Abstract
Hemodynamic forces are important effectors of endothelial cell phenotype and function. Because CD40-CD154 interactions between endothelial cells and mononuclear leukocytes or activated platelets play an important role in vascular dysfunction, we investigated the effects of cyclic stretch on CD40 expression in human cultured endothelial cells. Short-term stretch transiently up-regulated CD40 expression while long-term stretch resulted in a distinct decline in CD40 protein which was prevented by inhibition of the 20S proteasome or scavenging of peroxynitrite. Tyrosine nitration of CD40 also occurred under static conditions on addition of authentic peroxynitrite, and according to mass spectrometry analysis Tyr-82 but not Tyr-31 was its target in the native protein. Immunofluorescence analysis of endothelial cells transduced with a control or Tyr-82 to Ala mutated AAV9-CD40-eGFP expression construct confirmed a peroxynitrite-dependent redistribution of the protein from the cell membrane to the cytoplasm, which was prevented by methyl-β-cyclodextrin. Moreover, CD154-stimulated IL-12p40 and E-selectin expression markedly decreased after exposure to authentic peroxynitrite or cyclic stretch, respectively. Coimmunoprecipitation demonstrated a decreased binding of TRAF2 and TRAF6 to the CD40 protein after tyrosine nitration. Through this posttranslational oxidative modification of an important costimulatory molecule, endothelial cells are able to quickly adapt to unfavorable hemodynamics and maintain their anti-inflammatory phenotype.
Introduction
CD40-CD154 costimulation among APCs, Th cells, and activated platelets is of critical importance for correct functioning of the cellular and humoral immune response. On the other hand, exaggerated CD40-CD154 costimulation has been implicated in chronic inflammatory and autoimmune diseases like psoriasis or rheumatoid arthritis.1,2 Moreover, CD40 expression by nonimmune cells such as endothelial cells, fibroblasts, or vascular smooth muscle cells, and the proinflammatory response of these cells to CD40 ligation, suggest an important role for CD40-CD154 costimulation in atherosclerosis and other vasculopathies.3,4
Endothelial cells are constantly exposed to mechanical forces exerted by the flowing blood. While laminar shear stress, because of the resulting increase in NO formation, is considered to principally exert anti-atherosclerotic effects,5,6 cyclic stretch up-regulates proinflammatory gene expression in these cells through activation of NAD(P)H oxidase(s) and secondary formation of reactive oxygen species (ROS), namely superoxide anions (O2−) and hydrogen peroxide (H2O2).7,8 Moreover, NO and O2− rapidly react with each other to produce peroxynitrite. This reaction, which is only limited by diffusion, not only weakens the NO blockade of proinflammatory gene expression9 but, because of secondary tyrosine nitration of different target proteins, may exert a plethora of additional potentially deleterious effects.10
In native murine blood vessels, CD40 is strongly expressed in capillaries and postcapillary venules as well as at arterial bifurcations like the aortic arch but not by endothelial cells of straight unbranched arteries.11 Despite being constantly subjected to enhanced stretch and thus oxidative stress, these endothelial cells are capable of maintaining their anti-atherogenic phenotype hence pointing to a compensatory mechanism.12 Thus far, it is not known whether CD40 expression in human vascular cells is mechanosensitive. Therefore, we here have investigated the effects of cyclic stretch on CD40 expression, the putative role of ROS therein and potential consequences thereof in human cultured endothelial cells.
Methods
A full description of the methods can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell culture
In brief, human primary umbilical vein endothelial cells were kept under static conditions or exposed to cyclic stretch (FlexCell FX-3000 tension system). For CD40-eGFP fusion protein expression, human immortalized umbilical vein endothelial cells were transduced with an adeno-associated virus serotype 9 (AAV9)–based expression construct. CD40 stimulation was achieved by using the mouse myeloma cell line P3xTB.A7 stably transfected with human CD154. Human premonocytic THP-1 cells were maintained under standard conditions. Hydrogen peroxide formation in cells seeded onto gelatin-coated glass coverslips or BioFlex culture plates was monitored through changes in dichlorofluorescein (DCF) fluorescence.
Protein detection and mRNA analysis
Protein detection by Western blot, ELISA, FACS, immunocytochemistry, and immunofluorescence methods or electrophoretic mobility shift analysis was done according to standard protocols. Total RNA from cells was isolated by solid-phase extraction followed by RT and quantitative real-time PCR analyses. Ab-coated magnetic DynaBeads beads were used for immunoprecipitation to separate CD40 or tyrosine-nitrated proteins. Purified 20S and 26S proteasomal fractions (Biomol) were used for an in vitro proteasome assay with the immunoprecipitated native protein and a recombinant CD40/Fc chimeric protein. Selective nitration of Tyr-82 but not Tyr-31 in these proteins was verified by LTQ-FT tandem mass spectrometry analysis.
Internalization
Peroxynitrite-mediated internalization of endogenous nitrated CD40 protein was demonstrated by using a biotinylation-endocytosis assay in primary endothelial cells and transduction of immortalized endothelial cells with 2 different AAV9-based viral vectors.
Statistical analysis
Statistical calculations were done by 1-way ANOVA followed by exact, 2-sided Kruskal-Wallis tests or Wilcoxon-Mann-Whitney tests as required, with P < .05 considered to be significantly different.
Results
Cyclic stretch, ROS, and CD40 expression
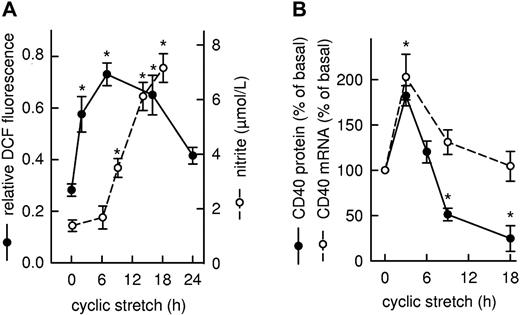
Exposure of the cultured endothelial cells to cyclic stretch resulted in a rapid increase in cell-associated DCF fluorescence, indicative of the formation of H2O2, which reached a maximum after 6 hours and returned to near baseline at 24 hours (Figure 1A). There was also a rise in the concentration of nitrite in the conditioned medium, indicative of cellular NO formation which, after a lag phase of 3-6 hours, steadily increased with time (Figure 1A). Concomitant with the increase in ROS formation, cyclic stretch also triggered a transient rise in CD40 expression on both the mRNA and protein level peaking at 3 hours (Figure 1B). Whereas CD40 mRNA levels returned to baseline within 9-18 hours, CD40 protein abundance reached the control level already after 6 hours and continued to decline to 25% of the level in nonstretched cells within 9-18 hours. Using an appropriate ROS scavenger and a protein kinase inhibitor revealed that short-term stretch-induced CD40 expression is ROS-dependent, occurs at the level of transcription, and is mediated via the JNK/AP-1 signaling pathway (supplemental Figure 1).
Time-dependent changes in intracellular ROS formation, extracellular nitrite concentration, and CD40 expression in human cultured endothelial cells exposed to cyclic stretch. (A) Time course of ROS formation shown as statistical summary of the relative changes in DCF fluorescence (●, n = 3-4, *P < .05 vs 0 hours). NOS-3 enzyme activity was estimated on the basis of the nitrite content of the conditioned medium (○, n = 4-8, *P < .05 vs 0 hours). (B) Time course of CD40 mRNA (○, n = 4-8, *P < .05 vs 0 hours) and CD40 protein abundance (●, n = 4-12; *P < .05 vs 0 hours) in the cultured cells, as judged by PCR and Western blot analysis, respectively. Changes in CD40 expression are calculated as percentage of the mRNA or protein level in nonstretched cells (basal).
Time-dependent changes in intracellular ROS formation, extracellular nitrite concentration, and CD40 expression in human cultured endothelial cells exposed to cyclic stretch. (A) Time course of ROS formation shown as statistical summary of the relative changes in DCF fluorescence (●, n = 3-4, *P < .05 vs 0 hours). NOS-3 enzyme activity was estimated on the basis of the nitrite content of the conditioned medium (○, n = 4-8, *P < .05 vs 0 hours). (B) Time course of CD40 mRNA (○, n = 4-8, *P < .05 vs 0 hours) and CD40 protein abundance (●, n = 4-12; *P < .05 vs 0 hours) in the cultured cells, as judged by PCR and Western blot analysis, respectively. Changes in CD40 expression are calculated as percentage of the mRNA or protein level in nonstretched cells (basal).
Stretch-induced degradation of CD40
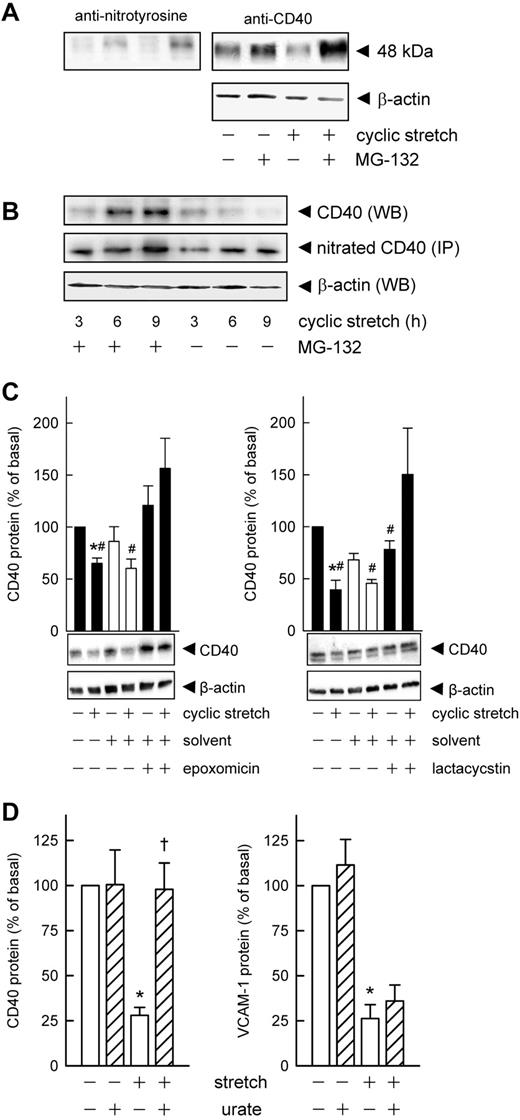
Next, down-regulation of CD40 protein in cells on prolonged exposure to cyclic stretch was examined. Direct and indirect blockade of NOS-3 activity with NG-nitro-l-arginine and the HSP-90 (NOS-3 chaperon) inhibitor geldanamycin suggested that the stretch-induced increase in NOS-3 activity was somehow linked to CD40 protein degradation (compare supplemental Figure 2A-C). Both RT-PCR and Western blot analyses revealed that neither NOS-3 nor HSP-90 expression was altered in the stretched endothelial cells, and there was no induction of NOS-2 (not shown). Moreover, the kinetics of the changes in ROS formation (presumably O2− dismutating to H2O2) and NOS-3 activity in response to cyclic stretch suggested a time-dependent increased formation of peroxynitrite, the reaction product of NO and O2−.9 By way of tyrosine nitration, peroxynitrite has been shown to enhance the susceptibility of both cytosolic (eg, GAPDH13 ) and membrane-associated proteins (eg, cystic fibrosis transmembrane conductance regulator14 ) for proteasomal degradation. To verify this assumption, colocalization experiments were performed in which endothelial cell protein was first probed for CD40 and then for nitrotyrosine. As shown in Figure 2A, the same immunoreactive band of ∼ 48 kDa was detected which disappeared on stretching of the cells for 9 hours, but was maintained in cells that had been treated with the proteasome inhibitor MG-132 before their exposure to cyclic stretch (supplemental Figure 2C). Subsequent immunoprecipation analyses confirmed that the 3-nitrotyrosine content of CD40 clearly is up-regulated in stretched cells treated with MG-132 to prevent degradation of the protein (supplemental Figure 2D). Moreover, both the kinetics and intensity of this tyrosine nitration not only matched the presumed stretch-induced rise in peroxynitrite levels, but also the stretch-induced degradation of CD40 protein (Figure 2B). MG-132 is also known to activate JNK and subsequently AP-1.15 However, endothelial cell expression of CD40 mRNA was not affected by MG-132 (not shown), suggesting that the observed maintenance by MG-132 of CD40 protein abundance in stretched cells is in fact because of proteasome inhibition. Because the ubiquitin-proteasome pathway is essential for many cellular processes, we checked whether CD40 is posttranscriptionally modified but failed to detect any ubiquitination or sumoylation of the protein (not shown).
Time-dependent tyrosine nitration causes CD40 degradation via the proteasome. (A) Colocalization experiment demonstrating the increase in 3-nitrotyrosine content of CD40 protein in endothelial cells treated with MG-132 (10μM, 30-minute preincubation) and exposed to cyclic stretch for 9 hours. Typical Western blot analysis, qualitatively identical results were obtained with at least 4 other batches of cells. (B) Time-dependent changes in the 3-nitrotyrosine content of immunoprecipitated (IP) CD40 protein compared with total CD40 protein (WB) in endothelial cells exposed to cyclic stretch with or without prior MG-132 treatment. Representative Western blot analysis, qualitatively identical results were obtained with at least 2 other batches of cells. (C) Lactacystin (50μM) and epoxomicin (10μM) reverse the stretch-induced decrease (16 hours, 15% elongation, 0.5 Hz) in CD40 protein content (n = 5; *P < .05 vs static control; #P < .05 vs cyclic stretch plus inhibitor). (D) Statistical summary (n = 6-11) of the effect of urate (10μM) on CD40 and VCAM-1 protein abundance in cells subjected to 16 hours of cyclic stretch (*P < .05 vs static control, †P < .05 vs cyclic stretch).
Time-dependent tyrosine nitration causes CD40 degradation via the proteasome. (A) Colocalization experiment demonstrating the increase in 3-nitrotyrosine content of CD40 protein in endothelial cells treated with MG-132 (10μM, 30-minute preincubation) and exposed to cyclic stretch for 9 hours. Typical Western blot analysis, qualitatively identical results were obtained with at least 4 other batches of cells. (B) Time-dependent changes in the 3-nitrotyrosine content of immunoprecipitated (IP) CD40 protein compared with total CD40 protein (WB) in endothelial cells exposed to cyclic stretch with or without prior MG-132 treatment. Representative Western blot analysis, qualitatively identical results were obtained with at least 2 other batches of cells. (C) Lactacystin (50μM) and epoxomicin (10μM) reverse the stretch-induced decrease (16 hours, 15% elongation, 0.5 Hz) in CD40 protein content (n = 5; *P < .05 vs static control; #P < .05 vs cyclic stretch plus inhibitor). (D) Statistical summary (n = 6-11) of the effect of urate (10μM) on CD40 and VCAM-1 protein abundance in cells subjected to 16 hours of cyclic stretch (*P < .05 vs static control, †P < .05 vs cyclic stretch).
Lactacystin and epoxomicin, 2 highly selective inhibitors of the chymotrypsin-like activity of the proteasome, abolished the stretch-dependent degradation of CD40 (Figure 2C). Unlike many other proteasome inhibitors, epoxomicin is specific for the 20S proteasome and does not inhibit other nonproteasomal proteases such as calpain, papain, cathepsin B, chymotrypsin, or trypsin.16 Conversely, neither the cathepsin C inhibitor Ca-074-Me (50μM), nor chymostatin (100μM) or the thiolpeptidase inhibitor iodoacetamide (10mM) affected the stretch-induced depletion of CD40 (n = 3, not shown).
Role of peroxynitrite and peroxidases in stretch-induced CD40 protein degradation
The putative role of peroxynitrite in CD40 protein degradation was subsequently investigated by using the peroxynitrite scavenger urate.17,18 Urate (10μM) significantly attenuated the stretch-induced degradation of CD40 protein but not that of VCAM-1 in the same series of experiments (Figure 2D, supplemental Figure 3A). Additional immunoprecipitation analyses confirmed that the 3-nitrotyrosine content of CD40 is diminished in stretched endothelial cells treated with urate while the native protein clearly is maintained (supplemental Figure 3B).
Furthermore, CD40 protein degradation under static conditions was mimicked by exposing the endothelial cells to authentic peroxynitrite (Figure 3A) which concomitantly elicited extensive protein nitration (supplemental Figure 4A). Preincubation of the cells with another peroxynitrite scavenger, FeTPPS, prevented both effects of peroxynitrite (supplemental Figure 4A-B). A potential role for peroxidases in CD40 protein nitration was ruled out because of the lack of effect of the myeloperoxidase inhibitor ABAH and the HRP inhibitor sodium azide (compare supplemental Figure 4B-C).
CD40 nitration enhances susceptibility to degradation by the 20S proteasome. (A) Effect of authentic peroxynitrite (160μM, 1 hour) and the proteasomal inhibitor lactacystin on the CD40 protein content of endothelial cells cultured under static conditions (n = 5; *P < .05 vs medium control), and representative Western blot analysis. Detection of CD40 tyrosine nitration (N-Tyr) with (B) immunoprecipitated native CD40 protein and (C) a recombinant CD40/Fc chimeric protein consisting of the extracellular domain of human CD40 and the Fc region of human IgG1. Nitration was achieved with the indicated concentrations of SIN-1 (peroxynitrite donor) and authentic peroxynitrite (1-hour exposure at 37°C). Representative Western blot analyses of at least 6 independent experiments. (D) Proteasome-dependent degradation of nitrated CD40 (N-Tyr) in a cell-free system. Native immunoprecipitated CD40 protein was incubated for 2 hours with 0.1 mg/mL recombinant 20S or 26S proteasome in the absence or presence of lactacycstin (50μM). Exemplary Western blot analysis, 2 repeat experiments produced the same result. (E) Time course of the degradation of nitrated CD40 protein (N-Tyr) by the 20S proteasome. Recombinant CD40/Fc chimeric protein was incubated with the recombinant 20S proteasome (0.1 mg/mL) for the indicated periods (n = 3; *P < .05 vs unmodified CD40).
CD40 nitration enhances susceptibility to degradation by the 20S proteasome. (A) Effect of authentic peroxynitrite (160μM, 1 hour) and the proteasomal inhibitor lactacystin on the CD40 protein content of endothelial cells cultured under static conditions (n = 5; *P < .05 vs medium control), and representative Western blot analysis. Detection of CD40 tyrosine nitration (N-Tyr) with (B) immunoprecipitated native CD40 protein and (C) a recombinant CD40/Fc chimeric protein consisting of the extracellular domain of human CD40 and the Fc region of human IgG1. Nitration was achieved with the indicated concentrations of SIN-1 (peroxynitrite donor) and authentic peroxynitrite (1-hour exposure at 37°C). Representative Western blot analyses of at least 6 independent experiments. (D) Proteasome-dependent degradation of nitrated CD40 (N-Tyr) in a cell-free system. Native immunoprecipitated CD40 protein was incubated for 2 hours with 0.1 mg/mL recombinant 20S or 26S proteasome in the absence or presence of lactacycstin (50μM). Exemplary Western blot analysis, 2 repeat experiments produced the same result. (E) Time course of the degradation of nitrated CD40 protein (N-Tyr) by the 20S proteasome. Recombinant CD40/Fc chimeric protein was incubated with the recombinant 20S proteasome (0.1 mg/mL) for the indicated periods (n = 3; *P < .05 vs unmodified CD40).
Authentic peroxynitrite increases nitration and 20S proteasome-dependent degradation of CD40 in cells and in a cell-free system
Peroxynitrite-induced degradation of CD40 in endothelial cells grown under static conditions was prevented by the proteasome inhibitor lactacystin (Figure 3A inset). In addition, concentration-dependent tyrosine nitration of CD40 could be demonstrated in a cell-free system using the peroxynitrite donor SIN-1 (0.1-2mM) and the immunoprecipitated native protein (Figure 3B) or a recombinant human CD40/Fc chimeric protein (Figure 3C). The recombinant protein comprises the extracellular N-terminal domain of CD40 (Met-1 to Arg-193) with the only 2 tyrosine residues present in the whole protein at positions 31 and 82. Figure 3D reveals that the nitrated native protein is mainly degraded by the 20S but not the 26S proteasome, and that this effect is prevented by lactacystin. Because of the long incubation period (2 hours) also the unmodified protein seems to be effectively degraded by the 20S proteasome. Therefore, an additional time-course experiment using the recombinant protein was performed (Figure 3E) which revealed a faster degradation of the nitrated protein compared with the unmodified protein.
CD40 is preferentially nitrated at Tyr-82
The native CD40 protein contains 2 tyrosine residues at positions 31 and 82 of the polypeptide chain which both might be nitrated by peroxynitrite. This was shown by treatment of the nitrated chimeric protein with activated factor Xa which cleaves the linker peptide between the extracellular CD40 domain and the Fc tag (supplemental Figure 5A-B). In an effort to distinguish between the 2 possibilities, mass spectrometry analysis was used which clearly demonstrated that Tyr-82 but not Tyr-31 is preferentially nitrated by authentic peroxynitrite (supplemental Table 1, supplemental Figure 5C-D). Moreover, calculation of the solvent accessible area of CD40 with the NACCESS program revealed that in fact only Tyr-82 is accessible by peroxynitrite while Tyr-31 is essentially buried in the polypeptide chain (supplemental Figure 5E). No other significant modifications by peroxynitrite in the CD40 moiety of the chimeric protein were detected.
Effect of tyrosine nitration on the subcellular localization of CD40
Because Tyr-82 is located in the extracellular domain of the membrane protein, it was unclear whether nitrated CD40 is internalized for proteasomal degradation or degraded by extracellular components of the 20S proteasome which can in fact be secreted.19 However, while the 20S proteasome was readily detectable in whole-cell lysates, it was not traceable in the conditioned medium of cells exposed to cyclic stretch for 16 hours (n = 3, not shown).
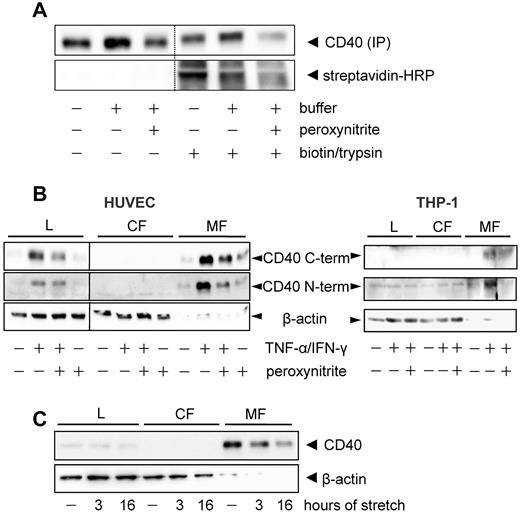
Using surface biotinylation followed by surface trypsin treatment and CD40 immunoprecipitation, we noticed that the membrane-bound protein is in fact internalized and that the amount of internalized protein is reduced after exposure to peroxynitrite (Figure 4A). Moreover, both peroxynitrite treatment and cyclic stretch resulted in a depletion of the protein in the microsomal fraction of the cultured endothelial cells (Figure 4B-C) and THP-1 monocytes (Figure 4B). Immunofluorescence analysis of the latter further revealed a peroxynitrite-dependent redistribution of the protein from the cell membrane to the cytoplasm with local cluster formation (supplemental Figure 6). Cholesterol depletion of the cell membrane with methyl-β-cyclodextrin prevented this redistribution (supplemental Figure 6) while siRNA knockdown of caveolin-1 had no such effect (n = 4, not shown). In addition, Hsp70-dependent uptake of CD4020 could be excluded by way of coimmunoprecipitation analysis (n = 3, not shown).
Peroxynitrite and cyclic stretch-mediated membrane depletion and internalization of CD40. (A) After biotinylation of surface proteins for 45 minutes, the cultured endothelial cells were exposed to peroxynitrite (160-200μM) for 1 hour. Thereafter, internalized biotinylated proteins and cells were separated by mild trypsinization and the labeled CD40 collected by immunoprecipitation. Relative amount of label was determined by Western blot analysis with streptavidin-HRP as detection agent. Shown is a representative blot of at least 5 independent experiments. (B) Peroxynitrite treatment (160μM, 1 hour) reduces the abundance of CD40 in the microsomal fraction of cytokine-stimulated (1000 U/mL IFN-γ + 100 U/mL TNF-α, 16 hours) human umbilical vein endothelial cells (HUVEC, left panel) and THP-1 cells (right panel), respectively. Detection was achieved with Abs recognizing the C or N terminus of human CD40 as indicated. L indicates whole-cell lysate; CF, cytosolic fraction; and MF, microsomal fraction. The Western blots are representative of at least 5 individual experiments. A vertical line has been inserted into the left blot to indicate that the whole-cell lysate samples of this experiment were run on a separate gel. Please note that the microsomal fraction naturally contains much less β-actin which nonetheless was rather evenly distributed among these samples. (C) Cyclic stretch (3 or 16 hours, 15% elongation, 0.5 Hz) time-dependently decreases CD40 abundance in the microsomal fraction of the cultured endothelial cells. The exemplary Western blot is representative of 4 independent experiments.
Peroxynitrite and cyclic stretch-mediated membrane depletion and internalization of CD40. (A) After biotinylation of surface proteins for 45 minutes, the cultured endothelial cells were exposed to peroxynitrite (160-200μM) for 1 hour. Thereafter, internalized biotinylated proteins and cells were separated by mild trypsinization and the labeled CD40 collected by immunoprecipitation. Relative amount of label was determined by Western blot analysis with streptavidin-HRP as detection agent. Shown is a representative blot of at least 5 independent experiments. (B) Peroxynitrite treatment (160μM, 1 hour) reduces the abundance of CD40 in the microsomal fraction of cytokine-stimulated (1000 U/mL IFN-γ + 100 U/mL TNF-α, 16 hours) human umbilical vein endothelial cells (HUVEC, left panel) and THP-1 cells (right panel), respectively. Detection was achieved with Abs recognizing the C or N terminus of human CD40 as indicated. L indicates whole-cell lysate; CF, cytosolic fraction; and MF, microsomal fraction. The Western blots are representative of at least 5 individual experiments. A vertical line has been inserted into the left blot to indicate that the whole-cell lysate samples of this experiment were run on a separate gel. Please note that the microsomal fraction naturally contains much less β-actin which nonetheless was rather evenly distributed among these samples. (C) Cyclic stretch (3 or 16 hours, 15% elongation, 0.5 Hz) time-dependently decreases CD40 abundance in the microsomal fraction of the cultured endothelial cells. The exemplary Western blot is representative of 4 independent experiments.
Tyr-82 nitration of CD40 is critical for peroxynitrite-induced internalization
Immunofluorescence analysis, as established for the THP-1 cells (supplemental Figure 6), or FACS analysis did not reveal an unambiguous association of CD40 with the endothelial cell membrane, presumably because of the rather low abundance of the protein in the plasma membrane of these cells.21 To overcome this limitation, adeno-associated virus (AAV9) expression constructs were generated encoding a CD40-enhanced GFP (eGFP) hybrid protein with (CD40Y82A-eGFP) or without a Tyr-82 to Ala mutation. Figure 5A demonstrates that expression of the unmodified hybrid protein in an immortalized human endothelial cell line resulted in its preferential localization to the plasma membrane from where it translocated to the cytoplasm on peroxynitrite exposure. This peroxynitrite-induced redistribution of the hybrid protein was not observed when these cells had been transduced with the CD40Y82A-eGFP construct (Figure 5B).
Nitration of Tyr-82 is crucial for peroxynitrite- induced internalization. (A) Effect of peroxynitrite (160μM, 1 hour) on the intracellular distribution of a CD40-eGFP hybrid protein expressed by a human endothelial cell line. Column 1, CD40-eGFP–positive cells (green); column 2, cells positive for the plasma membrane marker CD31 (red); and column 3, overlay of columns 1 and 2 with yellow fluorescence indicating localization of CD40 in the plasma membrane. The scale bar represents 10 μm. (B) Mutation of Tyr-82 to alanine abrogates translocation of the hybrid protein. Shown are representative fluorescent cells of at least 3 individual experiments each. The scale bar represents 20 μm (above) and 10 μm (below).
Nitration of Tyr-82 is crucial for peroxynitrite- induced internalization. (A) Effect of peroxynitrite (160μM, 1 hour) on the intracellular distribution of a CD40-eGFP hybrid protein expressed by a human endothelial cell line. Column 1, CD40-eGFP–positive cells (green); column 2, cells positive for the plasma membrane marker CD31 (red); and column 3, overlay of columns 1 and 2 with yellow fluorescence indicating localization of CD40 in the plasma membrane. The scale bar represents 10 μm. (B) Mutation of Tyr-82 to alanine abrogates translocation of the hybrid protein. Shown are representative fluorescent cells of at least 3 individual experiments each. The scale bar represents 20 μm (above) and 10 μm (below).
Functional consequences of the nitration of Tyr-82
Further investigations revealed that CD154-induced IL-12p40 and E-selectin expression in the cultured endothelial and THP-1 cells was markedly decreased after exposure to cyclic stretch or exogenous peroxynitrite (Figure 6, supplemental Figure 7A-E). This effect was reversed by preincubating the cells with the peroxynitrite scavenger urate. Methyl-β-cyclodextrin also reversed this peroxynitrite-mediated decrease in CD154-stimulated IL12-p40 (Figure 6C) and E-selectin expression (supplemental Figure 7B) in the endothelial cells but not in the THP-1 cells (supplemental Figure 7D-E). Cyclic stretch itself did not induce the expression of IL12-p40 and E-selectin (Figure 6D-E, supplemental Figure 7C).
Effect of authentic peroxynitrite and cyclic stretch on CD154-induced IL12-p40 mRNA and protein expression. (A-C) Urate (10μM, 30-minute preincubation) and methyl-β-cyclodextrine (MCD; 5μM, 30-minute preincubation) attenuate the peroxynitrite (160μM, 1 hour) mediated decrease in CD154-induced IL12-p40 mRNA (6-hour incubation) and protein (24-hour incubation) abundance in human umbilical vein endothelial cells (HUVEC) under static conditions (n = 3-5; *P < .05 vs CD154; #P < .05 vs CD154 + peroxynitrite). (D-E) Urate reverse the stretch-mediated (16 hours, 15% elongation, 0.5 Hz) decrease in CD154-stimulated IL12-p40 mRNA and protein levels (n = 3-4; *P < .05 vs CD154; #P < .05 vs CD154 + cyclic stretch). (F) Urate attenuates the peroxynitrite-mediated decrease in CD154-stimulated IL12-p40 protein abundance (24-hour incubation) in THP-1 cells (n = 3; *P < .05 vs CD154; #P < .05 vs CD154 + peroxynitrite). Specific stimulation of CD40 with mouse myeloma cells expressing human CD154 was verified (A,F) by preincubating these cells with a neutralizing anti-CD154 Ab (40 μg/mL).
Effect of authentic peroxynitrite and cyclic stretch on CD154-induced IL12-p40 mRNA and protein expression. (A-C) Urate (10μM, 30-minute preincubation) and methyl-β-cyclodextrine (MCD; 5μM, 30-minute preincubation) attenuate the peroxynitrite (160μM, 1 hour) mediated decrease in CD154-induced IL12-p40 mRNA (6-hour incubation) and protein (24-hour incubation) abundance in human umbilical vein endothelial cells (HUVEC) under static conditions (n = 3-5; *P < .05 vs CD154; #P < .05 vs CD154 + peroxynitrite). (D-E) Urate reverse the stretch-mediated (16 hours, 15% elongation, 0.5 Hz) decrease in CD154-stimulated IL12-p40 mRNA and protein levels (n = 3-4; *P < .05 vs CD154; #P < .05 vs CD154 + cyclic stretch). (F) Urate attenuates the peroxynitrite-mediated decrease in CD154-stimulated IL12-p40 protein abundance (24-hour incubation) in THP-1 cells (n = 3; *P < .05 vs CD154; #P < .05 vs CD154 + peroxynitrite). Specific stimulation of CD40 with mouse myeloma cells expressing human CD154 was verified (A,F) by preincubating these cells with a neutralizing anti-CD154 Ab (40 μg/mL).
Tyrosine nitration of CD40 attenuates CD154-stimulated recruitment of TRAF2 and TRAF6
Finally, the effects of CD40 nitration on the association of TRAF2 and 6 with the receptor were investigated as, presumably, the most proximal event in CD40 signaling. The 2 TRAF molecules were chosen as readouts because of reports on TRAF-deficient mice revealing TRAF2 and, in particular, TRAF6 as key regulators of neointima formation, arterial remodeling, and proinflammatory endothelial cell activation.22 Corresponding coimmunoprecipitation experiments in fact revealed a decreased association of TRAF2 and TRAF6 with CD40 in response to CD154 stimulation in endothelial cells exposed to peroxynitrite (Figure 7). Urate as well as methyl-β-cyclodextrin restored the CD154-stimulated association of both signaling molecules with CD40.
Tyrosine nitration of CD40 affects CD154-mediated TRAF protein recruitment. (A) Urate (10μM, 30-minute preincubation) and (B) methyl-β-cyclodextrine (MCD; 5μM, 30-minute preincubation) reverse the peroxynitrite (160μM, 15 minutes) mediated dissociation of TRAF2 and TRAF6 from CD40 in CD154-stimulated endothelial cells under static conditions (20-minute incubation). Statistical summary (n = 3; *P < .05 vs CD154; #P < .05 vs CD154 + peroxynitrite) and exemplary Western blot analyses of TRAF2 and TRAF6 coimmunoprecipitating with CD40.
Tyrosine nitration of CD40 affects CD154-mediated TRAF protein recruitment. (A) Urate (10μM, 30-minute preincubation) and (B) methyl-β-cyclodextrine (MCD; 5μM, 30-minute preincubation) reverse the peroxynitrite (160μM, 15 minutes) mediated dissociation of TRAF2 and TRAF6 from CD40 in CD154-stimulated endothelial cells under static conditions (20-minute incubation). Statistical summary (n = 3; *P < .05 vs CD154; #P < .05 vs CD154 + peroxynitrite) and exemplary Western blot analyses of TRAF2 and TRAF6 coimmunoprecipitating with CD40.
Discussion
The pivotal findings of this work are that the stretch-induced degradation of CD40 in endothelial cells is catalyzed by the chymotrypsin-like activity of the 20S proteasome and is the result of a peroxynitrite-dependent nitration of Tyr-82 located in the extracellular domain of the protein. Subsequent to this posttranslational modification, recruitment of TRAF proteins to CD40 is diminished, resulting in a blockade of CD154-stimulated IL12-p40 and E-selectin expression.
Arterial endothelial cells in particular are constantly exposed to 2 hemodynamic or biomechanical forces, that is, fluid shear stress which can be laminar or oscillatory and (cyclic) stretch as a result of the (pulsatile) changes in circumferential wall tension. Although both forces affect the endothelial cell phenotype in a major way,23 most in vitro studies with cultured endothelial cells are performed under static conditions. Depending on their localization in the vasculature, there are striking differences, for example, in proinflammatory gene expression in endothelial cells.24 These in situ differences may be preserved in organ culture models but typically are lost in (static) culture because of the rapid adaptation (dedifferentiation) of the endothelial cells to the altered environment. In the living organism, abundant expression of, for example, adhesion molecules, such as E-selectin or VCAM-1, is restricted to postcapillary venules or small veins, typical sites for leukocyte extravasation because of the low flow.23 In addition, CD40 expression in adult mice has been mapped to these regions as well as to bifurcations of conduit arteries where blood flow is disturbed and atherosclerosis develops.11
Here laminar shear stress, and thus endothelial cell NO formation, is diminished while cyclic stretch through activation of NADPH oxidase 2 (NOX-2) enhances the generation of O2−,25 which subsequently is converted to H2O2 by enzymatic or nonenzymatic dismutation. O2− rapidly reacts with NO to produce peroxynitrite.26 Consequently, the local concentration of NO is reduced to a level where it can no longer control proinflammatory gene expression which, on the other hand, is up-regulated by H2O2.7,8
A stretch-induced enhanced formation of ROS in endothelial cells, as shown herein, is thought to elicit a stress response involving the activation of several protein kinases including JNK,27 as well as the subsequent activation of “stress-sensitive ” transcription factors such as AP-1. Here we show that translocation of AP-1 to the nucleus precedes the transient stretch-induced rise in CD40 expression in the cultured endothelial cells which, in contrast, is abolished after JNK blockade, suggesting that it is in fact mediated through activation of the JNK–AP-1 signaling pathway.
Prolonged exposure to cyclic stretch may trigger compensatory mechanisms in endothelial cells aimed at normalizing ROS formation and ROS-dependent signaling such as, for example, an increased expression or activity of NOS-3.28 While in our work, NOS-3 expression did not change in response to cyclic stretch, there was a significant rise in activity of the enzyme, as judged by the accumulation of nitrite in the conditioned medium, which lagged several hours behind the stretch-induced increase in ROS formation. This stretch-induced rise in NO formation coincided with the fall in CD40 protein abundance, and both direct (NG-nitro-l-arginine) and indirect (geldanamycin) blockade of NOS-3 activity resulted in the maintenance of CD40 protein at baseline in endothelial cells stretched for extended periods of time. As O2− formation was still significantly enhanced at this point, we presumed that peroxynitrite, the reaction product of NO and O2−,10,26 is responsible for proteasomal degradation of the protein. In fact, transition metal or carbon dioxide-catalyzed peroxynitrite-dependent nitration of a single tyrosine residue in a protein may be sufficient to induce or accelerate its degradation by the chymotrypsin-like activity of the 20S proteasome.29
The broad-spectrum proteasome inhibitor MG-132 and the more selective proteasome inhibitors lactacystin and epoxomicin prevented the stretch-induced degradation of CD40 in the cultured endothelial cells. Briefly exposing them to authentic peroxynitrite essentially yielded the same effect, and this peroxynitrite-induced degradation of the protein was likewise prevented by the 20S proteasome inhibitor lactacystin.30 Western blot colocalization and immunoprecipitation analyses further revealed a stretch-dependent tyrosine nitration of the protein, the kinetics and intensity of which both matched the stretch-induced rise in NOS-3 activity, hence the formation of peroxynitrite and the stretch-induced fall in CD40 protein abundance. A role for peroxynitrite-mediated 3-nitrotyrosine formation in the loss of CD40 protein was substantiated by the finding that urate, a peroxynitrite scavenger,17,18 specifically attenuated the stretch-induced degradation of CD40 but not that of VCAM-1, which consequently must occur by a mechanism distinct from that of CD40 proteolysis. In this context, it may be important to note that this stretch-induced degradation of CD40 is not restricted to the “venous” endothelial cells used in this study, but reveals exactly the same kinetics both in human macrovascular and microvascular arterial endothelial cells (not shown).
3-nitrotyrosine residues in proteins can alternatively be formed through the one-electron oxidation of nitrite to the highly reactive nitrite radical,31 a reaction typically catalyzed by heme-containing peroxidases such as the myeloperoxidase of human neutrophils.32 The observed lack of effect of both a myeloperoxidase and a HRP inhibitor on the stretch-induced nitration and degradation of CD40 in the cultured endothelial cells, however, points to a peroxynitrite-dependent 3-nitrotyrosine formation, as previously shown for the tyrosine nitration, hence inactivation of prostacyclin synthase in these cells.33 This conclusion is also corroborated by the effective blockade of both stretch and peroxynitrite-induced degradation of CD40 by 2 prototypic peroxynitrite scavengers, urate and FeTPPS.
Moreover, exposure of the native protein (immunoprecipitated from the human cultured endothelial cells) or a recombinant human CD40/Fc chimera to authentic peroxynitrite or the peroxynitrite donor SIN-134 resulted in the nitration of both proteins. Using purified proteasomal fractions revealed that the nitrated native protein is degraded more rapidly by the 20S proteasome than the unmodified protein, and that this degradation in a cell-free system is lactacystin-sensitive, suggesting that it is in fact the chymotrypsin-like activity of the 20S proteasome which catalyzes its proteolysis.29
The recombinant N-terminal human CD40/Fc chimera like the native whole protein harbors only 2 tyrosine residues at position 31 and 82, at least one of which is nitrated on exposure to authentic peroxynitrite, as verified by splitting off the Fc tag with the aid of factor Xa, which solely retained the 3-nitrotyrosine immunoreactivity within the CD40 moiety of the chimeric protein. Subsequent computing of the solvent-accessible areas within this protein fragment by using the NACCESS program clearly revealed that only Tyr-82, but not Tyr-31, is readily accessible for peroxynitrite-mediated nitration; Tyr-31 is essentially buried in the polypeptide chain. This prediction was fully confirmed by subsequent high-resolution/high mass accuracy tandem mass spectrometry analysis of the peroxynitrite-treated native protein.
What are the functional consequences of this nitration of Tyr-82? A previous study has shown that Tyr-82 may be critical for binding of the ligand CD154.35 In fact, mutating the tyrosine to phenylalanine abrogates CD40-CD154 interaction, suggesting that it is the hydroxyl group of Tyr-82 that is critical for ligand-receptor interaction.35 Thus far, no oxidative modification of CD40 has been described except an endogenous S-nitrosylation in human monocyte/macrophages that may affect its spatial distribution on the cell surface, thereby preventing an optimum physical interaction of CD154 with CD40.36 The present data strongly suggest that nitration of Tyr-82 in stretched human cultured endothelial cells results in its subsequent degradation by the chymotrypsin-like activity of the 20S proteasome. The finding that these cells also synthesize CD40 de novo on exposure to cyclic stretch raised the question of whether it is the newly synthesized protein on its way to the membrane that is trapped by nitration, for example, in the trans-Golgi network, and then degraded by the proteasome or the mature membrane-bound protein whose nitration causes its internalization and subsequent degradation.
Unequivocally answering this question proved somewhat difficult, although the alternative explanation of an exteriorization of the 20S proteasome37 acting on the membrane-bound protein could be quickly ruled out. Biotinylation of surface proteins revealed that on exposure to peroxynitrite at least part of the membrane-bound CD40 is internalized and subsequently degraded by the proteasome. This, however, is only a qualitative method like assessing the intracellular distribution of the protein which after peroxynitrite treatment of the endothelial cells was clearly shifted from the membrane fraction to the cytosol. Our attempt to visualize and semi-quantitatively monitor this translocation of CD40 by immunofluorescence analysis failed because of low abundance of the protein on the endothelial cell surface.21 This was different for the monocyte/macrophage cell line THP-1 where we observed a nearly quantitative internalization and intracellular clustering of CD40 after exposure to peroxynitrite which was blocked by cholesterol depletion of the cell membrane but not by knockdown of caveolin-1.
An alternative approach to study the fate of a protein in a living cell is transduction of the cell with a viral vector expressing a fluorescent version of the protein. To this end, we used an AAV9-based expression system that resulted in the efficient transduction of a closely related human endothelial cell line with a vector encoding a CD40-eGFP fusion protein. The fusion protein in fact localized predominantly to the plasma membrane of these cells from where it translocated to the cytosol after peroxynitite treatment and clustered there in a manner similar to that observed for the native protein in THP-1 cells. Mutating Tyr-82 in the CD40 moiety of the fusion protein to alanine fully blocked its cellular redistribution in the presence of peroxynitrite, proving that one consequence of nitration of this tyrosine residue is internalization of the membrane-bound protein to make it available for degradation by the proteasome.
Does this internalization and subsequent degradation solely account for the observed abrogation of rather early alterations in the endothelial cell phenotype such as the CD154-induced changes in IL-12p40 and E-selectin mRNA abundance? In the human cultured endothelial cells, CD154-stimulated mRNA and protein expression of these 2 proinflammatory mediators was largely abolished after exposure to endogenous (cyclic stretch) or exogenous peroxynitrite, and these effects were reversed essentially by the same pharmacologic interventions (urate, methyl-β-cyclodextrin, lactacystin) that proved effective in preventing internalization and proteasomal degradation of the receptor. In fact, activated CD40 localizes to lipid rafts so that methyl-β-cyclodextrin disruption of the lipid raft structure impedes CD40 internalization through nonclathrin-mediated caveolae/raft-dependent endocytosis.38
Apparently, the first event after binding of the ligand to CD40 is association of different TRAF molecules with the receptor, and signaling via TRAF2 and TRAF6 in endothelial cells has been shown to be of particular relevance for their shift toward a proinflammatory phenotype.39 Subsequent coimmunoprecipitation experiments demonstrated that there is in fact a clearly reduced association of both TRAF molecules with CD40 in CD154-stimulated endothelial cells after prior exposure to peroxynitrite, and this effect was prevented through scavenging of peroxynitrite or cholesterol depletion of the plasma membrane. Notably, methyl-β-cyclodextrin disruption of lipid rafts and CD40 nitration per se did not affect recruitment of TRAF2 and TRAF6 but interrupted proximal CD40 signaling to the nucleus, suggesting that the nitrated protein is inoperable and thus disconnected from the subsequent signaling cascade in the endothelial cells. This may also explain the fairly rapid blockade of CD154-stimulated IL12-p40 and E-selectin mRNA expression in response to endogenous or exogenous peroxynitrite.
In our opinion, the aforementioned findings collectively suggest that on nitration of Tyr-82, CD40 is functionally inactivated and disconnected from the intracellular signal transduction cascade to the nucleus, followed by internalization of the nitrated protein and subsequent accelerated degradation by the chymotrypsin-like activity of the 20S proteasome. This may constitute a novel and smart double-safety mechanism by which endothelial cells escape the potentially deleterious changes in gene expression elicited by CD40 in unbranched arteries and arterioles where both shear stress-induced NO formation and stretch-induced O2− synthesis and thus peroxynitrite levels are elevated. In venules and veins where shear stress is comparatively low as well as at arterial bifurcations where shear stress-induced NO formation is nearly absent, CD40 expression is enabled because of insufficient degradation of the protein, and this may in fact make sense both in a physiologic (mononuclear leukocyte extravasation for immune defense) and pathophysiological contexts (atherosclerosis). Interestingly, CD40-CD154 interaction itself can lead to an increased formation of peroxynitrite,40 and CD40 nitration may therefore also constitute a self-inhibiting mechanism by which excessive stimulation of this immunologically important costimulatory pathway balances itself.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Anna Reifegerst and Manuela Höfer for expert technical assistance.
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (TRR 23 project C6; M.H.).
Authorship
Contribution: A.H.W. performed part of the experiments, contributed ideas, and was involved in designing experimental procedures, evaluating data, and writing the manuscript; A.H. was involved in designing the experimental procedures, performing part of the experiments, and evaluating data; S.B. performed part of the experiments and evaluated data; A.J. performed part of the experiments, contributed ideas, and was involved in designing the experimental procedures; V.S.S. contributed ideas, performed part of the experiments, and evaluated data; O.J.M. and C.S. contributed ideas and were involved in the writing of the manuscript; and M.H. supervised the research, contributed ideas, and was involved in designing the experimental procedures and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Markus Hecker, Institute of Physiology and Pathophysiology, Division of Cardiovascular Physiology, University of Heidelberg, Im Neuenheimer Feld 326, D-69120 Heidelberg, Germany; e-mail: hecker@physiologie.uni-hd.de.
References
Author notes
A.H.W. and A.H. contributed equally to this work.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal