Abstract
This review highlights a decade of investigations into the role of CD38 in CLL. CD38 is accepted as a dependable marker of unfavorable prognosis and as an indicator of activation and proliferation of cells when tested. Leukemic clones with higher numbers of CD38+ cells are more responsive to BCR signaling and are characterized by enhanced migration. In vitro activation through CD38 drives CLL proliferation and chemotaxis via a signaling pathway that includes ZAP-70 and ERK1/2. Finally, CD38 is under a polymorphic transcriptional control after external signals. Consequently, CD38 appears to be a global molecular bridge to the environment, promoting survival/proliferation over apoptosis. Together, this evidence contributes to the current view of CLL as a chronic disease in which the host's microenvironment promotes leukemic cell growth and also controls the sequential acquisition and accumulation of genetic alterations. This view relies on the existence of a set of surface molecules, including CD38, which support proliferation and survival of B cells on their way to and after neoplastic transformation. The second decade of studies on CD38 in CLL will tell if the molecule is an effective target for antibody-mediated therapy in this currently incurable leukemia.
The current view of chronic lymphocytic leukemia (CLL) is that of a disease characterized by a dynamic balance between cells circulating in the blood and cells located in permissive niches in lymphoid organs.1 The former are primarily mature-looking small lymphocytes resistant to apoptosis, whereas the latter are composed of lymphocytes that undergo either proliferation or apoptosis according to the environment.2 The heterogeneous clinical outcome of CLL patients is dictated, at least in part, by the nature of these microenvironmental signals and interactions that can promote or impair accumulation of genomic alterations.3 Detailed immunophenotypic and genetic analyses of different neoplastic clones have led to the identification of a number of molecular markers, some of which are gaining relevance in clinical practice to predict disease outcome.4-7 Cell surface CD38 is one of these markers.
This review highlights a decade of investigations into the role of CD38 in CLL. An association between CD38 expression by CLL cells and a more aggressive clinical behavior was first reported in 19994 and confirmed by several subsequent studies.8-12 Consequently, determination of the percentage of CD38-expressing cells within a clone has become part of the workup of CLL patients in many clinical centers,13 and it is generally accepted that CD38+ patients will have a shorter progression-free interval, require earlier and more frequent treatments, and ultimately die sooner.4
The underlying question behind these clinical observations is why expression of this molecule on the cell surface would correlate with a worse clinical outcome. The most obvious possibility is that CD38 expression reflects events occurring inside the cell. Indeed, the percentage of CD38+ cells within a leukemic clone was originally described as one of two independent indicators (along with IGHV gene mutations) of clinical outcome in CLL.4 Because both indicators marked somewhat overlapping populations, a link with IGHV mutation status, signals mediated by B-cell receptor (BCR), and CD38 was proposed.2,14,15 These two parameters are not obligatorily linked: an alternative (and not mutually exclusive) hypothesis is that the presence of cell surface CD38 provides a more global molecular bridge to the environment, shifting the balance between survival/proliferation and apoptosis in favor of the former.16 Evidence sustaining this hypothesis has been collected over the past decade and has contributed to the formulation of the current concept of CLL as a chronic disease in which the host physiologically provides essential elements and conditions leading to the acquisition and accumulation of genetic alterations by the leukemic cells. These changes begin at a preneoplastic stage (ie, monoclonal B-cell lymphocytosis),17 or possibly even before this stage, and continue to occur in a clone that progresses to a full-fledged CLL, notwithstanding that surface levels of CD38 are lower in monoclonal B-cell lymphocytosis than in CLL.18 This scheme accommodates the existence of structures that provide replicating and surviving signals to B cells on their way to neoplastic transformation.15,17 A key element in this view is that leukemic B cells can and do proliferate, with division taking place not in the blood, but primarily in specialized morphologically discrete structures of lymph nodes (LN) and bone marrow (BM) known as proliferation centers (PCs).3,19-21
Different sets of molecules may have different roles and confer at times the ability to localize in privileged sites, to receive signals from the external environment, to undergo apoptosis, or to modify surrounding cells.22 In this context, evidence so far uncovered on the role of CD38 expression provides new depth and relevance about the regulation of the social life of the neoplastic B cell.23 Here, we review the available evidence providing a mechanistic link between CD38 expression and CLL progression. Before delving into the CLL field, however, a short overview of the main features of the molecule is presented. In the final section, we discuss the pros and cons of using CD38 as a prognostic indicator and therapeutic target for selected CLL patients.
Biology of human CD38
Originally defined as a T-cell activation molecule, CD38 expression now does not appear to be dependent on cell lineage or activation.24 Within the B-cell compartment, CD38 is expressed at high levels by B lineage progenitors in BM and by B lymphocytes in germinal center, in activated tonsils, and by terminally differentiated plasma cells.24 On the contrary, mature virgin and memory B cells express low levels of the molecule. CD38 also has been found in pancreatic acinar cells, smooth muscle cells, osteoclasts, and in different areas of the brain, although in most of these instances, CD38 is located in the cytosol and/or in the nucleus and not on the cell membrane.24
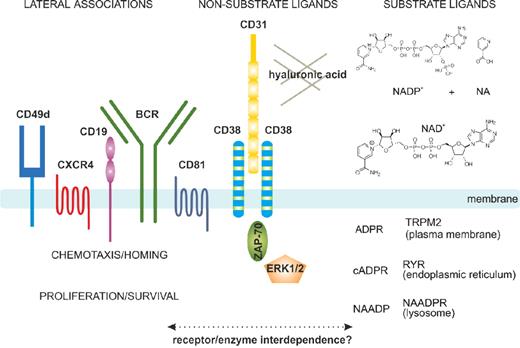
The protein encoded by CD38 is a single chain type II transmembrane molecule displaying a canonical molecular weight of ∼ 45 kDa. The crystal resolution of the extracellular portion of the molecule showed that the functional CD38 molecule is a dimer, with the central part hosting the catalytic site.25 The monomer to dimer transition modulates the functions of the molecule. Additional control is rendered by the dynamic localization of CD38 in lipid microdomains of the plasma membrane where the molecule has a tendency to associate with other proteins, forming large supramolecular complexes. CD38-associated molecules in human B cells include the CD19/CD81 complex, the chemokine receptor CXCR4, and adhesion molecules, such as CD49d23,26 (Figure 1). CD38 is also found in exosomes,27 membrane vesicles secreted by B cells, and is probably part of an intercellular communication network.
Structural and functional characteristics of the human CD38 molecule. In human B cells, CD38 is expressed as an integral surface membrane molecule, often in a dimeric conformation. As an enzyme, CD38 may interact with the substrate ligands NAD+ and NADP+, which are converted to cADPR, ADPR, and NAADP, all intracellular Ca2+-mobilizing agents. CD38 also interacts with non-substrate ligands, including CD31 and hyaluronic acid, which regulate cell-cell and cell-matrix contacts. On the plasma membrane, CD38 displays preferential localization in membrane lipid microdomains in close contact with the BCR complex (CD19/CD81) and with molecules regulating homing (CXCR4 and CD49d). CD38 engagement by means of the natural ligand CD31 (or surrogate agonistic mAb) triggers the activation of an intracellular signaling pathway, which includes ZAP-70 and ERK1/2 as major players. These signals increase chemotaxis as well as proliferation of neoplastic B cells. The interplay between the enzymatic and receptor activities still needs to be determined in the CLL context.
Structural and functional characteristics of the human CD38 molecule. In human B cells, CD38 is expressed as an integral surface membrane molecule, often in a dimeric conformation. As an enzyme, CD38 may interact with the substrate ligands NAD+ and NADP+, which are converted to cADPR, ADPR, and NAADP, all intracellular Ca2+-mobilizing agents. CD38 also interacts with non-substrate ligands, including CD31 and hyaluronic acid, which regulate cell-cell and cell-matrix contacts. On the plasma membrane, CD38 displays preferential localization in membrane lipid microdomains in close contact with the BCR complex (CD19/CD81) and with molecules regulating homing (CXCR4 and CD49d). CD38 engagement by means of the natural ligand CD31 (or surrogate agonistic mAb) triggers the activation of an intracellular signaling pathway, which includes ZAP-70 and ERK1/2 as major players. These signals increase chemotaxis as well as proliferation of neoplastic B cells. The interplay between the enzymatic and receptor activities still needs to be determined in the CLL context.
An initial function attributed to CD38 was the regulation of activation/proliferation of human T lymphocytes.28 Agonistic monoclonal antibodies (mAbs) specific for CD38 induce rapid Ca2+ fluxes and trigger the phosphorylation of a cascade of intracellular substrates, leading to activation of the nuclear factor-κB complex. Protracted effects include initiation of genetic programs causing cytokine secretion and proliferation of T lymphocytes.24 CD31 (also known as PECAM-1) is a non-substrate CD38 ligand that can start the signaling cascade and recapitulate the biologic events observed in vitro using surrogate agonistic mAbs.29
The functional properties of CD38 on human B cells appear to be strictly linked to the stage of maturation. The presence of blocking mAbs in cultures of CD19+ B-cell precursors on stromal layers markedly suppresses B-cell lymphopoiesis by inducing apoptosis.30 In addition, in murine systems, CD38 participates in cell selection of transitional B cells.31 Opposite effects are observed in mature circulating B lymphocytes and tonsillar germinal center B cells, where CD38 ligation is followed by activation, apoptosis inhibition, proliferation, and cytokine secretion.32,33 In both instances, the mechanisms are attributed to the activation of an intracellular signaling pathway ruled by CD38 and requiring an association with CD19. The ensuing phosphorylation cascade includes lyn and phosphatidylinositol 3-kinase, among other kinases.34
CD38 has a striking similarity (∼ 35% amino acid identity) to the enzyme adenosine diphosphate (ADP) ribosyl cyclase, which is present in a soluble form in the ovotestis of the mollusk Aplysia californica.35 This enzyme has the unique characteristic of cyclizing NAD+ to generate cyclic ADP ribose (cADPR), a second messenger that releases Ca2+ from internal stores, independently of the IP3 pathway.36 Under suitable conditions, a limited amount of cADPR is converted to ADPR. In mammals, CD38 maintained these enzymatic activities and became localized on the cell surface, thereby developing into an ecto-enzyme37 (Figure 1). However, evolution preserved the hydrolyzing function, which controls synthesis of ADPR. The human molecule conserves the ability to produce cADPR, even if at very low levels.38 cADPR is a ubiquitous second messenger in eukaryotic cells. Smooth muscle cells of different origins (vascular, bronchial, and uterine),39-41 along with epithelial and secretory cells (pancreas, kidney, and hypophysis the most studied),42 are the best examples. A further enzymatic activity recently attributed to CD38 is the pH-dependent conversion of NADP+ to NAADP+. These products bind different receptors and channels involved in the regulation of Ca2+ and activating critical signaling pathways, leading to muscle contraction (uterus and bronchi) or gland secretion (pancreas and neurohypophysis). These functions were initially described in mice genetically modified to lack or to overexpress CD3843 and confirmed in human disease models.44
The family of NAD+-consuming enzymes includes 4 classes of enzymes, of which CD38 and the ADP ribosyl transferases are extracellular, whereas poly(ADP-ribose) polymerases and sirtuins are intracellular. While consuming NAD+ as substrate, these enzymes generate nicotinamide, reinforcing the notion that CD38 is directly involved in maintaining NAD+ homeostasis.42 As the NAD+/NADH ratio is a direct measure of the energy status of a cell, the NAD+ dependence of these enzymes directly links cellular energy to metabolism, genomic stability, apoptosis, cell signaling, and stress tolerance.42 Additional novel roles in the control of immunity, inflammatory responses, and in shaping the regulatory T-cell compartment have now been assigned to extracellular NAD+.45
How these varied functional activities can be conducted by a single molecule is yet to be defined. A hypothesis is that they are completely unrelated as enzymatic mutants and enzyme inhibition of CD38 does not impact receptor functions46,47 in human B, T, and myeloid cells. An alternative is that the enzymatic functions are regulated through interactions taking place between CD38 and different proteins. The crystal structure of CD38 suggests that CD31 binding regulates accessibility of the substrate to the enzymatic site.25 If corroborated, one might conclude that the human enzyme is not only limited in function by the availability of the substrate, but it is further finely tuned by the interactions with other non-substrate ligands.
CD38 as a marker in CLL
CD38 expression identifies two subgroups of CLL patients with different clinical outcomes4 ; this distinction is based on the percentage of CD38+ leukemic cells within a CLL clone. In the majority of studies, the threshold is considered as ≥ 30% CD38+ clonal members.13 The 2 patient subgroups that result from this cut-off point differ clinically in several ways, including overall survival,4,9,10 time to first treatment,12,48 bias toward male gender,4 number of leukemic cells with atypical morphology,49 extent and level of adenopathy,9 lactate dehydrogenase and β2-microglobulin levels,9,50 and absolute lymphocyte counts.8 These subgroups also differ in responsiveness to various therapies.8 Combining CD38 with other prognostic markers, such as ZAP-70 and CD49d, provides complementary prognostic information.51-53 Because these molecules are components of the same functional network, their interrelated biologic roles probably explain these observations.23
The past decade has highlighted several molecular differences between CD38+ and CD38− members of the same clone, including expression of specific activation markers,54 which reflect quantitative and temporal differences in response to stimulation. CD38+ CLL cells express high levels of CD69 and HLA-DR,55,56 both indicative of recent activation. CD38 also marks a cellular subset enriched in Ki-67+ and ZAP-70+ cells, more efficiently responding to surface IgM cross-linking. Both features are apparently independent of the percentage of CD38-expressing cells within the original clone.20,57 In addition, CD38+ CLL clones display enhanced ability to migrate in response to CXCL12,58 to transduce BCR-mediated signals, and to respond to anti-IgM and anti-IgD antibody-mediated crosslinking.14,59,60 The genetic signature characterizing CD38+ and CD38− members of the same clone revealed higher VEGF and Mcl-1 levels in CD38+ cells,61 pointing to a survival advantage from microenvironmental interactions (Figure 2).
Proposed model explaining a role of CD38 in the pathogenesis and progression of CLL. (A) CD38+ CLL cells (red) are more sensitive to CXCL12 signals, with a higher propensity to home to lymphoid tissues than the CD38− counterpart (brown). (B) Once inside the LN PCs, CLL lymphocytes come into contact with accessory cells, such as nurse-like (NLC), follicular dendritic (FDC), stromal, endothelial, mesenchymal, and T cells. The presence of antigen and accessory signals leads to proliferation and potentially to acquisition of novel genetic lesions, which promote clonal evolution and disease progression. These events are more apparent in the CD38+ subsets. The C > G SNP occurring in a region of CD38 critical for transcriptional regulation adds an additional element of complexity, potentially affecting the levels of CD38 expression after microenvironmental interactions.
Proposed model explaining a role of CD38 in the pathogenesis and progression of CLL. (A) CD38+ CLL cells (red) are more sensitive to CXCL12 signals, with a higher propensity to home to lymphoid tissues than the CD38− counterpart (brown). (B) Once inside the LN PCs, CLL lymphocytes come into contact with accessory cells, such as nurse-like (NLC), follicular dendritic (FDC), stromal, endothelial, mesenchymal, and T cells. The presence of antigen and accessory signals leads to proliferation and potentially to acquisition of novel genetic lesions, which promote clonal evolution and disease progression. These events are more apparent in the CD38+ subsets. The C > G SNP occurring in a region of CD38 critical for transcriptional regulation adds an additional element of complexity, potentially affecting the levels of CD38 expression after microenvironmental interactions.
Other characteristics of the CD38+ and CD38− CLL subgroups are variable telomere lengths,20,56,62,63 telomerase levels,62 expression of COX-2 enzyme,64 and in vivo incorporation of 2H into DNA of dividing leukemic cells.20 Lastly, they differ in high-risk genomic abnormalities,65 in the development of new DNA mutations66 and copy number changes over time.67
These findings suggest that cellular proliferation might be at the root of the association between higher levels of CD38, aggressively growing CLL clones, and poor patient outcome.2 This suggestion is consistent with in vivo labeling experiments using 2H2O (“heavy water”) that demonstrated larger than anticipated rates of CLL birth, especially in patients with poor clinical course and outcome68 and a several-fold higher percentage of newly born cells in CD38+ fractions of CLL clones than in CD38− counterparts of the same clones.20 Furthermore, preliminary results from a large clinical study suggest that the percentage of CD38+ CLL cells strongly correlates with the level of leukemic cell turnover observed in vivo.69 Finally, a recent study using improved cell surface phenotyping and sorting techniques that incorporate expression densities of CXCR4 and CD5 indicates that the percentage of newly born cells is ∼ 10 times higher in the CD38+ than the CD38− fraction.70 Thus, the combined kinetic and CXCR4 expression analyses highlight relationships between CD38 expression, in vivo kinetics, cell migration to solid tissues, and clinical outcome.
Therefore, CD38 expression is at least a quantitative reflection of in vivo CLL cell activation. However, the activation status acquired in the PCs of lymphoid tissues may wane over time, leading to the conversion of CD38+ cells to CD38− quiescent cells. The circuit may be restarted when the cells are recruited into lymphoid tissues, where they may be reactivated.20 The initiating event for this activation is unclear, although the association of higher numbers of CD38+ cells with IGHV unmutated (U) CLL clones that often exhibit biased use and selection for specific IGHV/D/J rearrangements (stereotyped BCRs)71,72 suggests that one factor promoting cellular stimulation is BCR signaling.15
CD38-mediated signals and their connection with the BCR
Clones with higher numbers of CD38+ cells are generally those more responsive to BCR signaling in vitro, whereas CD38− clones are generally anergic, although exceptions exist.14,59,60 The same phenomena are observed at the intraclonal level as CD38+ cells respond more readily to BCR stimulation than CD38− cells of the same clone.57,73 However, evidence for involvement of CD38 in the BCR signaling pathway is indirect and linked to lateral associations with CD19 and CD81 (in turn part of the BCR complex) and colocalization in the same lipid rafts as the BCR.26
The most favorable conditions for expansion of CLL clones exist in discrete anatomic sites, such as PCs in LN and in the BM, where leukemic cells come into contact with accessory cells and the appropriate array of cytokines and chemokines. These sites contain more CD38+ CLL cells than detected in the periphery, implying that CD38 is involved in the expansion process and/or results from it.
These findings may indicate that a trafficking fraction of CLL cells may enter PCs of peripheral LNs, become activated, and express (more) CD38 as well as ZAP-70 and other activation markers. Additional stimulatory signals may also be delivered directly via CD38. The degree of activation would influence a cell's capacity to up-regulate the number of markers per cell and the percentage of positive cells. On re-entry into the peripheral circulation, CLL cells may express more activation markers (CD38, ZAP-70, CD49d, and others), thereby representing important predictors of disease outcome. Besides the BCR, CD38+ cells also respond better to signals coming through chemokine and other receptors. Therefore, signals emanating initially and subsequently from this pathway probably provide molecular bridges to the extended CLL microenvironment, thereby favoring survival/proliferation of CLL cells.
CD38 is part of a molecular hub integrating proliferative and migratory signals of CLL cells
In vitro activation of CD38 elicited by agonistic mAbs induces a portion of the CLL clone (10%-30%) to proliferate, giving rise to an immunoblast-like component that does not secrete Igs74 ; these effects are dependent on significant amounts of soluble IL-2, in keeping with the observation that T lymphocytes and accessory cells are needed in vivo.19 This was a first indication that CD38 transduces signals, leading or contributing to CLL cell growth, even in the absence of stimuli coming from the BCR.
First indications that CD38 acts as a molecular compass that routes leukemic cells to specialized niches arose from in vivo findings that the number of CD38 molecules expressed in BM and LN is higher than in circulating lymphocytes.75 In addition, nurse-like cells, differentiated in vitro from circulating CD14+ cells and also presumably present in solid lymphoid tissues in vivo,22 express CD31, the CD38 ligand.76 Interactions between CD38 and CD31 in this system promote proliferation and survival through a molecular circuit that can be interrupted by blocking anti-CD31 or anti-CD38 mAbs.76 CD38/CD31 interaction results in a genetic signature that includes more than 1500 modulated sequences. Pathway analysis drastically reduces this complexity, with proliferation and migration emerging as the main elements characterizing this receptor/ligand system.77
CD38 signaling may be influenced by the presence of ZAP-70. Patients with CD38+ZAP-70+ clones are more responsive to activation of intracellular proteins than CD38+ZAP-70− persons.78 This may explain why the simultaneous expression of the two molecules offers a more efficient identification of the high-risk patient subset.51 CD38+ZAP-70+ CLL cells also migrate better in response to the CXCL12 chemokine and exhibit a genetic signature primarily consisting of genes involved in cell locomotion.78 A functional cooperation between CD38 and CXCR4 was demonstrated by showing that binding of agonistic anti-CD38 mAbs and de novo expression of CD38 by lentiviral infection increases chemotaxis in response to CXCL12.58
These effects on proliferation and chemotaxis are perturbed by blocking reagents, which interfere with CXCL12-mediated migration in vitro and block CLL homing in an NOD/SCID mouse model.58 A possible explanation is that CD38 and CXCR4 are associated on the same membrane patches; and hence, agonistic and blocking anti-CD38 mAbs have the potential to interfere positively or negatively with CXCL12 binding to the CXCR4 receptor, modulating functional responses. Recent data indicate that this signaling platform of molecules is more complex and includes adhesion molecules, such as CD49d and matrix metalloproteases, such as MMP-9.79,80 Targeting the CLL invadosome blocks trafficking to LN and BM, suggesting potential translation of these observations in a therapeutic setting.23
Role of genetic elements
Human CD38, located on the short arm of chromosome 4 (4p15), exhibits a number of unique features. First, the gene is relatively large, with > 98% of the genetic material consisting of introns. The promoter region is atypical, lacking a canonical TATA box, but containing a CpG island, pointing to epigenetic regulatory mechanisms, yet to be demonstrated.81 CD38 expression by peripheral blood mononuclear cells behaves as a polymorphic trait in the general population, in keeping with the notion that the gene is under the pressure of constant and intense regulatory activity. Retinoids, vitamin D, and a variety of cytokines are the most known inducers.24
Besides being variable among CLL patients, CD38 expression can change at anatomic sites, being higher in BM and in areas of intense CLL/T lymphocyte contact.19 Accordingly, during CLL cell activation in vitro, CD38 expression can be up-regulated by a number of different signals, such as IL-2,74 CD40L plus IL-4,82 CpG oligonucleotides,83 and coculture with mesenchymal stem cells.84
A single nucleotide polymorphism (C > G, rs6449182) is located at the 5′ end of intron 1 of CD38,81 and allelic frequencies of the polymorphism in healthy Italian, Spanish, Irish, and Polish populations have been defined.24 The frequency of the G allele is significantly higher in a subset of CLL patients exhibiting clinical and molecular markers of poor prognosis. The highest frequency is found in patients with Richter syndrome (RS),85 with the G allele representing an independent risk factor for RS. The same G allele is a susceptibility factor for CLL development in the Polish population.86 Furthermore, there are no significant differences in the percentage of CD38+ cells or intensity of expression in circulating CLL lymphocytes of G carriers and CC homozygotes in these populations, whereas environmental signals lead to greater increases in CD38 expression selectively in G carriers. The latter suggests involvement of the single nucleotide polymorphism (SNP) in transcription,85 possibly because the C > G SNP is located within a binding site for E47, the predominantly active isoform of E2A in human B lymphocytes that binds canonical E-box elements and activates gene transcription.87 These events are essential in the programs regulating B-cell differentiation by controlling Ig gene transcription and recombination as well as expression of other B-lineage genes.87 E47 is effectively recruited to the regulatory region of CD38 and the CD38 genotype in the E-box conditions the strength of the binding. E2A shows a comparatively higher affinity for the G allele, linking CD38 genotype to the dynamic regulation of surface expression of the molecule.88 The finding that E2A directly drives transcription of AICDA,89 in turn responsible for class switch recombination and somatic hypermutation,90 opens the possibility that the interplay between E47 and CD38 may lead to increased susceptibility to the insurgence of RS.
In line with the presence of a CpG island, the CD38 promoter can be methylated and methylation negatively correlates with surface expression. In a study of 168 CLL patients and using a cut-off of 7% for surface CD38 expression, 96% of CD38− persons exhibited methylation, whereas only 25% of CD38+ samples were methylated. Methylation was not observed in peripheral blood mononuclear cells of healthy controls.91 Given that the defect appears to be site-specific and not a global event in CLL patients, it is foreseeable that it will become a useful predictor of outcome.
Pros and cons in using CD38 as a marker and a therapeutic target
Why do the numbers of CD38-expressing cells in a CLL clone predict clinical course?
A definite answer to this question is not available at this point; the following is our view of this issue. CD38 expression is dynamic and indicates the proliferative activity of members of the leukemic clone at the time of analysis.20,56 Therefore, it is a “real-time” indicator of the level of leukemic proliferation and thereby actual or potential clonal evolution, which ultimately determines the clinical course and outcome for an individual patient. This evolutionary change can be affected by many parameters84 ; in particular, it can be influenced by stimulation through cell surface receptors for antigens,83 cytokines, chemokines,19,74,82 etc, within the microenvironment. Simplistically viewed, the more CD38+ cells in a clone, the greater the number of dividing cells and hence the greater the chance for occurrence of new DNA lesions, enhanced clonal aggressiveness, and worse clinical outcome.
Why and how do the numbers of CD38-expressing cells and IGHV mutation status of a CLL clone interrelate and herald clinical course?
In our view, IGHV mutation status is a more static marker, indicative of the cell of origin and the maturational events that occurred in the life of the B cell before its leukemic transformation.15 In particular, the absence or presence of IGHV mutations could report the ability and type of antigen binding that the BCR can accomplish (eg, polyreactive vs oligoreactive vs monoreactive).92-94 Antigen binding specificity correlates with outcome because it indicates the breadth of antigenic epitopes that the BCR can engage, the affinity of these engagements, and therefore the likelihood that survival/proliferation signals will be delivered to the CLL cell. It is for these reasons that CD38 expression, which is a reflection of the existing level of cellular activation within a leukemic clone, often correlates with a lack of IGHV somatic mutations, which is more likely to lead to polyspecific binding, cell signaling, and eventual cellular proliferation. Thus, both prognostic indicators can be linked by the common thread of leukemic cell proliferation: IGHV mutations indicating the likelihood of binding multiple antigens and of cellular stimulation and CD38 expression representing the consequence of such binding and stimulation.
What are the advantages of using CD38 clonal percentages as a prognostic marker?
As mentioned, the number of CD38-expressing cells can be considered a real-time indicator of the proliferative activity of a patient's CLL clone. As such, it is a reflection or harbinger of new genomic lesions,66,67 which require DNA replication and subsequently of clonal evolution to a more dangerous leukemic variant.
What are the disadvantages of using CD38 clonal percentages as a prognostic marker?
It has been documented that CD38 levels can change over time.95 Although in our experience4 and that of others96 this change is usually not large (∼ 10%) and more often than not does not overstep the boundaries that mark “better” or “worse” clinical outcome, it can occur. However, when it does, the suggested downside can be actually viewed as a positive feature of this prognostic indicator as an upward trend in CD38-expressing cells may signal clonal evolution to a more aggressive state.97 Therefore, serial analyses of the percentage of CD38+ cells, real-time indicators of leukemic cell proliferation, can have an additional advantage of indicating a change in clonal behavior.
Is there a role for CD38 SNP analysis?
The C > G SNP identified in the regulatory region is localized within an E-box and conditions the affinity of E2A binding.88 The consequence is that G carriers up-regulate CD38 in response to environmental signals with a higher efficiency than CC homozygotes.85 It would be of interest to test, in a retrospective cohort, whether the C > G SNP affects the stability of CD38 expression over time. The prediction based on in vitro observations would be that G carriers up-regulate CD38 more readily in response to specific external signals and then become CD38+. CC homozygotes, on the other hand, would be less prone to do so. If confirmed, this observation would support the clinical usefulness of identifying the CD38 SNP at diagnosis, with closer monitoring of CD38 expression over time selectively in G carriers. In addition, clinical testing for the G allele, one of the few risk factors for RS transformation,85 may highlight patients more susceptible to this dangerous event.
How can CD38 expression be best used as a prognostic indicator?
The current National Cancer Institute guidelines recommend that treatment be initiated at disease progression (ie, in patients at Binet stage C or Rai stage III or IV).98 This strategy is suggested because patients are heterogeneous in clinical course and certain patients never progress, often dying of a different disease, whereas others do progress, at times quite rapidly. Thus, to prevent unnecessary treatment of a large segment of affected persons, a watch-and-wait attitude is chosen and treatment is generally delayed until patients become symptomatic. The disadvantage of this understandably prudent approach is that treatment may come too late for those persons requiring it, when a number of unfavorable cytogenetic lesions have already accumulated in CLL subclones. Therefore, the option of early treatment should be carefully considered if markers were available to precisely predict clinical course and progression to select patients based on real treatment requirement. So far, none of the available predictors alone is sufficiently precise to mandate a change in the present therapeutic strategy. In addition, most of the available studies with marker combinations have been carried out retrospectively and in studies where the precise assessment of the value of CD38 can be hampered by the time of marker determination during the clinical course, perhaps because of change that can occur longitudinally.99
Despite these limitations, scoring systems involving a multiplicity of markers (eg, IGHV mutations), CD38 and/or ZAP-70 levels, chromosomal abnormalities, p53 mutations, and serum molecules such as β2-microglobulin, can be effective risk assessors and group stratifiers.1 The issue of markers is covered by a wide literature.98,100 These approaches may require further prospective testing to refine and simplify the methods. At this point, the watch-and-wait strategy should be compared with that of early, potentially aggressive treatments in patients randomized into a formal clinical trial and selected for expression of unfavorable prognostic markers.
Is there a role for CD38 as a therapeutic target?
Targeted immunotherapy with mAbs has become critical for the successful treatment of many forms of cancer. This is exemplified by rituximab, a chimeric anti-CD20 mAb, which has revolutionized the treatment of several B-cell malignancies. The main concern about in vivo use of anti-CD38 mAbs is its widespread expression in multiple cell types and differentiation stages, such as early committed BM precursors, activated T cells, and cells of the innate immune response. Another serious drawback is the presence of CD38 in the brain, pancreas, and retina,24 although the earliest hematopoietic stem cells do not appear to express CD38. Another potentially useful approach could rely on the use of inhibitors of the enzymatic activities of CD38, even if experience is so far limited.101
These intrinsic limits, however, have not prevented the design of models for in vivo applications. In the past, several Abs to human CD38 have been generated that induce killing of neoplastic B-cell lines.102,103 Two CD38 mAbs are currently in clinical development: a humanized mAb (SAR650984, http://clinicaltrialsfeeds.org/clinical-trials/show/NCT01084252) and a human mAb (daratumumab).104,105 Ongoing clinical trials will determine whether these reagents are effective (alone or in combination) and at the same time safe.
Acknowledgments
The Fondazione Internazionale Ricerca in Medicina Sperimentale (FIRMS) provided valuable assistance.
Work from the Torino group was supported in part by Associazione Italiana Ricerca Cancro (Special Program Molecular Clinical Oncology “5 per Mille” 19980, 2010/15, and IG #8590), by the Italian Ministries of Health (Bando Giovani Ricercatori 2008) and University (Bando FIRB Giovani 2008 and Progetto di Rilevanza Nazionale [PRIN] 2009). Studies from the US group were supported in part by RO1 grant CA81554 from the National Cancer Institute, The Karches Foundation, The Prince Foundation, The Marks Foundation, The Jerome Levy Foundation, and The Leon Levy Foundation. Work from the Genova group was supported by grants from the Associazione Italiana Ricerca sul Cancro (AIRC, Special Program Molecular Clinical Oncology “5 per Mille” and IG 10492), Ministries of Health (2006), and University and Compagnia di San Paolo.
Authorship
Contribution: All authors synthesized ideas and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Malavasi, Laboratory of Immunogenetics, Department of Genetics, Biology and Biochemistry, University of Torino, Via Santena 19, 10126 Torino, Italy; e-mail: fabio.malavasi@unito.it.