Abstract
Hematopoiesis is the process leading to the sustained production of blood cells by hematopoietic stem cells (HSCs). Growth, survival, and differentiation of HSCs occur in specialized microenvironments called “hematopoietic niches,” through molecular cues that are only partially understood. Here we show that agrin, a proteoglycan involved in the neuromuscular junction, is a critical niche-derived signal that controls survival and proliferation of HSCs. Agrin is expressed by multipotent nonhematopoietic mesenchymal stem cells (MSCs) and by differentiated osteoblasts lining the endosteal bone surface, whereas Lin−Sca1+c-Kit+ (LSK) cells express the α-dystroglycan receptor for agrin. In vitro, agrin-deficient MSCs were less efficient in supporting proliferation of mouse Lin−c-Kit+ cells, suggesting that agrin plays a role in the hematopoietic cell development. These results were indeed confirmed in vivo through the analysis of agrin knockout mice (Musk-L;Agrn−/−). Agrin-deficient mice displayed in vivo apoptosis of CD34+CD135− LSK cells and impaired hematopoiesis, both of which were reverted by an agrin-sufficient stroma. These data unveil a crucial role of agrin in the hematopoietic niches and in the cross-talk between stromal and hematopoietic stem cells.
Introduction
Hematopoiesis is a dynamic process in which multipotent, hematopoietic stem cells (HSCs) give rise to all hematopoietic lineage cells: neutrophils, eosinophils, basophils, monocytes, macrophages, megakaryocytes, platelets, and erythrocytes, which constitute the myeloid lineage, and T and B cells, which compose the lymphoid lineage.1 After birth, sustained hematopoiesis in the bone marrow depends on the self-renewal of the resident HSCs in the stem cell niche, where signaling molecules, extracellular matrix (ECM), and cell adhesion molecules that regulate stem cell fates are produced. Indeed, hematopoiesis involves the coordination of several signal transduction pathways, which are induced by extracellular stimuli through cell-cell and cell-ECM interactions.2 Among ECM components, heparan sulfate proteoglycans (HSPGs) are crucial controllers of the structural and functional organization of the bone marrow HSC niche,3 where they regulate skeletal-hematopoietic interactions4 by mediating cell adhesion of hematopoietic progenitors to stromal cells5 and by binding and thus modulating the activity of cytokines.6
Agrin, an ECM protein belonging to the heterogeneous family of HSPGs expressed by motor neurons, is a critical regulator of neuromuscular synapses where it binds to skeletal muscle Lrp4, leading to activation of Musk, a receptor tyrosine kinase essential for transmitting the agrin signal.7 However, the function of nonneuronal isoforms of agrin, expressed in numerous cell types, is poorly understood. Previous studies indicated a role for agrin at the T-cell immunologic synapse with antigen presenting cells.8 The agrin receptor at the immunologic synapse has been defined as α-dystroglycan (α-DG),9 a broadly expressed cell surface receptor with a high affinity for ECM proteins.10 Dystroglycans are critical in the early stages of development and mice deficient for α-DG show embryonic lethality at embryonic day (E) 6.5, probably arising from defects in extra-embryonic structures and their association with the extracellular matrix.11 Interestingly, α-DG is expressed in human hematopoietic CD34+ cells,12 but the in vivo functional significance of such expression has not been determined. Here, we examined the role of agrin in postnatal hematopoiesis and found that agrin is a nonredundant component of the osteoblast endosteal niche providing signals essential for HSC survival.
Methods
Mice
Agrin-deficient mice have been described elsewhere.13 Musk-LAgrn+/− mice (on C57BL/6 background) were bred at the animal facility of the Humanitas Clinical Institute. Mutant and control mice were genotyped by PCR of tail DNA as already described.13 Congenic B6(CD45.1) mice, purchased from The Jackson Laboratory, were maintained in the Charles River animal facility and used as recipients of bone marrow (BM) transplantation experiments. Congenic B6(CD45.2) mice were crossed with B6(CD45.1) mice to obtain B6(CD45.1/45.2) recipients for competition BM transfer experiments. Procedures involving animals and their care conformed to institutional guidelines in compliance with national (4D.L. N.116, G.U., suppl. 40, 18-2-1992) and international (EEC Council Directive 86/609, OJ L 358,1,12-12-1987; National Institutes of Health Guide for the Care and Use of Laboratory Animals) laws and policies. All efforts were made to minimize the number of animals used and their suffering.
BM transfer assays
For long-term competition experiments, 0.6 × 106 BM cells from P5 control or agrin-deficient mice (Musk-LAgrn−/−; CD45.2) were coinjected with 0.6 × 106 BM cells from an adult B6(CD45.1/2) donor into lethally irradiated (950 cGy), 8- to 12-week-old B6(CD45.1) recipients, that were placed under antibiotic treatment 1 week before and 2 weeks after irradiation. Repopulating unit (RU) values were calculated from percentages of donor-type PB leukocytes in the recipients, where the relative repopulating ability of 100 000 competitor marrow cells is defined as 1 RU.14 Mice were killed 16 weeks after transfer.
Short-term reconstitution experiments were performed by modifying a previous published protocol15 : 1200 donor bone marrow sorted CD34+ CD135− LSK cells (CD45.2) were transferred into lethally irradiated congenic recipients (CD45.1), together with 800 sorted CD34+ CD135− LSK cells from adult CD45.1/45.2 mice and 1 × 105 Lin+ feeder BM cells from control adult mice (CD45.1). C1-INH (15 U/mouse) was administered intravenously to the lethally irradiated congenic recipients 2 hours before transfer. C1-INH (1 U corresponded to the activity of 1 mL of normal plasma) was from Baxter-Immuno. The reconstitution ability of donor cells (CD45.2) was evaluated 8 days after the transfer.
For BM transfer experiments, 1 × 106 BM cells from P5 Musk-LAgrn−/− or control mice were transferred into 8- to 12-week-old B6(CD45.1) and recipients were sacrificed and analyzed 8 weeks after transfer.
Histology
Tissues were fixed in 4% (weight/volume) formalin, embedded in paraffin, sectioned, and stained with H&E.
Immunohistochemistry and immunofluorescence
Five-micron sections of OCT-embedded kidneys were incubated with anti-agrin (1:2000; gift from Prof M. Ferns, UC Davis School of Medicine, Davis, CA) and the appropriate secondary antibody, was used. Staining was visualized by 3,3′-diaminobenzidine (DAB; Dako Cytomation) and counterstained with hematoxylin.
Five-micron sections of OCT-embedded femur from control and Musk-L;Agrn−/− mice fixed in 4% (weight/volume) paraformaldehyde and decalcified as previously reported16 were incubated with anti-osteopontin (1:200; R&D Systems); anti-CD31 (1:1000; R&D Systems); anti–ER-TR717 (1:200; AbCam), an antibody that detects an antigen present in and produced by BM reticular fibroblasts; and anti-cathepsin K18 (1:400; gift from Prof D. Bromme, The University of British Columbia, Vancouver, BC) antibodies and the appropriate secondary antibody. Staining was visualized by 3,3′-diaminobenzidine (DAB; Dako Cytomation) and counterstained with hematoxylin. Images were acquired with an Olympus BX51 microscope equipped with a Colorview III digital camera (Olympus).
Femurs of 4-6 days were embedded in OCT compound and snap-frozen in liquid nitrogen–cooled isobutane. Two percent formaldehyde–fixed 5-μm sections were incubated with anti-agrin (1:2000; gift from M. Ferns) anti-osteopontin (1:200; R&D Systems), anti-CD31 (1:1000; R&D Systems), anti-ER-TR717 (1:200; AbCam), or anti-cathepsin K18 (1:400; gift from Prof D. Bromme) antibodies. The appropriate Alexa-conjugated secondary antibodies were used (Molecular Probes). The nuclei were counterstained with Hoechst 33342 (Molecular Probes).
LSK were fixed with 1% or 4% formaldehyde, blocked with BSA 1%, and incubated with the primary antibodies anti-agrin (1:2000; gift from Prof M. Ferns) and anti–α-DG (1:250; AbCam). The appropriate Alexa-conjugated secondary antibodies were used (Molecular Probes). The nuclei were counterstained with Hoechst 33342 (Molecular Probes). Images were acquired by a confocal microscope Fluoview FV1000 (Olympus) with an oil-immersion objective (40× or 60×/1.4 NA Plan-Apochromat; Olympus) using laser excitation at 405, 488, 594, or 633 nm. Images were processed using Adobe Photoshop 9.0.2. Colocalization was measured on single confocal sections using the colocalization module of Imaris 5.0.1, 64-bit version (Bitplane AG).
Proliferation analysis
Proliferation of BM cells was evaluated by immunohistochemical analysis of minichromosome maintenance protein 2 (MCM2) expression and by in vivo bromodeoxyuridine (BrdU) analysis. For MCM2 analysis,19 formalin fixed, paraffin-embedded, 2-μm sections were deparaffinated, rehydrated, and incubated with anti-MCM2 Ab (1:100; Santa Cruz Biotechnology) followed by the incubation with the appropriate secondary antibody. Staining was visualized by 3,3′-diaminobenzidine (Dako Cytomation) and counterstained with hematoxylin. The proliferation index was quantified as total number of positive cells on total number of cells counted in different fields. For in vivo cell proliferation, mice were injected intraperitoneally with BrdU in PBS (50 μg/g body weight) and, after 2 hours, BM cells were isolated and BrdU incorporation was evaluated using a commercially available kit (BD Biosciences).
FACS sorting and analysis
BM cell suspensions were obtained by flushing of multiple bones (femur, tibia, humerus, ulna) in PBS containing 3% FCS (sterile buffer). Peripheral blood was obtained by retro-orbital blood withdrawal. Cell surface, 4/6 color stainings were performed in staining buffer for 30 minutes, on ice, in the dark, with the appropriate combinations of saturating concentrations of the following conjugated monoclonal antibodies (mAbs) obtained from BD Pharmingen, eBioscience, or BioLegend: CD45.2 (104), CD45.1 (A20), Mac1/CD11b (M1/70), CD3e (145-2C11), CD127/IL-7Rα (A7R34), CD25 (PC61.5), CD115 (AFS98), CD19 (MB19-1), CD45 (R0-F11), Ly6G (1A8), Ly-6C (AL-21), CD4 (GK1,5), CD8a (53-6,7), B220 (RA3–6B2), TER119/Ly-76 (TER-119), Sca-1 (D7), CD16/32 (2.4G2), CD44 (IM7), CD62e (10E9.6), CD29 (HMbeta1-1), CD34 (RAM34), CD41 (MWReg30), CD48(HM48.1), CD150(TC15-12F12.2), CD135(A2F10.1), and CD106 (429MVCAM.A). The lineage cocktail that included the Mac1, Gr1, B220, TER119, CD5, 7/4 biotin-conjugated mAbs and CD117/c-Kit (3C1) were purchased from MACS Miltenyi. F4/80 (A3–1) was purchased from Serotech. Phospho-ERK expression was detected using anti-ERK1/2(T202/Y204) mAb (BD Pharmingen). Dead cells were excluded by low angle and orthogonal light scatter.
Absolute count of leukocytes was performed using TruCount tubes (BD Biosciences) according to the manufacturer's instructions. For annexin V staining, freshly isolated BM cells were first stained with the appropriate mAbs, then washed in binding buffer and incubated with annexin V (BD Pharmigen).
Stained cells were analyzed with a FACSCanto I (2 lasers) or FACSCanto II (3 lasers) flow cytometer (BD Biosciences). Cell sorts were performed using a FACSAria (3 lasers; BD Biosciences). Diva Version 6.1.1 software (BD Biosciences) was used for data acquisition and analysis. HSC analysis was performed according to published protocols.15,20
Osteoblast isolation
Osteoblast-lineage cells were isolated using minor modifications of published procedures.21 Briefly, bone fragments were dissected from soft tissue, flushed to remove BM cells, progressively “minced” to a fine granular consistency, and digested for 60 minutes at 37°C with collagenase type IV (Sigma-Aldrich) into DMEM-Ham's F12 mixture supplemented with 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Collagenase was than inactivated with 10% FBS. Cells were used for subsequent immunofluorescence analyses.
Primary MSC-enriched cell isolation
Endosteal cells were isolated with minor modifications according to published procedures.22 BM cells were flushed of femurs and tibias of control mice and the bones were minced with scissors. Bone fragments were incubated at 37°C with 3 mg/mL type I collagenase in Dulbecco-modified Eagle medium with 10% FCS, and gently agitated for 90 minutes at 37°C. Subsequently, the cells were stained for CD45, CD31, Ter119, Sca1, anti-ALCAM, and the CD45−CD31−Ter119−ALCAM−Sca-1+ MSC-enriched cell fraction was sorted for immunofluorescence analysis.
Isolation and culture of MSCs from mouse BM
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
SYBR-green RT-PCR
See supplemental Methods.
Statistical analysis
Statistical analyses were performed using the unpaired Student t test (GraphPad Prism 4.0).
Differences were considered significant when P ≤ .05 (*), very significant when P ≤ .01 (**), and extremely significant when P ≤ .0001 (***).
Results
Agrin is expressed by osteoblasts at the stem cell niche
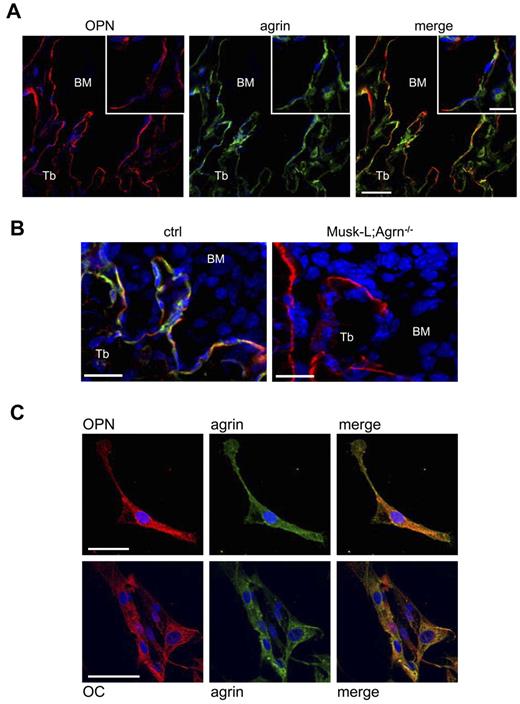
Within the BM, HSCs are found in close contact with the endosteum, the inner surface of the bone. The endosteum is lined by undifferentiated bone-lining cells, as well as bone-resorbing osteoclasts and bone-forming osteoblasts, which provide an important niche for HSCs.23 To investigate the expression of agrin at the hematopoietic stem cell niche we performed immunofluorescence staining of cryostat sections of femurs from C57BL/6 mice. Agrin-positive cells were detected within the bone trabeculae and along the endosteum, which also expressed osteopontin, a marker of osteoblasts and a key component of the hematopoietic niche24 (Figure 1A-B). Agrin expression in osteoblasts was confirmed by immunofluorescence analysis of osteopontin-positive and osteocalcin-positive cells enzymatically isolated from long bones (Figure 1C). In contrast, osteoclasts, endothelial cells, and reticular cells, other important components of the hematopoietic niche,25,26 were negative for agrin expression (supplemental Figure 1).
Agrin is expressed by osteoblast at the hematopoietic niche. (A) Confocal images of methapyseal sections of leg bones obtained from control mice and stained for osteopontin (red), agrin (green) and nuclear DNA (blue). Scale bar, 40 μm; inset scale bar, 20 μm. (B) Z-stack projection of 26 BM confocal sections (0.5 μm each) of trabecular bones obtained from control and Musk-L;Agrn−/− mice stained for osteopontin (red), agrin (green), and nuclear DNA (blue). Scale bar, 20 μm. (C) Osteopontin-positive cells enzymatically isolated from long bones of control mice and stained for osteopontin (red), agrin (green) and nuclear DNA (blue; top row) or osteocalcin (red), agrin (green), and nuclear DNA (blue; bottom row). Scale bar, 50 μm.
Agrin is expressed by osteoblast at the hematopoietic niche. (A) Confocal images of methapyseal sections of leg bones obtained from control mice and stained for osteopontin (red), agrin (green) and nuclear DNA (blue). Scale bar, 40 μm; inset scale bar, 20 μm. (B) Z-stack projection of 26 BM confocal sections (0.5 μm each) of trabecular bones obtained from control and Musk-L;Agrn−/− mice stained for osteopontin (red), agrin (green), and nuclear DNA (blue). Scale bar, 20 μm. (C) Osteopontin-positive cells enzymatically isolated from long bones of control mice and stained for osteopontin (red), agrin (green) and nuclear DNA (blue; top row) or osteocalcin (red), agrin (green), and nuclear DNA (blue; bottom row). Scale bar, 50 μm.
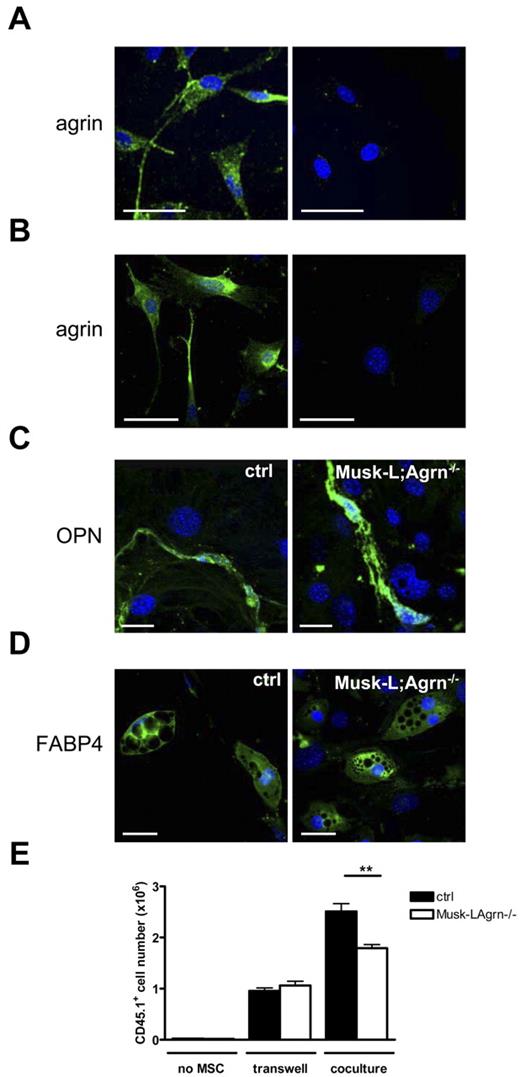
Bone-forming osteoblasts originate from mesenchymal stem cells (MSCs), a heterogeneous subset of stromal stem cells that can differentiate into cells of the mesenchymal lineage, such as adipocytes, osteoblasts, and chondrocytes.27 To evaluate agrin expression in MSCs, we first isolated bone-associated endosteal cells according to a published protocol.22 Femur and tibia fragments were treated with collagenase and the CD45−CD31−Ter119−ALCAM−Sca-1+ cell population, primary MSC-enriched cells,22 were sorted, and agrin expression was analyzed by immunofluorescence (Figure 2A). Agrin expression in MSCs was confirmed using BM-derived MSCs obtained by in vitro culture, and the analysis indicated that agrin is expressed both at the cell surface and intracellularly (Figure 2B).
Agrin is expressed by MSCs and stimulates in vitro hematopoiesis. (A-B) Confocal images of primary mMSCs isolated from the endosteum (A) or in vitro cultured mMSCs (B) stained for agrin (green) and nuclear DNA (blue). Right panel shows the isotype control. Scale bars, 40 μm. The images are representative of ∼ 95% of the plated mMSCs. (C) Confocal images of osteogenically differentiated mMSCs stained for osteopontin (green). Scale bar, 20 μm. (D) Confocal images of adipogenically differentiated mMSCs stained for FABP4. Scale bar, 50 μm (green). (E) Sorted Lin− c-Kit+ BM cells (45.1) were cultured either directly or separated by transwell assay on MSC monolayers from control (ctrl) and Musk-L;Agrn−/− mice. Hematopoietic proliferation of CD45.1+ cells was measured 5 days later. Data are shown as mean ± SEM; n = 4; **P < .01
Agrin is expressed by MSCs and stimulates in vitro hematopoiesis. (A-B) Confocal images of primary mMSCs isolated from the endosteum (A) or in vitro cultured mMSCs (B) stained for agrin (green) and nuclear DNA (blue). Right panel shows the isotype control. Scale bars, 40 μm. The images are representative of ∼ 95% of the plated mMSCs. (C) Confocal images of osteogenically differentiated mMSCs stained for osteopontin (green). Scale bar, 20 μm. (D) Confocal images of adipogenically differentiated mMSCs stained for FABP4. Scale bar, 50 μm (green). (E) Sorted Lin− c-Kit+ BM cells (45.1) were cultured either directly or separated by transwell assay on MSC monolayers from control (ctrl) and Musk-L;Agrn−/− mice. Hematopoietic proliferation of CD45.1+ cells was measured 5 days later. Data are shown as mean ± SEM; n = 4; **P < .01
These results prompted us to analyze MSCs derived from the BM of mice that are null for agrin and carry a transgene that increases Musk expression selectively in skeletal muscle. Previous studies have shown that this skeletal muscle actin: Musk transgene (Musk-L) increases Musk expression by 3-fold and is sufficient to achieve an adequate level of Musk activation in muscle, in the absence of agrin, to restore neuromuscular synapse formation and prevent neonatal lethality of agrin mutant mice.13
At postnatal day 5 (P5), agrin knockout mice (Musk-L;Agrn−/−) showed a 2-fold reduction in body weight compared with Musk-L;Agrn+/+, and Musk-L;Agrn+/− littermates (supplemental Figure 2A). Musk-L;Agrn+/+, or Musk-L;Agrn+/− (referred to hereafter as control mice) did not differ in any of the analyzed phenotypes, and hence, will not be further distinguished. Most Musk-L;Agrn−/− mice died between P7 and P10; for this reason, experiments were performed with P4 to P6 mice. We confirmed complete deletion of agrin in Musk-L;Agrn−/− mice by RT-PCR in hematopoietic organs (data not shown) and by immunohistochemical analysis of the kidney glomerular basement membrane (supplemental Figure1B), a normal site of agrin expression.
MSCs from P5 control and Musk-L;Agrn−/− littermates were isolated from the BM and further characterized by analyzing the expression of several cell surface markers, such as Sca-1, β1 integrin (CD29), VCAM(CD106), CD44, CD45, Lin, c-Kit, CD31, CD62E, and CD34. Flow cytometric analysis showed that both control and agrin knockout MSCs represented a homogeneous population unequivocally negative for the hematopoietic markers CD45, Lin, CD34, and c-Kit and the endothelial CD31 and CD62E (supplemental Figure 3 and not shown), but positive for MSC-associated markers, such as Sca-1, CD44, VCAM, and CD29.
To determine whether agrin-deficient MSCs cells could differentiate into multiple mesenchymal lineages, we examined osteoblast and adypocyte differentiation. Differentiation into the 2 mesoderm lineages was confirmed by immunofluorescence analysis of markers for the osteoblast (osteopontin and osteocalcin) and the adypocyte (FABP4) lineages and it resulted comparable between agrin-deficient and control MSCs (Figure 2C-D and supplemental Figure 4A). Bone-nodule formation assay, a key test of osteoblast function in vitro, was performed with differentiated osteoblasts from control and agrin-deficient mice, and the results indicated that the agrin deficiency does not affect the osteogenic differentiation potential of these cells (supplemental Figure 4B).
To determine whether agrin expression in MSCs plays a role in supporting hematopoiesis, we examined the ability of a confluent monolayer of MSCs, from control and Musk-L;Agrn−/− B6 (CD45.2) mice, to support the differentiation of Lin−c-Kit+ bone marrow progenitor cells [derived from adult B6(45.1) mice] to Lin+CD45.1+c-Kit− lineage-committed cells. Flow cytometric analysis showed that after 5 days of Lin−c-Kit+ BM progenitor-MSC cocultures, 99% of the cells were CD45.1+, independently of agrin expression in MSCs (data not shown). However, the number of hematopoietic cells obtained after 5 days of coculture was significantly reduced when MSCs did not express agrin (Figure 2E), thus suggesting that agrin expressed at the MSC membrane provides signals that sustain progenitor cells proliferation or survival. In accordance, the proliferation was strongly dependent on direct contact with MSCs, as shown by the fact that the interposition of a porous membrane (transwell cocultures) between Lin−c-Kit+ bone marrow progenitors and control MSCs resulted in a reduced expansion (Figure 2E). Notably, in the absence of cell-cell contacts (transwell cocultures), no difference in cell yield was observed on coincubation with control and agrin-deficient MSCs (Figure 2E), indicating that MSCs derived from agrin-deficient mice produce soluble factors required for in vitro progenitor cell expansion.
Hematopoietic alterations in agrin-deficient mice
The expression of agrin at the bone marrow HSC niche, together with the in vitro data reported, prompted us to analyze in vivo the role of agrin in hematopoiesis using Musk-L;Agrn−/− mice.
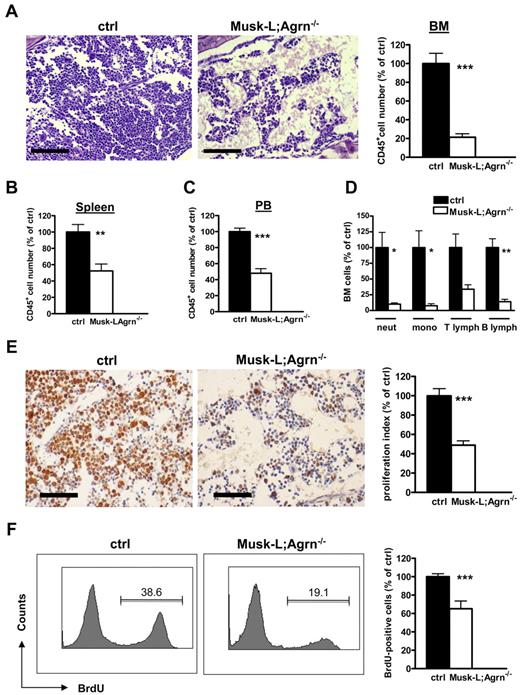
We were strikingly impressed by major histologic alterations in the bone marrow of Musk-L;Agrn−/− mice. HE-stained sections from femurs revealed BM hypoplasia, and flow cytometric analysis indicated that the number of CD45+ bone marrow cells was reduced 5-fold in Musk-L;Agrn−/− mice compared with control mice (Figure 3A). Analysis of spleen and blood cell counts showed a 2-fold reduction in absolute number of CD45+ cells in Musk-L;Agrn−/− mice compared with control mice (Figure 3B-C). A similar (3-fold) reduction of cell number was observed in the thymus of agrin-deficient mice, and HE analysis did not reveal major structural alterations of the organ (not shown). Flow cytometry showed that all T-lineage subpopulations in the thymus were reduced by 2-fold, beginning from the most immature DN1 stage (data not shown).
Hematopoietic defects in agrin-deficient (Musk-L;Agrn−/−) mice. (A) Representative images of hematoxylin and eosin–stained sections of bone marrow from P5 control (ctrl) and Musk-L;Agrn−/− mice. Scale bar, 200 μm. The histogram on the right shows the average absolute numbers of total CD45+ cells (ctrl, n = 20; Musk-L;Agrn−/−, n = 10; ***P < .0001) (B-C) Absolute numbers of CD45+ cells in spleen (B) or blood (C) of control and Musk-L;Agrn−/− mice (spleen: ctrl, n = 12; Musk-L;Agrn−/−, n = 7; **P < .01; blood: ctrl, n = 7; Musk-L;Agrn−/agn−/−, n = 4; ***P < .0001). (D) Absolute numbers of CD45+ hematopoietic cells in control and Musk-L;Agrn−/− bone marrows (ctrl, n = 12; Musk-L;Agrn−/agn−/−, n = 8; *P < .05, **P < .01). (E) MCM2 immunoreactivity of bone marrow sections of P5 control (ctrl; n = 10) and agrin-deficient (Musk-L;Agrn−/−; n = 7) mice. Scale bar, 100 μm. The graph shows the analysis of the proliferation index (***P < .0001). (F) In vivo BrdU incorporation experiments. Bone marrow cells from control or Musk-L;Agrn−/− mice were analyzed 2 hours after intraperitoneal injection. Left, representative flow cytometry profiles; right, statistical analysis. (ctrl, n = 10; Musk-L;Agrn−/−, n = 4; ***P < .0001). In all panels, error bars represent SEM.
Hematopoietic defects in agrin-deficient (Musk-L;Agrn−/−) mice. (A) Representative images of hematoxylin and eosin–stained sections of bone marrow from P5 control (ctrl) and Musk-L;Agrn−/− mice. Scale bar, 200 μm. The histogram on the right shows the average absolute numbers of total CD45+ cells (ctrl, n = 20; Musk-L;Agrn−/−, n = 10; ***P < .0001) (B-C) Absolute numbers of CD45+ cells in spleen (B) or blood (C) of control and Musk-L;Agrn−/− mice (spleen: ctrl, n = 12; Musk-L;Agrn−/−, n = 7; **P < .01; blood: ctrl, n = 7; Musk-L;Agrn−/agn−/−, n = 4; ***P < .0001). (D) Absolute numbers of CD45+ hematopoietic cells in control and Musk-L;Agrn−/− bone marrows (ctrl, n = 12; Musk-L;Agrn−/agn−/−, n = 8; *P < .05, **P < .01). (E) MCM2 immunoreactivity of bone marrow sections of P5 control (ctrl; n = 10) and agrin-deficient (Musk-L;Agrn−/−; n = 7) mice. Scale bar, 100 μm. The graph shows the analysis of the proliferation index (***P < .0001). (F) In vivo BrdU incorporation experiments. Bone marrow cells from control or Musk-L;Agrn−/− mice were analyzed 2 hours after intraperitoneal injection. Left, representative flow cytometry profiles; right, statistical analysis. (ctrl, n = 10; Musk-L;Agrn−/−, n = 4; ***P < .0001). In all panels, error bars represent SEM.
Next, we examined in more detail the bone marrow composition of Musk-L;Agrn−/− mice. Flow cytometric analysis revealed a dramatic reduction in the number of the myeloid (13-fold reduction for monocytes, 10-fold reduction for neutrophils) and lymphoid (3-fold reduction for T lymphocytes, 7-fold reduction for B lymphocytes) components in agrin-deficient mice (Figure 3D). Cell proliferation was analyzed by staining for minichromosome maintenance protein 2 (MCM2) on BM sections (Figure 3E) and evaluating in vivo bromodeoxyuridine (BrdU) incorporation in bone marrow cells (Figure 3F). Both experiments indicated that agrin deficiency impairs proliferation of bone marrow cells.
Agrin is required for HSC functions
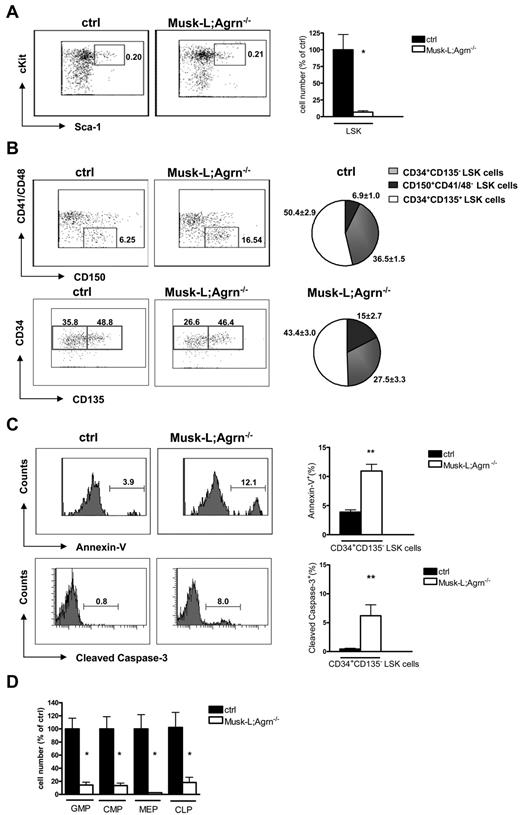
Sustained hematopoiesis in the BM depends on the self-renewal of resident HSCs in the stem cell niche. In Musk-L;Agrn−/− mice, in agreement with the observed BM hypoplasia, we estimated a 6-fold reduction of the Lin−IL7R−c-Kit+Sca-1+ (LSK) HSC population (Figure 4A), indicating a defect at the earliest stages of hematopoietic development. Analysis of the LSK population in the fetal liver at embryonic day 12 (E12) did not reveal any difference between Musk-L;Agrn−/− and control mice (ctrl: 100.0 ± 14.31, n = 8; Musk-L;Agrn−/− 95.79 ± 24.64, n = 4; P = .88), indicating that hematopoietic dysfunctions are restricted to postnatal life or to the bone marrow environment.
BM hypoplasia and stem cell impairment in agrin-deficient (Musk-L;Agrn−/−) mice. (A) Representative flow cytometric analysis of the LSK population for control and Musk-L;Agrn−/− mice and mean numbers relative to controls are shown. Dot plots correspond to the Lin−/IL7R− gate and the indicated values represent the percentage among total BM cells (ctrl, n = 26; Musk-L;Agrn−/−, n = 11; *P < .05). (B) Representative flow cytometric analysis on LSK population: the analysis of CD150 versus CD41/48 expression and the analysis of CD135 versus CD34 expression were done on the Lin−IL7R−c-Kit+Sca-1+ population; the indicated values represent the percentage among total LSK cells. Pie charts represent average frequencies of the 3 subsets within LSK population (ctrl, n = 6; Musk-L;Agrn−/−, n = 4;). CD150+CD41/48− LSK and CD34+CD135− LSK population frequencies are statistically different (P < .05). (C) Annexin V (top row) and cleaved caspase-3 (bottom row) analysis on CD34+CD135− LSK cells. Left, representative flow cytometry profiles; right, statistical analysis (annexin: ctrl, n = 6; Musk-L;Agrn−/−, n = 4; cleaved caspase-3: ctrl, n = 5; Musk-L;Agrn−/−, n = 3; **P < .01). (D) Mean numbers relative to controls of the hematopoietic progenitors for control and Musk-L;Agrn−/− mice are shown. CMPs are defined as Lin−IL7R−Sca-1−c-Kit+CD34+CD16/32low, GMPs as Lin−IL7R−Sca-1−c-Kit+ CD34+CD16/32+, MEPs as Lin−IL7R−Sca-1−c-Kit+CD34−CD16/32−, and CLPs are defined as Lin−IL7R+Sca-1loc-Kit+ (ctrl, n = 15; Musk-L;Agrn−/−, n = 8; *P < .05). In all panels, error bars represent SEM.
BM hypoplasia and stem cell impairment in agrin-deficient (Musk-L;Agrn−/−) mice. (A) Representative flow cytometric analysis of the LSK population for control and Musk-L;Agrn−/− mice and mean numbers relative to controls are shown. Dot plots correspond to the Lin−/IL7R− gate and the indicated values represent the percentage among total BM cells (ctrl, n = 26; Musk-L;Agrn−/−, n = 11; *P < .05). (B) Representative flow cytometric analysis on LSK population: the analysis of CD150 versus CD41/48 expression and the analysis of CD135 versus CD34 expression were done on the Lin−IL7R−c-Kit+Sca-1+ population; the indicated values represent the percentage among total LSK cells. Pie charts represent average frequencies of the 3 subsets within LSK population (ctrl, n = 6; Musk-L;Agrn−/−, n = 4;). CD150+CD41/48− LSK and CD34+CD135− LSK population frequencies are statistically different (P < .05). (C) Annexin V (top row) and cleaved caspase-3 (bottom row) analysis on CD34+CD135− LSK cells. Left, representative flow cytometry profiles; right, statistical analysis (annexin: ctrl, n = 6; Musk-L;Agrn−/−, n = 4; cleaved caspase-3: ctrl, n = 5; Musk-L;Agrn−/−, n = 3; **P < .01). (D) Mean numbers relative to controls of the hematopoietic progenitors for control and Musk-L;Agrn−/− mice are shown. CMPs are defined as Lin−IL7R−Sca-1−c-Kit+CD34+CD16/32low, GMPs as Lin−IL7R−Sca-1−c-Kit+ CD34+CD16/32+, MEPs as Lin−IL7R−Sca-1−c-Kit+CD34−CD16/32−, and CLPs are defined as Lin−IL7R+Sca-1loc-Kit+ (ctrl, n = 15; Musk-L;Agrn−/−, n = 8; *P < .05). In all panels, error bars represent SEM.
The LSK population comprises a CD150+CD41/48− long-term repopulating subset (LT-HSCs) that has lifelong self-renewing potential and gives rise to a CD34+CD135− population with limited self-renewing potential (short-term, ST-HSCs), but able to generate CD34+CD135+ multipotent progenitors (MPP) on commitment.15 The analysis of the LSK population in Musk-L;Agrn−/− mice indicated that the absence of agrin did not affect the LT-HSC population, that was 2-fold reduced compared with the same population in control mice (data not shown), in agreement with the observed weight reduction already mentioned. However, agrin deficiency affected the proliferation or survival of CD34+CD135− LSK cells, which were significantly reduced (P < .05) in terms of relative frequency (Figure 4B). Further analysis revealed that this selective reduction may be explained by increased apoptosis, as detected with either annexin V or cleaved caspase-3 staining, of CD34+CD135− LSK cells in vivo, and that no difference in apoptosis was observed among LT-HSCs of control and agrin-deficient mice (Figure 4C and supplemental Figure 5). However, it is important to note that in neonatal mice CD34 expression on LT-HSC has been reported,28 thus we cannot formally exclude the possibility that a fraction of LT-HSC included in the CD34+ gate may also be dependent on agrin-mediated survival signals.
As expected, common myeloid progenitors (CMP), granulocyte-monocyte progenitors (GMP), megakaryocyte-erythroid progenitors (MEP), and common lymphoid progenitors (CLP) were likewise reduced in number in Musk-L;Agrn−/− mice compared with control littermates (Figure 4D).
It was important, however, to exclude major structural or cellular alterations in the bone marrow niche that would account for the impaired HSC functions observed in the agrin-deficient mice. The number and morphology of osteoblasts, endothelial and reticular cells in Musk-L;Agrn−/− mice were comparable with their control littermates (supplemental Figure 6A). Furthermore, the serum concentration of osteocalcin, a marker for bone formation and thus for osteoblast function, was not significantly different between the 2 genotypes (supplemental Figure 6B). Finally, real-time PCR analysis of c-Kit ligand (SCF), Flt-3 ligand (FL), thrombopoietin (TPO), IL-7, and the chemokine CXCL12 showed that cytokines involved in the regulation of the HSC niche were normally expressed at the bone marrow of Musk-L;Agrn−/− mice (supplemental Figure 7).
Nonredundant role of agrin at the HSC niche
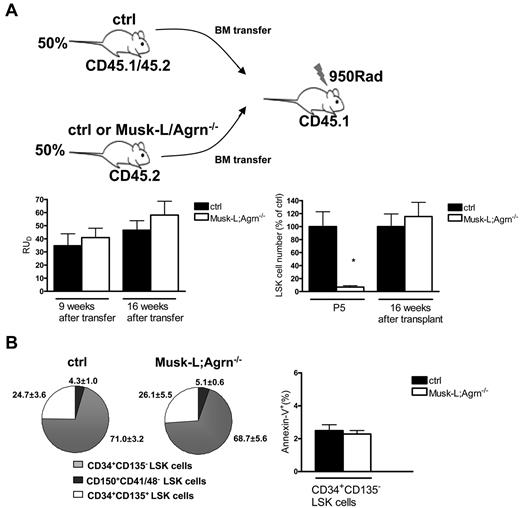
To confirm that the observed defects in the BM of agrin-deficient mice were not stem cell autonomous but because of the loss of agrin expression on the stromal component of the niche, we performed BM transfer assays, in which lethally irradiated B6(CD45.1) recipient mice were reconstituted with BM cells from B6(CD45.2) control or Musk-L;Agrn−/− mice. The majority of lethally irradiated host animals injected with either control or agrin-deficient BM cells survived, indicating successful engraftment. Eight weeks after the transfer, the frequency of BM hematopoietic cells expressing CD45.2 was quantified by flow cytometry, demonstrating comparable reconstitution in all recipients (91 ± 3.7% for mice that received control bone marrow cells and 87.8 ± 4.6% for mice that received Musk-L;Agrn−/− cells). Furthermore, we performed competitive long-term repopulation assays, where the same number of donor BM cells, from either control or Musk-L;Agrn−/− P5 mice (CD45.2), were transferred into lethally irradiated congenic recipients (CD45.1), together with the same number of competitor BM cells from adult CD45.1/CD45.2 wild-type mice. FACS analysis of blood leukocytes from transplanted animals was performed 9 and 16 weeks after transfer, and the analysis of donor repopulating units (RUD) indicated absence of cell-autonomous defects in agrin-deficient HSCs (Figure 5A). Moreover, the experiments confirmed that agrin deficiency does not affect LT-HSC viability. RUD values for both types of P5 donors were higher than those for adult competitors because neonatal HSCs are known to have increased renewal capacity compared with adult ones.29,30 Analysis of LSK cells of control or agrin-deficient donor origin at 16 weeks after transfer revealed no differences in terms of absolute numbers (Figure 5A), frequency of CD150+CD41/48−, CD34+CD135−, and CD34+CD135+ subpopulations (Figure 5B), and CD34+CD135− LSK cells apoptosis levels (Figure 5B).
Agrin nonredundant role at the hematopoietic niche. (A) Total bone marrow cells from either control or Musk-L;Agrn−/− P5, CD45.2 mice were transplanted into lethally irradiated CD45.1 recipients in competition with BM cells from CD45.1/45.2 adult mice, at a 1:1 ratio. Donor repopulating units (RUD) in the PB of recipient mice 9 and 16 weeks after transfer are shown (left). Data are from 3 independent experiments (ctrl, n = 8; Musk-L;Agrn−/−, n = 6). Right: mean numbers relative to controls of the LSK population in the bone marrow of P5 Musk-L;Agrn−/− mice or of mice transplanted as described and analyzed 16 weeks after transfer (CD45.1−Lin−IL7R−c-Kit+Sca-1+ gate; ctrl n = 8, Musk-L;Agrn−/−, n = 6; *P < .05). (B) Pie charts (right) represent average percentages of CD150+CD41/48−, CD34+ CD135−, and CD34+CD135+ subsets within the LSK population (ctrl, n = 8; Musk-L;Agrn−/−, n = 6). Annexin V analysis (left) on CD34+CD135− LSK cells of donor origin (ctrl, n = 8; Musk-L;Agrn−/−, n = 6; differences are not statistically significant). In all panels, error bars represent SEM.
Agrin nonredundant role at the hematopoietic niche. (A) Total bone marrow cells from either control or Musk-L;Agrn−/− P5, CD45.2 mice were transplanted into lethally irradiated CD45.1 recipients in competition with BM cells from CD45.1/45.2 adult mice, at a 1:1 ratio. Donor repopulating units (RUD) in the PB of recipient mice 9 and 16 weeks after transfer are shown (left). Data are from 3 independent experiments (ctrl, n = 8; Musk-L;Agrn−/−, n = 6). Right: mean numbers relative to controls of the LSK population in the bone marrow of P5 Musk-L;Agrn−/− mice or of mice transplanted as described and analyzed 16 weeks after transfer (CD45.1−Lin−IL7R−c-Kit+Sca-1+ gate; ctrl n = 8, Musk-L;Agrn−/−, n = 6; *P < .05). (B) Pie charts (right) represent average percentages of CD150+CD41/48−, CD34+ CD135−, and CD34+CD135+ subsets within the LSK population (ctrl, n = 8; Musk-L;Agrn−/−, n = 6). Annexin V analysis (left) on CD34+CD135− LSK cells of donor origin (ctrl, n = 8; Musk-L;Agrn−/−, n = 6; differences are not statistically significant). In all panels, error bars represent SEM.
Altogether, these data indicate that agrin-deficient HSCs differentiate normally in an agrin-sufficient microenvironment and that agrin is a novel and essential niche factor that delivers survival signals to CD34+ CD135− LSK cells.
α-DG, an agrin receptor, delivers survival signals to HSCs
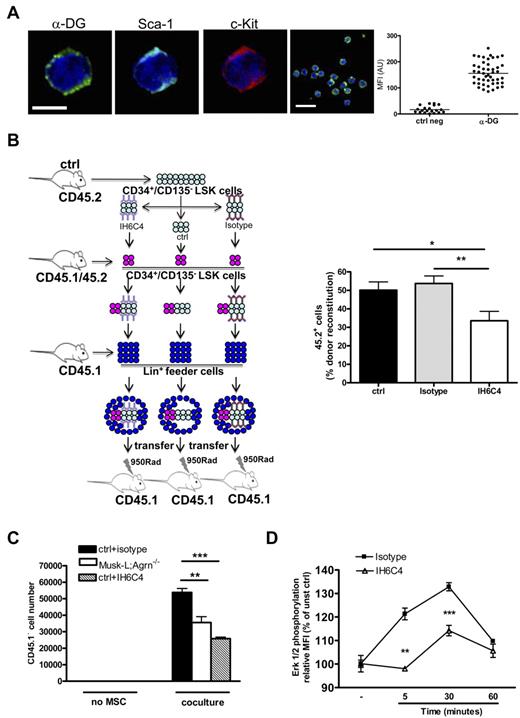
The agrin receptor at the immunologic synapse has been defined as α-DG,9 a broadly expressed cell-surface receptor with high affinity for ECM proteins.10 As already observed in humans,12 we found that mouse c-Kit+Sca+ cells express α-DG at their surface (Figure 6A).
Agrin controls ST-HSC functions through the α-DG receptor. (A) Representative confocal images of LSK cells obtained from the BM of control mice and stained for α-DG (green), Sca-1 (light blue), c-kit (red), and nuclear DNA (blue). Scale bars, 5 and 10 μm. The graph shows α-DG MFI analysis on a representative LSK sample (ctrl neg, n = 20; α-DG, n = 45).(B) In vivo competitive reconstituting ability of sorted CD34+CD135− LSK cells (CD45.2) pretreated with anti–α-DG blocking antibody IH6C or with isotype control or untreated. A mixture of 60% (CD45.2) in competition with 40% (CD45.1/45.2) untreated sorted CD34+CD135− LSK cells was transferred into lethally irradiated recipients (CD45.1) together with 1 × 105 Lin+ feeder BM cells from control adult mice mice (CD45.1). BM cells were analyzed by flow cytometry 8 days after the transfer; data are expressed as the relative percentage of CD45.2+ cells on total CD45.2+ plus CD45.1/45.2+ gated cells; n = 14, *P < .05, **P < .01. (C) Sorted control CD34+CD135− LSK cells (45.1) were cultured on MSC monolayers from either control (ctrl) or Musk-L;Agrn−/− mice, or, where indicated, control CD34+CD135− LSK cells were pretreated with isotype or blocking antibody IH6C4 (+IH6C4) and cultured on control MSC monolayers. Proliferation of CD45.1+ cells was measured 5 days later. Data are shown as mean, n = 3; **P < .01, ***P < .0001. (D) Total BM cells from control mice were preincubated with α-DG blocking antibody IIH6C4, or its isotype control, for 40 minutes at room temperature. Cells were than stimulated with 10 μg/mL recombinant agrin (R&D) and the ERK phosphorylation was detected on gated CD34+CD135− LSK cells. Changes in ERK phosphorylation were expressed as fold increase of mean fluorescence intensity (MFI) of stimulated over unstimulated cells (time 0); n = 3. Statistical analysis was performed comparing isotype versus IIH6C4 treated cells (**P < .01, ***P < .0001). In all panels, error bars represent SEM.
Agrin controls ST-HSC functions through the α-DG receptor. (A) Representative confocal images of LSK cells obtained from the BM of control mice and stained for α-DG (green), Sca-1 (light blue), c-kit (red), and nuclear DNA (blue). Scale bars, 5 and 10 μm. The graph shows α-DG MFI analysis on a representative LSK sample (ctrl neg, n = 20; α-DG, n = 45).(B) In vivo competitive reconstituting ability of sorted CD34+CD135− LSK cells (CD45.2) pretreated with anti–α-DG blocking antibody IH6C or with isotype control or untreated. A mixture of 60% (CD45.2) in competition with 40% (CD45.1/45.2) untreated sorted CD34+CD135− LSK cells was transferred into lethally irradiated recipients (CD45.1) together with 1 × 105 Lin+ feeder BM cells from control adult mice mice (CD45.1). BM cells were analyzed by flow cytometry 8 days after the transfer; data are expressed as the relative percentage of CD45.2+ cells on total CD45.2+ plus CD45.1/45.2+ gated cells; n = 14, *P < .05, **P < .01. (C) Sorted control CD34+CD135− LSK cells (45.1) were cultured on MSC monolayers from either control (ctrl) or Musk-L;Agrn−/− mice, or, where indicated, control CD34+CD135− LSK cells were pretreated with isotype or blocking antibody IH6C4 (+IH6C4) and cultured on control MSC monolayers. Proliferation of CD45.1+ cells was measured 5 days later. Data are shown as mean, n = 3; **P < .01, ***P < .0001. (D) Total BM cells from control mice were preincubated with α-DG blocking antibody IIH6C4, or its isotype control, for 40 minutes at room temperature. Cells were than stimulated with 10 μg/mL recombinant agrin (R&D) and the ERK phosphorylation was detected on gated CD34+CD135− LSK cells. Changes in ERK phosphorylation were expressed as fold increase of mean fluorescence intensity (MFI) of stimulated over unstimulated cells (time 0); n = 3. Statistical analysis was performed comparing isotype versus IIH6C4 treated cells (**P < .01, ***P < .0001). In all panels, error bars represent SEM.
To verify the involvement of α-DG in the survival of CD34+CD135− LSK cells, we decided to inhibit α-DG–agrin interactions in vivo using an antibody to α-DG (IIH6C4) known to block its interaction with agrin.31 We performed short-term competitive reconstitution experiments by modifying a previously published protocol15 to determine the self-renewal potential of tested cells. CD34+CD135− LSK cells were sorted from the bone marrow of control, adult mice (donor, CD45.2), preincubated with IIH6C4, its isotype control, or nothing, and injected together with untreated CD34+CD135− LSK cells sorted from the BM of other control, adult mice (competitor, CD45.1/45.2) into lethally irradiated (CD45.1) control mice. Eight days after the transfer, bone marrow cells from recipient mice were harvested and the reconstitution ability of donor cells (CD45.2) was evaluated by flow cytometry (Figure 6B). The results indicated that CD34+CD135− LSK cells pretreated with blocking antibody to α-DG displayed reduced reconstitution ability compared with untreated or isotype-treated ones, thus confirming the hypothesis that α-DG is an HSC receptor that receives survival/proliferation signals from the agrin-expressing stromal niche.
Next, to further confirm the specific effect of the loss of agrin-mediated signaling on the CD34+CD135− LSK population, we took again advantage of the in vitro coculture system previously described (Figure 2E) and plated CD34+CD135− LSK cells, isolated from the bone marrow of control mice (CD45.1), with either control or agrin-deficient MSCs. In addition, to further verify the role of α-DG, control CD34+CD135− LSK cells were preincubated with blocking antibody to α-DG (IIH6C4), or its isotype control. After 5 days of coculture, the number of CD45.1+ hematopoietic cells recovered was significantly reduced in the presence of agrin-deficient MSCs, as well as when agrin–α-DG interactions were prevented (Figure 6C).
The DG complex is a transmembrane heterodimer of α and β subunits that links the extracellular matrix to the cell cytoskeleton and is known to bind the growth factor receptor binding protein 2 (Grb2),32 a widely expressed signaling protein associated with Son of Sevenless (SOS), an exchange factor of Ras GTPase. The Grb2-SOS-Ras signaling pathway is involved in development, proliferation, and survival of hematopoietic cells and, in particular, it is required for monocyte/macrophage differentiation.33
Activation of the agrin-induced Grb2 signaling pathway was therefore analyzed by measuring Erk phosphorylation in control CD34+CD135− LSK cells. Total bone marrow cells, preincubated with the α-DG blocking antibody (IIH6C4) or its isotype control, were stimulated with recombinant, soluble agrin and the phosphorylation of Erk was evaluated in the CD34+CD135− LSK population by flow cytometry (Figure 6D). Cells stimulated with PMA were used as positive control (supplemental Figure 8). The results showed that agrin induced detectable Erk phosphorylation in CD34+CD135− LSK cells and that this signaling was inhibited by α-DG blocking antibody (Figure 6D).
Discussion
Within the BM, hematopoietic stem cells dynamically regulate their number, while sustaining hematopoiesis, through interactions with specialized microenvironments called niches.34 The endosteal niche comprises bone-lining cells, including osteoblasts and their precursor mesenchymal stem cells which are heterogeneous in terms of functions and degree of differentiation.35 The endosteum has an important role in HSC instruction: many HSCs reside at, or near, the endosteum where osteoblasts express and secrete factors, such as angiopoietin, thrombopoietin, osteopontin, and the chemokine CXCL12, that regulate HSC biology.24,36-38
In addition, HSCs that reside adjacent to sinusoidal blood vessels in the bone marrow receive signals by perivascular cells.25 At the perivascular niche, endothelial and CXCL12-abundant reticular (CAR) cells contribute to HSC maintenance through both direct interactions and secreted factors.26,39
The contribution of the endosteal and perivascular niches to HSC functions, and whether these are 2 distinct and anatomically separate environments is still under debate.40
Indeed, in spite of the recent advances in the understanding of the cellular and molecular interactions at the HSC niches the sophisticated mechanisms required to control mantainance of stem cell number, while sustaining hematopoiesis, are only partially understood.
Our study has identified agrin as a critical and novel component of the hematopoietic niche. We found that agrin is expressed by MSCs and osteoblasts, whereas no expression was detected in reticular and endothelial components of the vascular microenviroment. Furthermore, our results show that agrin-mediated cell-cell contacts between HSCs and endosteal osteoblasts in the BM are crucial for CD34+CD135− LSK cell survival and expansion. Thus, agrin appears to oppose osteopontin, a matrix protein produced by osteoblasts, which limits HSC proliferation.24
The bone marrow is the site of adult hematopoiesis and HSC maintenance in mammals.41 HSCs start to shift from the fetal liver to the bone marrow during late gestation/first days of postnatal life in mice42 and their properties and requirements change after they engraft in the bone marrow.43 Interestingly, agrin-deficient mice had no defects in fetal HSCs (E12), indicating that agrin is specifically required for the regulation of HSCs in the BM niche. Several examples of mutations that specifically affect hematopoiesis in the bone marrow (without altering the fetal liver hematopoiesis) have been already described, including the transcriptional repressors Tel,44 Gfi1,44 and Bmi1,45 and the calcium-sensing receptor.46 All together, these data indicate that both HSCs and their microenvironmental requirements change when stem cells engraft in the bone marrow niche. Although we cannot formally exclude that agrin deficiency might alter some specific osteoblast function in vivo, our results do not support the possibility that agrin deficiency might induce dramatic changes in the BM niche and thus be indirectly responsible of the HSC phenotype in agrin knockout mice. Indeed, the niche of agrin-deficient mice is very similar to the one of control mice, in terms of both cellular and soluble factors. Moreover, in vitro experiments confirmed that, when cocultured with MSCs obtained from agrin-deficient mice, wild-type CD34+CD135− LSK cells have defective proliferation. It is important to note that, in the absence of cell-cell contacts (transwell cocultures), control and agrin-deficient MSCs supported CD34+CD135− LSK cell proliferation equally, indicating that MSCs derived from agrin-deficient mice are able to produce all soluble factors required for their in vitro expansion. Finally, the defects observed in CD34+CD135− LSK cells of agrin-deficient mice were reproduced in short-term reconstitution experiments, where the lethally irradiated recipients were reconstituted with control CD34+CD135− LSK cells that could not sense agrin because of preincubation with a blocking antibody for its receptor, the α-DG.9
Together, these data indicate that agrin is a novel key player of the stem cell niche at the BM, where it is expressed by MSCs and osteoblasts, and provides survival signals to CD34+CD135− LSK cells through its receptor.
The agrin receptor α-DG docks with β-DG, a transmembrane protein which interacts with dystrophin in muscle cells and has a key role in maintaining the integrity of the sarcolemmal membrane. In addition to this muscle-specific function, β-DG binds the growth factor receptor binding protein 2 (Grb2),32 a widely expressed signaling protein associated with Son of Sevenless (SOS), an exchange factor of Ras GTPase. The Grb2-SOS-Ras signaling pathway is involved in development, proliferation, and survival of hematopoietic cells.47 Although for obvious reasons we could not perform biochemical experiments with CD34+CD135− LSK cells, we found that soluble agrin induces Erk phosphorylation in CD34+CD135− LSK cells, thus demonstrating the involvement of the Ras pathway (well known for being required for hematopoietic cell survival and proliferation)47 in agrin signaling.
The reason that CD150+CD41/48− LSK cells (LT-HSCs) are less dependent on agrin-induced survival signals than the CD34+CD135− LSK population is still to be explored. However, we can speculate that this may be in part because of the different intrinsic properties of these 2 populations.48,49 Although LT-HSC are mainly in a quiescent status, ST-HSC are proliferating cells with some, albeit limited, self-renewing potential15 and agrin is indeed required to allow their expansion supporting rapid replenishment of progenitors.
This study provides an updated picture of the microenviromental cues that support HSC development by identifying a novel molecular interaction involved in the enhancement of HSC survival. Although the importance of agrin in human hematopoiesis remains to be confirmed, it is known that the α-DG receptor is expressed by human hematopoietic CD34+ cells12 and that human BM-derived MSCs express agrin (our unpublished data, 2009), thus suggesting that agrin–α-DG functional interactions may also occur between human MSCs and HSCs. The identification of agrin as a novel, nonredundant player involved in the enhancement of HSC survival and proliferation could allow for the manipulation of niche components and their signaling pathways, thus providing new insight to the field of regenerative medicine.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marta Lezama, Lucia Zanotti, Yeni Martinez de la Torre, Stefano Mantero, and Gabriele Campi for assistance; Michael Ferns and Dieter Bromme for providing reagents; and Alberto Mantovani and Francesca Ficara for helpful discussions and critical reading of the paper.
This work was funded by grants from the Italian Association for Cancer Research (AIRC), Ministero dell'Università e della Ricerca and Ministero della Salute and by EU-FP7 “Sybilla” n° 201106 (A.V.), and by grants from the National Institutes of Health (M.L.D. and S.B.). A.S. was funded by Inserm (France) and CARIPLO Foundation (Italy).
National Institutes of Health
Authorship
Contribution: C.M. and A.A. designed and performed experiments, analyzed the data, and wrote the manuscript; J.C. performed in vivo experiments; C.S. performed confocal microscopy experiments; A.D. and M.R. helped with immunohistochemical analysis; N.K. and S.J.B. provided the animal model; S.J.B. and M.L.D. reviewed the manuscript; A.S. supervised the study; and A.V. supervised the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cristina Mazzon, Istituto Clinico Humanitas IRCCS, via Manzoni 113, Rozzano, Milan, Italy; e-mail: cristina.mazzon@humanitasresearch.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal