Abstract
Biologic and clinical observations suggest that combining imatinib with IFN-α may improve treatment outcome in chronic myeloid leukemia (CML). We randomized newly diagnosed chronic-phase CML patients with a low or intermediate Sokal risk score and in imatinib-induced complete hematologic remission either to receive a combination of pegylated IFN-α2b (Peg–IFN-α2b) 50 μg weekly and imatinib 400 mg daily (n = 56) or to receive imatinib 400 mg daily monotherapy (n = 56). The primary endpoint was the major molecular response (MMR) rate at 12 months after randomization. In both arms, 4 patients (7%) discontinued imatinib treatment (1 because of blastic transformation in imatinib arm). In addition, in the combination arm, 34 patients (61%) discontinued Peg–IFN-α2b, most because of toxicity. The MMR rate at 12 months was significantly higher in the imatinib plus Peg–IFN-α2b arm (82%) compared with the imatinib monotherapy arm (54%; intention-to-treat, P = .002). The MMR rate increased with the duration of Peg–IFN-α2b treatment (< 12-week MMR rate 67%, > 12-week MMR rate 91%). Thus, the addition of even relatively short periods of Peg–IFN-α2b to imatinib markedly increased the MMR rate at 12 months of therapy. Lower doses of Peg–IFN-α2b may enhance tolerability while retaining efficacy and could be considered in future protocols with curative intent.
Introduction
Imatinib (Gleevec, Glivec, Novartis Pharma) 400 mg daily is with significant efficacy and safety the current standard first-line therapy for chronic myeloid leukemia (CML).1 Used as monotherapy, however, up to 35% of patients have adverse effects, unsatisfactory response, or disease progression.2 Thus, for a substantial number of patients, there is a need for an alternative therapy. To improve outcome, imatinib has been combined with conventional chemotherapeutic agents or biologic modifiers.3
Before the introduction of imatinib, IFN-α–based regimens were preferred for upfront treatment of patients not eligible for allogeneic stem cell transplantation.4-7 In vitro, IFN-α has a synergistic effect in combination with imatinib.8 IFN-α is, however, barely detectable in the serum 24 hours after its administration, thus warranting frequent administration (daily to 2 or 3 times weekly) for sustained efficacy. To overcome this limitation, 2 forms of pegylated (covalent attachement of polyethylene glycol [Peg]) IFN-α have been developed: Peg–IFN-α2a and Peg–IFN-α2b. The pegylation results in modified properties, including sustained absorption/exposure and prolonged half-life, allowing for administration once weekly.9 Peg–IFN-α2a (40 kDa), 450 μg once weekly, compared with IFNα-2a, 9 MIU once daily, resulted in higher rates of hematologic and cytogenetic response and improved overall survival.10 Therapy with Peg–IFN-α2a was also safe and better tolerated than conventional IFN-α. In a randomized phase 3 study, weekly Peg–IFN-α2b was compared with daily IFN-α2b. The adverse events (AEs), efficacy, and safety profiles were comparable.11 Thus, Peg–IFN-α represented an excellent candidate for clinical development in CML.
We now report the results from an academic multicenter randomized phase 2 trial comparing the efficacy of imatinib in combination with Peg–IFN-α2b and imatinib monotherapy in newly diagnosed chronic phase (CP) CML patients with an intermediate or low Sokal risk score who have achieved a complete hematologic remission (CHR) with 3 months of imatinib monotherapy.
Methods
Definitions of risk, disease phase, and response
The relative risk for disease progression was calculated and defined as low, intermediate, or high, according to the Sokal risk score12 and assessed by a web-based calculator at http://www.roc.se/hematologi/KML/Sokal.asp. Accelerated and blastic phases were defined by at least one of the following criteria: blood myeloblasts ≥ 10%, blood myeloblasts and promyelocytes ≥ 30%, blood basophils > 20%, and any extramedullary involvement (apart from liver or spleen; eg, lymph node, skin, bone, and central nervous system). All cases who did not meet any of these criteria were defined as CP. CHR was defined as a normal blood count without immature forms in peripheral blood and without splenomegaly.
Cytogenetic responses were determined by calculation of percentage of Ph+ metaphases (at least 20 mitoses should be analyzed) in bone marrow by karyotyping (G-band): complete cytogenetic response (CCgR) = 0%; partial cytogenetic response (PCgR) = 1%-34%; minor cytogenetic response = 35%-65%, minimal cytogenetic response 66%-95%, and no cytogenetic response > 95% Ph+ cells. Cytogenetic response loss was defined as any regression from a condition of CCgR or PCgR to any other response type.
Study design
This is a randomized phase 2 study performed by the Nordic CML Study Group in collaboration with the Chaim Sheba Medical Center in Israel. Patients with low or intermediate Sokal risk CML in early CP received an induction therapy with imatinib 400 mg daily for 3 months. Those who achieved CHR after induction treatment were randomized to imatinib 400 mg daily or imatinib 400 mg daily plus Peg–IFN-α2b (PegIntron, Merck & Co Inc, formerly Schering Plough) 50 μg weekly.
Sokal high-risk patients were concurrently enrolled in a separate, randomized comparison of imatinib 400 mg versus 800 mg daily.13
The primary objective of the study was to compare and demonstrate superiority of the combination arm versus the monotherapy arm for the rate of major molecular response (MMR) at 12 months after randomization (ie, 15 months after the start of imatinib treatment). Other objectives were comparisons in the timing of molecular response, of the rates of CCgR, and treatment safety and tolerability. After 12 months on study, decision of the subsequent treatment was left to the discretion of the treating physician.
The study was approved by ethics committees for all participating centers, sponsored by the Nordic CML Study Group and supported by free Peg–IFN-α2b from the drug manufacturer. Imatinib was prescribed. Additional financial support was received from Schering-Plough (currently Merck & Co Inc) and Novartis.
Patients
Patients were eligible for registration if they had Sokal intermediate- or low-risk CML in early CP (< 6 months of duration), were previously treated with no other chemotherapy than hydroxyurea, were > 17 years old with performance status (Eastern Cooperative Oncology Group) 0 to 2, and had provided a written informed consent.
Exclusion criteria were impaired liver or renal function (as defined by bilirubin or aspartate aminotransferase/alanine aminotransferase > 3 times upper normal limits and by creatinine > 20 mg/L = 177 μM), alcohol or drug addiction and severe unrelated disease.
Patients were eligible for the randomization if they fulfilled the registration criteria and had achieved at least a CHR after 3 months of imatinib.
Disease burden assessment
Blood counts, clinical status, conventional cytogenetics (karyotyping), and standardized quantitative real-time quantitative polymerase chain reaction (RQ-PCR) for BCR-ABL1 were continuously monitored.
Visits were weekly during the first 3 months, every 2 weeks during the fourth, fifth, and sixth months, and monthly thereafter. The karyotyping with standard G-banding technique on bone marrow cells and RQ-PCR studies on whole blood were performed before therapy start and then every 6 months during treatment.
Dosage modifications for AEs
AEs were identified and graded as defined in the National Cancer Institute/National Institutes of Health Common Toxicity Criteria Version 3.0 and were divided into hematologic and nonhematologic AEs. In case of concurrent hematologic and nonhematologic AEs, dose adaptation was regulated based on which AE was more severe and required more dose reduction. The basic principle of dose adaptation was to save and maintain the dose of imatinib over that of Peg–IFN-α2b.
Hematologic AEs
In case of AE grade 1 or 2, no action was taken. If a patient developed grade 3 or 4 neutropenia, it was allowed to add granulocyte colony-stimulating factor (G-CSF).
Arm imatinib.
With grade 3 (absolute neutrophil count [ANC], 0.5-1.0 × 109/L or a platelet count 25-50 × 109/L), imatinib was discontinued. Blood counts and ANC were checked weekly. When toxicity resolved to grade 0 or 1 (ANC > 1.0 × 109/L, platelet count > 75 × 109/L), imatinib was resumed at 400 mg. In case of > 3 episodes, imatinib treatment was adapted to the maximum tolerated dose (MTD). With grade 4 (ANC < 0.5 × 109/L or a platelet count < 25 × 109/L), imatinib was discontinued. Blood counts and ANC were checked weekly. When toxicity resolved to grade 0 or 1, imatinib was resumed at 300 mg for 2 weeks, hence at 400 mg. In case of > 2 episodes, imatinib treatment was adopted to MTD.
Arm imatinib plus Peg–IFN-α2b.
With grade 3 (defined as in “Arm imatinib”), Peg–IFN-α2b was stopped. Blood counts and ANC were checked weekly. When toxicity resolved to grade 0 to 2, Peg–IFN-α2b was resumed at the dosage given before grade 3 toxicity occurred. In case of increasing grade 3 thrombocytopenia (platelet count < 50 × 109/L), imatinib was stopped but resumed as soon as grade 3 toxicity had resolved. In case of > 3 episodes of hematologic toxicity grade 3, Peg–IFN-α2b was discontinued permanently, but in case of grade 3 neutropenia G-CSF was given, Peg–IFN-α2b continued and imatinib adapted to MTD. Grade 4: First time, both drugs discontinued. Blood counts and ANC checked weekly. Platelet transfusions, G-CSF and antibiotics could be used. When toxicity had recovered to grade 0 to 2, imatinib was resumed at 300 mg for 2 weeks, hence at 400 mg. Peg–IFN-α2b was skipped for 2 weeks and then resumed to the dosage given before grade 4 toxicity evolved. With grade 4, second time, both drugs were discontinued. Blood counts and ANC were checked weekly. When toxicity had resolved to grade 0 to 2, imatinib was resumed at 300 mg for 2 weeks, hence at 400 mg. Peg–IFN-α2b was discontinued permanently. In case of > 2 episodes, imatinib treatment was adopted as to MTD.
Nonhematologic AEs
In case of grade 1, no action was taken.
Arm imatinib.
With grade 2, if probably related to imatinib, imatinib was discontinued. Toxicity was then checked weekly. When toxicity had resolved to grade 0 or 1, imatinib was resumed at 400 mg. With grade 3, if probably related to imatinib, imatinib was discontinued. When toxicity had resolved to grade 0 or 1, imatinib was resumed at 300 mg for 2 weeks, hence at 400 mg. In case of > 2 episodes, imatinib was adapted to MTD. With grade 4, if probably related to the study drug, imatinib was discontinued permanently.
Arm imatinib plus Peg–IFN-α2b.
With grade 2 attributed to imatinib, only imatinib was discontinued. Toxicity was checked weekly. When toxicity resolved to grade 0 or 1, imatinib was resumed at 400 mg. When attributed to Peg–IFN-α2b, only Peg–IFN-α2b was discontinued. Toxicity was checked weekly. When toxicity had resolved to grade 0 or 1, Peg–IFN-α2b was resumed at 15-30 μg. When attributed to both drugs or uncertain, both drugs were discontinued. Toxicity was checked weekly. When toxicity had resolved to grade 0 or 1, imatinib was resumed at 400 mg. Peg–IFN-α2b was resumed at 15 (→ 30) μg the following week. With grade 3 attributed to imatinib, only imatinib was discontinued. Toxicity was checked weekly. When toxicity had recovered to grade 0 or 1, imatinib was resumed at 300 mg for 2 weeks, hence at 400 mg. When attributed to Peg–IFN-α2b, only Peg–IFN-α2b was discontinued. Toxicity was checked weekly. When toxicity had recovered to grade 0 or 1, Peg–IFN-α2b was resumed at 15 (→ 30) μg. When attributed to both drugs or uncertain, both drugs were discontinued. Toxicity was checked weekly. When toxicity had recovered to grade 0 or 1, both drugs were resumed, imatinib at 300 mg for 2 weeks, thereafter at 400 mg, and Peg–IFN-α2b at 15 (→ 30) μg the following week. With grade 3, second time, attributed to imatinib, both drugs were discontinued. Toxicity was checked weekly. When toxicity had recovered to grade 0 or 1, imatinib was resumed at 300 mg for 2 weeks, hence at 400 mg. Peg–IFN-α2b was resumed at 15 (→ 30) μg. When attributed to Peg–IFN-α2b, both drugs were discontinued. Toxicity was checked weekly. When toxicity had recovered to grade 0 or 1, only imatinib was resumed, at 400 mg. Peg–IFN-α2b was discontinued permanently. When attributed to both drugs or uncertain, both drugs were discontinued. Toxicity was checked weekly. When toxicity was recovered to grade 0 or 1, only imatinib was resumed, at 300 mg for 2 weeks, hence at 400 mg. Peg–IFN-α2b was discontinued permanently. In case of > 2 episodes of grade 3, imatinib was adapted as appropriate to MTD. With grade 4 attributed to imatinib, both drugs were discontinued. Toxicity was checked weekly. When toxicity had recovered to grade 0 or 1, only Peg–IFN-α2b was resumed (at 15-30 μg). Imatinib was discontinued permanently. If the treatment was temporarily discontinued or reduced for nonhematologic AE, hydroxyurea could be given, if white blood cells > 20 × 109/L or platelets > 1000 × 109/L or if the disease was symptomatic. In case of grade 3 or 4 neutropenia, adding G-CSF was permitted.
Neuropsychiatric AEs
Based on previous experience from IFN-α therapy, neuropsychiatric AEs (eg, depression) were attributed to Peg–IFN-α2b. With grade 1, there was no dose reduction, but a weekly check for toxicity. With grade 2, first and second time, Peg–IFN-α2b was discontinued. When toxicity had recovered to grade 0 or 1, Peg–IFN-α2b was resumed at 30 μg. With grade 2, third time or grade 3, first time, Peg–IFN-α2b was permanently discontinued.
Molecular monitoring of BCR-ABL1
Molecular analyses were performed at registration, at randomization, and then at 6 and 12 months. Molecular response was evaluated by blood RQ-PCR for BCR-ABL1 transcripts and expressed on the international scale, as previously described.14 The primary endpoint MMR was defined as BCR-ABL1 ≤ 0.1% on the international scale. At the time of study initiation, the national reference laboratories in Finland (Turku) and Sweden (Uppsala) already had established conversion factors for expressing BCR-ABL1 values on the international scale.15 Secondary Nordic quality control rounds were run to allow the establishment of conversion factors for all participating laboratories.16
Statistics
The primary endpoint (the rate of MMR at 12 months after randomization) was considered as a binary variable. The power calculation was based on the assumption of 60 patients in each group. An assumed absolute difference of 25% in response rate would be possible to detect with a power of 80% (uncorrected χ2 test, α = 0.05 2-sided). Differences between the treatment arms with respect to categorical variables were tested using Yates corrected χ2 test. For continuous variables, the Mann-Whitney test was used. The proportion of patients in MMR at week 52 in each treatment arm was compared as per an intention-to-treat analysis. To investigate the influence of other characteristics besides treatment arm on the primary endpoint, a multivariate logistic regression model was fitted. All tests were 2-sided, and a P value < 5% was considered statistically significant.
Results
Patients
From September 2004 until March 2008, 130 patients were registered from 27 centers in the Nordic countries (Denmark, Finland, Norway, and Sweden) and Israel. The inclusion rate was approximately 4 patients per month for the first 2 years, but then decreased, mainly because of concurrent competitively recruiting industry-sponsored studies. The number of patients who fulfilled the criteria for randomization after 3 months was 112 (56 in each treatment arm). The reasons for not randomizing 18 patients were AEs (2), protocol violation (13), drug addiction (1), and no CHR at 3 months (2). Characteristics at diagnosis of the randomized patients are shown in Table 1. Treatment groups were well balanced. At the time of enrollment, all patients were in early CP (< 3 months from diagnosis) and 20 patients (10 in each treatment arm, 18%) had been pretreated with hydroxyurea; 46% and 54% (in both treatment arms) of patients were in Sokal intermediate- and low-risk groups, respectively.
Characteristics of randomized patients at diagnosis
| . | Arm A: imatinib (N = 56) . | Arm B: imatinib + Peg–IFN-α2b (N = 56) . | P . | ||
|---|---|---|---|---|---|
| Sex, % | .849 | ||||
| Male | 31 | (55.4) | 33 | (58.9) | |
| Female | 25 | (44.6) | 23 | (41.1) | |
| Median age, y (range) | 51 | (17-74) | 49 | (19-74) | .675 |
| White blood cells, × 109/L, median (range) | 92.2 | (1.5-411.0) | 83.7 | (4.6-285.0) | .880 |
| Platelets, × 109/L, median (range) | 376 | (102-1294) | 386 | (154-1236) | .359 |
| % blasts in peripheral blood, median (range) | 1.0 | (0.0-5.0) | 0.8 | (0.0-4.0) | .328 |
| Spleen (cm) below costal margin, median (range) | 0 | (0-20) | 0 | (0-11) | .758 |
| Median Sokal score (range) | 0.8 | (0.5-1.2) | 0.8 | (0.5-1.1) | .778 |
| . | Arm A: imatinib (N = 56) . | Arm B: imatinib + Peg–IFN-α2b (N = 56) . | P . | ||
|---|---|---|---|---|---|
| Sex, % | .849 | ||||
| Male | 31 | (55.4) | 33 | (58.9) | |
| Female | 25 | (44.6) | 23 | (41.1) | |
| Median age, y (range) | 51 | (17-74) | 49 | (19-74) | .675 |
| White blood cells, × 109/L, median (range) | 92.2 | (1.5-411.0) | 83.7 | (4.6-285.0) | .880 |
| Platelets, × 109/L, median (range) | 376 | (102-1294) | 386 | (154-1236) | .359 |
| % blasts in peripheral blood, median (range) | 1.0 | (0.0-5.0) | 0.8 | (0.0-4.0) | .328 |
| Spleen (cm) below costal margin, median (range) | 0 | (0-20) | 0 | (0-11) | .758 |
| Median Sokal score (range) | 0.8 | (0.5-1.2) | 0.8 | (0.5-1.1) | .778 |
Additional cytogenetic abnormalities were found in 8 patients (7%; 5 in the imatinib and 3 in the combination arm) and included variant translocations (2 cases) and others (4 cases).
AEs
The number of patients with reported AEs for both treatment arms during the first 12 months is shown in Table 2. Overall, there were more patients with grade 3 or 4 hematologic and nonhematologic AEs in the imatinib plus Peg–IFN-α2b combination therapy arm than in the imatinib monotherapy arm (n = 33 vs n = 16, respectively, P = .002) Musculoskeletal pain, rash, and fatigue were more common in the combination therapy than in the monotherapy arm. Neutropenia occurred in 7 patients in the monotherapy and 21 in the combination therapy arm (P = .005). Biochemical AEs were uncommon. Neuropsychiatric AEs occurred in 9 patients on monotherapy and 17 on combination therapy (P = .017).
Number of patients with AEs
| . | Imatinib . | Imatinib + Peg–IFN-α2b . | ||
|---|---|---|---|---|
| Any . | Grade 3 or 4 . | Any . | Grade 3 or 4 . | |
| Nonhematologic AE | ||||
| Superficial edema | 7 | 0 | 3 | 0 |
| Nausea | 2 | 0 | 5 | 1 |
| Muscle cramps | 9 | 1 | 3 | 1 |
| Musculoskeletal pain | 3 | 0 | 9 | 3 |
| Rash | 9 | 0 | 15 | 4 |
| Fatigue | 6 | 1 | 13 | 2 |
| Diarrhea | 5 | 1 | 7 | 1 |
| Headache | 1 | 0 | 3 | 0 |
| Abdominal pain | 1 | 0 | 1 | 0 |
| Vomiting | 2 | 1 | 1 | 0 |
| Joint pain | 1 | 0 | 0 | 0 |
| Dyspepsia | 0 | 0 | 1 | 0 |
| Dizziness | 0 | 0 | 1 | 1 |
| Upper respiratory tract infection | 0 | 0 | 3 | 1 |
| Weight gain | 1 | 0 | 1 | 0 |
| Pyrexia | 0 | 0 | 1 | 0 |
| Insomnia | 0 | 0 | 1 | 0 |
| Depression | 2 | 0 | 1 | 0 |
| Other | 11 | 5 | 17 | 4 |
| Hematologic AE | ||||
| Neutropenia | 7 | 7 | 21 | 21 |
| Thrombocytopenia | 1 | 1 | 2 | 2 |
| Biochemical AE | ||||
| ALAT | 1 | 1 | 2 | 2 |
| . | Imatinib . | Imatinib + Peg–IFN-α2b . | ||
|---|---|---|---|---|
| Any . | Grade 3 or 4 . | Any . | Grade 3 or 4 . | |
| Nonhematologic AE | ||||
| Superficial edema | 7 | 0 | 3 | 0 |
| Nausea | 2 | 0 | 5 | 1 |
| Muscle cramps | 9 | 1 | 3 | 1 |
| Musculoskeletal pain | 3 | 0 | 9 | 3 |
| Rash | 9 | 0 | 15 | 4 |
| Fatigue | 6 | 1 | 13 | 2 |
| Diarrhea | 5 | 1 | 7 | 1 |
| Headache | 1 | 0 | 3 | 0 |
| Abdominal pain | 1 | 0 | 1 | 0 |
| Vomiting | 2 | 1 | 1 | 0 |
| Joint pain | 1 | 0 | 0 | 0 |
| Dyspepsia | 0 | 0 | 1 | 0 |
| Dizziness | 0 | 0 | 1 | 1 |
| Upper respiratory tract infection | 0 | 0 | 3 | 1 |
| Weight gain | 1 | 0 | 1 | 0 |
| Pyrexia | 0 | 0 | 1 | 0 |
| Insomnia | 0 | 0 | 1 | 0 |
| Depression | 2 | 0 | 1 | 0 |
| Other | 11 | 5 | 17 | 4 |
| Hematologic AE | ||||
| Neutropenia | 7 | 7 | 21 | 21 |
| Thrombocytopenia | 1 | 1 | 2 | 2 |
| Biochemical AE | ||||
| ALAT | 1 | 1 | 2 | 2 |
n = 56 in each study arm. AEs were recorded during 52 weeks of treatment.
ALAT indicates alanine aminotransferase.
Treatment discontinuation, dose reduction, and disease progression
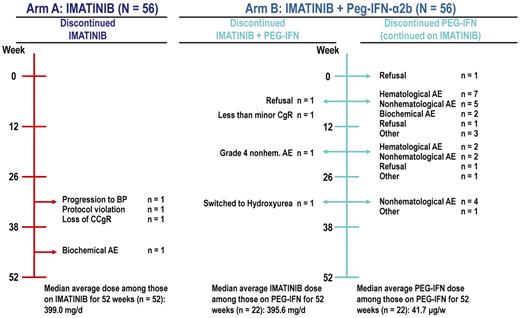
Imatinib treatment was permanently discontinued in 7% of patients (4 patients in each treatment arm), and Peg–IFN-α2b was discontinued in 34 (30 only Peg–IFN-α2b and 4 Peg–IFN-α2b+imatinib) of 56 patients (61%) in the combination arm. The reasons for treatment discontinuation can be found in Figure 1.
Causes for therapy discontinuation by study arm. BP indicates blastic phase.
As per protocol, the starting dose of Peg–IFN-α2b was at the beginning of the study 50 μg weekly but was reduced to 30 μg weekly according to a protocol amendment because of an excess of AEs (neutropenia, constitutional symptoms, such as pain and fever). Depending on tolerability, the dose could then be escalated to 50 or reduced down to 15 μg weekly. The median administered dose of Peg–IFN-α2b in patients who did not discontinue treatment (n = 22) was 42 μg weekly. The median dose of imatinib in patients who continued the scheduled treatment for 12 months was close to 400 mg in each treatment arm (Figure 1). Only 2 patients (both in the imatinib arm) discontinued treatment because of disease progression (one because of loss of CCgR and one because of progression to blastic phase).
Response and course
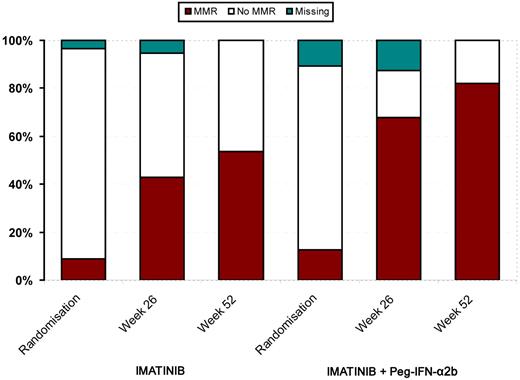
The rates of CCgR and MMR at week 52 are shown in Table 3. No statistically significant difference between the treatment arms regarding CCgR was observed. However, there was a highly significant difference in MMR rates between the imatinib monotherapy and the imatinib plus Peg–IFN-α2b arms (53.6% vs 82.1%, respectively, P = .002, intention-to-treat analysis). The outcome in the intermediate-risk group was even more marked (n = 52; ie, 42.3 vs 84.6%, P = .004), whereas the difference in the low-risk group was not statistically significant (63.3% vs 80.0%, respectively, P = .25). A per-protocol analysis was performed on the 22 patients who continued Peg–IFN-α2b for the whole study period and showed that 91% achieved MMR versus 58% in patients who completed 12 months in the imatinib monotherapy arm (P = .012).
Response at week 52
| . | Arm A: imatinib (N = 56) . | Arm B: imatinib + Peg–IFN-α2b (N = 56) . | P . |
|---|---|---|---|
| Cytogenetic response, % | .391 | ||
| CCgR | 47 (83.9) | 51 (91.1) | |
| Molecular response, % | .002 | ||
| MMR | 30 (53.6) | 46 (82.1) |
| . | Arm A: imatinib (N = 56) . | Arm B: imatinib + Peg–IFN-α2b (N = 56) . | P . |
|---|---|---|---|
| Cytogenetic response, % | .391 | ||
| CCgR | 47 (83.9) | 51 (91.1) | |
| Molecular response, % | .002 | ||
| MMR | 30 (53.6) | 46 (82.1) |
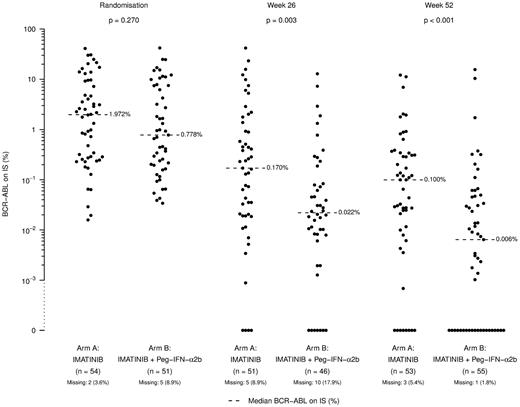
The MMR rate at 12 months was the same for all patients who were treated with Peg–IFN-α2b for > 12 weeks (Table 4), indicating that potentially a short period of PegIFN-α2b treatment may be enough to achieve patient benefit. Figure 2 illustrates that the MMR was achieved more rapidly in the combination arm with individual RQ-PCR values shown in Figure 3. In this figure, an apparent difference in undetectable transcripts in the experimental arm is apparent; however, no strict definition of CMR (including sensitivity criteria) was defined in the study. Our data are in line with data from the French study,17 in which higher CMR and superior response rates were observed in the experimental arm. Among the patients with additional cytogenetic abnormalities at diagnosis, 3 of 3 in the combination arm and 3 of 5 in monotherapy arm achieved a MMR.
MMR rates according to time of Peg–IFN-α2b exposure
| Duration of Peg–IFN-α2b treatment . | MMR at week 52, % . |
|---|---|
| < 12 weeks (n = 21) | 67 |
| 12-25 weeks (n = 7) | 86 |
| 26-37 weeks (n = 6) | 100 |
| ≥ 38 weeks (n = 22) | 91 |
| Duration of Peg–IFN-α2b treatment . | MMR at week 52, % . |
|---|---|
| < 12 weeks (n = 21) | 67 |
| 12-25 weeks (n = 7) | 86 |
| 26-37 weeks (n = 6) | 100 |
| ≥ 38 weeks (n = 22) | 91 |
Calculation according to an intention-to-treat analysis.
Individual blood BCR-ABL1 RQ-PCR values at randomization, and at weeks 26 and 52 from randomization by study arm. IS indicates international scale.
Individual blood BCR-ABL1 RQ-PCR values at randomization, and at weeks 26 and 52 from randomization by study arm. IS indicates international scale.
In a multivariate model, including treatment arm, sex, age, blood counts, and spleen size at diagnosis as variables, only treatment arm emerged as a predictive factor for MMR at 12 months of therapy (odds ratio = 4.77; 95% confidence interval, 1.76-12.97, P = .002; data not shown).
Discussion
The present study demonstrates a benefit of adding Peg–IFN-α2b to standard imatinib treatment in newly diagnosed low or intermediate Sokal risk CML patients as judged by a significantly higher MMR rate at 52 weeks. There were no unpredictable complications or AEs reported. Apparently, the benefit of Peg–IFN-α2b addition could be achieved with a relatively short Peg–IFN-α2b exposure (3-6 months).
Three other European groups have presented studies on the effect of combining IFN-α and imatinib as an upfront therapy for CML.
In the Italian GIMEMA Working Party phase 2, open, single-arm study with imatinib plus Peg–IFN-α2b, 66 of 76 patients had discontinued Peg–IFN-α2b, mainly because of toxicity. The cytogenetic and molecular responses at 5 years were excellent. The intended dosages of Peg–IFN-α2b were 50, 100, and 150 μg weekly and in retrospect, too high in combination with imatinib (the average administered dose was only 33 μg weekly).18 A trial by the German CML Study group was initiated in 2002 as a randomized controlled comparison of imatinib versus imatinib plus IFN-α (pegylated or nonpegylated) versus imatinib plus low-dose cytarabine after IFN-α failure. In a preliminary report, MMR at 12 months was reached in significantly more patients with imatinib 800 mg daily than with imatinib 400 mg daily or imatinib 400 mg plus IFN-α daily (P < .001), and no clear effect of IFN-α was seen. In the assessment of overall and progression-free survival, there was no difference between the 3 treatment arms.19
In the French SPIRIT study, 636 patients were randomized to imatinib 400 mg daily, imatinib 600 mg daily, imatinib 400 mg plus low-dose cytarabine daily, or imatinib 400 mg daily plus Peg–IFN-α2a (Pegasys) 90 μg weekly.17 Although 46% stopped Peg–IFN-α2a treatment for toxicity reasons within 12 months, this arm had faster cytogenetic and molecular responses at 6 and 12 months and was the superior arm in the study. Hence, it was selected to remain as the single experimental arm for the second half of the study. Rates of MMR at 24 months (intention-to-treat) for imatinib plus Peg–IFN-α2a, imatinib 400 mg daily, imatinib 600 mg daily, and imatinib 400 mg daily plus low-dose cytarabine were 71%, 48%, 62%, and 63%, respectively (P < .001). Subgroup analysis also showed a superior MMR rate if patients could tolerate 12 months of full dose therapy.20 Thus, despite the relatively small sample size and thus limited statistical power, the data presented in the current study are in accordance with previous studies.
If one summarizes the reports from these 4 study groups, it seems that only Peg–IFN-α2b and Peg–IFN-α2a have an additional value for imatinib treatment of CML. Standard IFN-α has not yet been demonstrated to have such an effect. The study by Lipton et al previously also pointed at a benefit for use of a more active and tolerable Peg–IFN-α2b in combination with tyrosine kinase inhibitors for the eradication of minimal residual disease in CML.10 Possible explanations for the Peg–IFN-α2a and Peg–IFN-α2b superiority to conventional IFN-α could be longer presence in blood because of sustained absorption and prolonged half-life.
Our clinical impression was that Peg–IFN-α2b treatment was challenging to tolerate, in the same manner as reported in the Italian and French studies. An average weekly dose of 42 μg was achieved in the 39% of our patients who continued the scheduled treatment for 12 months. Compared with the Italian study, our study showed comparatively less toxicity, probably because of the lower starting dose. Interestingly, the French Group amended their protocol for tolerability reasons, halving the dose of Peg–IFN-α2a from 90-45 μg weekly for similar reasons.17 The average administered dose in the first part of the study was 54 μg weekly. Despite tolerability problems, there is still apparently a positive effect of these drugs at the given dose, a lesson for future studies.
Imatinib and IFN-α are both effective in CML. The mode of action and biologic effects of imatinib and IFN-α are quite different and therefore may explain why a combination treatment is better than treatment with either drug alone. The IFNs are proteins with antiproliferative, immunomodulatory, and antiviral effects. Studies in vitro with leukocyte IFN-α show cytostatic effects on leukemic cells. However, the mechanism of action in vivo is probably multifactorial. IFN-α can induce recognition and elimination of CML cells by the immune system.21-25 The presence and expansion of PR-1 specific cytotoxic T cells have been linked to favorable therapeutic responses during IFN-α therapy.26 There is one report that describes a comparison of immunologic parameters in CML patients in CCgR after IFN-α versus imatinib therapy. Although treatment duration was very different, it was reported that the number of T cells was significantly lower in both patient groups compared with normal persons, the number of NK cells was unaffected, whereas the absolute numbers of B cells and monocytes and serum IgA and IgG concentrations were significantly lower after treatment with imatinib.27
In conclusion, our results from a randomized phase 2 study indicate potential benefit of Peg–IFN-α2b in combination with standard-dose imatinib in CML patients with a low or intermediate Sokal risk score. Approximately 40% of patients in prolonged (< 2 years) complete molecular remissions do not relapse after imatinib discontinuation.28 Thus, currently, a rational primary objective of future CML studies is cure with drug therapy. The proportion of deep molecular responses is higher in patients treated with second-generation tyrosine kinase inhibitors,29,30 which may translate into higher cure rates. Combination of low-dose Peg–IFN-α2b (15-30 μg/week of) with a second-generation tyrosine kinase inhibitor is a particularly rational approach, bearing in mind the multifaceted mode of action of IFN-α.
The advent of imatinib and the second-generation tyrosine kinase inhibitors, with impressive efficacy and advantageous side effect profiles, overshadowed IFN-α treatment in CML. However, our data reported here and the recent experience from the French study17 suggest that the era of IFN-α treatment in CML is not over. Its unique mechanism of action could be used both in combination regimens as well as in monotherapeutic approaches, such as in the maintenance phase before therapy discontinuation. Whether this may result in increased cure rates needs to be evaluated in future prospective treatment protocols.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients for participating in this study and all the study personnel (physicians, study nurses, and laboratory technicians) facilitating the successful conduct of the NordCML002 study.
This work was supported by the European LeukemiaNet, Novartis Pharma, Merck & Co (formerly Schering-Plough), and Regional Oncological Center, University of Uppsala.
Authorship
Contribution: B.S. and K.P. designed and performed research, analyzed data, and wrote the paper; T.G.-D., B.M., K.R., J.S., A.A., M.B., M.F., P.K., A.L., C.M., K.M.-E., L.O., A.R., M.S., A.S., U.S., A.N., and J.L.N. performed research; S.M. and O.W.B. performed research and wrote the paper; H.E. and V.K. performed research and contributed vital analytical tools; F.G. contributed vital analytical tools; K.O. and F.S. analyzed data; and H.H.-H. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: B.S. received honoraria from BMS and Novartis. J.S. received honoraria from BMS and Novartis. P.K. received honoraria from Novartis and BMS. S.M. received honoraria from BMS and Novartis. U.S. received honoraria from BMS. O.W.B. received honoraria from BMS and Novartis. J.L.N. honoraria from Novartis. H.H.-H. received honoraria from Novartis and BMS. K.P. received honoraria and research funding from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Bengt Simonsson, Department of Hematology 50C, University Hospital, S-751 85 Uppsala, Sweden; e-mail: bengt.simonsson@medsci.uu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal