Abundant data in the past decade have convincingly demonstrated that microenvironmental clues are crucial determinants of the fate of hematopoietic stem cells (HSCs). However, as we have learned more about the HSC niche, its extraordinary intricacy has begun to emerge. In the current issue of Blood, Mazzon et al add to this complexity by identifying agrin as a novel noncellular element of the HSC niche.1
Agrin is an extracellular matrix component that belongs to the heterogeneous family of heparan sulfate proteoglycans. Heparan sulfate proteoglycans as a class were initially discovered to regulate stromal-hematopoietic interactions by seminal ex vivo experiments demonstrating their ability to adsorb growth factors and present them in their biologically active form to immature hematopoietic cells.2 Later studies used in vitro functional human HSC assays (the long-term culture-initiating cell assay) to demonstrate that heparan sulfate proteoglycans are an essential factor in stromal-HSC interactions,3 by binding matrix component and cytokines and colocalizing them with immature hematopoietic cells in supportive niches.4 Heparan sulfate proteoglycans also participate in adhesion of HSCs with niche cells.5
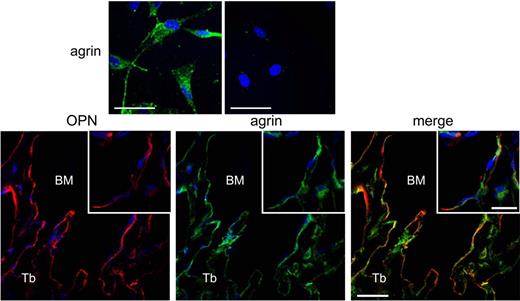
Agrin is an essential regulator of neuromuscular synapses.6 Its nonneuronal isoforms have been shown to contribute importantly to T-cell interaction with antigen-presenting cells,7 but had not been previously implicated in the regulation of immature hematopoietic cells. In the current article, Mazzon et al convincingly demonstrate that within the marrow microenvironment, agrin is selectively expressed in cells of mesenchymal stem cells (MSCs; top panels of figure) and the osteoblastic lineage (bottom panels of figure), but not endothelial, osteoclastic, and reticular cells (see supplemental Figure 1 to Mazzon et al1 ). The authors then evaluate hematopoiesis in mice lacking agrin where the lethal neuromuscular defects are rescued by skeletal muscle over expression of one of the agrin receptors.8 Interestingly, no abnormalities in osteoblastic cells or MSCs were detected in these genetically altered mice, in spite of confirmation of agrin deletion in MSCs; therefore, agrin is not required for cell-autonomous MSC functions. In contrast, postnatal lack of agrin resulted in severe global hematopoietic defects.
Top panels: Primary endosteal MSCs express agrin (from Figure 2A, Mazzon et al1 ). Bottom panels: In vivo endosteal ostepontin-expressing cells of the osteoblastic lineage express agrin (from Figure 1A, Mazzon et al1 ).
When the phenotypic HSC population was quantified, phenotypic HSCs with limited self-renewal (or short-term HSCs, ST-HSCs) were decreased, without changes in the more quiescent long-term HSCs. Notably, there was no cell-autonomous HSC defect in the mice lacking agrin. In these agrin-deficient mice, no HSC abnormalities were noted until the postnatal period, and transplantation assays using HSCs lacking agrin demonstrated normal engraftment. In contrast, agrin-mediated cell-cell contact between HSCs and endosteal osteoblastic/MSCs was crucial for ST-HSC survival and expansion. Interestingly, lack of agrin did not change local expression of HSC-supportive cytokines, again excluding an indirect mode of disruption of HSC-stromal interactions. Indeed, use of a blocking antibody to the agrin receptor that is expressed on HSCs decreased the ability of normal ST-HSCs to engraft, suggesting that interactions of stromal/osteoblastic agrin with its receptor on HSCs are crucial for normal ST-HSC function.
Thus, this report by Mazzon et al contributes a new molecular target to the osteoblastic HSC niche, and is one of very few reports highlighting differential microenvironmental regulation of individual HSC subsets. The compartmentalized expression of agrin in MSCs and osteoblastic but not endothelial and osteoclastic cells furthers our understanding of differential effects of individual niche components on specific HSC behaviors. Because the agrin receptor is present on human CD34+ cells,9 this novel therapeutic target may be of use in clinical scenarios where specific expansion of ST-HSCs may reduce morbidity, such as in recovery from iatrogenic or toxic myeloablative injury.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal