Abstract
A pathogenic role for high-mobility group box 1 (HMGB1) protein has been postulated in severe sepsis. Activated protein C (APC) is the only drug approved by the Food and Drug Administration for severe sepsis; however, its effect on HMGB1 signaling has never been investigated. Here, we monitored the effect of APC on the lipopolysaccharide-mediated release of HMGB1 and the HMGB1-mediated modulation of proinflammatory responses in HUVECs. APC potently inhibited the release of HMGB1 and down-regulated the adhesion of the monocytic cell line, THP-1, to HMGB1-activated endothelial cells. HMGB1 up-regulated proinflammatory responses by interacting with 3 pathogen-related pattern recognition receptors: TLR2 and TLR4 and the receptor for advanced glycation end products. APC not only inhibited HMGB1 release but also down-regulated the cell surface expression of all 3 HMGB1 receptors in endothelial cells. The protective effects of APC were mediated through endothelial cell protein C receptor (EPCR) and protease-activated receptor 1 (PAR-1). Interestingly, a thrombin derivative containing the Gla-domain of APC recapitulated all protective effects of APC with a 20- to 50-fold higher efficacy. These results suggest that the EPCR- and PAR-1–dependent protective effects of APC in severe sepsis may partially be mediated through the inhibition of HMGB1 signaling and that the chimeric thrombin mutant has potential therapeutic utility for severe sepsis.
Introduction
The nonhistone chromatin-associated nuclear protein, high-mobility group box 1 (HMGB1), has been identified as a potent proinflammatory extracellular cytokine and a late mediator of endotoxin lethality in mice.1,2 It can be secreted into intravascular spaces by cells of the innate immune system or passively released by damaged tissues and necrotic cells in response to bacterial endotoxin or trauma or both.1-4 HMGB1 is known to be secreted or released to plasma at high levels with late kinetics in severe sepsis and in experimental models of endotoxemia.5 It triggers the activation of the endothelium and leukocytes by binding to ≥ 3 pathogen-associated cell surface pattern recognition receptors: TLR2 and TLR4 and the receptor for advanced glycation end products (RAGE), thereby inducing TNF-α expression and NF-κB activation in target cells.6-9 Binding of HMGB1 to these receptors on endothelial cells induces the expression of adhesion molecules and stimulates the production of an array of proinflammatory cytokines that are involved in mediating leukocyte adherence, increased vascular permeability, coagulation activation, and microvascular thrombosis.9-11 A high concentration of HMGB1 in the plasma of patients with severe sepsis correlates with a poor prognosis and high mortality, and its pharmacologic inhibition improves survival in animal models of acute inflammation and severe sepsis in response to endotoxin challenge.5,12
Activated protein C (APC) is a vitamin K–dependent plasma anticoagulant serine protease that has been approved by the Food and Drug Administration for treating adult patients with severe sepsis.13 Results from several laboratories have shown that APC elicits potent cytoprotective and anti-inflammatory responses when it binds to endothelial protein C receptor (EPCR) to activate protease-activated receptor 1 (PAR-1) on endothelial cells.14-18 The EPCR- and PAR-1–dependent anti-inflammatory effect of APC is mediated through its ability to suppress the NF-κB–dependent expression of proinflammatory cytokines and to inhibit the interaction and migration of leukocytes across the endothelium.19,20 APC also inhibits apoptosis and protects the endothelium from the hyperpermeability effect of inflammatory mediators.20-22 The EPCR- and PAR-1–dependent cytoprotective and anti-inflammatory activities of APC have been confirmed in several acute animal models of inflammation and severe sepsis.14,16-19,23 Nevertheless, a recent study also reported a potent protective activity for APC in an lipopolysaccharide (LPS)–induced murine model of endotoxemia, which appeared to be mediated through APC proteolytically degrading intravascularly released nuclear histones independent of its interaction with EPCR and PAR-1.24 A similar protective effect has been observed for the thrombin-thrombomodulin (TM) complex through the proteolytic degradation and inhibition of HMGB125 ; however, the effect of APC on HMGB1 release or HMGB1 signaling or both has never been investigated.
In this study, we monitored the effect of APC on HMGB1 signaling with the use of primary HUVECs. We discovered that APC inhibits the LPS-mediated release of HMGB1 as well as the HMGB1-mediated proinflammatory signaling responses in endothelial cells through down-regulation of the cell surface expression of HMGB1 receptors TLR2, TLR4, and RAGE by EPCR- and PAR-1–dependent mechanisms. Interestingly, a meizothrombin derivative containing the γ-carboxyglutamic acid (Gla) domain of APC potently inhibited HMGB1 signaling pathways in endothelial cells with a 20- to 50-fold higher efficacy. The meizothrombin derivative also effectively cleaved HMGB1 when it was bound to TM.
Methods
Regents
Bacterial LPS, 2-mercaptoethanol, and antibiotics (penicillin G and streptomycin) were purchased from Sigma-Aldrich. Human recombinant HMGB1 was purchased from Abnova. FBS and Vybrant DiD were purchased from Invitrogen. The cleavage-blocking monoclonal anti–PAR-1 Ab (H-111) was purchased from Santa Cruz Biotechnology Inc. The function-blocking anti-EPCR Ab was purchased from Cell Sciences. All recombinant proteins, including APC, and the thrombin activation intermediate meizothrombin (MeizoTh) and its derivative containing the Gla-domain of APC (PC-gla/MeizoTh) were prepared as described.26
Cell culture
Primary HUVECs were obtained from Cambrex Bio Science Inc and maintained as described. Human monocytic leukemia cell line, THP-1, was maintained at a density of 2 × 105 to 1 × 106 cells/mL in RPMI 1640 with l-glutamine and 10% heat-inactivated FBS supplemented with 2-mercaptoethanol (55μM) and antibiotics (penicillin G and streptomycin) as described.27
ELISAs for HMGB1, NF-κB, and TNF-α
Commercially available ELISA kits were used to measure the concentrations of HMGB1 (Shino-Test Corporation), NF-κB (Cell Signaling Technology), and TNF-α (R&D Systems) in cell culture supernatants according to the manufacturers' protocols.
Cell adhesion assay
THP-1 cell adherence to endothelial cells was evaluated by fluorescent labeling of THP-1 cells as described.28 Briefly, THP-1 cells were labeled with the Vybrant DiD dye followed by their addition to the washed and stimulated HUVECs. Cells were allowed to adhere, and the nonadherent THP-1 cells were washed off and the fluorescence of the adherent cells was measured. The percentage of adherent THP-1 cells was calculated by the formula: percentage of adherence = (adherent signal/total signal) × 100 as described.28 The data are expressed as means ± SDs from ≥ 3 independent experiments.
Migration assay
The migration assay was performed in trans-well plates of 6.5-mm diameter, with 8-μm pore size filters as described.27,29 HUVECs (6 × 104) were cultured for 3 days to obtain confluent endothelial cell monolayers. The monolayers were treated for 3 hours with indicated proteases followed by HMGB1 (1 μg/mL for 16 hours) and washed 3 times with PBS, and THP-1 cells were immediately added to the upper compartment. After trans-well plates were incubated for 2 hours, cells in the upper chamber of the filter were aspirated, and the nonmigrating cells on top of the filter were removed with a cotton swab. THP-1 cells on the lower side of the filter were fixed with 8% glutaraldehyde and stained with 0.25% crystal violet in 20% methanol (wt/vol). Each experiment was repeated in duplicate wells, and within each well counting was done in 9 randomly selected microscopic high-power fields.
Analysis of expression of cell surface receptors
The expression of VCAM-1, ICAM-1, and E-selectin on HUVECs was determined by a whole-cell ELISA as described.29 Briefly, cell monolayers, which were treated for 3 hours with indicated proteases, were incubated with either HMGB1 (1 μg/mL for 16 hours) or LPS (100 ng/mL for 4 hours) and then fixed in 1% paraformaldehyde. After washing 3 times, mouse anti–human mAbs to VCAM-1, ICAM-1, and E-selectin (Chemicon International) were added. After 1 hour (37°C, 5% CO2), cells were washed, and peroxidase-conjugated anti–mouse IgG (Sigma-Aldrich) was added for 1 hour. Cells were washed again and developed using o-phenylenediamene substrate (Sigma-Aldrich). All measurements were performed in triplicate wells and repeated at least twice.
The same experimental procedures were used to monitor the cell surface expression of TLR2, TLR4, and RAGE receptors with the use of specific Abs (A-9, H-80, and A-9, respectively) obtained from Santa Cruz Biotechnology Inc.
RNA interference
The expression of inflammatory mediators (NF-κB and TNF-α) by endothelial cells in response to HMGB1 (1 μg/mL for 16 hours) was evaluated after the knockdown of TLR2, TLR4, and RAGE expression by pools of target-specific 20- to 25-nucleotide small interfering RNAs (siRNAs) obtained from Santa Cruz Biotechnology Inc, according to the manufacturer's instruction and as described.27 A nontargeting 20- to 25-nucleotide siRNA obtained from the same company was used as a negative control.
HMGB1 degradation assay
The degradation of HMGB1 by PC-gla/MeizoTh and APC was monitored by SDS-PAGE as described.25 Briefly, HMGB1 (400 nmol/L) was incubated with 2nM PC-gla/MeizoTh with or without 400 nmol/L recombinant TM for 15-120 minutes at 37°C in 50mM Tris-HCl (pH 8.0), 2mM CaCl2, and 0.1M NaCl in a total volume of 50 μL.25 These samples were then run on SDS-PAGE (10%, reducing) followed by immunoblot analysis with the use of a rabbit polyclonal Ab against HMGB1 (Abcam). The same methods were used to monitor the cleavage of HMGB1 by 100nM APC in the absence and presence of 120 nmol/L soluble EPCR (kindly provided by Dr Charles Esmon, Oklahoma Medical Research Foundation) and 25μM phospholipid vesicles composed of 40% phosphatidylcholine, 20% phosphatidylserine, and 40% phosphatidylethanolamine (PCPSPE), and 120nM/L recombinant TM.
Statistical analysis
Data are expressed as means ± SDs from ≥ 3 independent experiments. Statistical significance between 2 groups was determined by Student t test. The significance level was set at P < .05.
Results
Effect of APC on the LPS-induced HMGB1 release
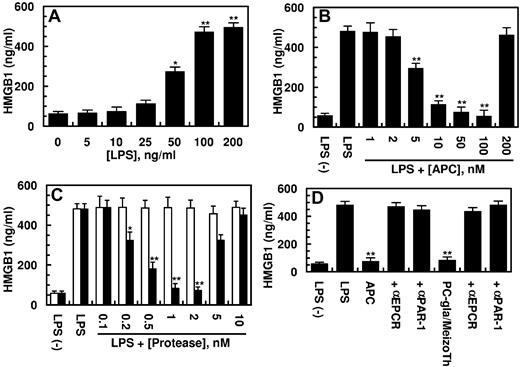
Previous studies have showm that HMGB1 can be released from human endothelial cells in response to both endotoxin and TNF-α.9-11 After its release to intravascular spaces, HMGB1 is known to interact with specific cell surface receptors to amplify inflammatory responses by inducing the expression of proinflammatory cytokines.8-11 In agreement with previous results, we show that LPS stimulates the HMGB1 release by HUVECs by a concentration-dependent manner (Figure 1A). The LPS-mediated HMGB1 release occurred with late kinetics of 8 hours after stimulation by LPS and reached its peak level after 16 hours (data not shown). To investigate the effect of APC on the LPS-mediated HMGB1 release, endothelial cells were pretreated with increasing concentrations of APC for 3 hours before stimulation of cells with 100 ng/mL LPS for 16 hours. The results presented in Figure 1B indicated that APC inhibits the HMGB1 release by endothelial cells by a concentration-dependent manner. Significant inhibition of the LPS-mediated HMGB1 release could be observed at 5nM APC; however, the optimal inhibition required an APC concentration of 100nM (Figure 1B). We recently replaced the Gla domain of the thrombin activation intermediate product, meizothrombin, with the corresponding domain of APC and showed that the resulting mutant protease (PC-gla/MeizoTh) elicits a potent barrier-protective effect in endothelial cells in response to proinflammatory mediators, including LPS and TNF-α.20,26 The results presented in Figure 1C show that PC-gla/MeizoTh was also a potent inhibitor of HMGB1 release by endothelial cells with its optimal effect occurring at a concentration of < 1nM. By contrast, wild-type MeizoTh exhibited no protective activity in this assay (Figure 1C white bars), suggesting that the interaction of the Gla-domain of APC with EPCR is a prerequisite for the protective activity of the mutant protease. The HMGB1 inhibitory function of APC was eliminated at a supraphysiologic concentration of the protease > 200nM (Figure 1B). This was also true for the PC-gla/MeizoTh concentration of > 10nM (Figure 1C). These results are consistent with previous observations that the cytoprotective activity of APC in endothelial cells is also diminished at high and nonphysiologic concentrations of the protease.22,26 The inhibitory effects of both APC and PC-gla/MeizoTh toward HMGB1 required the EPCR-dependent activation of PAR-1 as evidenced by the function-blocking Abs to both receptors abrogating the protective effects (Figure 1D).
Effect of APC on the LPS-mediated release of HMGB1. (A) HUVECs were stimulated with indicated concentrations of LPS for 16 hours, and the release of HMGB1 was measured by an ELISA as described in “Methods.” (B) The LPS (100 ng/mL) mediated HMGB1 release by HUVECs was monitored after treating the cell monolayer with indicated concentrations of APC for 3 hours. (C) The same as panel B, except that cells were incubated with increasing concentrations of meizothrombin (MeizoTh; white bars) or PC-gla/MeizoTh (black bars). (D) The same as panels B or C, except that cells were preincubated with function-blocking Abs to PAR-1 or EPCR (25 μg/mL for 30 minutes) before treating cells with each protease (100nM APC, 2nM PC-gla/MeizoTh). All results are shown as means ± SDs of 5 different experiments. *P < .05 and **P < .01 compared with 0 (A) or LPS (B-D).
Effect of APC on the LPS-mediated release of HMGB1. (A) HUVECs were stimulated with indicated concentrations of LPS for 16 hours, and the release of HMGB1 was measured by an ELISA as described in “Methods.” (B) The LPS (100 ng/mL) mediated HMGB1 release by HUVECs was monitored after treating the cell monolayer with indicated concentrations of APC for 3 hours. (C) The same as panel B, except that cells were incubated with increasing concentrations of meizothrombin (MeizoTh; white bars) or PC-gla/MeizoTh (black bars). (D) The same as panels B or C, except that cells were preincubated with function-blocking Abs to PAR-1 or EPCR (25 μg/mL for 30 minutes) before treating cells with each protease (100nM APC, 2nM PC-gla/MeizoTh). All results are shown as means ± SDs of 5 different experiments. *P < .05 and **P < .01 compared with 0 (A) or LPS (B-D).
Effects of APC on the HMGB1-mediated CAM expression, THP-1 adhesion, and migration
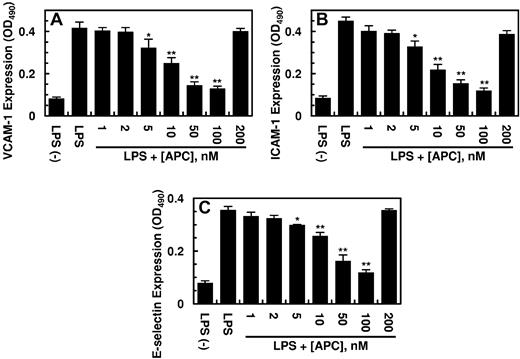
Previous results have indicated that HMGB1 mediates proinflammatory responses by increasing the expression of cell adhesion molecules ICAM-1, VCAM-1, and E-selectin on the surface of endothelial cells, thereby promoting the adhesion and migration of leukocytes across the endothelium.9-11 As presented in Figure 2, HMGB1 up-regulated the cell surface expression of all 3 adhesion molecules (CAM), and APC inhibited this effect of HMGB1 by a concentration-dependent manner. The inhibitory effect of APC toward the expression of CAMs was mediated through APC down-regulating the HMGB1 signaling pathway. The cell surface expression of CAMs was also up-regulated by LPS, and APC inhibited the expression of all 3 adhesion molecules (Figure 3). The elevated expression of CAMs correlated well with an enhanced binding of THP-1 cells to the HMGB1-activated endothelial cells and their subsequent migration across the monolayer (Figure 4A-B). APC down-regulated the adherence of THP-1 cells and their migration across activated endothelial monolayer by a concentration-dependent manner (Figure 4A-B). These results suggest that APC not only inhibits the endotoxin-mediated release of HMGB1 by endothelial cells but also down-regulates the proinflammatory signaling effect of the released HMGB1, thereby inhibiting the amplification of the proinflammatory pathways by the nuclear cytokine. Consistent with the data presented earlier, PC-gla/MeizoTh, but not wild-type MeizoTh, inhibited both LPS- and HMGB1-mediated cell surface expressions of all 3 CAMs (data not shown) and inhibited the adherence and migration of THP-1 cells across HMGB1-activated endothelial cell monolayer with ∼ 20- to 50-fold higher efficacy (Figure 4C-D). A similar 20- to 50-fold higher efficacy for PC-gla/MeizoTh was observed in inhibition of LPS- and HMGB1-mediated up-regulation of CAMs on endothelial cells (data not shown). The anti-inflammatory effects of both APC and PC-gla/MeizoTh required the EPCR-dependent cleavage of PAR-1 because the function-blocking Abs to both receptors abrogated these effects (data not shown).
Effect of APC on the HMGB1-mediated expression of cell adhesion molecules in HUVECs. Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) after treating cells with indicated concentrations of APC for 3 hours. The cell surface expression of VCAM-1 (A), ICAM-1 (B), and E-selectin (C) on HUVECs was measured by a cell-based ELISA as described in “Methods.” All results are shown as means ± SDs of 5 different experiments. *P < .05 and **P < .01 compared with HMGB1.
Effect of APC on the HMGB1-mediated expression of cell adhesion molecules in HUVECs. Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) after treating cells with indicated concentrations of APC for 3 hours. The cell surface expression of VCAM-1 (A), ICAM-1 (B), and E-selectin (C) on HUVECs was measured by a cell-based ELISA as described in “Methods.” All results are shown as means ± SDs of 5 different experiments. *P < .05 and **P < .01 compared with HMGB1.
Effect of APC on the LPS-mediated expression of cell adhesion molecules in HUVECs. Confluent HUVECs were incubated with LPS (100 ng/mL for 4 hours) after treating cells with indicated concentrations of APC for 3 hours. The cell surface expression of VCAM-1 (A), ICAM-1 (B), and E-selectin (C) on HUVECs was measured by a cell-based ELISA as described in “Methods.” All results are shown as means ± SDs of 2 different experiments with triplicate wells. *P < .05 and **P < .01 compared with LPS.
Effect of APC on the LPS-mediated expression of cell adhesion molecules in HUVECs. Confluent HUVECs were incubated with LPS (100 ng/mL for 4 hours) after treating cells with indicated concentrations of APC for 3 hours. The cell surface expression of VCAM-1 (A), ICAM-1 (B), and E-selectin (C) on HUVECs was measured by a cell-based ELISA as described in “Methods.” All results are shown as means ± SDs of 2 different experiments with triplicate wells. *P < .05 and **P < .01 compared with LPS.
Analysis of the HMGB1-mediated THP-1 adhesion and migration in HUVECs. (A) Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) after treating cells with indicated concentrations of APC for 3 hours, and the THP-1 adherence to HUVECs was monitored as described in “Methods.” (B) The HMGB1 (1 μg/mL for 16 hours) mediated migration of THP-1 across HUVEC monolayers was analyzed after treating cells with indicated concentrations of APC. (C-D) The same as panels A and B, except that MeizoTh (white bars) or PC-gla/MeizoTh (black bars) were used to treat cells for 3 hours before stimulation by LPS. All results are shown as means ± SDs of 5 different experiments. *P < .05 and **P < .01 compared with HMGB1.
Analysis of the HMGB1-mediated THP-1 adhesion and migration in HUVECs. (A) Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) after treating cells with indicated concentrations of APC for 3 hours, and the THP-1 adherence to HUVECs was monitored as described in “Methods.” (B) The HMGB1 (1 μg/mL for 16 hours) mediated migration of THP-1 across HUVEC monolayers was analyzed after treating cells with indicated concentrations of APC. (C-D) The same as panels A and B, except that MeizoTh (white bars) or PC-gla/MeizoTh (black bars) were used to treat cells for 3 hours before stimulation by LPS. All results are shown as means ± SDs of 5 different experiments. *P < .05 and **P < .01 compared with HMGB1.
Effect of APC on the HMGB1-mediated NF-κB activation and TNF-α expression
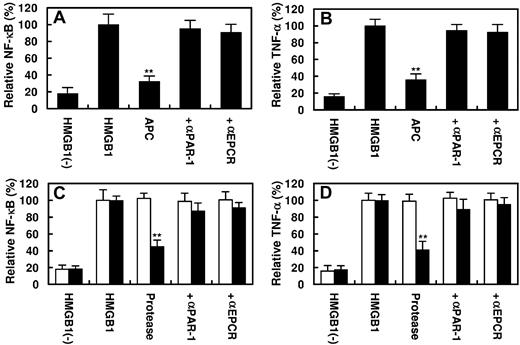
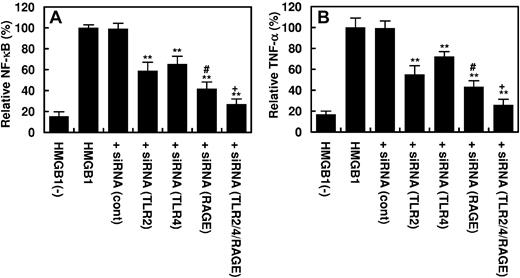
It is known that HMGB1 up-regulates inflammatory pathways by activating NF-κB and promoting the expression of TNF-α by endothelial cells and monocytes.4,8,9 As presented in Figure 5, HMGB1 activated NF-κB and stimulated the expression of TNF-α by endothelial cells. APC inhibited both signaling pathways by EPCR- and PAR-1–dependent mechanisms (Figure 5A-B). The PC-gla/MeizoTh mutant elicited a similar protective effect but required a significantly lower concentration of the protease to inhibit the HMGB1-mediated activation of NF-κB as well as the expression of TNF-α by endothelial cells (Figure 5C-D). Further studies showed that the proinflammatory activity of HMGB1 is mediated through its interaction and subsequent signaling through ≥ 3 cell surface receptors TLR2, TLR4, and RAGE because specific siRNAs for each receptor significantly inhibited both NF-κB activation and TNF-α expression by endothelial cells in response to HMGB1 (Figure 6A-B). All 3 receptors appeared to be involved in HMGB1 signaling in endothelial cells because transfecting cells with the combination of all 3 siRNAs exhibited a statistically significant additive effect in inhibiting the inflammatory mediators (Figure 6A-B). Relative to TLR2 and TLR4, the siRNA for RAGE exhibited a significantly higher inhibitory effect, suggesting that RAGE may play a dominant role in mediating the HMGB1 signaling in endothelial cells.
Effect of APC or PC-gla/MeizoTh on the HMGB1-mediated NF-κB activation and TNF-α expression. Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) after treating cells with APC (100nM) for 3 hours. The activation of NF-κB (A) or the induction of TNF-α (B) in HUVECs was analyzed as described in “Methods.” In the presence of Abs, cells were first preincubated with function-blocking Abs to PAR-1 or EPCR (25 μg/mL for 30 minutes) before treating cells with APC. (C-D) The same as panels A and B, except that instead of 100nM APC, 2nM MeizoTh (white bars) or 2nM PC-gla/MeizoTh (black bars) was used in the experiments. All results are shown as means ± SDs of 5 different experiments. **P < .01 compared with HMGB1.
Effect of APC or PC-gla/MeizoTh on the HMGB1-mediated NF-κB activation and TNF-α expression. Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) after treating cells with APC (100nM) for 3 hours. The activation of NF-κB (A) or the induction of TNF-α (B) in HUVECs was analyzed as described in “Methods.” In the presence of Abs, cells were first preincubated with function-blocking Abs to PAR-1 or EPCR (25 μg/mL for 30 minutes) before treating cells with APC. (C-D) The same as panels A and B, except that instead of 100nM APC, 2nM MeizoTh (white bars) or 2nM PC-gla/MeizoTh (black bars) was used in the experiments. All results are shown as means ± SDs of 5 different experiments. **P < .01 compared with HMGB1.
The Effect of siRNA knockdown of pattern recognition receptors on the HMGB1-mediated NF-κB activation and TNF-α expression in HUVECs. Confluent HUVECs were transfected with the control siRNA (1 μg for 3 days) or siRNA (1 μg for 3 days) specific for TLR2, TLR4, and RAGE individually or in combination of 3 before incubating cells with HMGB1 (1 μg/mL for 16 hours). The activation of NF-κB (A) or the induction of TNF-α (B) in HUVECs was analyzed as described in “Methods.” **P < .01 compared with HMGB1; #P < .05 compared with TLR2; #P < .02 compared with TLR4; +P < .05 compared with RAGE; and +P < .02 compared with either TLR2 or TLR4.
The Effect of siRNA knockdown of pattern recognition receptors on the HMGB1-mediated NF-κB activation and TNF-α expression in HUVECs. Confluent HUVECs were transfected with the control siRNA (1 μg for 3 days) or siRNA (1 μg for 3 days) specific for TLR2, TLR4, and RAGE individually or in combination of 3 before incubating cells with HMGB1 (1 μg/mL for 16 hours). The activation of NF-κB (A) or the induction of TNF-α (B) in HUVECs was analyzed as described in “Methods.” **P < .01 compared with HMGB1; #P < .05 compared with TLR2; #P < .02 compared with TLR4; +P < .05 compared with RAGE; and +P < .02 compared with either TLR2 or TLR4.
APC down-regulates the expression of HMGB1 receptors
We next investigated the effect of HMGB1 on the stimulation of its own receptors and the effect of APC on modulation of the expression of these receptors in endothelial cells. As presented in Figure 7A, HMGB1 induced the expression of all 3 receptors TLR2, TLR4, and RAGE by endothelial cells ∼ 2.5- to 3-fold. Interestingly, APC significantly inhibited the stimulatory effect of HMGB1 on all 3 receptors (Figure 7A). The same results were observed with PC-gla/MeizoTh, except that a markedly lower concentration of the protease was required to obtain a similar inhibitory effect on the expression of the receptors (Figure 7A). The stimulatory effect of HMGB1 on the expression of RAGE was higher than the other 2 receptors, and the inhibitory effect of the proteases on the expression of this receptor was also more pronounced. Similar to HMGB1, LPS also stimulated the expression of TLR2, TLR4, and RAGE on the surface of endothelial cells, and both APC and PC-gla/MeizoTh inhibited the LPS-mediated expression of all 3 receptors (Figure 7B). Similar to the results presented earlier, a significantly lower concentration of PC-gla/MeizoTh was required to effectively inhibit the LPS-mediated expression of these proinflammatory receptors on endothelial cells.
Effect of APC on the HMGB1- and LPS-mediated expression of pattern recognition receptors on HUVECs and the proteolytic cleavage of HMGB1 by PC-gla/MeizoTh. (A) Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) with or without pretreating cells with protein C (100nM), APC (100nM), MeizoTh (2nM), and PC-gla/MeizoTh (2nM) for 3 hours as described in “Methods.” The expression of TLR2 (white bars), TLR4 (gray bars), and RAGE (black bars) on HUVECs was measured by a cell-based ELISA as described in “Methods.” (B) The same as panel A except that, instead of HMGB1, LPS (100 ng/mL for 4 hours) was used to stimulate HUVECs. All results are shown as means ± SDs of 5 different experiments. **P < .01 compared with HMGB1 in panel A and LPS in panel B.
Effect of APC on the HMGB1- and LPS-mediated expression of pattern recognition receptors on HUVECs and the proteolytic cleavage of HMGB1 by PC-gla/MeizoTh. (A) Confluent HUVECs were incubated with HMGB1 (1 μg/mL for 16 hours) with or without pretreating cells with protein C (100nM), APC (100nM), MeizoTh (2nM), and PC-gla/MeizoTh (2nM) for 3 hours as described in “Methods.” The expression of TLR2 (white bars), TLR4 (gray bars), and RAGE (black bars) on HUVECs was measured by a cell-based ELISA as described in “Methods.” (B) The same as panel A except that, instead of HMGB1, LPS (100 ng/mL for 4 hours) was used to stimulate HUVECs. All results are shown as means ± SDs of 5 different experiments. **P < .01 compared with HMGB1 in panel A and LPS in panel B.
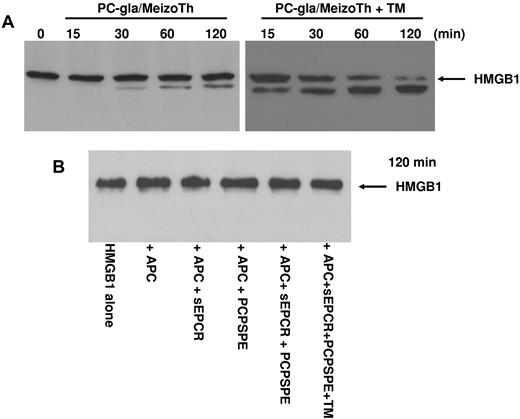
PC-gla/MeizoTh in complex with TM cleaves HMGB1
Previous results have indicated that thrombin in complex with TM can inhibit the proinflammatory effect of HMGB1 by cleaving the protein.25 Because the TM-binding exosite-1 of thrombin is also expressed on meizothrombin,30 we wondered whether PC-gla/MeizoTh can cleave HMGB1 in the presence of TM. The results presented in Figure 8A indicated that PC-gla/MeizoTh can proteolytically cleave HMGB1 and that TM can function as a cofactor to markedly accelerate the cleavage reaction. By contrast, APC had no direct proteolytic effect on HMGB1 because its incubation with 100nM APC in the absence and presence of soluble EPCR or PCPSPE vesicles or both and TM did not lead to degradation of HMGB1 even after 2 hours of incubation at 37°C (Figure 8B).
Analysis of the proteolytic activity of PC-gla/MeizoTh and APC toward HMGB1. (A) The time course of cleavage of HMGB1 by PC-gla/MeizoTh (2nM) was monitored at 37°C in the absence and presence of TM by SDS-PAGE (10% under reducing conditions) followed by immunoblotting as described in “Methods.” (B) The same as panel A except that APC (100nM) was used to monitor the cleavage of HMGB1 for 120 minutes in the absence and presence of soluble EPCR (sEPCR; 120nM); PCPSPE (25μM); sEPCR + PCPSPE; or sEPCR + PCPSPE + recombinant TM (120nM).
Analysis of the proteolytic activity of PC-gla/MeizoTh and APC toward HMGB1. (A) The time course of cleavage of HMGB1 by PC-gla/MeizoTh (2nM) was monitored at 37°C in the absence and presence of TM by SDS-PAGE (10% under reducing conditions) followed by immunoblotting as described in “Methods.” (B) The same as panel A except that APC (100nM) was used to monitor the cleavage of HMGB1 for 120 minutes in the absence and presence of soluble EPCR (sEPCR; 120nM); PCPSPE (25μM); sEPCR + PCPSPE; or sEPCR + PCPSPE + recombinant TM (120nM).
Discussion
In this study, we have demonstrated for the first time that APC inhibits the LPS-mediated secretion of HMGB1 by endothelial cells as well as the HMGB1-mediated proinflammatory signaling responses in endothelial cells by EPCR- and PAR-1–dependent mechanisms. The inhibitory effect of APC was concentration dependent with 5nM APC significantly down-regulating the HMGB1 secretion and signaling. The inhibitory effect of APC was optimal at a concentration of 100nM. APC also down-regulated both the LPS- and HMGB1-mediated expression of the cell surface endothelial cell adhesion molecules, ICAM-1, VCAM-1, and E-selectin, thereby inhibiting both the interaction of the monocytic THP-1 cells with the activated endothelial cells and their subsequent migration across the monolayer. The in vitro anti-inflammatory effects of APC were mediated through the protease inhibiting the HMGB1-mediated activation of the NF-κB pathway as well as suppressing the induction of TNF-α in endothelial cells in response to HMGB1. APC not only down-regulated the expression of HMGB1 and its proinflammatory signaling through the inhibition of the NF-κB pathway, but it also down-regulated the cell surface expression of the 3 receptors, TLR2, TLR4, and RAGE, that are known to bind HMGB1 to initiate proinflammatory responses in endothelial cells.6-8 In support of these receptors mediating the intracellular signaling activities of HMGB1, the specific siRNA for each receptor significantly inhibited the signaling function of HMGB1. The simultaneous transfection of endothelial cells with siRNA for all 3 receptors resulted in a statistically significant additive inhibitory effect, suggesting that all 3 receptors contribute to HMGB1 signaling, alhough the specific siRNA for RAGE appeared to be the most effective inhibitor of HMGB1 signaling in endothelial cells. APC also inhibited the expression of RAGE more effectively than the expression of the other 2 receptors. Similar to HMGB1, LPS also up-regulated the expression of all 3 pattern recognition receptors on endothelial cells and APC down-regulated this effect, suggesting that APC can inhibit the amplification of proinflammatory pathways propagated by LPS and HMGB1 during infection.
Activation of the endothelium by proinflammatory cytokines during infection plays an important pathophysiologic role in the chain of events that may lead to the septic shock/severe sepsis syndrome. The results presented here, together with those previously reported by others,9-11 suggest that vascular endothelial cells may be rich sources of HMGB1, which can be released in response to bacterial endotoxin and endogenously expressed proinflammatory cytokines, thereby contributing to the pathology of severe sepsis. In support of HMGB1 playing an important pathologic role in severe sepsis, it has been found that high plasma levels of HMGB1 in patients with severe sepsis and in animal models of endotoxemia correlate with higher mortality.5,12 Moreover, the administration of exogenous HMGB1 to experimental animals has led to an elaboration of severe inflammatory responses, tissue injury, and death.1,5,11 Further support for a deleterious effect for HMGB1 in severe sepsis is provided by the observations that neutralizing Abs, chemical inhibitors, and antagonists of HMGB1 release have all protected animals from the lethality of endotoxemia.5 Thus, the finding that APC inhibits the LPS-mediated secretion of HMGB1 and its proinflammatory signaling function through the 3 receptors TLR2, TLR4, and RAGE strongly suggests that the APC inhibition of this late-acting inflammatory mediator may contribute to its mortality-reducing protective activity against severe sepsis.
It was interesting to note that, unlike meizothrombin that up-regulated the HMGB1 signaling pathway, the chimeric meizothrombin mutant containing the Gla-domain of protein C (PC-gla/MeizoTh) inhibited the expression of HMGB1 and its signaling function through the same 3 cell surface receptors with ∼ 20- to 50-fold higher efficacy than APC. These results are in agreement with our previous observation showing that PC-gla/MeizoTh efficiently protects HUVECs from the barrier disruptive and cytotoxic effects of LPS and TNF-α, supporting the hypothesis that EPCR modulates the PAR-1–dependent signaling specificity of coagulation proteases.20,26 We previously demonstrated that PC-gla/MeizoTh binds EPCR with an affinity (KD ∼ 30nM) that is essentially identical to that observed with APC.26 However, similar to thrombin, PC-gla/MeizoTh can cleave the cell surface PAR-1 with ∼ 3 orders of magnitude higher efficiency than APC,26 possibly accounting for its markedly improved anti-inflammatory signaling activity in endothelial cells. These results provide further support for our hypothesis that, when EPCR is occupied by the Gla-domain of protein C, the activation of PAR-1 by coagulation proteases initiates protective responses in endothelial cells exposed to proinflammatory mediators.20,26 Noting its 20- to 50-fold improved efficacy, PC-gla/MeizoTh may have therapeutic superiority over APC in treating patients with severe sepsis. In this context, it is also of interest to note that meizothrombin has minimal procoagulant activity because, unlike thrombin, it cannot effectively cleave fibrinogen, but similar to thrombin it can bind to TM to rapidly catalyze the activation of protein C to APC.30 Thus, a further advantage of PC-gla/MeizoTh as a potential therapeutic molecule is that it can activate protein C when it binds to TM, thereby amplifying the anti-inflammatory responses through the APC pathway. Moreover, similar to the thrombin-TM complex,25 PC-gla/MeizoTh in complex with TM can cleave HMGB1 to down-regulate its proinflammatory signaling activities by a proteolytic pathway. This is in agreement with previous results showing that the thrombin-cleaved HMGB1 has significantly decreased proinflammatory properties.25 Thus, in vivo studies with PC-gla/MeizoTh in animal models of endotoxemia is warranted to assess the anti-inflammatory properties of this highly interesting molecule.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Audrey Rezaie for proofreading the manuscript.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0003410 and 2010-0022296, J.-S.B.) and the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants HL 101917 and HL 68571, A.R.R.).
National Institutes of Health
Authorship
Contribution: J.-S.B. designed experiments, performed research, and analyzed data; and A.R.R. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jong-Sup Bae, College of Pharmacy, Research Institute of Pharmaceutical Sciences, Kyungpook National University, 1370 Sankyuk-dong, Buk-gu, Daegu 702-701, Republic of Korea; e-mail: baejs@knu.ac.kr; and Alireza R. Rezaie, Department of Biochemistry and Molecular Biology, St Louis University School of Medicine, 1100 S Grand Blvd, St Louis, MO 63104; e-mail: rezaiear@slu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal