applied Biosystems) according to the manufacturer's instructions. Random hexamers and an MMLV Reverse Transcriptase kit (Stratagene) were used for cDNA synthesis. Transcripts were quantified by real-time quantitative PCR on an ABI PRISM 7700 Sequence Detector (Perkin-Elmer Applied Biosystems) with Applied Biosystems predesigned TaqMan Gene Expression Assays and reagents according to the manufacturer' s instructions. The following probes were used (identified by Applied Biosystems assay identification number): RORC, Hs01076112_m1; TBX21, Hs00203436_m1; IL17A, Hs99999082_m1; IFNG, Hs99999041_m1; IL1BR, Hs00168392_m1 IL6R, Hs00169842_m1; IL23R, Hs00332759_m1; TGFBR, Hs00188614_m1; and AHR, HS00169233_m1. For each sample, mRNA abundance was normalized to the amount of 18S rRNA.
Statistics
A standard 2-tailed t test or a t test with the Welch correction was used for statistical analysis. P values < .05 were considered significant.
Results
Factors inducing the differentiation of IL-17+ Vγ9Vδ2 T cells
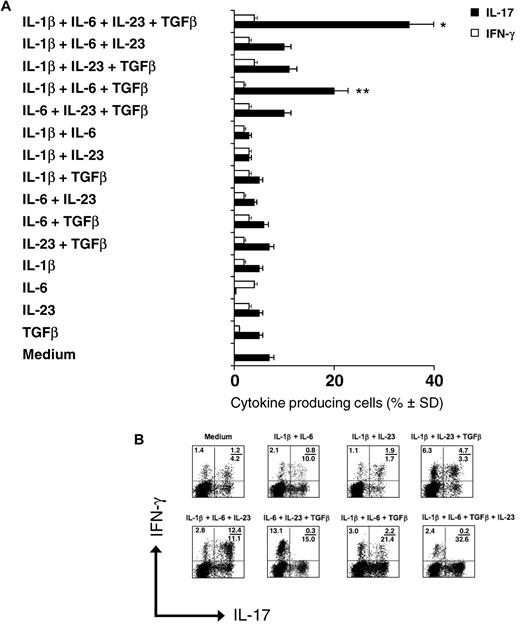
To identify conditions that permit the polarization of human Vγ9Vδ2 T cells to IL-17 production, we stimulated highly purified subsets of naive (Tnaive, CD45RA+CD27+), central memory (TCM, CD45RA−CD27+), effector memory (TEM, CD45RA−CD27−), and terminally differentiated effector memory (TEMRA, CD45RA+CD27−) Vγ9Vδ2 T cells for 6 days with irradiated autologous DCs and antigen (IPP), together with different cytokines either alone or in combination. We allowed the cells to proliferate for an additional 6 days in the presence of low doses of IL-2 (see “Cell purification and culture” for details), and analyzed their capacity to produce IL-17 and/or IFN-γ by intracellular cytokine staining on stimulation with IPP for 6 hours, and by ELISA on stimulation with IPP for 24 hours. Moreover, because promotion of Th17 polarization requires stimulation of the aryl hydrocarbon receptor (AhR),29 a ligand-dependent transcription factor that responds to a wide range of ligands, including metabolites of tryptophan and other aromatic amino acids, we performed cultures in the presence of either standard RPMI-1640 medium or IMDM medium, which is enriched in aromatic amino acids.29 Cumulative data from 15 different healthy subjects are shown in Figure 1A (intracellular staining), representative FACS plots are shown in Figure 1B, and the results of the ELISA assay are shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Polarization of IL-17+ Vγ9Vδ2 T cells is induced by antigen and IL-1β, IL-6, IL-23, and TGF-β. Intracellular cytokine staining for IL-17 and IFN-γ in naive (CD45RA+CD27+) human Vγ9Vδ2 T cells primed for 6 days with an equal number of irradiated DCs and IPP, in the presence of various combinations of cytokines, then incubated for 6-7 days more in IL-2 and stimulated for 6 hours with IPP. (A) Cumulative results (mean values ± SD) of 3 different experiments each performed with 5 different healthy donors. *P < .01 and **P < .05 compared with the medium group. (B) Typical flow cytometry panels of a representative experiment. Numbers in quadrants indicate percent cells in each.
Polarization of IL-17+ Vγ9Vδ2 T cells is induced by antigen and IL-1β, IL-6, IL-23, and TGF-β. Intracellular cytokine staining for IL-17 and IFN-γ in naive (CD45RA+CD27+) human Vγ9Vδ2 T cells primed for 6 days with an equal number of irradiated DCs and IPP, in the presence of various combinations of cytokines, then incubated for 6-7 days more in IL-2 and stimulated for 6 hours with IPP. (A) Cumulative results (mean values ± SD) of 3 different experiments each performed with 5 different healthy donors. *P < .01 and **P < .05 compared with the medium group. (B) Typical flow cytometry panels of a representative experiment. Numbers in quadrants indicate percent cells in each.
In the absence of exogenous cytokines, only a small percentage (≤ 7%) of antigen-primed Vγ9Vδ2 T cells produced IL-17 but not IFN-γ. The addition of IL-1β, IL-23, and TGF-β alone did not enhance IL-17 production, whereas IL-6 suppressed the production of IL-17 and enhanced the production of IFN-γ, even if such inhibitory effect was evident only when measuring IL-17 intracellularly but not by ELISA (supplemental Figure 1). Similarly, the addition of 2 cytokines in various combinations failed to induce production of IL-17 by Vγ9Vδ2 T cells. In contrast, combinations of IL-1β, IL-6, and TGF-β strongly induced the differentiation of IL-17+ Vγ9Vδ2 T cells (20%), most of which did not produce IFN-γ (Figure 1A-B); the combinations of IL-1β, IL-23, and TGF-β and IL-1β, IL-6, and IL-23 caused high frequencies of double IFNγ+/IL-17+ cells (Figure 1B and data not shown). Furthermore, the combination of IL-1β, IL-6, TGF-β, and IL-23 augmented the overall percentage of IL-17+ Vγ9Vδ2 T cells (35%) but did neither influenced single IFN-γ+ Vγ9Vδ2 T cells nor induced detectable double IFNγ+/IL-17+ cells (Figure 1A-B). Similar results were obtained by the measurement of IL-17 and IFN-γ concentrations in culture supernatants by ELISA (supplemental Figure 1).
Like CD4 T cells,6 Vγ9Vδ2 T cells with a Tnaive phenotype were the only subset that can be polarized to IL-17 production, whereas TCM, TEM, and TEMRA Vγ9Vδ2 T cells failed to polarize to IL-17 production under similar cytokine conditions (supplemental Figure 2A). Moreover, differentiation of IL-17+ Vγ9Vδ2 T cells only occurred on culture with IMDM medium but not in RPMI-1640 medium (supplemental Figure 2B), indicating that, similarly to CD4 T cells,29 also Vγ9Vδ2 T cells require AhR stimulation to efficiently polarize to IL-17 production. Most notably, AHR expression was found in Vγ9Vδ2 T cells differentiating under IL-17 polarizing conditions (supplemental Figure 2C).
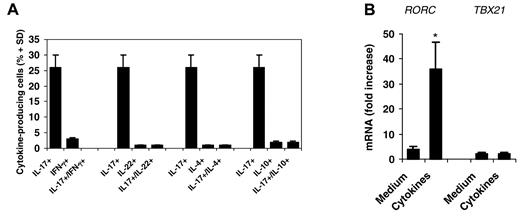
To further characterize Vγ9Vδ2 T cells differentiated in vitro, we measured the production of additional cytokines. Vγ9Vδ2 T cells did not produce IFN-γ, IL-4, and IL-10 (Figure 2A). Unexpectedly, and differently than CD4 T cells,6,30 Vγ9Vδ2 T cells did not produce IL-22, a Th17-related cytokine.
Priming with antigen and IL-1β, IL-6, IL-23, and TGF-β induces exclusive IL-17 production and RORC expression in differentiating Vγ9Vδ2 T cells. (A) Intracellular cytokine staining for IL-17, IFN-γ, IL-22, IL-4, and IL-10 in naive (CD45RA+CD27+) human Vγ9Vδ2 T cells primed for 6 days with an equal number of irradiated DCs and IPP in the presence of a cocktail of 4 cytokines (IL-1β, IL-6, IL-23, and TGF-β) then incubated for 6-7 days more in IL-2 and stimulated for 6 hours with IPP. (B) RT-PCR of the expression of RORC and TBX21 in cells primed with antigen in the presence or absence (medium) of cytokines, as described in panel A. Data represent the mean values ± SD of 3 separate experiments, each performed with 2 different healthy donors. *P < .001 compared with the medium group.
Priming with antigen and IL-1β, IL-6, IL-23, and TGF-β induces exclusive IL-17 production and RORC expression in differentiating Vγ9Vδ2 T cells. (A) Intracellular cytokine staining for IL-17, IFN-γ, IL-22, IL-4, and IL-10 in naive (CD45RA+CD27+) human Vγ9Vδ2 T cells primed for 6 days with an equal number of irradiated DCs and IPP in the presence of a cocktail of 4 cytokines (IL-1β, IL-6, IL-23, and TGF-β) then incubated for 6-7 days more in IL-2 and stimulated for 6 hours with IPP. (B) RT-PCR of the expression of RORC and TBX21 in cells primed with antigen in the presence or absence (medium) of cytokines, as described in panel A. Data represent the mean values ± SD of 3 separate experiments, each performed with 2 different healthy donors. *P < .001 compared with the medium group.
Differentiation of Th17 cells involves the coordinated up-regulation of the key transcription factors RORγt and RORα.31 We therefore measured the expression of mRNA encoding the human orthologs of mouse RORγt (RORC) and T-bet (TBX21) in IL-17+ Vγ9Vδ2 T cells. Culture of naive Vγ9Vδ2 T cells under IL-17 polarizing conditions (IL-1β, IL-6, TGF-β, and IL-23) induced high expression of RORC, whereas expression of TBX21 was induced only slightly or not at all (Figure 2B). Collectively, these data indicate that IL-1β, IL-6, TGF-β, and IL-23 induce expression of RORC in antigen-primed Vγ9Vδ2 T cells, which is consistent with their ability to promote IL-17 production.
The relative role of antigen and cytokines in the regulation of lineage-specifying factors
The development of a polarized Th17-cell subset takes up to 5 days in vivo6 and requires stimulation by a specific antigen in the presence of IL-1β, IL-6, IL-23, and TGF-β. This initial activation results in the up-regulation of STAT3 and RORC expression, which enhance IL-23 responsiveness and induce IL-17 production.31 By contrast, studies in the mouse have shown that innate γδ T cells that reside in peripheral tissues can be activated by IL-23 and IL-1β alone or in combination with microbial antigens recognition by TLR1 and TLR2 or through their TCR.10,11,15,16 Because these cells constitutively express transcriptional regulators for IL-17 production, they can produce IL-17 within few hours of stimulation.
To investigate early events in the differentiation of IL-17+ Vγ9Vδ2 T cells and the relative role of antigen and polarizing cytokines, we assessed the kinetics of expression of mRNA encoding for different cytokine receptors, as well as RORC and IL17A. Data are shown in Figure 3. Resting, unstimulated Vγ9Vδ2 T cells did not express constitutively either receptors for IL-17–polarizing cytokines (IL1BR, IL6R, TGFBR, and IL23R) or IL2R, IL17A, and RORC (data not shown). However, Vγ9Vδ2 TCR stimulation by antigen induced expression of IL1BR, IL6R, TGFBR and, but at a lower extent, IL23R mRNA, as early as 6 hours after stimulation. Expression of IL1BR, IL6R, TGFBR, and IL23R mRNA peaked on day 3 and consistently decreased on day 6. Antigen stimulation alone was not sufficient to induce detectable RORC and IL17A mRNA, indicating that up-regulation of lineage-specifying transcription factors requires combination of antigen and IL-17–polarizing cytokines. Accordingly, RORC and IL17A were significantly induced by antigen in the presence of IL-1β, IL-6, and TGF-β (data not shown), and in a more sustained way, by antigen and the combination of IL-1β, IL-6, TGF-β, and IL-23. In contrast, the addition of a single cytokine or 2 of the aforementioned cytokines in various combinations failed to induce or induced low expression of RORC and IL-17A, a finding that is consistent with their inability to induce the differentiation of IL-17+ Vγ9Vδ2 T cells. In Vγ9Vδ2 T cells exposed to antigen and the combination of IL-1β, IL-6, TGF-β, and IL-23, RORC and IL17A mRNA peaked on days 3-6 and decreased by day 9 onward.
Antigen and cytokines differently regulate expression of lineage-specifying transcription factors in IL-17–differentiating Vγ9Vδ2 T cells. RT-PCR of the expression of IL1βR, IL6R, TGFβR, IL23R, IL17, and RORC in naive (CD45RA+CD27+) human Vγ9Vδ2 T cells primed for various times (horizontal axes) with antigen, or left unprimed, in the presence or absence of cytokines. Data represent the mean values ± SD of 4 separate experiments, each performed with 5 different donors.
Antigen and cytokines differently regulate expression of lineage-specifying transcription factors in IL-17–differentiating Vγ9Vδ2 T cells. RT-PCR of the expression of IL1βR, IL6R, TGFβR, IL23R, IL17, and RORC in naive (CD45RA+CD27+) human Vγ9Vδ2 T cells primed for various times (horizontal axes) with antigen, or left unprimed, in the presence or absence of cytokines. Data represent the mean values ± SD of 4 separate experiments, each performed with 5 different donors.
These results indicate that the coordinated combination of TCR triggering by antigen and the presence of IL-1β, IL-6, TGF-β, and IL-23 induces sustained expression of RORC and IL17A in human Vγ9Vδ2 T cells, which is consistent with their ability to promote differentiation and polarization toward IL-17 production.
Phenotype of IL-17+ Vγ9Vδ2 T cells
Similarly to CD8 T cells, human peripheral blood Vγ9Vδ2 T cells can be subdivided into distinct populations (Tnaive, TCM, TEM, and TEMRA) that can be distinguished on the basis of surface marker expression and effector functions.32 To characterize the memory status of IL-17+ Vγ9Vδ2 T cells, we performed staining for CD27 and CD45RA on human Vγ9Vδ2 T cells that had been cultured under IL-17–polarizing conditions. Although IFN-γ+ Vγ9Vδ2 T cells had a predominant TEM, and at a lower extent TEMRA, phenotype (Figure 4A), the majority of IL-17+ Vγ9Vδ2 T cells had a TEMRA-like, CD27−CD45RA+ phenotype, and only a few cells had a Tnaive phenotype. Thus, and differently than IL-17+ CD8 T cells, which are almost exclusively restricted to the Tearly and Tintermediate subsets,33 IL-17 production is mostly restricted to Vγ9Vδ2 T cells with a TEMRA-like phenotype.
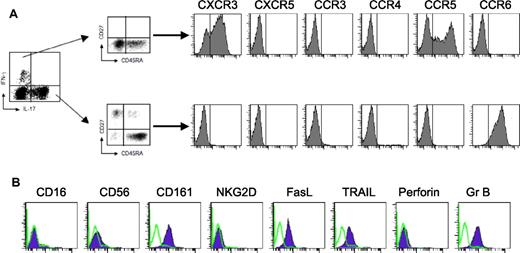
Surface phenotype of IL-17+ Vγ9Vδ2 T cells. Vγ9Vδ2 T cells were primed for 6 days with an equal number of irradiated DCs and IPP in the presence of cytokines and then incubated for 6-7 days more in IL-2 and stimulated for 6 hours with IPP. After intracellular staining for IL-17 and IFN-γ, cells were surface stained for several different markers. (A) Chemokine receptor expression is shown on gating on IL-17+ or IFN-γ+ Vγ9Vδ2 T cells. The vertical line in each panel indicates the negative cutoff as determined by staining with isotype-control mAbs. (B) Surface markers expression on IL-17+ Vγ9Vδ2 T cells (violet-filled lines). Green open lines indicate staining with isotype-control mAbs.
Surface phenotype of IL-17+ Vγ9Vδ2 T cells. Vγ9Vδ2 T cells were primed for 6 days with an equal number of irradiated DCs and IPP in the presence of cytokines and then incubated for 6-7 days more in IL-2 and stimulated for 6 hours with IPP. After intracellular staining for IL-17 and IFN-γ, cells were surface stained for several different markers. (A) Chemokine receptor expression is shown on gating on IL-17+ or IFN-γ+ Vγ9Vδ2 T cells. The vertical line in each panel indicates the negative cutoff as determined by staining with isotype-control mAbs. (B) Surface markers expression on IL-17+ Vγ9Vδ2 T cells (violet-filled lines). Green open lines indicate staining with isotype-control mAbs.
In addition, IL-17+ Vγ9Vδ2 T cells expressed CCR6 (Figure 4A), a chemokine receptor that has been identified as a marker of human memory Th17 cells,6,11,14 but they did not express CCR3, CCR4, CCR5, CXCR3, and CXCR5. Conversely, IFN-γ+ Vγ9Vδ2 T cells similarly differentiated in vitro reciprocally expressed high levels CXCR3 and CCR5 but low levels CCR6 (Figure 4A). Moreover, IL-17+ Vγ9Vδ2 T cells expressed granzyme B, TRAIL, FasL, and CD161 but did not express perforin, NKG2D, CD16, and CD56 (Figure 4B).
IL-17+ Vγ9Vδ2 T cells induce neutrophils migration and enhance their phagocytic activity: role of CXCL8
Th17 cells directly or indirectly induce the recruitment of neutrophils.34 We investigated the effects of IL-17+ Vγ9Vδ2 T cells on distinct functional properties of neutrophils. To study the capability of IL-17+ Vγ9Vδ2 T cells to induce chemotaxis of neutrophils, we used a Boyden chamber, in which neutrophils were placed in the top well and IL-17+ Vγ9Vδ2 T cells were placed in the lower chamber. After 3 hours, migrated neutrophils adherent to the lower side of the membrane were stained and counted. As shown in Figure 5A, antigen-activated IL-17+ Vγ9Vδ2 T cells induced significant neutrophils migration, which was abrogated by addition to the lower chamber of a neutralizing mAb to CXCL8. IL-17+ Vγ9Vδ2 T cells that had been cultured with medium alone failed to induce neutrophils migration.
IL-17+ Vγ9Vδ2 T cells promote CXCL8-mediated neutrophil migration and phagocytic activity. (A) Neutrophils were placed in the top well of a Boyden Chamber, and IL17+ Vγ9Vδ2 T cells were placed in the lower chamber in the presence or absence of IPP. In some experiments, neutralizing mAb to CXCL8 or isotype control mAbs were added to the lower chamber. After 3 hours of incubation at 37°C, migrated neutrophils adherent to the lower side of the membrane were stained and counted. Data are expressed as percentage of migrated cells among input. (B) Neutrophils were incubated with PE-fluorescent beads in the presence of IL17+ Vγ9Vδ2 T cells and IPP. In some experiments, neutralizing mAbs to CXCL8 or isotype control mAbs were added to cultures. After 2 hours, the percentage of PE+ neutrophils was determined FACS. Data are expressed as percentage PE+ neutrophils. *P < .01 and **P < .02 compared with all other groups.
IL-17+ Vγ9Vδ2 T cells promote CXCL8-mediated neutrophil migration and phagocytic activity. (A) Neutrophils were placed in the top well of a Boyden Chamber, and IL17+ Vγ9Vδ2 T cells were placed in the lower chamber in the presence or absence of IPP. In some experiments, neutralizing mAb to CXCL8 or isotype control mAbs were added to the lower chamber. After 3 hours of incubation at 37°C, migrated neutrophils adherent to the lower side of the membrane were stained and counted. Data are expressed as percentage of migrated cells among input. (B) Neutrophils were incubated with PE-fluorescent beads in the presence of IL17+ Vγ9Vδ2 T cells and IPP. In some experiments, neutralizing mAbs to CXCL8 or isotype control mAbs were added to cultures. After 2 hours, the percentage of PE+ neutrophils was determined FACS. Data are expressed as percentage PE+ neutrophils. *P < .01 and **P < .02 compared with all other groups.
We also discovered that activated IL-17+ Vγ9Vδ2 T cells potentiate neutrophil phagocytosis. Results from 8 independent experiments (Figure 5B) showed that when incubated in the presence of antigen-activated IL-17+ Vγ9Vδ2 T cells, neutrophils acquired an increased capability to phagocyte PE-labeled beads, and this was reversed by the addition of neutralizing mAb to CXCL8 to the cultures. Cultures of neutrophils with IL-17+ Vγ9Vδ2 T cells but in the absence of antigen or with antigen but in the absence of IL-17+ Vγ9Vδ2 T cells failed to increase the phagocytic activity of neutrophils. Together, these results reveal a novel function of IL-17+ Vγ9Vδ2 T cells, namely their ability to produce chemokines (CXCL8) that induce recruitment and potentiate phagocytosis of neutrophils.
IL-17+ Vγ9Vδ2 T cells up-regulate production of β-defensin by epithelial cells
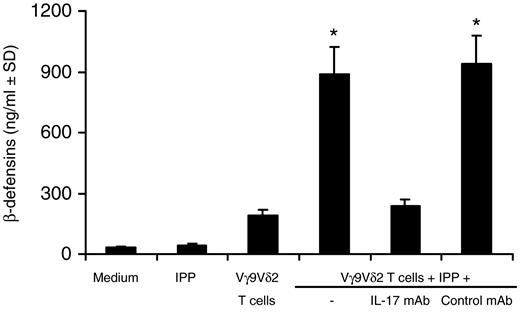
Th17 cells induce the production of antibacterial proteins and peptides by epithelial cells.1 We evaluated the release of β-defensin from epithelial cells on culture with IL-17+ Vγ9Vδ2 T cells. As shown in Figure 6, antigen-activated IL-17+ Vγ9Vδ2 T cells significantly up-regulated β-defensin production by epithelial cells, whereas IL-17+ Vγ9Vδ2 T cells that had been cultured with medium alone (ie, in the absence of antigen) failed to up-regulate β-defensin production. The addition to cultures of a neutralizing mAb to IL-17 significantly abrogated the capability of IL-17+ Vγ9Vδ2 T cells to up-regulate β-defensin production (Figure 6). Collectively, these results indicate that IL-17+ Vγ9Vδ2 T cells display several function-promoting host defenses against infectious agents and contribute to immune responses occurring at mucosal surfaces.
IL-17+ Vγ9Vδ2 T cells promote IL-17–dependent production of β-defensin by epithelial cells. HT29 epithelial cells were incubated with IL-17+ Vγ9Vδ2 T cells in the presence or absence of IPP. In some experiments, neutralizing mAbs to IL-17 or isotype control mAbs were added to cultures. After 24 hours, supernatants were collected and β-defensins quantitated by commercially available ELISA. *P < .005 compared with all other groups.
IL-17+ Vγ9Vδ2 T cells promote IL-17–dependent production of β-defensin by epithelial cells. HT29 epithelial cells were incubated with IL-17+ Vγ9Vδ2 T cells in the presence or absence of IPP. In some experiments, neutralizing mAbs to IL-17 or isotype control mAbs were added to cultures. After 24 hours, supernatants were collected and β-defensins quantitated by commercially available ELISA. *P < .005 compared with all other groups.
IL-17+ Vγ9Vδ2 T cells are rarely found in healthy subjects but significantly increase in patients with bacterial meningitis
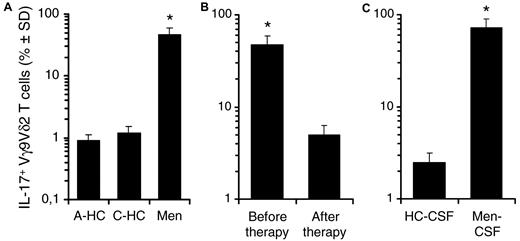
It has been recently reported that IL-17+ Vγ9Vδ2 T cells can be detected in the peripheral blood at a frequency of approximately 1% of all Vγ9Vδ2 T cells, on polyclonal stimulation of whole PBMCs, although the proportions varies widely.24 Accordingly, we rarely found IL-17–producing cells among Vγ9Vδ2 T cells from the peripheral blood of healthy donors (n = 30) on short-term stimulation with either antigen (Figure 7A) or anti-CD3 (data not shown).
Frequencies of IL-17+ Vγ9Vδ2 T cells in control subjects and in patients with bacterial meningitis. (A) Frequencies of IL-17–producing cells among Vγ9Vδ2 T cells from the peripheral blood of adult healthy donors (A-HC; n = 30), control children (C-HC; n = 8), and children affected by bacterial meningitis (Men; n = 12) on short-term stimulation with antigen (IPP). (B) The frequency of IL-17+ Vγ9Vδ2 T cells in the peripheral blood of children affected by bacterial meningitis (n = 12), before and after successful therapy, determined on antigen stimulation, as above described. (C) Frequencies of IL-17+ Vγ9Vδ2 T cells in the cerebrospinal fluid (CSF) of control children (HC-CSF; n = 8) and children affected by bacterial meningitis (Men-CSF; n = 12) on short-term stimulation with antigen (IPP). *P < .001 compared with all other groups.
Frequencies of IL-17+ Vγ9Vδ2 T cells in control subjects and in patients with bacterial meningitis. (A) Frequencies of IL-17–producing cells among Vγ9Vδ2 T cells from the peripheral blood of adult healthy donors (A-HC; n = 30), control children (C-HC; n = 8), and children affected by bacterial meningitis (Men; n = 12) on short-term stimulation with antigen (IPP). (B) The frequency of IL-17+ Vγ9Vδ2 T cells in the peripheral blood of children affected by bacterial meningitis (n = 12), before and after successful therapy, determined on antigen stimulation, as above described. (C) Frequencies of IL-17+ Vγ9Vδ2 T cells in the cerebrospinal fluid (CSF) of control children (HC-CSF; n = 8) and children affected by bacterial meningitis (Men-CSF; n = 12) on short-term stimulation with antigen (IPP). *P < .001 compared with all other groups.
It has become apparent that Th17 responses are important for the host defense against extracellular bacteria.1 Therefore, we asked whether frequencies of IL-17+ Vγ9Vδ2 T cells increased during such infection. To this aim we studied the frequencies of IL-17+ Vγ9Vδ2 T cells in the peripheral blood of children affected by bacterial meningitis (Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis) before and after successful therapy and in control children: moreover, we also investigated whether IL-17+ Vγ9Vδ2 T cells could be detected at the site of disease by measuring their frequency in the CSF of the same patients. As shown in Figure 7A and supplemental Figure 3A-B, the frequency of circulating IL-17+ Vγ9Vδ2 T cells was significantly (P < .001) increased in all patients with bacterial meningitis compared with control subjects, but this pattern reversed after the administration of successful antibacterial therapy (Figure 7B). In addition, the γδ T-cell repertoire from the CSF of patients was characterized by the predominance of IL-17+ Vγ9Vδ2 T lymphocytes (Figure 7C), which accounted for > 70% of γδ T cells. In these patients, the cytokine profile of CD4 T cells from either PBMCs or CSF was dominated by IL-17+ cells over IFN-γ+ cells (supplemental Figure 3A-B). Moreover, IL-17+ Vγ9Vδ2 T lymphocytes isolated from the PBMCs of children with bacterial meningitis expressed CD45RA, CD161, CCR6, and TRAIL but not CD27 and perforin, thus matching the phenotype of in vitro differentiated IL-17+ Vγ9Vδ2 T cells (supplemental Figure 3C). Unfortunately, we did not obtain enough cells from the CSF of children with meningitis to perform a detailed phenotypic analysis of the IL-17+ Vγ9Vδ2 T lymphocytes accumulating at the site of disease. Thus, the increased percentage of circulating IL-17+ Vγ9Vδ2 T lymphocytes and their localization at the site of active disease suggest that IL-17+ Vγ9Vδ2 T lymphocytes may play an important role in the pathogenesis and inflammatory response during bacterial meningitis.
Discussion
Vγ9Vδ2 T cells display in vitro a certain degree of plasticity in their function that is reminiscent, and may even exceed, that of conventional CD4 T cells. In analogy with CD4 T cells, where a plethora of specialized subsets affects the host's response, Vγ9Vδ2 T cells may readily and rapidly assume distinct Th1-, Th2-like, and/or follicular B-cell helper T (TFH)-like effector functions,35 suggesting that they profoundly influence immune response.
Here we show that in human naive Vγ9Vδ2 T cells, RORγt expression and polarization toward IL-17 production are efficiently induced by the coordinated antigen stimulation of the specific TCR; a combination of polarizing cytokines, IL-1β, IL-6, TGF-β, and IL-23, and AhR ligands. The IL17+ Vγ9Vδ2 T cells exhibit a TEMRA phenotype, illustrated by the expression of CD45RA in the absence of CD27. Thus, and differently than IL-17+ CD8 T cells that are almost exclusively restricted to the Tearly and Tintermediate subsets,33 IL-17 production is restricted to Vγ9Vδ2 T cells with a TEMRA-like phenotype.
Vγ9Vδ2 TEMRA cells initially were identified by our group in the ascites and the CSF of patients with tuberculosis and considered a distinct and critical pool of cytotoxic effectors corresponding to a late stage of Vγ9Vδ2 T-cell differentiation.32 However, significant phenotypic differences exist between these previously described cytotoxic Vγ9Vδ2 TEMRA cells and the IL-17+ Vγ9Vδ2 T population, as these latter cells express granzyme B, TRAIL, FasL, and CD161 but lack the expression of perforin, NKG2D, CD16, and CD56. The NKG2D− phenotype of IL-17+ Vγ9Vδ2 T cells is surprising, given that NKG2D is expressed by the majority of circulating Vγ9Vδ2 T cells. It is likely that TGFβ, present in our culture conditions, down-regulates NKG2D expression on Vγ9Vδ2 T cells, as demonstrated for NK and CD8 T cells,36 although additional studies are required to confirm this possibility. Lack of perforin expression, in the presence of granzyme B, is intriguing, although dissociation between expression of these 2 molecules has been previously reported in the CD8 compartment and has been correlated with absence of or poor cytolytic activity.37 Human and murine IL-17+ CD8 T cells do not express perforin and granzyme B and lack cytolytic activity, although the authors of a most recent study have found that mouse Tc17 cells mediate immunity to vaccinia virus by acquisition of a cytolytic potential that correlates with FasL expression.38 Accordingly, IL-17+ Vγ9Vδ2 T cells similarly express FasL and exert potent TRAIL-mediated cytotoxic activity against epithelial tumor cells (supplemental Figure 4). This functional aspect further distinguishes IL-17+ T Vγ9Vδ2 cells from the cytotoxic Vγ9Vδ2 TEMRA population, as these latter preferentially exploit the granule/exocytosis pathway to kill targets.32
Expression of CD161 by IL-17+ Vγ9Vδ2 T cells is in agreement with the finding that CD161 is a marker of IL-17–producing cells.25,39 CD161 is the human homologue of the mouse NK1.1,34 which is expressed not only on almost all NK and NK T cells40 but also on all circulating lymphocytes (including γδ T cells) able to produce IL-17, as well as precursors of IL-17–producing T cells, and this feature is RORC dependent.25 CD161 was previously found on Vγ9Vδ2,41 but the memory status and the cytokine-secreting or cytotoxic capacities of CD161+ cells were not investigated. Interestingly, CD161+ Vγ9Vδ2 T cells are greatly increased in patients with multiple sclerosis,41 a finding that is highly suggestive of an hitherto-unrecognized role of IL-17+ Vγ9Vδ2 T cells in autoimmune inflammation.
IL-17+ Vγ9Vδ2 T cells distinctively express CCR6, a chemokine receptor that has been identified as a marker of Th17 cells,6,11,14 but they do not express CCR3, CCR4, CCR5, CXCR3, and CXCR5. Conversely, Th1-like, IFN-γ+ Vγ9Vδ2 T cells express CXCR3 and CCR5 but not CCR6.32
Thus, the selective expression of the characteristic markers of the Th17 program (RORC, IL-17, CCR6) on IL-17+ Vγ9Vδ2 T cells, and the requirement for medium rich in aromatic amino acids (and subsequently for AhR), provide further support to the concept that there is a coordinate regulation of migratory capabilities and effector functions in differentiating IL-17+ Vγ9Vδ2 T cells. Notably, the CCR6 agonist CCL20, which is constitutively expressed in normal skin and mucosa-associated tissues, is up-regulated by IL-1742 and mediates the recruitment of T cells and DCs to inflamed sites. In addition, IL-17+ Vγ9Vδ2 T cells rapidly induce IL-17–dependent production of β-defensin by epithelial cells, which is another CCR6 agonist,43,44 and CXCL8-mediated recruitment and up-regulation of phagocytosis of neutrophils. This last finding is in full agreement with the recent report that activated Th17 cells could directly chemoattract neutrophils via the release of biologically active CXCL8.45
Thus, IL-17 produced by migrating Vγ9Vδ2 T cells may trigger a positive loop that further attracts Th17 and Th1 cells, as well as DCs and neutrophils, that amplifies host inflammatory responses. In previous studies in mice, investigators have shown that γδ T cells are an innate source of IL-177-9 without the need for TCR engagement.15,16 A striking consequence of these findings is that the role of the TCR in IL-17+ γδ T cells could be redundant, in line with their predetermined phenotype in the thymus without positive or negative selection. Accordingly, murine γδ T cells acquire IL-17A potential in the neonatal thymus12,13,46 and, at least in the T10/T22 antigen model, this is not dependent on encountering the specific antigen in the thymus.47
In contrast to mouse studies, we have found that TCR engagement is required in the differentiation of human IL-17+ Vγ9Vδ2 T cells. Thus, the deciphering of relative roles of cytokines and of TCR-dependent or TCR-independent activation of human IL-17+ Vγ9Vδ2 T cells and their role in protective immune response or in pathology is a great challenge for their potential use in clinical settings.
Although it is increasingly appreciated that effector T cells are extremely heterogeneous in terms of cytokine production, we found that after antigenic stimulation in vitro IL-17+ Vγ9Vδ2 T cells do not produce either IL-22 or IFN-γ. However, and in partial contrast to our results, a recently published study of human IL-17+ Vγ9Vδ2 T cells has shown that they also produce IL-22 and/or IFN-γ, whereas IL-17+ single producers are rarely found.24 This finding is consistent with the idea that polarized T cells, although they retain memory of the imprinted cytokine, may undergo further differentiation in response to polarizing cues.48 Alternatively, it is also possible that some IL-17–producing Vγ9Vδ2 T cells may be programmed to differentiate to IL-22– and/or IFN-γ–producing cells, as it has been shown for cells precommitted to Th1 or Th2 differentiation.49
The commitment and flexibility of effector T-cell populations are probably controlled by the expression and balance of lineage-specifying transcription factors.50 It is plausible that in certain conditions of antigenic stimulation, or cytokine microenvironment, or both, Vγ9Vδ2 T cells may differentiate into multifunctional cells able to trigger additional responses in the periphery.
Similar to our findings, in studies in human CD4 T cells differentiating under IL-17–polarizing conditions, authors have found production of IL-17 and expression of AHR in the absence of IL-22 production.29,51 Although we do not have any explanation for the dissociation between IL-17 and IL-22 production, in the presence of AHR expression, one important consideration is that culture conditions may have a profound influence on the outcome of the response. For instance, it appears that an excess of TGF-β, most likely compounded by extra TGF-β in serum, irrespective of the presence of IL-6, still give rise to Th17 cells but determines inhibition of IL-22 production.52
It has become apparent that Th17 responses are important for the host defense against microorganisms, particularly extracellular bacteria.1 Accordingly, the population of IL-17+ Vγ9Vδ2 T cells was significantly increased in patients with bacterial meningitis, and this pattern reversed after successful antibacterial therapy. In addition, and most important, IL-17+ cells were the predominant Vγ9Vδ2 T-cell population from the CSF of these patients. The IL-17+ Vγ9Vδ2 T lymphocytes isolated from PBMCs of children with bacterial meningitis expressed CD45RA, CD161, CCR6, and TRAIL but not CD27 and perforin, thus matching the phenotype of in vitro differentiated IL-17+ Vγ9Vδ2 T cells. Thus, the increased percentage of circulating IL-17+ Vγ9Vδ2 T lymphocytes and their localization at the site of active disease suggest that IL-17+ Vγ9Vδ2 T lymphocytes may actually play an important role in inflammatory response during bacterial meningitis.
In conclusion, our studies delineate a novel subset of human IL-17+ Vγ9Vδ2 T lymphocytes that participates in inflammatory responses during bacterial infections and emphasizes the well-known plasticity of human Vγ9Vδ2 T cells in their ability to exert different effector functions on the basis of the influence of polarizing cytokines.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ministry of University and Research (MIUR-PRIN; to F.D.), the Ministry of Health (to F.D. and G. Stassi) and the University of Palermo. J.J.F. was supported by grants from INSERM, Institut National contre le Cancer, and Association pour la Recherche sur le Cancer. V.O. is a student of the International PhD Program in Immunopharmacology at the University of Palermo.
Authorship
Contribution: N.C., J.J.F., and F.D. conceived and designed research; C.L.M., V.O., S.M., M.T., G. Stassi, and G. Sireci performed research; J.J.F., provided comments and support; N.C. and F.D., wrote the manuscript; and J.J.F. reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Dieli, Dipartimento di Biopatologia e Biotecnologie Mediche e Forensi, Università di Palermo, Corso Tukory 211, Palermo 90134, Italy; e-mail: dieli@unipa.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal