Abstract
In SIV/HIV infection, the gastrointestinal tissue dominates as an important site because of the impact of massive mucosal CD4 depletion and immune activation-induced tissue pathology. Unlike AIDS-susceptible rhesus macaques, natural hosts do not progress to AIDS and resolve immune activation earlier. Here, we examine the role of dendritic cells (DCs) in mediating immune activation and disease progression. We demonstrate that plasmacytoid DCs (pDCs) in the blood up-regulate β7-integrin and are rapidly recruited to the colorectum after a pathogenic SIV infection in rhesus macaques. These pDCs were capable of producing proinflammatory cytokines and primed a T cytotoxic 1 response in vitro. Consistent with the up-regulation of β7-integrin on pDCs, in vivo blockade of α4β7-integrin dampened pDC recruitment to the colorectum and resulted in reduced immune activation. The up-regulation of β7-integrin expression on pDCs in the blood also was observed in HIV-infected humans but not in chronically SIV-infected sooty mangabeys that show low levels of immune activation. Our results uncover a new mechanism by which pDCs influence immune activation in colorectal tissue after pathogenic immunodeficiency virus infections.
Introduction
Human/simian immunodeficiency virus (HIV/SIV) infections are marked by the rapid depletion of mucosal CD4 T cells, particularly in the gastrointestinal (GI) tract, a major component of the immune system and one of the few sites exposed to foreign entities from the environment.1-4 The consequences of HIV/SIV infection not only include a deficit in mucosal CD4 T cells but also tissue damage and increased vulnerability to opportunistic infections.5 HIV/SIV-associated pathology in the GI tract includes epithelial hyperplasia-induced villous atrophy, abnormal cellular infiltrates, disrupted lymphoid architecture, and increased apoptotic cells, mostly attributed to an overt and chronic inflammatory immune response during HIV/SIV infection.6-8
By contrast, SIV-infected natural nonhuman primate hosts such as sooty mangabeys (SMs) and African green monkeys do not progress to simian AIDS, unlike nonnatural hosts such as infected rhesus macaques (RMs, Macaca mulatta).9,10 Prolonged immune activation is thought to contribute to disease progression in nonnatural hosts because natural hosts quickly dampen generalized immune activation after the acute phase of SIV infection. Notably, immune activation occurs independently of viremia and CD4 T-cell depletion because natural hosts display comparably high viral load11 and face similar depletion of mucosal CD4 T cells.12-15 Often, this state of chronic immune activation in nonnatural hosts and HIV+ patients is associated with the presence of inflammatory cytokines, such as type 1 and 2 interferons (IFNs), TNF-α and activation and proliferation of nonvirus-specific T cytotoxic (Tc)1 CD8 T-cell responses.16-21 These characteristics are obvious within tissues where the impact of chronic inflammation is visible from the damaged cellular microarchitecture.22,23 In both SIV- and HIV-infected individuals, activated CD8 T cells in GI tract are increased and could certainly impede the processes of CD4 T-cell immune reconstitution and intestinal repair and regeneration.24-26 Some studies attribute CD8 T-cell immune activation to type 1 IFN signaling,27,28 a chronic feature of pathogenic SIV infections.20,21 Moreover, higher levels of immune activation and expression of IFN-stimulated genes were observed in gut-associated tissues of SIV+ progressors compared with long-term nonprogressors.29 Therefore, prolonged type 1 IFN-signaling in tissues is thought to be an important factor contributing to disease progression in HIV/SIV infection.
Being major producers of type 1 IFN in response to TLR7, one of the pathways mediating the response to HIV-1,30 plasmacytoid dendritic cells (pDCs) are important in virus control31 ; however in SIV/HIV infection, a few roles related to pathogenesis and disease progression have been proposed. For example, (1) pDC production of type 1 IFN contributes to chronic immune activation,32 (2) TRAIL-expressing pDCs induce death of CD4 T cells that express TRAIL-associated death receptors,33 (3) pDCs inhibit T-cell proliferation through a indoleamine (IDO)–dependent pathway,34 and (4) pDCs rapidly migrate to the vaginal site of infection where they attract target CD4 T cells.35
At present, there is no direct association between pDCs and CD8 T-cell immune activation in the gut, and it is unknown whether pDC are recruited to the GI tract where there is increased CD8 T-cell immune activation and tissue damage as a result of HIV/SIV infection. Here, we address the role of pDCs during a pathogenic SIV infection, particularly in the GI tract, and the extent of pDC involvement in chronic immune activation within the context of nonvirus-specific CD8 T-cell responses and type 1 IFN responses. For the first time, we show that blood pDCs up-regulate β7-integrin and are rapidly recruited to the colorectum after a pathogenic SIV infection. We also demonstrate that blockade of α4β7-integrin after an intrarectal pathogenic SIV infection dampens pDC migration to colorectum and reduces immune activation in the colorectum. These results highlight an important role for pDCs in contributing to immune activation in pathogenic SIV infections through type 1 IFN responses and nonvirus-specific CD8 T-cell responses.
Methods
Animals and human subjects
Samples were taken from adult Indian RMs and SMs that were housed at the Yerkes Primate Center and cared for under guidelines established by the Animal Welfare Act and the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” using protocols approved by the Emory University Institutional Animal Care and Use Committee. Rhesus macaques were infected intravenously with SIVmac251 (stock provided by Dr Nancy Miller, National Institutes of Health) at a dose of 100 median tissue culture infective dose. Some of the SIV-infected macaques were treated with antiretroviral drugs 9-[2-(R)-(phosphonomethoxy)propyl]adenine (20 mg/kg), emtricitabine (30 mg/kg), Kaletra (lopinavir, 12 mg/kg; ritonavir 3 mg/kg), and 3′-azido-2′,3′-dideoxythymidine (5 mg/kg) at ∼ 18 weeks after infection. Sooty mangabeys (sm) were housed in colonies of 50 to 60 animals and SIVsm is endemic in this population. None of the animals used in the study were experimentally infected. The SIV-negative animals used here were killed because of neurologic and mental problems but were checked to ensure no history of bowel problems. Samples from HIV-infected individuals were described previously36 or provided by the Emory Clinical Research Core Specimen Repository. Blood samples from humans were collected after approvals from Emory Institutional Review Board. All HIV+ individuals were highly active antiretroviral therapy (ART)–naive. Patient sample data on viral load and CD4 T-cell counts are presented in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Anti-α4β7 antibody treatment
Two groups of adult Indian RMs (n = 4/group) comprised this study. One group was administered the simianized α4β7 monoclonal antibody37 at 50 mg/kg intravenously in sterile PBS on day −2 and the same dose on day 28 after SIV infection. All 8 RMs were each infected intrarectally on day 0 with 500 median tissue culture infective dose of a stock of SIVmac251.
Tissue cell processing and isolation
Peripheral blood mononuclear cells were obtained as described previously.36 Spleen and lymph nodes were cut into small pieces and digested with 100 U/mL collagenase IV (Worthington Biochemicals) for 15 minutes at 37°C and 20 minutes at room temperature with shaking. The supernatant was filtered through a cell strainer and kept, whereas the undigested pieces were further ground in medicons (BD Biosciences). Small and large intestinal tissue pieces were washed thoroughly in HBSS supplemented with 100mM dithioerythritol (Sigma-Aldrich), and tiny pieces were cut and incubated in HBSS supplemented with 2mM EDTA and 5% FBS, shaking vigorously for two 20-minute intervals. The supernatant was kept and the procedure repeated. The remaining pieces were washed in RPMI 1640 (Lonza) and digested with 200 U/mL collagenase IV (Worthington Biochemicals), shaking vigorously for 2 hours at 37°C. Remaining intestinal pieces were shredded using a syringe with 18- and 16-guage blunt-ended needles. All remaining supernatant was filtered through a cell strainer, and cells were isolated using 44%/67.5% Percoll (GE Healthcare) and gradient spun at 600g for 25 minutes at room temperature. Approximately 15-20 colorectal pinch biopsies were processed similarly but without the dithioerythritol treatment and Percoll gradient steps.
DC stimulation and T-cell assays
For DC intracellular cytokine staining, cells were stimulated with 5μM imiquimod (InvivoGen) or 2.85 μg/mL ariththiol (AT)-2–inactivated SIVmac251 (provided by Jeffrey Lifson, National Institutes of Health) for 12 hours at 37°C and 5% CO2. Brefeldin A (10 μg/mL) was added 1 hour after stimulation. For T-cell assays, naive T cells from SIV− macaques were enriched using a pan T-cell isolation kit and anti-CD45RA microbeads (Miltenyi Biotec). Purity was at least 90% (supplemental Figure 3A). Between 1000 and 2500 DCs and 5 × 104 T cells were cocultured in a 96-well plate over 3.5 days. Where indicated, DCs were pretreated for 30 minutes with imiquimod or AT-2–inactivated SIVmac251 before addition of T cells. For mixed lymphocyte reaction (MLR) assays, allogeneic naive T cells from an SIV-negative macaque were stained with 3μM CFSE (Invitrogen) and cocultured with DCs for 4.5 days. For cytokine assays, 96-well plates were precoated with 0.4 μg/mL anti-CD3 (clone FN18; BioSource International) before DC–T-cell coculture. After 3.5 days, the T-cell cytokine profiles were examined by restimulating T cells with phorbol myristate acetate (25 ng/mL) and ionomycin (1 μg/mL) for 4 hours with brefeldin A (10 μg/mL) and GolgiStop (1 μg/mL; BD Biosciences) added during the last 2 hours of incubation. For intracellular staining, cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) according to manufacturer's instructions.
Flow cytometry
See supplemental Table 2 for additional antibody information. At least 1 million cells were stained and between 3500 and 10 000 HLA-DR+ lineage (LIN; CD3, CD20, CD14−) events were acquired on an LSRII flow cytometer (BD Immunocytometry) and analyzed using FlowJo (TreeStar). All tissue-derived cells were stained with the amine-reactive Alexa Fluor 430 carboxylic acid, succinimidyl ester dye (Invitrogen) to exclude dead cells.
Cell sorting
Cells were first depleted of CD20+ and CD3+ cells using anti-CD20 microbeads, PE anti-CD3 antibody (clone SP-34-2), and anti-PE microbeads (all from Miltenyi Biotec). Sorting was done on an FACSAria-1 flow cytometer (BD Biosciences), and purity was at least 90% (supplemental Figure 3B).
Quantitative real-time PCR
Total RNA was extracted from cells using the RNeasy kit (QIAGEN) and reverse-transcribed using a High-Capacity cDNA Archive kit protocol (Applied Biosystems). cDNA was preamplified using the TaqMan PreAmp Master Mix kit protocol (Applied Biosystems). Quantitative real-time PCR reactions were carried out using TaqMan Gene Expression Master Mix (Applied Biosystems), and sequences were amplified using the 7900HT Sequence Detection system (Applied Biosystems) under the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, followed by 45 cycles at 95°C for 15 seconds, and 60°C for 1 minute. Alternatively, qPCR reaction was performed using the SYBR Green PCR Master Mix.20 Each primer pair was tested with a logarithmic dilution of cDNA to generate a standard curve that was then used to calculate the starting quantity of target RNA. The housekeeping genes GUSB and/or GAPDH were used for normalization. Primers for gene expression assays were specific for RM and were obtained from Applied Biosystems. StatMiner software (Applied Biosystems) was used.
Affymetrix GeneChip Analysis
Rhesus GeneChip assays were performed in the Yerkes Microarray Core Facility using RNA from RM and SM colorectal biopsy tissues. RNA was run on Rhesus Macaque GeneChip that consists of 52 865 imprinted probe sets that recognize > 47 000 rhesus transcripts and > 20 000 genes (Affymetrix). Target RNA labeling, hybridization, and posthybridization processing were performed according to Affymetrix GeneChip Expression standard protocols. GeneChips were scanned on an Affymetrix scanner 3000, and analysis was carried out with GeneSpring GX10 by using CEL and CHP files originating from the Affymetrix GCOS software. Data files were preprocessed and normalized using the GC robust multiarray analysis. Gene changes of at least 1.5-fold and with a P value of < .05 were considered significant. All microarray data are available at the Gene Expression Omnibus under accession GSE29980.
Statistical analysis
To compare the frequencies of DCs in blood and tissues, data were checked for parametric assumptions, and if they satisfied the assumptions, we used Student t test. If the data were log-normal, we used Student t test on log-transformed data. Whenever the data failed to meet the parametric assumptions, we used Wilcoxon rank-sum test. The P values reported here are before any adjustments for multiple comparisons. A 2-sided P < .05 was considered significant. Statistical analyses were performed using Spotfire S-PLUS 8.1 (TIBCO).
Results
Blood pDCs up-regulate β7-integrin after pathogenic SIV infection
Using a model of acute SIVmac251 infection, we monitored the dynamics of pDCs and myeloid (m)DCs in the blood of Indian RMs infected intravenously. We classified pDCs and mDCs using the respective markers CD123+ HLA-DR+ LIN− (CD3− CD20− CD14−) and CD11c+ HLA-DR+ LIN− (supplemental Figure 1A). Consistent with previous reports,38,39 the pDC frequency in the blood increased in some monkeys at 1 week postinfection (PI) and declined at 2 weeks PI (supplemental Figure 1B). Generally, the pDC frequency remained lower than the preinfection frequency for each individual animal at least up to 18 weeks PI. In contrast, the mDC frequency did not increase at 1 week PI but declined dramatically by 2 week PI (supplemental Figure 1C). This decline may suggest recruitment to lymph nodes (LNs) as we observed some CCR7 up-regulation on these mDCs (data not shown). Unlike for the pDCs, the mDC frequency returned to preinfection levels by 18 weeks PI. A similar pattern was also observed for the absolute numbers of pDCs and mDCs in the blood.
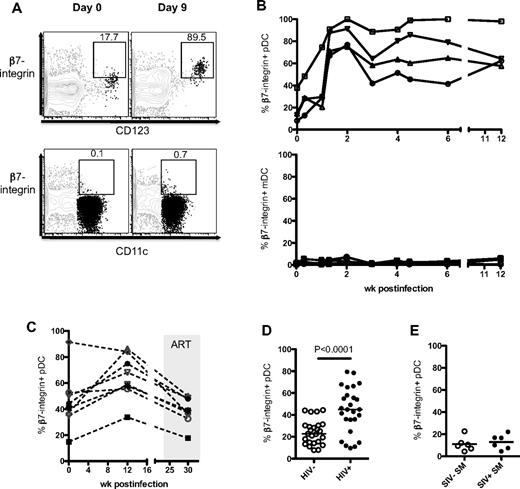
The decline of pDCs in the blood indicated a possible recruitment to the LNs as pDCs up-regulate CCR7 during SIV infection (supplemental Figure 1D; also demonstrated by others32,38,39 ). However, it is unknown whether any DCs were recruited to the GI tract, a site of viral replication and early CD4 T-cell depletion. To address this, we studied the expression α4β7, a gut-homing integrin, by pDCs after SIV infection. In RMs, the expression of β7-integrin on pDCs in blood increased rapidly after SIV infection, peaked by day 9 to 14 PI (Figure 1A-B), and was sustained for at least 12 weeks PI (last point of analysis). These pDCs did not express CD103 (αE) but costained for CD49 days (α4); hence, the heterodimer expressed is that of α4β7 and not αEβ7 (supplemental Figure 2A). Longitudinal analysis of β7-integrin expression on pDCs showed no significant increase over time in uninfected RMs (supplemental Figure 2B). In SIV-infected RMs, the expression of β7-integrin on blood pDCs decreased after ART (Figure 1C). We did not observe an increase in β7-integrin expression on mDCs after early infection (Figure 1B). We checked whether β7-integrin on pDCs was a marker of activation but found little up-regulation when pDCs were cultured in vitro with aldrithiol-2–inactivated SIVmac251 (AT-2 SIV) or imiquimod (data not shown).
pDCs up-regulate β7-integrin during SIV infection. (A) FACS plots depict the gating used and the proportion of β7-integrin+ pDCs (CD123+) and mDCs (CD11c+) at day 0 and day 9 PI from the same RM. FACS plots are an overlay of pDCs or mDCs (black) on the HLA-DR+ LIN− population (gray). (B) Kinetics of β7-integrin+ pDCs and mDCs in the blood of RM after intrarectal SIV infection. (C) Kinetics of β7-integrin+ pDCs in the blood of RM after intravenous SIV infection and ART. (D) Proportion of β7-integrin+ pDCs in the blood of healthy (HIV−) and HIV+ individuals. Human blood pDCs were defined as CD123hi HLA-DR+ CD3− CD20− CD14− cells. (E) Proportion of β7-integrin+ pDCs in the blood of uninfected or SIV-infected SM.
pDCs up-regulate β7-integrin during SIV infection. (A) FACS plots depict the gating used and the proportion of β7-integrin+ pDCs (CD123+) and mDCs (CD11c+) at day 0 and day 9 PI from the same RM. FACS plots are an overlay of pDCs or mDCs (black) on the HLA-DR+ LIN− population (gray). (B) Kinetics of β7-integrin+ pDCs and mDCs in the blood of RM after intrarectal SIV infection. (C) Kinetics of β7-integrin+ pDCs in the blood of RM after intravenous SIV infection and ART. (D) Proportion of β7-integrin+ pDCs in the blood of healthy (HIV−) and HIV+ individuals. Human blood pDCs were defined as CD123hi HLA-DR+ CD3− CD20− CD14− cells. (E) Proportion of β7-integrin+ pDCs in the blood of uninfected or SIV-infected SM.
We next checked whether pDCs in the blood of HIV+ individuals up-regulate β7-integrin during chronic HIV infection. Consistent with SIV-infected RMs, the proportion of pDCs expressing β7-integrin was significantly higher in HIV+ individuals (mean ± SEM, 44.3 ± 4.0%) compared with healthy individuals (24.1 ± 2.2%; Figure 1D). We asked whether the up-regulation of β7-integrin on blood pDCs was an attribute of pathogenic SIV infections or that it also occurred in natural hosts such as SMs that do not progress to AIDS despite high viremia. The frequencies of β7-integrin+ pDCs in the blood of SIV− and chronically infected SIV+ SMs were comparable, further suggesting that induction of β7-integrin expression on pDCs may be a property of immunodeficiency virus infections in their nonnatural hosts (Figure 1E). In general, the frequency of β7-integrin+ pDCs in SMs was low but was not attributed to poor antibody staining because SM T cells stain for β7-integrin at a similar intensity as RM and human T cells (supplemental Figure 2C-D).
pDCs are recruited to the colorectum after SIV infection
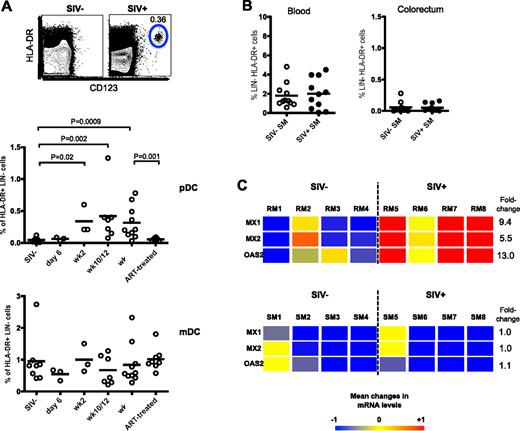
To determine that up-regulation of β7-integrin on pDCs promotes their migration to GI tract, we conducted a cross-sectional study of colorectal biopsies from SIV+ and SIV− RM and checked for pDCs and mDCs. In SIV− RMs, there were few or negligible number of pDCs and a low frequency of mDCs (∼ 1% of HLA-DR+ LIN−; Figure 2A). However in SIV+ RM, we found a significant increase in pDC frequency as early as 2 weeks PI that persisted until 18 weeks PI (last time point of analysis). Consistent with reduced β7-integrin expression on blood pDCs after ART (Figure 1C), the ART-treated SIV-infected RMs had a significantly lower frequency of pDCs in the colorectal tissue than chronically infected (week 18) RMs (Figure 2A). Longitudinal analysis of colorectal biopsies taken from RMs also showed a consistent absence or low frequency of pDCs before infection and a significant increase after infection (see Figure 6A). In contrast to pDCs, the frequency of mDCs was not significantly altered in the colorectum of RMs either after SIV infection or ART (Figure 2A).
pDCs are recruited to the colorectum after SIV infection. (A) Frequency of pDCs and mDCs in the colorectum of uninfected and SIV-infected RMs at indicated times PI. ART-treated macaques received 22 weeks of therapy at 18 weeks PI. FACS plots depict representative data from an uninfected and SIV-infected animal. (B) Frequency of pDCs in the blood and colorectum of SIV− or SIV+ SMs. (C) Affymetrix GeneChip heat map with linear fold change values of MX1, MX2, and OAS2 gene expression in the colorectum of uninfected and chronically SIV-infected RMs and SMs. Numbers indicate individual animals. SIV+ RMs were at 11 weeks PI and SIV+ SMs were naturally infected for at least 1 year. Genes represented here are considered significant if they were regulated at least 1.5-fold and have a P value cut-off of .05 (adjusted for multiple hypothesis testing using the Benjamini-Hochberg false discovery rate method). Color scale represents level of gene expression, where −1 indicates very low level of expression, 0 indicates median level of expression, and +1 indicates high level of expression. Fold change values were calculated against uninfected RMs or uninfected SMs.
pDCs are recruited to the colorectum after SIV infection. (A) Frequency of pDCs and mDCs in the colorectum of uninfected and SIV-infected RMs at indicated times PI. ART-treated macaques received 22 weeks of therapy at 18 weeks PI. FACS plots depict representative data from an uninfected and SIV-infected animal. (B) Frequency of pDCs in the blood and colorectum of SIV− or SIV+ SMs. (C) Affymetrix GeneChip heat map with linear fold change values of MX1, MX2, and OAS2 gene expression in the colorectum of uninfected and chronically SIV-infected RMs and SMs. Numbers indicate individual animals. SIV+ RMs were at 11 weeks PI and SIV+ SMs were naturally infected for at least 1 year. Genes represented here are considered significant if they were regulated at least 1.5-fold and have a P value cut-off of .05 (adjusted for multiple hypothesis testing using the Benjamini-Hochberg false discovery rate method). Color scale represents level of gene expression, where −1 indicates very low level of expression, 0 indicates median level of expression, and +1 indicates high level of expression. Fold change values were calculated against uninfected RMs or uninfected SMs.
Next, we asked whether the recruitment and presence of pDCs to the colorectum was an attribute of pathogenic SIV infections or whether colorectal pDCs were also present in SMs. Examination of the blood and colorectum of uninfected and chronically infected SMs showed no significant difference in pDC frequency in the blood and colorectum of both SIV+ and SIV− SMs (Figure 2B). This difference between SMs and RMs suggests a role for pDC recruitment to colorectal tissues in disease progression during a pathogenic SIV infection.
One of the mechanisms associated with disease progression in pathogenic SIV infection may be the prolonged production of type 1 IFN by pDCs. Because we detected increased migration of pDCs to the colorectum during pathogenic SIV infection, we also investigated whether there was an increase in type 1 IFN-stimulated gene expression in the colorectum. We compared the colorectum of uninfected and chronically infected RMs and SMs and found that the type 1 IFN-stimulated genes MX1, MX2, and OAS2 were significantly up-regulated in SIV+ RMs (P < .05), whereas in SIV+ SMs, these genes were not increased (Figure 2C). This seems consistent with the increased colorectal pDC frequency found in chronically infected RMs and the lack of colorectal pDC increase in chronically infected SMs.
Macaques with AIDS have increased pDC frequency in the colon and rectum
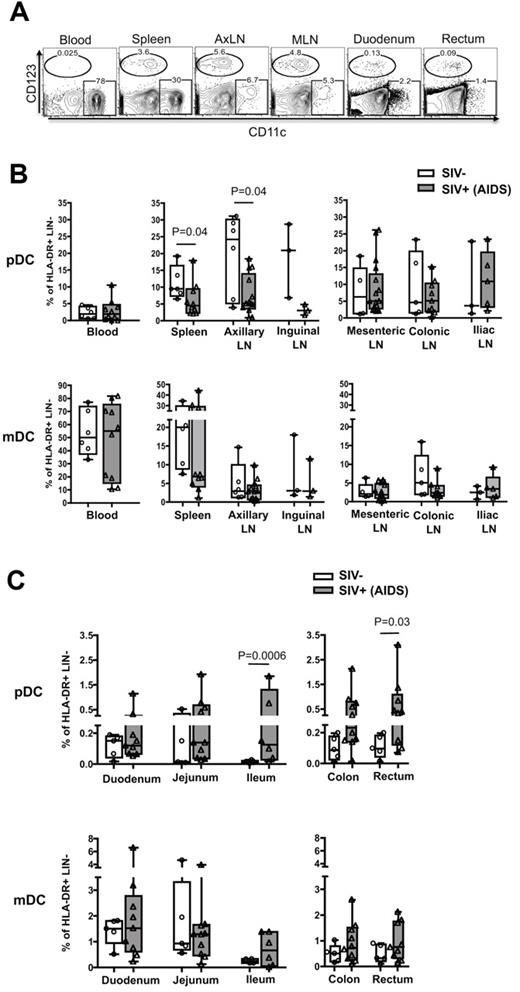
Next, we asked whether the enhanced recruitment of pDCs was specific to the colorectum and whether it was a long-term phenomenon? We characterized the frequency of pDCs and mDCs in various lymphoid and GI tissues of SIV− RMs and SIV+ RMs that progressed to AIDS (Figure 3). In SIV− RMs, the pDCs were predominant in lymphoid tissue and blood and were rare in both small and large intestines (Figure 3A-C). The mDC frequency correlated inversely with pDCs, tending to be higher in the spleen and blood but not the peripheral or gut-associated lymph nodes (Figure 3A-B), and the mDC were present at low levels in the small and large intestines (Figure 3C). In SIV+ RMs, the pDC frequency in the peripheral lymphoid tissue (spleen, axillary, and inguinal LNs) tended to be lower than that of SIV− RMs, whereas there was no difference in the gut-associated lymphoid tissue (GALT; mesenteric, colonic, and iliac LNs; Figure 3B). Contrary to SIV− RMs, pDC frequency in SIV+ RMs was higher along the length of the GI tract, particularly near (ileum) and in the large intestinal region (colon and rectum; Figure 3C). Significant differences were only seen in the ileum and rectum. In contrast, we did not see significant differences in the mDC frequency of both peripheral and GALT and the GI tract in the same SIV+ RMs. These results suggest that the enhanced recruitment of pDCs after SIV infection could be specific to the ileum and large intestine and may persist throughout infection.
Macaques with AIDS have increased pDC frequency in the ileum, colon, and rectum. (A) FACS plots depict the gating used and the frequency of pDCs and mDCs as a percentage of HLA-DR+ LIN− in different tissues from an SIV+ RM with AIDS. AxLN represents axillary LNs. Frequency of pDCs and mDCs as a percentage of HLA-DR+ LIN− cells in the peripheral and GALT (B) and gastrointestinal tract (C). Each symbol represents a RM.
Macaques with AIDS have increased pDC frequency in the ileum, colon, and rectum. (A) FACS plots depict the gating used and the frequency of pDCs and mDCs as a percentage of HLA-DR+ LIN− in different tissues from an SIV+ RM with AIDS. AxLN represents axillary LNs. Frequency of pDCs and mDCs as a percentage of HLA-DR+ LIN− cells in the peripheral and GALT (B) and gastrointestinal tract (C). Each symbol represents a RM.
pDC in the blood, GALT, and rectum of SIV-infected RMs displayed a proinflammatory phenotype
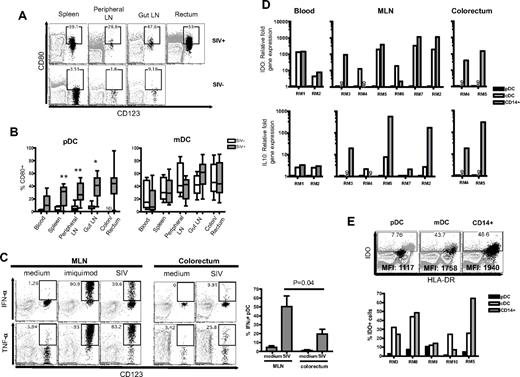
We went on to phenotype pDC in SIV− and SIV+ RMs for their activation status and production of proinflammatory cytokines. We analyzed markers of activation (CD80) on pDCs and mDCs, and we found increased CD80 expression on pDCs throughout all tissues after SIV infection (Figure 4A-B). We could not determine the CD80 expression on pDCs from the colorectum of SIV− RMs because they were rare. The mDCs displayed a heterogenic expression of CD80 throughout different tissues regardless of SIV status, and there was no significant difference in all tissues between SIV+ and SIV− RMs.
pDCs in SIV+ RM reflect a more inflammatory rather than tolerogenic phenotype. (A) FACS plots depict gating used and the proportion of CD80+ pDCs in different tissues. FACS plots are an overlay of pDCs (black) on the HLA-DR+ LIN− population (gray). The SIV+ and SIV− animals represented here were examined at different times hence the differences in gating. The gates were placed based on CD80 expression on total lymphocytes. (B) Proportion of CD80+ pDCs or mDCs in the blood, spleen, peripheral LNs, gut LNs, and colon/rectum. *P < .01, **P < .005. ND indicates not done. (C) Intracellular cytokine expression of IFN-α and TNF-α in pDCs from the MLN and colorectum after stimulation with imiquimod or AT-2–inactivated SIVmac251. Bar chart shows the proportion of IFNα+ pDCs in the MLN or colorectum after stimulation with AT-2–inactivated SIVmac251 from 3 SIV+ RMs. (D) Relative fold expression of IDO and IL-10 mRNA in pDCs, mDCs, and CD14+ cells from individual SIV+ RMs. Fold expression was calculated using the δCt method and compared against the same gene mRNA levels in pDC from the same animal. ND indicates not done. (E) Proportion of IDO+ cells from the MLN of individual SIV+ RMs. FACS plots show pDCs, mDCs, or CD14+ cells (black) overlaid on total cells (gray background). Mean fluorescence intensity (MFI) values of IDO expression in pDCs, mDCs, or CD14+ cells are indicated on the plots.
pDCs in SIV+ RM reflect a more inflammatory rather than tolerogenic phenotype. (A) FACS plots depict gating used and the proportion of CD80+ pDCs in different tissues. FACS plots are an overlay of pDCs (black) on the HLA-DR+ LIN− population (gray). The SIV+ and SIV− animals represented here were examined at different times hence the differences in gating. The gates were placed based on CD80 expression on total lymphocytes. (B) Proportion of CD80+ pDCs or mDCs in the blood, spleen, peripheral LNs, gut LNs, and colon/rectum. *P < .01, **P < .005. ND indicates not done. (C) Intracellular cytokine expression of IFN-α and TNF-α in pDCs from the MLN and colorectum after stimulation with imiquimod or AT-2–inactivated SIVmac251. Bar chart shows the proportion of IFNα+ pDCs in the MLN or colorectum after stimulation with AT-2–inactivated SIVmac251 from 3 SIV+ RMs. (D) Relative fold expression of IDO and IL-10 mRNA in pDCs, mDCs, and CD14+ cells from individual SIV+ RMs. Fold expression was calculated using the δCt method and compared against the same gene mRNA levels in pDC from the same animal. ND indicates not done. (E) Proportion of IDO+ cells from the MLN of individual SIV+ RMs. FACS plots show pDCs, mDCs, or CD14+ cells (black) overlaid on total cells (gray background). Mean fluorescence intensity (MFI) values of IDO expression in pDCs, mDCs, or CD14+ cells are indicated on the plots.
Next, we studied the ability of pDCs from the gut-associated LNs and colorectum of SIV+ RMs to produce proinflammatory cytokines IFN-α and TNF-α in response to imiquimod or AT-2 SIV. IFN-α and TNF-α were readily produced by mesenteric lymph node (MLN) and colorectal pDCs, although the latter produced relatively less cytokines (Figure 4C). This may be a sensitized response because of the prior activated status of colorectal pDCs since the majority of colorectal pDCs were CD80+ and HLA-DRhi (data not shown). Consistent with previous reports,38,40 we also observed that the pDCs from blood of SIV+ RMs produce IFN-α and TNF-α after stimulation with imiquimod (data not shown). We could not compare the ability of colorectal pDCs to produce proinflammatory cytokines from SIV+ RMs with SIV− RMs because the latter population was rare.
pDCs can possess either immunostimulatory or immunosuppressive properties. It is suggested that pDCs from HIV-infected individuals can suppress CD4 T-cell proliferation by inducing CD4 regulatory T cells through an indoleamne 2,3-dioxygenase (IDO)–dependent pathway.34 To understand the immunosuppressive potential of pDCs from SIV+ RMs, we measured the relative levels of IDO and IL-10 mRNAs. In our studies, IDO mRNA expression in purified pDCs from the blood, MLN, or rectum of SIV+ RMs was much lower than that of purified mDCs and/or CD14+ cells found in the same tissues (Figure 4D). The level of IDO mRNA expression coincided with intracellular IDO protein expression, demonstrating that only a small fraction of pDCs expressed IDO and a much higher proportion of mDCs and/or CD14+ cells expressed IDO compared with pDCs in the same tissue (Figure 4E). Stimulation of pDCs with AT-2 SIV also did not enhance IDO expression to the same level of mean fluorescence intensity as in mDCs and CD14+ cells (data not shown). In addition, there were negligible levels of IL-10 mRNA in pDCs compared with mDCs and/or CD14+ cells from the same tissues (Figure 4D).
pDCs in the blood and GALT of SIV-infected RMs are potent inducers of CD8 T cell proliferation and Tc1 differentiation
We next tested the hypothesis that pDCs contribute to immune activation during a pathogenic SIV infection (Figure 5). We sorted DCs from the blood of SIV+ RMs and cocultured them with naive T cells from SIV− RMs. Our goal was to examine the ability of pDCs from SIV-infected macaques to promote naive T-cell proliferation and -differentiation. We used naive T cells to ensure that the responding cells were not yet committed to a specific lineage. Using an MLR assay, AT-2 SIV-stimulated pDCs or mDCs were cocultured with CFSE-stained allogeneic naive T cells for 4 days. We prestimulated DCs with AT-2 SIV because we found that the unstimulated pDCs could activate T cells but not as effectively as the stimulated pDCs (data not shown). The same was seen with pDCs from SIV-negative macaques. At a DC:T-cell ratio of 1:50, the CD8 T cells from AT-2 SIV-stimulated pDC cocultures proliferated more than those in the mDC cocultures (Figure 5A). Even at a ratio of 1:20, the mDCs were still not as efficient as pDCs at inducing naive T-cell allogeneic proliferation. Similarly, pDCs were more efficient than mDCs at inducing CD4 T-cell proliferation. The pDCs isolated from the MLN of SIV+ RMs were also capable of inducing naive T-cell allogeneic proliferation (Figure 5B).
pDCs are efficient stimulators of CD8 T-cell proliferation and Tc1 differentiation. (A) FACS plots depict the gating used and the proportion of divided (CFSElow) CD8 or CD4 T cells in a mixed leukocyte assay at a DC:T cell ratio of 1:50. Naive T cells were enriched and cocultured with pDCs or mDCs prestimulated with AT-2–inactivated SIVmac251 (AT-2 SIVmac251) at a DC:T cell ratio of 1:50 or 1:20 in a mixed leukocyte assay. Bar charts show fold increase in CFSElow CD8 or CD4 T cells induced by blood-derived DCs prestimulated with AT-2 SIVmac251. Each number represents an individual experiment. ND indicates not done. (B) Bar charts show fold increase in CFSElow CD8 or CD4 T cells induced by MLN-derived pDCs prestimulated with AT-2 SIVmac251. (C) FACS plots depict gating for IFN-γ+ CD8 T cells. Bar charts show cell counts of IFN-γ+ CD8 or CD4 T cells stimulated with anti-CD3 in the presence or absence of blood-derived DCs stimulated with AT-2 SIVmac251at a DC:T cell ratio of 1:50. These cell counts represent the difference between DC and no DC controls. Each number represents an individual experiment.
pDCs are efficient stimulators of CD8 T-cell proliferation and Tc1 differentiation. (A) FACS plots depict the gating used and the proportion of divided (CFSElow) CD8 or CD4 T cells in a mixed leukocyte assay at a DC:T cell ratio of 1:50. Naive T cells were enriched and cocultured with pDCs or mDCs prestimulated with AT-2–inactivated SIVmac251 (AT-2 SIVmac251) at a DC:T cell ratio of 1:50 or 1:20 in a mixed leukocyte assay. Bar charts show fold increase in CFSElow CD8 or CD4 T cells induced by blood-derived DCs prestimulated with AT-2 SIVmac251. Each number represents an individual experiment. ND indicates not done. (B) Bar charts show fold increase in CFSElow CD8 or CD4 T cells induced by MLN-derived pDCs prestimulated with AT-2 SIVmac251. (C) FACS plots depict gating for IFN-γ+ CD8 T cells. Bar charts show cell counts of IFN-γ+ CD8 or CD4 T cells stimulated with anti-CD3 in the presence or absence of blood-derived DCs stimulated with AT-2 SIVmac251at a DC:T cell ratio of 1:50. These cell counts represent the difference between DC and no DC controls. Each number represents an individual experiment.
Another factor differentiating pathogenic and nonpathogenic SIV infection is the sustained expression of IFN-γ in tissues, a cytokine mostly expressed by CD8 and CD4 T cells. To understand the influence of pDCs from SIV-infected macaques on naive CD8 T-cell differentiation into Tc1 response, we stimulated naive CD8 T cells with anti-CD3 for 3.5 days in the presence or absence of pDCs or mDCs. To determine the Tc1/T helper 1 phenotype of the responding CD8 or CD4 T cells, we then restimulated the T cells with phorbol 12-myristate 13-acetate/ionomycin for 4 hours and measured production of IFN-γ. Anti-CD3 stimulation was used because it provided a higher frequency of cytokine-producing cells, thereby allowing a better assessment of the T-cell phenotypes induced by DC-derived cytokines and/or costimulation. Apart from one individual experiment, SIV-stimulated pDCs were more efficient than mDCs in inducing CD8 Tc1 phenotype because there were higher numbers of IFN-γ+ CD8 T cells in the pDC cocultures (Figure 5C). Fewer IFN-γ+ CD4 T cells were induced in either pDC or mDC cocultures. The Tc1 response induced by pDCs from SIV+ RMs was comparable to the Tc1 response induced by pDCs from SIV− RMs (supplemental Figure 4), indicating that pDC functions remain relatively intact in SIV+ RMs. We note that pDCs can potentially inhibit T-cell responses through IDO-dependent mechanisms, but in our MLR assay we did not see any significant enhancement of T-cell proliferation (CFSE-dilution) in the presence of 1-methyl tryptophan nor did we see any expansion of CD25+FOXP3+ Tregs (data not shown).
In vivo blockade of α4β7-integrin dampens pDC recruitment and reduces immune activation in the colorectum
To further address the role of migration of blood pDCs to the colorectal tissue and mediating immune activation, we performed an in vivo blockade of α4β7-integrin during the acute phase of SIV infection in RMs by administering 50 mg/kg of a simianized anti-rhesus α4β7 monoclonal antibody 2 days before and 28 days after intrarectal SIV infection. Our previous study in SIV-negative animals demonstrated that this antibody can persist up to 4 weeks and blocks α4β7-integrin.37 As expected, we observed recruitment of pDCs to the colorectum in all 4 RMs of the untreated group after SIV challenge (Figure 6A). On the contrary, 3 of the 4 RMs treated with anti–α4β7-integrin antibody did not show a significant increase in colorectal pDCs during the first 6 weeks of infection. However, in the blood, we observed the typical expansion (by day 9 PI) and contraction (by week 2 PI) of pDCs in both untreated and treated groups, except a transient rebound of pDCs at week 6 PI in 3 of the treated animals (supplemental Figure 5A).
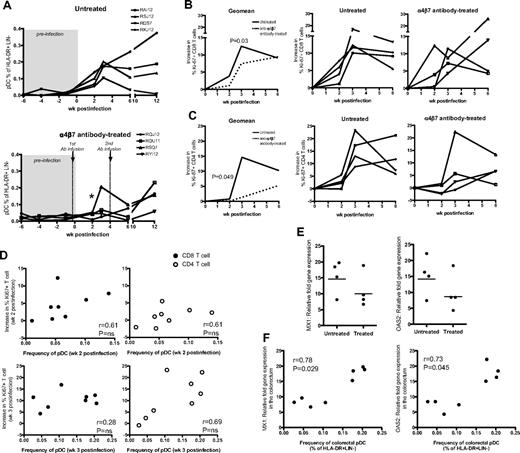
In vivo blockade of α4β7-integrin dampens pDC recruitment and reduces immune activation in the colorectum of SIV-infected RMs after intrarectal infection. (A) Frequency of pDCs in colorectum of the untreated control group and anti–α4β7-integrin antibody-treated group. Asterisk (*) indicates significant difference between groups at week 2 PI (P = .02). Frequency of Ki-67+ CD8 T cells (B) and Ki-67+ CD4 T cells (C) in the colorectum post-SIV infection. Charts show the geomean and individual data from the treated and untreated groups. An average of 3 preinfection time points was used for week 0. The increase in Ki-67+ CD8 or CD4 T cells was calculated by subtracting the proportion of these cells at week 0 from the proportion of Ki-67+ CD8 or CD4 T cells at a specific postinfection time point. P values (0.03 or 0.049) indicate significant difference between groups at week 3 PI for CD8 T cells or at week 2 PI for CD4 T cells. (D) Association between pDC frequency in the colorectum and the increase in Ki-67+ CD8 or CD4 T-cell levels in the colorectum at week 2 or week 3 postinfection. Data are obtained from treated and untreated groups. Nonparametric correlation was analyzed using the Spearman 2-tailed test. (E) Relative fold increase of MX1 or OAS2 mRNA levels in the colorectum of untreated and treated RMs at week 3 PI. Relative fold increase gene expression is calculated using week 3 PI levels over preinfection levels. (F) Association between pDC frequency in the colorectum at week 3 PI and MX1 or OAS2 fold-increase gene expression levels in the colorectum at week 3 PI. Data are obtained from treated and untreated groups. Nonparametric correlation was analyzed using the Spearman 2-tailed test.
In vivo blockade of α4β7-integrin dampens pDC recruitment and reduces immune activation in the colorectum of SIV-infected RMs after intrarectal infection. (A) Frequency of pDCs in colorectum of the untreated control group and anti–α4β7-integrin antibody-treated group. Asterisk (*) indicates significant difference between groups at week 2 PI (P = .02). Frequency of Ki-67+ CD8 T cells (B) and Ki-67+ CD4 T cells (C) in the colorectum post-SIV infection. Charts show the geomean and individual data from the treated and untreated groups. An average of 3 preinfection time points was used for week 0. The increase in Ki-67+ CD8 or CD4 T cells was calculated by subtracting the proportion of these cells at week 0 from the proportion of Ki-67+ CD8 or CD4 T cells at a specific postinfection time point. P values (0.03 or 0.049) indicate significant difference between groups at week 3 PI for CD8 T cells or at week 2 PI for CD4 T cells. (D) Association between pDC frequency in the colorectum and the increase in Ki-67+ CD8 or CD4 T-cell levels in the colorectum at week 2 or week 3 postinfection. Data are obtained from treated and untreated groups. Nonparametric correlation was analyzed using the Spearman 2-tailed test. (E) Relative fold increase of MX1 or OAS2 mRNA levels in the colorectum of untreated and treated RMs at week 3 PI. Relative fold increase gene expression is calculated using week 3 PI levels over preinfection levels. (F) Association between pDC frequency in the colorectum at week 3 PI and MX1 or OAS2 fold-increase gene expression levels in the colorectum at week 3 PI. Data are obtained from treated and untreated groups. Nonparametric correlation was analyzed using the Spearman 2-tailed test.
To understand the influence of in vivo α4β7 blockade on immune activation, we measured the frequency of CD8 and CD4 T cells coexpressing Ki-67. We observed an increase in the frequency of Ki-67+ CD8 T cells by week 3 PI in both groups; however, the increase was significantly lower in the anti-α4β7 antibody-treated group (P = .03; Figure 6B). In contrast, in blood, a similar increase in the frequency of Ki-67+ CD8 T cells was noted in both groups (supplemental Figure 5B). We note that the in vivo blockade also could dampen CD8 T-cell migration into the colorectum and thus may contribute to lower immune activation in the colorectum. To address this in part, we examined the overall CD8 T-cell frequency in the colorectum and did not observe a significant difference in colorectal CD8 T-cell frequency between treated and untreated groups (supplemental Figure 5C). Regarding CD4 T cells in the colorectum (Figure 6C), we observed an increase in the frequency of Ki-67+ CD4 T cells in the colorectum of both untreated and anti-α4β7 antibody-treated groups. However, at 2 weeks (P = .049) and 3 weeks PI (P = NS, because of an outlier monkey that showed recruitment of pDCs to colorectum in the treated group), the levels of Ki-67+ CD4 T cells in the anti-α4β7 antibody-treated group was lower than the untreated group. To understand the association between pDC migration and T-cell activation, we correlated the frequency of pDCs and Ki-67+ CD8 and CD4 T cells in the colorectum at week 2 or 3 PI (Figure 6D). We observed a significant positive correlation between the frequency of pDCs and activated T cells (both CD8 and CD4 combined). This was true either at 2 weeks (P = .04, r = 0.51) or 3 weeks PI (P = .02, r = 0.55). However, we observed only a trend for direct correlation when we used either CD8 or CD4 subsets for correlations. We think this is because of small group size (n = 4). These results suggest that enhanced recruitment of pDCs may contribute to enhanced T-cell activation in the colorectum.
In addition to measuring T-cell proliferation, we also measured for type 1 IFN-induced genes MX1, OAS2 in the colorectum tissue at week 3 PI and found that 3 of 4 RMs in the untreated group had higher gene expression levels compared with 3 of 4 RMs from the antibody-treated group (Figure 6E). We observed a significant positive correlation between the frequency of colorectal pDCs and MX1 or OAS2 expression (Figure 6F), suggesting an association between pDCs and immune activation. The lower levels of immune activation was not because of lower levels of viral load because we did not observe a significant correlation between immune activation and viral load in the colorectal tissue (supplemental Figure 5D). By 6 to 12 weeks PI, we noticed a reduced effect of α4β7-integrin blockade because there was detectable pDC recruitment and increased CD8 immune activation in the colorectum of RMs from the antibody-treated group.
Discussion
The effects of immune activation become counterproductive when prolonged and chronic inflammation overwhelms and disrupts the processes of tissue repair and regeneration. Much of the mechanisms leading to chronic immune activation during pathogenic SIV/HIV infections could be initiated by the innate immune system and microbial translocation and perpetuated by the absence of CD4 regulatory T cells36 and T helper 17 cells41 that are depleted.42,43 Here, we focused on the role of pDCs in the pathogenesis of SIV infection and demonstrated that pDCs in blood rapidly up-regulate β7-integrin expression and migrate to colorectal tissue after a pathogenic SIV infection in RMs. These pDCs were capable of producing proinflammatory cytokines and primed Tc1 response after stimulation with SIV in vitro. Furthermore, we demonstrated that in vivo blockade of α4β7-integrin dampens pDC recruitment to the colorectal tissue and that was associated with reduced CD8 and CD4 immune activation, type 1 IFN-induced gene expression. Higher expression of β7-integrin on pDCs also was observed in HIV+ individuals, suggesting that induction of β7-integrin on pDCs may be a characteristic of pathogenic immunodeficiency virus infections. Collectively, our results uncover a new mechanism by which pDCs influence SIV disease progression by contributing to immune activation in the colorectum, a site also vulnerable to opportunistic infections.
The mechanisms by which SIV infection influences gut-homing potential on pDCs are not clear. Our attempts to stimulate pDCs with either AT-2 SIV or live SIV in vitro failed to up-regulate β7-integrin. In addition, SIVsm infection in SMs did not enhance β7-integrin expression on blood pDCs, although we observed both SIVmac and SIVsm (data not shown) infections in RMs enhanced β7-integrin expression on blood pDCs. These results suggested that the induction of β7-integrin expression on blood pDCs is not solely mediated by the virus. On the contrary, suppression of viremia following ART of SIV+ RMs resulted in down-regulation of β7-integrin on pDCs. This also suggests that factors other than virus components also may be involved and most likely could depend on the cytokine environment within the blood or bone marrow and host factors.
The induction of β7-integrin seems to be unique to pDCs because mDCs did not up-regulate β7-integrin during pathogenic SIV infection. In the blood of HIV+ individuals, the pDC frequency is often lower than that of healthy individuals,44,45 and in our study we demonstrated that the pDCs from HIV+ individuals express higher β7-integrin, suggesting an ongoing dissemination of pDCs to tissues and perhaps the GI tract. It is also possible that blood pDCs from HIV+ individuals express higher CCR7 (as we observed in SIV+ RMs) and migrate to secondary lymphoid tissues. It would be ideal to investigate whether β7-integrin up-regulation on pDCs in HIV+ individuals relate to the frequency of pDC in the colorectal samples where perhaps β7-integrin+ pDCs in blood could be used as a marker of immune activation in the gut.
SIV-infected SMs do not progress to simian AIDS, despite having high viremia,11 and this in part has been attributed to their ability to dampen generalized immune activation during chronic infection. However, mechanisms by which SIV+ SMs control immune activation are still under active investigation. Importantly, our results demonstrate that the chronic SIV infection in SMs does not induce up-regulation of β7-integrin on pDCs, and this was associated with very low levels of pDCs in the colorectum of these animals. These results suggest that one of the ways that SMs dampen immune activation could be by regulating the induction of β7-integrin on pDCs by SIV. Such regulatory mechanisms are unknown but could involve CD4 regulatory T cells and/or the presence of tolerogenic DCs. It is also important to note that the basal levels of β7-integrin expression on pDCs of SIV-negative SMs were lower compared with SIV-negative RMs. This raises the possibility that the low β7-integrin expression on pDCs may be a property of the species itself. It also would be ideal to compare the copy number of α4β7 transcripts expressed by pDCs from RMs and SMs to understand whether the low β7-integrin expression is regulated at the level of transcription or translation. Understanding the basis for the failure to express α4β7 on the pDCs of SMs is important and further experiments are needed to address this phenomenon.
In conclusion, we believe that when pDCs are left unchecked in pathogenic SIV infection, they add considerable burden to SIV+ animals already immunocompromised and susceptible to opportunistic infections. For HIV+ individuals, immune reconstitution, tissue repair, and regeneration in the GI tract are critical and chronic CD8 immune activation seems only to impede these processes.26,46 Whereas antiretroviral treatment failed to fully suppress CD8 T-cell immune activation in the GI tract of HIV+ individuals,25,47 it becomes more essential to target the sources of chronic CD8 T-cell immune activation. The significant positive association that we observed in this study between pDCs and immune activation highlights the important role of pDCs in regulating chronic immune activation and thereby disease progression. One of the important findings of our study is that in vivo blockade of α4β7-integrin does not prevent systemic immune activation. We think this is because of the fact that pDCs also express CCR7 after SIV infection that promotes their migration to secondary lymphoid tissues and therefore may contribute to systemic immune activation. Thus, our results strongly suggest that therapeutic interventions that down-modulate proinflammatory functions of pDCs in both the GI tract and secondary lymphoid tissues may slow down HIV disease progression.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to the veterinary staff at the Yerkes Division of Research Resources for support and donors who participated in this study. They thank Mark Mulligan and Jeffery Lennox for providing human samples from the Emory Clinical Research Core Specimen Repository; Lakshmi Chennareddi for statistical support and Helen Drake-Perrow for administrative support; Emory Continuing and Facilitating AIDS Research Virology and Drug Discovery Core, in particular Natalia Kozyr, for gene expression studies, and Bali Pulendran for helpful discussion.
This work was supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases grants R01 AI057029 and R01 AI071852 (to R.R.A.), Yerkes National Primate Research Center base grant P51 RR00165, and Emory Continuing and Facilitating AIDS Research grant P30 AI050409.
National Institutes of Health
Authorship
Contribution: S. Kwa contributed to the design of the study, carried out the experiments, and wrote the manuscript; S. Kannaganat contributed to the data and analyses; P.N. and M.S. provided technical assistance in tissue processing and flow cytometry; R.D. contributed to GeneChip data; W.A. contributed to human data; A.A. contributed to the in vivo blockade experiment; S.E.B. and G.S. contributed to qPCR data; and R.R.A. supervised the project and contributed to the design of the study and to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rama Rao Amara, Emory Vaccine Center, Department of Microbiology and Immunology, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30329; e-mail: ramara@emory.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal