Abstract
Replacement of the missing factor VIII (FVIII) is the current standard of care for patients with hemophilia A. However, the short half-life of FVIII makes frequent treatment necessary. Current efforts focus on the development of longer-acting FVIII concentrates by introducing chemical and genetic modifications to the protein. Any modification of the FVIII protein, however, risks increasing its immunogenic potential to induce neutralizing antibodies (FVIII inhibitors), and this is one of the major complications in current therapy. It would be highly desirable to identify candidates with a high risk for increased immunogenicity before entering clinical development to minimize the risk of exposing patients to such altered FVIII proteins. In the present study, we describe a transgenic mouse line that expresses a human F8 cDNA. This mouse is immunologically tolerant to therapeutic doses of native human FVIII but is able to mount an antibody response when challenged with a modified FVIII protein that possesses altered immunogenic properties. In this situation, immunologic tolerance breaks down and antibodies develop that recognize both the modified and the native human FVIII. The applicability of this new model for preclinical immunogenicity assessment of new FVIII molecules and its potential use for basic research are discussed.
Introduction
Hemophilia A is an X-linked bleeding disorder that is caused by reduced function or lack of clotting factor VIII (FVIII).1 Replacement of the missing protein is the current standard of care for patients. However, the short half-life of FVIII, ∼ 7-17 hours,2 makes frequent treatment necessary. Current efforts focus on the development of longer-acting FVIII concentrates, which should decrease the required treatment frequency and therefore improve the quality of life for patients. Recently described approaches in the development of longer-acting concentrates are based on strategies that have been successfully applied to other therapeutic proteins: chemical modifications such as the addition of polyethylene glycol (PEG) polymers, polysialic acids, or hydroxyethyl starch3,4 ; alternative formulations with PEG-modified liposomes3 ; and fusion to the Fc part of human IgG.5 In addition, molecular modifications of the FVIII protein aimed at increasing the duration of its cofactor activity or reducing its clearance in vivo have been reported.3
Any chemical or molecular modification of the FVIII protein can potentially increase its immunogenic potential. Modifications could generate neo-epitopes for both B and T cells or may induce altered structures that could bind and trigger receptors expressed on cells of the innate immune system, thereby amplifying potential anti-FVIII antibody responses.6 Finally, modifications could generate repetitive epitopes for B cells that might cause the activation and differentiation of B cells and the subsequent production of antibodies without the requirement for T-cell help.7,8 Therefore, the potential impact that any modification of an FVIII concentrate might have on its immunogenic potential requires comprehensive assessment based on immunogenicity data obtained during clinical development. However, it would be highly desirable to identify candidates with a high risk for increased immunogenicity before entering clinical development to minimize the risk of exposing patients to such candidates. So far, assessing the immunogenicity of modified FVIII proteins in preclinical animal models has been difficult because of the xenogenic nature of human FVIII in conventional models. Human FVIII induces antibodies when injected into mice, rats, dogs, or monkeys,9-12 which makes it difficult to distinguish between the immunogenicity of native human FVIII and the immunogenicity of modified human FVIII. Previously, we reported the generation of new transgenic mouse lines that express a human F8 cDNA as a transgene.13,14 The transgene is randomly integrated into the murine genome, and its expression is driven by an albumin promoter and α-fetoprotein enhancer elements. The human F8 transgene should result in the expression of native human FVIII during embryonic development, when the T-cell repertoire in the thymus is shaped and high-avidity “self-reactive” T cells are deleted from the repertoire.15,16 Expression of human FVIII during this period should therefore lead to the depletion of high-avidity human FVIII-specific T cells and result in specific immune tolerance toward human FVIII. Similar genetically engineered mouse models were described for human insulin analogs,17 modified human tissue plasminogen activator variants,18 human IFNs,19,20 human Fas ligand inhibitory protein (decoy receptor 3, DcR3),21 and a mutant of human FVIII.22 Transgenic mouse models for human IFNα2b (hIFNα2b) and human IFNβ (hIFNβ)19,20 were shown to be predictive of the human immune response, because commercially available hIFNβ drugs that were found to induce antibodies in a large number of patients were shown to break immune tolerance in these mice.19
In the present study, we describe the selection and characterization of a transgenic mouse line that is immunologically tolerant to native human FVIII but is still able to mount an antibody response to human FVIII when challenged with a modified FVIII protein that possesses increased immunogenicity. In this situation, immunologic tolerance breaks down and antibodies develop that recognize both the modified human FVIII and the native human FVIII.
Methods
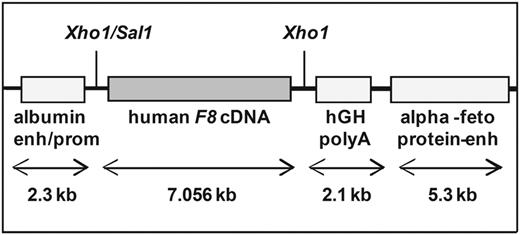
Human F8 transgenic mice
Hemophilic mice that express a transgene of a native human F8 cDNA were generated by random integration of the transgene, as described previously.13,14 Briefly, we used a liver-specific expression vector,23 which was a generous gift from G. Schuetz (German Cancer Research Center, Heidelberg, Germany), to direct F8 expression to the liver. The vector contains an albumin promoter, a poly A signal derived from the human growth hormone, and α-fetoprotein enhancer elements (Figure 1). An Xhol restriction site between the albumin promoter and the poly A tail was used for insertion of the human F8 cDNA, as described previously.13 The vector was linearized and microinjected into the male pronucleus of fertilized oocytes obtained from mated female C57BL/6J mice after superovulation. Founder mice carrying the human F8 cDNA transgene were crossed with hemophilic E17 mice (characterized by a targeted disruption of exon 17 of the murine F8 gene24 ) and bred to homozygosity for the murine hemophilic E17 genotype and the human F8 transgene.
Structure of the linearized human F8 cDNA construct. The human F8 cDNA was expressed under the control of the mouse albumin promoter and enhancer and the mouse α-fetoprotein enhancer. A 2.1-kb fragment of the human growth hormone gene (hGH) was cloned downstream of the human F8 cDNA to ensure correct polyadenylation of the human F8 mRNA.
Structure of the linearized human F8 cDNA construct. The human F8 cDNA was expressed under the control of the mouse albumin promoter and enhancer and the mouse α-fetoprotein enhancer. A 2.1-kb fragment of the human growth hormone gene (hGH) was cloned downstream of the human F8 cDNA to ensure correct polyadenylation of the human F8 mRNA.
Confirmation of complete transgene integration
Correct and complete integration of the human F8 cDNA into the mouse genome was confirmed by PCR. For this purpose, genomic DNA was isolated using the nexttec DNA isolation kit (Genomic DNA Isolation Kit; Biotechnologie). The following 9 primer pairs were designed, which produce overlapping F8 DNA products within the human F8 cDNA: pair (P) 1 primer forward (P1for), 5′-TTCTTTCTGTGCCTTTTGCGATTC-3′; P1 primer reverse (P1rev), 5′-TCATTCGTAGTTGGGGTTCCTCTG-3′; P2for, 5′-CAGGCGTCCTTGGAAATCTCGC-3′; P2rev, 5′-TGTGAGGTACCAGCTTCGGTTC-3′; P3for, 5′-CAGTGACTGTAG-AAGATGGGCCAAC-3′; P3rev, 5′-TACCTGCTGCCAAATTGTCTGATG-3′; P4for, 5′-TCCATCAGACAATTTGGCAGCAG-3′; P4rev, 5′-CTTGCCCAGAGTTCAGAGAGTTCT-3′; P5for, 5′-GCCAGAATCAGCAAGGTGGAT-3′; P5rev, 5′-TTGTGGTGCATGCATATTTCTCTAC-3; P6for, 5′-TAGAGAAATATGCATGCACCACAAG-3′; P6rev, 5′-CACAAGCGTTCAGGGACAAAATG-3′; P7for, 5′-TGTTCTCCCGAAACCAGACTTGC-3′; P7rev, 5′-TTCTGCTCCTTGCCTCTGATCTTC-3′; P8for, 5′-AGGAAGATCAGAGGCAAGGAGCAG-3; P8rev, 5′-TCTCTAATGTG-TCCAGAAGCCATTC-3′; P9for, 5′-GGAATGGCTTCTGGACACATTAGAG-3′; and P9rev, 5′-GAACCTCCATCCTCAGGGCAATC-3′. To prove complete integration of the human F8 cDNA into the mouse genome, additional primer pairs were designed to cover the regions flanking the human F8 cDNA: primer pair A (PA, including FVIII ATG) PAfor, 5′-CCAGATGGCAAACATACGCAAG-3′; PArev, 5′- CAGGCAGCTCACCGAGATCAC-3′; primer pair B (PB, including FVIII TGA) PBfor, 5′-CGTTACTGACTCGCTACCTTCGA-3′; and PBrev, 5′-ACTCTCCCTGCTCCAGGAGC-3′. The accuracy of the PCR products was confirmed by sequencing using an ABI Prism 3100 capillary sequencer (Applied Biosystems) and alignment using Vector NTI Advance 11.0.
Finally, integration of F8 cDNA in full-length was confirmed by long-range PCR (QIAGEN) using the following primer pair: primer forward (Pfor), 5′- GTCATGGGACTATATGCAAAGTGATCTCG-3′, and primer reverse (Prev), 5′- AGAACCTCCATCCTCAGGGCAATCT-3′. The PCR product was partially sequenced to confirm specificity. The consensus of the ∼ 2000 bases that were sequenced was > 99%.
Analysis of F8 gene expression
F8 mRNA levels of various organs were analyzed by a 2-step RT-PCR. RNA was isolated from organ samples using RNeasy Mini Kit (QIAGEN) and then reverse transcribed into cDNA using QuantiTect Reverse Transcription (QIAGEN). This procedure included a DNA wipeout step. TaqMan real-time quantitative PCR for human F8 cDNA was done using TaqMan GeneExpressionMasterMix (Applied Biosystems) and a specific human F8 primer pair and probe: forward primer,13 5′-GGCACTGTACAATCTCTATCCAGGT-3′; reverse primer,13 5′-GATGCTCGCCAATAAGGCAT-3 and the F8-specific dual-labeled Black Hole Quencher probe,13 FAM-5′-CCAAAGCTGGAATTTGGCGGGTG-3′-BHQ. Murine F8 cDNA was analyzed using a set of primers from Applied Biosystems that covers the murine F8 13-14 exon boundary. In addition, as a normalization control, the expression of murine β-actin was determined in all samples using the TaqMan mouse ACTB (actin, beta) endogenous control (VIC/MGB Probe, Primer Limited) from Applied Biosystems.
The expression of full-length human F8 mRNA in liver samples was analyzed using the 9 primer pairs described in “Confirmation of complete transgene integration.”
FISH analysis of the integration sides of human F8 cDNA
FISH analysis was done as described previously.25,26 Briefly, spleen cells obtained from human F8 transgenic mice were stimulated with concanavalin A to induce cell division. Chromosomes were prepared and stained with 4′,6-diamidino-2-phenylindole (DAPI). DAPI-stained metaphases were imaged before hybridization. The human F8-specific probe for hybridization was generated using the 9 human F8-specific primer pairs described in “Confirmation of complete transgene integration.” The probe was labeled with dioxigenin-dUTP and biotin-dUTP. DAPI-stained chromosomes and hybridization signals were analyzed by fluorescence microscopy. Chromosome and FISH signals were displayed in false colors and images were merged on the computer.
Treatment of mice with human FVIII and human VWF
All mouse studies were approved by the local authority in Vienna, Austria in accordance with Austrian federal law (Act BG 501/1989) regulating animal experiments and were approved by the institutional animal care and use committee.
Male and female mice 8-17 weeks of age were treated intravenously with up to 9 weekly doses of 0.2 μg of recombinant human FVIII (∼ 80 IU/kg) or 4 weekly doses of 5 μg of recombinant human VWF (all from Baxter BioScience).
In some experiments, mice were treated with PEG-modified FVIII preparations (mFVIII1 and mFVIII2) from Baxter BioScience. mFVIII1 and mFVIII2 are full-length recombinant FVIII preparations that were chemically modified by coupling 20-kDa PEG reagents using preferential amine coupling to lysine residues at neutral pH, as described by Zaplinski et al.27 The PEG reagents used to prepare mFVIII1 and mFVIII2 were described previously.28-30 mFVIII1 and mFVIII2 mainly differ in their degree of modification: mFVIII1 contains ∼ 2 mol of PEG/mol FVIII and mFVIII2 contains 12 mol of PEG/mol FVIII. Because of the higher degree of modification, mFVIII2 shows reduced procoagulant activity and reduced binding to VWF (Table 1). Analysis of aggregates in native and modified human FVIII by asymmetrical flow field-flow fractionation31 and high-performance size-exclusion chromatography using an Agilent SEC-5 500 Å column (Agilent Technologies) with fluorescence detection (excitation at 280 nm and emission at 340 nm) did not reveal any relevant formation of FVIII protein aggregates in any of the preparations.
Comparison of mFVIII1 and mFVIII2 with respect to PEGylation degree, specific activity, and VWF binding
| . | PEGylation degree, mol PEG/mol FVIII . | Specific activity, FVIII:C/mg protein . | VWF binding, KD, nM . |
|---|---|---|---|
| mFVIII1 | 2 | 5773 | 0.82 |
| mFVIII2 | 12 | 153 | 7.23 |
| . | PEGylation degree, mol PEG/mol FVIII . | Specific activity, FVIII:C/mg protein . | VWF binding, KD, nM . |
|---|---|---|---|
| mFVIII1 | 2 | 5773 | 0.82 |
| mFVIII2 | 12 | 153 | 7.23 |
Blood samples were taken before the first treatment, 1 week after the fourth treatment, and 1 week after the last treatment. Blood samples were added at a 4:1 (vol/vol) ratio to 0.1M sodium citrate and plasma was separated by centrifugation. Plasma samples were stored at −20°C until further analysis. Samples used for the analysis of FVIII antigen or FVIII activity were kept at −80°C.
Analysis of antibodies and antibody-secreting cells
Binding antibodies against human FVIII and human VWF were measured by ELISA as described previously.32 ELISA plates were coated with 1 μg/mL of one of the following proteins: recombinant human FVIII, PEGylated human FVIII (mFVIII1 or mFVIII2), or recombinant human VWF.
Inhibitory antibodies against FVIII were analyzed by Bethesda assay.33
Antibody-secreting cells (ASCs) were detected by enzyme-linked immunospot (ELISPOT) assays as described previously.34 ELISPOT plates were coated with 10 μg/mL of either recombinant human FVIII or recombinant human VWF.
Restimulation of FVIII-specific T cells in vitro
Spleens were obtained 7 days after the last dose of FVIII and spleen cell suspensions were prepared and restimulated as described previously.34 Cytokines released into cell culture supernatants were analyzed using a multiplex bead-based assay (Bio-Plex) from Bio-Rad Laboratories. Proliferation of FVIII-specific T cells was analyzed as described previously.34
Analysis of FVIII antigen, FVIII activity, and binding of FVIII to VWF
Human FVIII antigen in mouse plasma was measured using the human VIIIC:Ag ELISA kit (Diagnostica Stago). FVIII activity in mouse plasma and the potencies of mFVIII1 and mFVIII2 were analyzed using the chromogenic activity assay Immunochrom FVIII (Baxter Diagnostics). The specific activity was determined by calculating the potency of mFVIII1 and mFVIII2 per milligram of protein. Protein was quantified by conventional protein determination using the Bradford method.
Binding of FVIII to VWF was analyzed by surface plasmon resonance technology using a Biacore 3000 system. Briefly, a plasma-derived VWF (Diagnostica Stago) was immobilized on the flow cells of a CM5 biosensor chip. The samples to be analyzed were diluted to 20nM FVIII with a 10mM HEPES buffer containing 150mM NaCl and 0.005% surfactant P20, pH 7.4, according to their protein content calculated with a molecular mass of 280 kDa. Diluted samples were then applied to the chip using the “kinject” mode with a constant flow rate of 5 μL/min. Time for association was 10 minutes and time for dissociation was 60 minutes.
Determination of blood loss after tail cut
Blood loss after tail cut was determined using the tail-cut model.35 At the end of the observation period of 60 minutes, mice were killed by cervical dislocation before recovery from anesthesia.
Statistics
Group statistics were performed by 1-way ANOVA with the Dunnett posttest using Prism 5 software (GraphPad). P < .05 was considered statistically significant.
Results
Generation and selection of a human F8 transgenic mouse line that does not develop antibodies against native human FVIII
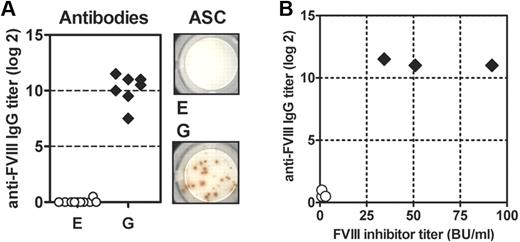
Previously, we reported the generation of new transgenic mouse lines that express a human full-length F8 cDNA as a transgene on the hemophilic E17 background.13,14 Initially, we obtained 7 transgenic lines. To select a line that does not develop antibodies after repeated intravenous application of therapeutic doses of human native FVIII, we treated all sublines with 8 IV doses of human FVIII at weekly intervals, and analyzed antibody responses against human FVIII. We identified one subline that did not develop antibodies under these conditions. The remaining 6 sublines developed different levels of antibody responses ranging from low-titer to high-titer antibodies. Examples are shown in Figure 2A and B, illustrating results obtained with subline E (no antibody response) and subline G (highest levels of antibody response).
Transgenic mice of sublines E and G differ in their antibody responses to human FVIII. Mice were treated with 8 weekly intravenous doses of human FVIII. Blood for antibody analysis and spleens for the analysis of antibody-secreting cells (ASC) were collected 1 week after the last dose. (A) Titers of anti–human FVIII IgG antibodies were detected by ELISA. Antibody titers were shown for individual mice. Anti-FVIII antibody-secreting cells (ASC) were analyzed by ELISPOT. Results obtained from a representative mouse are shown. Each spot corresponds to 1 ASC. (B) Plasma samples from 3 mice of each subline were analyzed for both titers of anti–human FVIII IgG antibodies by ELISA and FVIII inhibitor titers by Bethesda assay.
Transgenic mice of sublines E and G differ in their antibody responses to human FVIII. Mice were treated with 8 weekly intravenous doses of human FVIII. Blood for antibody analysis and spleens for the analysis of antibody-secreting cells (ASC) were collected 1 week after the last dose. (A) Titers of anti–human FVIII IgG antibodies were detected by ELISA. Antibody titers were shown for individual mice. Anti-FVIII antibody-secreting cells (ASC) were analyzed by ELISPOT. Results obtained from a representative mouse are shown. Each spot corresponds to 1 ASC. (B) Plasma samples from 3 mice of each subline were analyzed for both titers of anti–human FVIII IgG antibodies by ELISA and FVIII inhibitor titers by Bethesda assay.
The question arose as to why different transgenic sublines would respond differently to challenges with human FVIII. We chose the 2 sublines presented in Figure 2A and B and investigated whether differences in integration sites of the human F8 cDNA, incomplete integration of the human F8 cDNA, and/or differences in transcription of the human F8 cDNA could explain the differences in the phenotype that we observed.
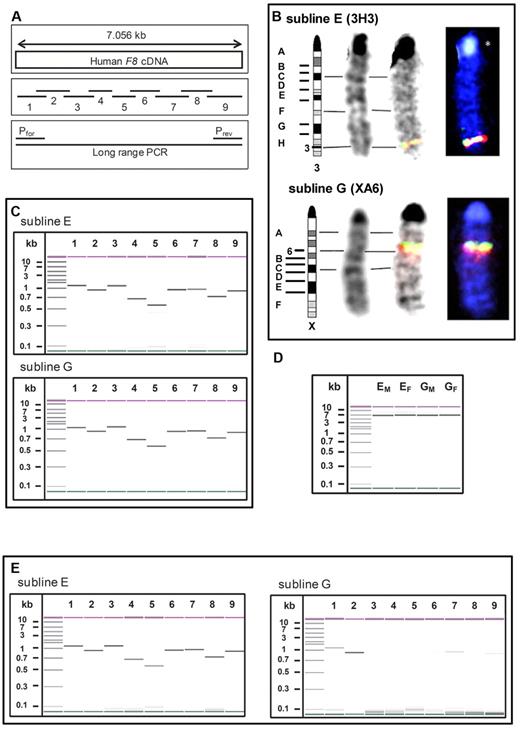
Using FISH analysis, we specified the integration sites of the human F8 cDNA in sublines E and G. Whereas subline E has the transgene integrated into chromosome 3H3, subline G has it integrated into chromosome XA6 (Figure 3B). We only identified one integration site for each subline.
Differences in integration sites and transcription of human F8 cDNA between sublines E and G. (A) Schematic pictures of 9 overlapping primer pairs spanning the whole human F8 cDNA and of a primer pair designed for long-range PCR. (B) Identification of the integration sites of human F8 cDNA by FISH analysis: chromosomes for FISH analysis were prepared from spleen cells activated with concanavalin A. F8-specific probes were prepared using the 9 overlapping primer pairs shown in panel A and labeled with 2 different fluorochromes: dioxigenin-dUTP and biotin-dUTP. The hybridization signals of the F8-specific probes and the corresponding chromosome mapping for samples obtained from mice of sublines E and G are demonstrated. (C) Detection and sizing of all PCR products obtained from genomic DNA of sublines E and G with the 9 overlapping primer pairs shown in panel A using an Agilent 2100 Bioanalyzer. The molecular size is indicated in the first lane in kilobases. (D) Confirmation of the integration of the full-length human F8 cDNA into the genome of mice of sublines E and G (EM is a male mouse from subline E; EF is a female mouse from subline E; GM is a male mouse from subline G; and GF is a female mouse from subline G) by long-range PCR on genomic DNA using an Agilent 2100 Bioanalyzer. The molecular size is indicated in the first lane in kilobases. (E) Detection and sizing of all PCR products obtained from RNA after reverse transcription into cDNA using an Agilent 2100 Bioanalyzer. The molecular size is indicated in the first lane in kilobases. RNA was prepared from liver samples obtained from mice of sublines E and G.
Differences in integration sites and transcription of human F8 cDNA between sublines E and G. (A) Schematic pictures of 9 overlapping primer pairs spanning the whole human F8 cDNA and of a primer pair designed for long-range PCR. (B) Identification of the integration sites of human F8 cDNA by FISH analysis: chromosomes for FISH analysis were prepared from spleen cells activated with concanavalin A. F8-specific probes were prepared using the 9 overlapping primer pairs shown in panel A and labeled with 2 different fluorochromes: dioxigenin-dUTP and biotin-dUTP. The hybridization signals of the F8-specific probes and the corresponding chromosome mapping for samples obtained from mice of sublines E and G are demonstrated. (C) Detection and sizing of all PCR products obtained from genomic DNA of sublines E and G with the 9 overlapping primer pairs shown in panel A using an Agilent 2100 Bioanalyzer. The molecular size is indicated in the first lane in kilobases. (D) Confirmation of the integration of the full-length human F8 cDNA into the genome of mice of sublines E and G (EM is a male mouse from subline E; EF is a female mouse from subline E; GM is a male mouse from subline G; and GF is a female mouse from subline G) by long-range PCR on genomic DNA using an Agilent 2100 Bioanalyzer. The molecular size is indicated in the first lane in kilobases. (E) Detection and sizing of all PCR products obtained from RNA after reverse transcription into cDNA using an Agilent 2100 Bioanalyzer. The molecular size is indicated in the first lane in kilobases. RNA was prepared from liver samples obtained from mice of sublines E and G.
We then investigated whether the human F8 cDNA is completely integrated into the genomes of sublines E and G using 2 different approaches. First, we designed 9 overlapping primer pairs covering the whole F8 cDNA (Figure 3A) and ran PCRs. Results indicated that both sublines have the complete human F8 cDNA integrated into their genome (Figure 3C). To further confirm complete integration, additional primer pairs were designed to cover the regions flanking the human F8 cDNA. The accuracy of the PCR products was confirmed by sequencing.
Our second approach was a long-range PCR that amplified the entire humane F8 cDNA (Figure 3A). The results proved that the complete human F8 cDNA is integrated into the mouse genome of both subline E and G (Figure 3D). Sequencing of ∼ 2000 bases at both the 3′and 5′ ends of the PCR products revealed a homology of > 99% with the original human F8 cDNA for both sublines.
Based on these data, we conclude that the observed differences in the phenotype of sublines E and G were not caused by incomplete integration of the human F8 cDNA into the genome of one of the sublines.
We then investigated whether incomplete transcription of the human F8 cDNA might be associated with differences in the phenotype of sublines E and G. We prepared RNA from the liver, reversely transcribed it into cDNA, and ran PCRs using the 9 overlapping primer pairs that covered the whole human F8 cDNA (Figure 3A). Results obtained with subline E indicated that the complete human F8 cDNA was transcribed into mRNA. However, results obtained with subline G suggested that transcription of F8 cDNA into F8 mRNA was very weak and only partially detectable (Figure 3E).
Based on these results, we selected subline E for all subsequent studies.
Lack of antibody development against FVIII in subline E is caused by specific immune tolerance
To exclude the possibility that the human F8 cDNA transgene resulted in some form of general immune suppression, we investigated whether mice of subline E could still respond to an unrelated human protein. We treated mice with 8 weekly IV doses of native human FVIII and subsequently with 4 weekly IV doses of human VWF, which was shown to induce high titer antibodies in hemophilic E17 mice.36 We included conventional hemophilic E17 mice as controls.
All conventional hemophilic E17 mice developed high-titer anti-FVIII antibodies that were correlated with a high frequency of anti-FVIII ASCs in the spleen (Figure 4A). In contrast, human F8 transgenic mice did not develop detectable antibodies in the circulation, which correlated with the absence of anti-FVIII ASCs in the spleen (Figure 4A). However, both conventional E17 mice and human F8 transgenic mice developed high-titer anti-VWF antibodies in the circulation and high frequencies of anti-VWF ASCs in the spleen after treatment with human VWF (Figure 4B).
Lack of antibody development against human FVIII in mice of subline E is caused by specific immune tolerance. Mice were treated with 8 weekly IV doses of human FVIII (A), followed by 4 weekly IV doses of human VWF (B). Blood for antibody analysis was drawn 1 week after the fourth (4xFVIII, A) and the eighth (8xFVIII, A) dose of FVIII, as well as 1 week after the fourth dose of VWF (4xVWF, B). Spleens for the analysis of ASCs were collected from 3 mice 1 week after the eighth dose of FVIII (A) and from the remaining mice 1 week after the fourth dose of VWF (B). Titers of anti–human FVIII IgG antibodies (A) and anti–human VWF IgG antibodies (B) were detected by ELISA. Antibody titers are shown for individual mice as follows: human F8 transgenic mice, male (▴) and female (●); conventional E17 F8-knockout mice: male (♢). Anti–human FVIII ASCs (A) and anti–human VWF ASCs (B) in the spleens of human F8 transgenic mice (huF8) and conventional E17 F8-knockout mice (E17F8ko) were analyzed by ELISPOT. Results obtained from a representative animal are shown. Each spot represents 1 ASC.
Lack of antibody development against human FVIII in mice of subline E is caused by specific immune tolerance. Mice were treated with 8 weekly IV doses of human FVIII (A), followed by 4 weekly IV doses of human VWF (B). Blood for antibody analysis was drawn 1 week after the fourth (4xFVIII, A) and the eighth (8xFVIII, A) dose of FVIII, as well as 1 week after the fourth dose of VWF (4xVWF, B). Spleens for the analysis of ASCs were collected from 3 mice 1 week after the eighth dose of FVIII (A) and from the remaining mice 1 week after the fourth dose of VWF (B). Titers of anti–human FVIII IgG antibodies (A) and anti–human VWF IgG antibodies (B) were detected by ELISA. Antibody titers are shown for individual mice as follows: human F8 transgenic mice, male (▴) and female (●); conventional E17 F8-knockout mice: male (♢). Anti–human FVIII ASCs (A) and anti–human VWF ASCs (B) in the spleens of human F8 transgenic mice (huF8) and conventional E17 F8-knockout mice (E17F8ko) were analyzed by ELISPOT. Results obtained from a representative animal are shown. Each spot represents 1 ASC.
Based on these data, we conclude that transgenic mice of subline E express specific immune tolerance toward native human FVIII.
Mice of subline E express human F8 mRNA in a range of different organs but do not express FVIII protein in the circulation
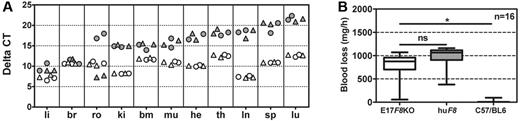
We then assessed the transcription of human F8 cDNA in various murine tissues, including liver, brain, reproductive organs, kidneys, bone marrow, muscle, heart, thymus, lymph nodes, spleens, and lungs, and compared the expression of the human F8 cDNA with the expression of the murine F8 gene in the same organs in conventional C57BL/6 mice. Expression of both the human F8 cDNA and the murine F8 gene was normalized to the expression of the murine β-actin gene. Because we observed differences in the transcription of the murine β-actin gene between different organs, we compared levels of F8 mRNA only within the same organ. Human F8 mRNA was detected in all tissues studied, predominantly in the liver (Figure 5A). In comparison, expression of the murine F8 gene was also found in all tissues obtained from conventional C57BL/6 mice (Figure 5A). Analysis of liver and brain revealed similar expression levels for the human F8 cDNA in transgenic mice and the murine F8 gene in C57BL/6 mice. All other organs studied showed higher expression levels of the murine F8 gene in C57BL/6 mice compared with the expression levels of the human F8 cDNA in transgenic mice of subline E.
Transgenic mice of subline E express human F8 mRNA in a variety of different organs but express a hemophilic phenotype. (A) Expression of F8 mRNA in transgenic male (▴) and female (●) mice compared with the expression of murine F8 mRNA in wild-type C57BL/6 male (▵) and female mice (○). Total RNA was isolated from liver (li), brain (br), reproductive organs (ro), kidneys (ki), bone marrow (bm), muscle (mu), heart (he), thymus (th), lymph nodes (ln), spleen (sp), and lungs (lu) and reverse transcribed into cDNA. Expression of human and murine F8 mRNA was analyzed in relation to murine β-actin by TaqMan real-time quantitative PCR. Each data point represents results obtained from an individual mouse. (B) Blood loss after tail cut was determined over a period of 60 minutes using the tail-cut model.35 Presented are box plots representing the results obtained with groups of 16 mice. Compared are transgenic mice expressing the human F8 cDNA (huF8), conventional hemophilic E17 F8-knockout mice (E17 F8 ko), and wild-type mice (C57BL/6). ns indicates that the difference was not significant. *P < .001.
Transgenic mice of subline E express human F8 mRNA in a variety of different organs but express a hemophilic phenotype. (A) Expression of F8 mRNA in transgenic male (▴) and female (●) mice compared with the expression of murine F8 mRNA in wild-type C57BL/6 male (▵) and female mice (○). Total RNA was isolated from liver (li), brain (br), reproductive organs (ro), kidneys (ki), bone marrow (bm), muscle (mu), heart (he), thymus (th), lymph nodes (ln), spleen (sp), and lungs (lu) and reverse transcribed into cDNA. Expression of human and murine F8 mRNA was analyzed in relation to murine β-actin by TaqMan real-time quantitative PCR. Each data point represents results obtained from an individual mouse. (B) Blood loss after tail cut was determined over a period of 60 minutes using the tail-cut model.35 Presented are box plots representing the results obtained with groups of 16 mice. Compared are transgenic mice expressing the human F8 cDNA (huF8), conventional hemophilic E17 F8-knockout mice (E17 F8 ko), and wild-type mice (C57BL/6). ns indicates that the difference was not significant. *P < .001.
Based on these data, we investigated whether the expression of human F8 mRNA results in the expression of human FVIII protein in the circulation of transgenic mice. We analyzed plasma samples for human FVIII antigen and human FVIII activity. The levels of human FVIII were below the detection limits for both assays (below 0.005 IU/mL and 0.006 IU/mL, respectively) indicating a lack of circulating FVIII protein.
We next investigated whether the lack of circulating human FVIII protein would correspond to a hemophilic phenotype in transgenic mice. We used the tail-cut model35 and determined the resulting amount of blood loss. Both conventional hemophilic E17 mice and human F8 transgenic mice of subline E expressed a hemophilic phenotype. In contrast, normal wild-type C57BL/6 mice expressed normal hemostasis (Figure 5B).
We conclude that transgenic mice of subline E express human F8 mRNA in a variety of different organs but do not express detectable levels of human FVIII protein in the circulation. This lack of circulating human FVIII protein corresponds to a hemophilic phenotype.
Mice of subline E develop antibodies against FVIII when treated with a modified FVIII that possesses increased immunogenicity
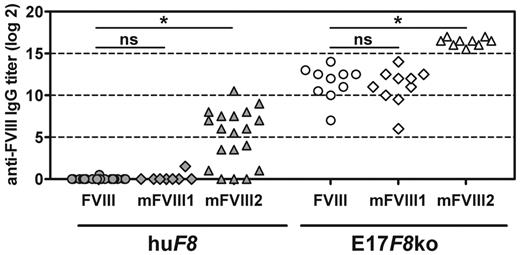
After demonstrating specific immune tolerance to native human FVIII, we investigated whether transgenic mice of subline E would still be able to develop antibodies against human FVIII when treated with a modified human FVIII protein that exhibits increased immunogenicity. We studied 2 PEG-FVIII proteins that contained different amounts of PEG residues (mFVIII1 and mFVIII2). From earlier studies, we already knew that mFVIII2 expresses increased immunogenicity compared with native human FVIII in conventional hemophilic E17 mice. We treated mice with 8 IV doses of either human native FVIII or one of the PEGylated FVIII preparations, and analyzed antibodies against FVIII after 4 and 8 doses. We included conventional hemophilic E17 mice as controls. Conventional hemophilic mice developed anti-FVIII antibodies after treatment with each of the 3 FVIII preparations tested (Figure 6). Native human FVIII and mFVIII1 induced similar titers of anti-FVIII antibodies, whereas mFVIII2 induced significantly higher titers of anti-FVIII antibodies, confirming our earlier observation. Anti-FVIII antibodies induced by mFVIII1 and mFVIII2 recognized both native human FVIII and the PEGylated FVIII that was used for treatment. Titers measured against native human FVIII and against the respective PEGylated FVIII were comparable. For simplicity, we only show antibody titers against native human FVIII in Figure 6.
Transgenic mice of subline E develop antibodies against human FVIII when treated with a modified human FVIII with high immunogenic potential. Transgenic mice (huF8) and conventional hemophilic E17 F8-knockout mice (E17F8 ko) were treated with 8 doses of either native human FVIII (FVIII) or 1 of 2 PEGylated human FVIII proteins (mFVIII1 and mFVIII2). Titers of antibodies against native human FVIII were determined by ELISA 1 week after the eighth dose. Results for individual mice are shown. ns indicates that the difference was not significant. *P < .001.
Transgenic mice of subline E develop antibodies against human FVIII when treated with a modified human FVIII with high immunogenic potential. Transgenic mice (huF8) and conventional hemophilic E17 F8-knockout mice (E17F8 ko) were treated with 8 doses of either native human FVIII (FVIII) or 1 of 2 PEGylated human FVIII proteins (mFVIII1 and mFVIII2). Titers of antibodies against native human FVIII were determined by ELISA 1 week after the eighth dose. Results for individual mice are shown. ns indicates that the difference was not significant. *P < .001.
In contrast to conventional hemophilic E17 mice, human F8 transgenic mice of subline E did not develop antibodies against human FVIII when treated with either native human FVIII or mFVIII1 (Figure 6). However, transgenic mice treated with mFVIII2 developed antibodies against human FVIII that recognized both native human FVIII (Figure 6) and mFVIII2 (results not shown). These findings indicate a break of immune tolerance in mice of subline E by mFVIII2.
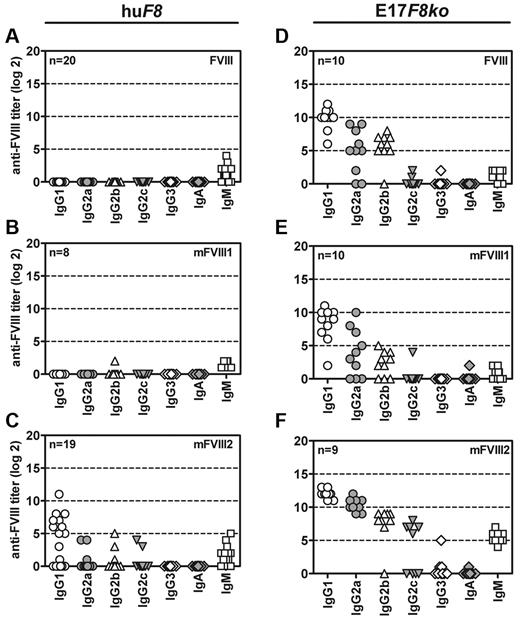
We then investigated whether the isotype and subclass distribution of anti-FVIII antibodies seen in transgenic mice after break of immune tolerance by mFVIII2 would be similar or different to the isotype and subclass distribution of anti-FVIII antibodies induced by native human FVIII, mFVIII1, or mFVIII2 in conventional hemophilic E17 mice. Results presented in Figure 7 indicate that the isotype and subclass distribution of anti-FVIII antibodies induced in conventional hemophilic E17 mice were similar for FVIII, mFVIII1, and mFVIII2 (Figure 7D-F). All antibody titers in conventional hemophilic E17 mice revealed a clear ranking of IgG1 → IgG2a → IgG2b → IgG2c (Figure 7D-F). However, the subclass distribution after break of immune tolerance induced by mFVIII2 in human F8 transgenic mice was different (Figure 7C). The major IgG subclass was IgG1, whereas there was no difference in titers between IgG2a, IgG2b, and IgG2c.
Break of immune tolerance against human FVIII in mice of subline E is not isotypically restricted. Transgenic mice expressing human F8 cDNA (huF8, A-C) and conventional hemophilic E17 F8-knockout mice (E17F8ko, D-F) were treated weekly with 9 doses of either native human FVIII (FVIII, A and D) or 1 of 2 PEGylated human FVIII proteins (mFVIII1, B and E; mFVIII2, C and F). Subclasses of IgG, IgA, and IgM antibodies specific for native human FVIII were analyzed by ELISA 1 week after the last treatment. Results of individual mice are shown.
Break of immune tolerance against human FVIII in mice of subline E is not isotypically restricted. Transgenic mice expressing human F8 cDNA (huF8, A-C) and conventional hemophilic E17 F8-knockout mice (E17F8ko, D-F) were treated weekly with 9 doses of either native human FVIII (FVIII, A and D) or 1 of 2 PEGylated human FVIII proteins (mFVIII1, B and E; mFVIII2, C and F). Subclasses of IgG, IgA, and IgM antibodies specific for native human FVIII were analyzed by ELISA 1 week after the last treatment. Results of individual mice are shown.
Both human F8 transgenic mice and conventional hemophilic E17 mice developed similar levels of low-titer IgM antibodies against FVIII when treated with human native FVIII or mFVIII1, which might indicate a T cell–independent, low-titer, low-affinity antibody response (Figure 7A-F).
We also investigated whether break of immune tolerance by mFVIII2 in human F8 transgenic mice is associated with an altered pattern of cytokines released into cell-culture supernatants or with an increased proliferation after in vitro restimulation of FVIIII-specific T cells. For this purpose, we isolated spleen cells from mice treated with the different FVIII preparations and restimulated them in vitro for up to 6 days with either native human FVIII or the modified FVIII preparation that was used for treating the mice. We did not observe significant differences in cytokines (IL-4, IL-5, IL-6, IL-10, IL-17, and IFN-γ) released into culture supernatants from spleen cells obtained from human F8 transgenic mice treated with native human FVIII, mFVIII1, or mFVIII2. A similar lack of differences was seen when spleen cell proliferation was compared between the groups (Table 2). The situation was different in conventional hemophilic E17 mice, in which the results revealed a significant increase in proliferation when spleen cells obtained from mice treated with mFVIII2 were in vitro restimulated with native human FVIII (Table 2).
Incorporation of 3H-thymidin in spleen cells restimulated for 3 days with human FVIII in vitro
| Mouse strain . | Treatment . | FVIII in culture, μg/mL . | 3H incorporation, cpm . | Stimulation index . | |
|---|---|---|---|---|---|
| mean (n = 3) . | SD . | ||||
| E17F8ko | FVIII | 0 | 5.020 | 460 | 1 |
| 1 | 8.586 | 2.075 | 1.70 | ||
| 10 | 9.252 | 2.333 | 1.84 | ||
| E17F8ko | mFVIII1 | 0 | 4.758 | 401 | 1 |
| 1 | 6.272 | 2.210 | 1.32 | ||
| 10 | 5.518 | 999 | 1.16 | ||
| E17F8ko | mFVIII2 | 0 | 5.666 | 939 | 1 |
| 1 | 34.257 | 2.788 | 6.05* | ||
| 10 | 31.402 | 2.170 | 5.54* | ||
| huF8 | FVIII | 0 | 3.647 | 671 | 1 |
| 1 | 4.002 | 452 | 1.10 | ||
| 10 | 4.297 | 1.721 | 1.18 | ||
| huF8 | mFVIII1 | 0 | 5.041 | 505 | 1 |
| 1 | 6.215 | 502 | 1.23 | ||
| 10 | 4.714 | 530 | 0.94 | ||
| huF8 | mFVIII2 | 0 | 4.757 | 268 | 1 |
| 1 | 4.723 | 466 | 0.99 | ||
| 10 | 5.767 | 1.167 | 1.21 | ||
| Mouse strain . | Treatment . | FVIII in culture, μg/mL . | 3H incorporation, cpm . | Stimulation index . | |
|---|---|---|---|---|---|
| mean (n = 3) . | SD . | ||||
| E17F8ko | FVIII | 0 | 5.020 | 460 | 1 |
| 1 | 8.586 | 2.075 | 1.70 | ||
| 10 | 9.252 | 2.333 | 1.84 | ||
| E17F8ko | mFVIII1 | 0 | 4.758 | 401 | 1 |
| 1 | 6.272 | 2.210 | 1.32 | ||
| 10 | 5.518 | 999 | 1.16 | ||
| E17F8ko | mFVIII2 | 0 | 5.666 | 939 | 1 |
| 1 | 34.257 | 2.788 | 6.05* | ||
| 10 | 31.402 | 2.170 | 5.54* | ||
| huF8 | FVIII | 0 | 3.647 | 671 | 1 |
| 1 | 4.002 | 452 | 1.10 | ||
| 10 | 4.297 | 1.721 | 1.18 | ||
| huF8 | mFVIII1 | 0 | 5.041 | 505 | 1 |
| 1 | 6.215 | 502 | 1.23 | ||
| 10 | 4.714 | 530 | 0.94 | ||
| huF8 | mFVIII2 | 0 | 4.757 | 268 | 1 |
| 1 | 4.723 | 466 | 0.99 | ||
| 10 | 5.767 | 1.167 | 1.21 | ||
Spleen cells were obtained from conventional hemophilic E17 F8-knockout mice or human F8-transgenic mice at 1 week after the last dose of 9 weekly treatments with either human FVIII or 1 of 2 PEGylated FVIII preparations (mFVIII1 and mFVIII2).
P < .01.
Summarizing our data, we conclude that human F8 transgenic mice that are immunologically tolerant to native human FVIII are still able to develop antibodies against human FVIII when treated with a modified FVIII protein that exhibits increased immunogenicity. The break of immune tolerance involves the development of different IgG subclasses with a dominance of IgG1.
Discussion
Previously, we reported the development of new transgenic mouse lines that express a human F8 cDNA as a transgene.13,14 In the present study, we describe the selection of one particular transgenic mouse line that does not develop antibodies against native human FVIII given IV in therapeutic doses, but is still able to respond to human FVIII when treated with a modified FVIII preparation expressing increased immunogenicity. The lack of antibody response against native human FVIII was caused by specific immune tolerance and was not the result of a more general immune suppression. The development of specific immune tolerance is most likely caused by the deletion of high-avidity human FVIII-reactive CD4+ T cells during embryonic development, when the T-cell repertoire in the thymus is shaped and central immune tolerance is established.15,16 In addition, peripheral tolerance mechanisms such as anergy, clonal deletion, and active suppression by regulatory T cells37,38 could contribute to the immune tolerance. Both pathways of tolerance induction are initiated by the presentation of peptides through MHC class II expressed on antigen-presenting cells that requires intracellular protein or peptides. Therefore, it seems clear that human F8 mRNA is translated into FVIII protein, even though human FVIII protein cannot be detected in the circulation of transgenic mice. The question arises as to why human FVIII protein is present intracellularly but is not found in the circulation. We were able to show that human F8 cDNA is completely integrated into the mouse genome and is completely transcribed into F8 mRNA. However, the integration site of the human F8 cDNA is different from the location of the murine F8 gene, which could influence the function of the transgene and might suppress its effective transcription and translation.39 Furthermore, we used a cDNA for the construction of the transgene because the genomic sequence of the human F8 locus is very large and difficult to handle. The artificial structure of the cDNA construct could have a strong impact on its function. The loss of introns in the transgenic sequence can result in disturbances of the nucleosome alignment, which is important for the transcription of the transgene. Therefore, the expression of cDNA constructs is usually rather low.40-42 Although we observed levels of human F8 mRNA in the liver that were similar to levels of murine F8 mRNA in the livers of wild-type mice, these levels might not be sufficient to produce enough human protein to achieve detectable levels in the circulation. Furthermore, it is most likely that the human FVIII protein is mainly expressed in hepatocytes because its expression is driven by a murine albumin promoter. Hepatocytes do not produce VWF, which could facilitate a rapid degradation of FVIII after the secretion or the clearance of FVIII by clearance receptors such as members of the low-density lipoprotein receptor family.43 It has been shown previously that FVIII requires stabilization by VWF after secretion.44,45 Successful gene therapy approaches using viral vectors containing a full-length human F8 cDNA driven by an albumin promoter to achieve detectable levels of circulating human FVIII protein in hemophilic mice were described previously.46 However, stable integration of human F8 cDNA into the mouse genome cannot be compared with gene-therapy approaches that achieve reasonable levels of circulating human FVIII. Stably integrated transgenes are transmitted to the next generation via the germline. This process includes DNA demethylation and de novo methylation. Transgenic constructs are often silenced by cytosine methylation.47 This phenomenon is especially true for cDNA constructs.39 Therefore, the difference in the levels of human F8 cDNA and human F8 mRNA found after successful gene-therapy approaches and after transgenic expression of human F8 cDNA are likely to be substantial. There are several other examples of transgenic mouse models that are immunologically tolerant to specific human proteins such as insulin,17 IFNs,19,20 or factor IX,48 but lack detectable human protein in the circulation. Moreover, Connely et al49 demonstrated that the lack of circulating FVIII protein observed after gene therapy using a vector containing a human F8 cDNA driven by an albumin promoter was not necessarily associated with a lack of the production of functional human FVIII protein. Therefore, we believe that the transgenic mouse line that is immunotolerant to human FVIII does produce human FVIII protein but does not produce sufficient quantities of protein to be detectable in the circulation.
An important objective in the selection of the transgenic mouse line was the requirement that mice should still be able to develop antibodies against human FVIII when challenged with a modified human FVIII protein expressing increased immunogenicity. We tested 2 different PEGylated FVIII preparations, mFVIII1 and mFVIII2, which showed different immunogenicity profiles in conventional hemophilic E17 mice. mFVII1 and mFVIII2 mainly differed in their degree of modification. An increase in the formation of protein aggregates as the cause for the different immunogenicity profiles can be excluded. When we tested mFVIII1, mFVIII2, and native human FVIII in our new transgenic mice, mFVIII1 and native FVIII did not induce anti-FVIII antibodies, whereas mFVIII2 induced antibodies that recognized both native human FVIII and mFVIII2. mFVIII1 and mFVIII2 differed substantially in their specific activity and in their binding to VWF, which might be explained by the difference in their degree of modification. PEGylation could cause a reduced ability of mFVIII2 to function as a cofactor in the “tenase” complex and could block binding sites for VWF. It remains to be determined whether these 2 modifications to FVIII function caused mFVIII2 to break tolerance in the transgenic mouse model. Several other mechanisms could explain the break of immune tolerance by mFVIII2. Protein modification could induce new epitopes (neo-epitopes) for T cells, possibly through different processing of mFVIII2 in endo-lysosomal compartments of antigen-presenting cells. PEGylation could shield certain cleavage sites of proteolytic enzymes responsible for the generation of peptides within endo-lysosomal compartments, resulting in altered peptide patterns, which could eventually lead to the presentation of new FVIII peptide epitopes to CD4+ T cells. Alternatively, PEGylation could generate altered structures that trigger certain pattern recognition receptors expressed on innate immune cells,50 thereby activating these cells and disturbing peripheral mechanisms of immune tolerance. Our results demonstrate that treatment with mFVIII2 caused an amplification of the anti-FVIII antibody response in conventional hemophilic E17 mice, which could indicate a stimulation of the innate immune system. Break of immune tolerance by mFVIII2 could also be driven by B cells if PEGylation would induce structural changes giving rise to novel B-cell epitopes. In this case, however, some T-cell help would probably be required. Antibody production without T-cell help would only be expected if PEGylation would induce repetitive B-cell epitopes that could induce T-cell–independent antibody responses by cross-linking and triggering B-cell receptors.7,8 The induction of several subclasses of IgG antibodies, however, would suggest that the break of tolerance involved antibody class switch, which would be expected to require T-cell help.7,8 The exact mechanisms for the break of immune tolerance by mFVIII2 are currently being studied.
In summary, in the present study, we created a new transgenic mouse line with a hemophilic phenotype in which specific immune tolerance towards native human FVIII is achieved by transgenic expression of a full-length human F8 cDNA. Immune tolerance breaks down when mice are challenged with a modified human FVIII molecule that expresses increased immunogenicity. We believe that this new mouse line provides an important advantage over existing hemophilic mouse models in the preclinical immunogenicity assessment of new FVIII molecules with chemical or molecular modifications. Furthermore, this model seems suitable to study mechanisms causing break of immune tolerance against human FVIII.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Elisabeth Hopfner, Ingrid Neunteufl, Katharina Steinitz, Azra Trbic, Nicole Pfeffer, Thomas Wurz, Fatima Al-Awadi, Nidha Abrar, and Monika Grewal for technical assistance; to Maurus de la Rosa for critical discussion; and to John-Philip Lawo for statistical analysis. Furthermore, they acknowledge the contribution of Maria Sasgary to the development of the construct containing the human F8 cDNA and the contribution of Thomas Rülicke, University of Veterinary Medicine Vienna, who generated the first transgenic founder mouse. They also thank Elise Langdon-Neuner for editing the manuscript.
This study was supported by Baxter BioScience.
Authorship
Contribution: P.M.v.H. designed research, performed the immunologic in vitro analysis, analyzed and interpreted the data, and wrote the manuscript; S.U. designed the research, did the molecular biological in vitro analysis, and analyzed and interpreted the data; C.H. designed the research and analyzed and interpreted the molecular biological data; M.S. designed and supervised the breeding and cross-breeding of transgenic mice; R.U.A. designed, supervised, and performed the animal experiments; A.N.S. designed, performed, and interpreted the tail-cut experiments; M.W. designed and performed the animal experiments; G.A. designed the molecular biological studies and interpreted these data; P.L.T. supervised the generation and analysis of mFVIII1 and mFVIII2; E.M.M. supervised the breeding of mice and the animal experiments; H.P.W. interpreted the data; and B.M.P. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors are employees of Baxter BioScience.
Correspondence: Birgit M. Reipert, Baxter BioScience, Industriestrasse 72, A-1220 Vienna, Austria; e-mail: birgit_reipert@baxter.com.