Before the introduction of imatinib, biologic therapy with IFN-α was the most promising treatment option for CML because it induced durable remissions in a subset of chronic-phase patients.1 However, because of its toxicity as well as its limited and unpredictable efficacy, IFN-α therapy has been displaced by Bcr-Abl kinase inhibitors, which have limited toxicity and marked efficacy in the treatment of most CML patients.
While imatinib is clearly a remarkable drug, it also has limitations. The most obvious are the development of resistant disease (which can largely be overcome by second- and third-generation kinase inhibitors) and the limited effects of imatinib (as well as other kinase inhibitors) on the leukemic stem or initiating cell.2,3 Some preliminary clinical observations suggested that prior IFN-α therapy suppressed relapsing leukemia on termination of imatinib.4,5 Recently, combination therapy with imatinib/IFN-α (pegylated IFN-α-2a/b) has been explored as a possible means of deepening the molecular response (4-log reduction in Bcr-Abl/c-Abl ratio) in newly diagnosed CML patients. Two recent studies showed that IFN-α therapy does have this potential, albeit with predicted, but partially manageable, side effects.6,7 Although these studies support the clinical benefit of adding IFN-α to imatinib therapy, several unanswered questions remain. First, does IFN-α primarily act against the leukemic cell or are other effectors (ie, immune cells) playing a prominent yet indirect role? Second, what are the potential biochemical mechanisms and intersecting cascades that underlie the improved activity of imatinib in the presence IFN-α? The article by Bhattacharya et al in this issue of Blood provides some insight and answers to both of these questions.8
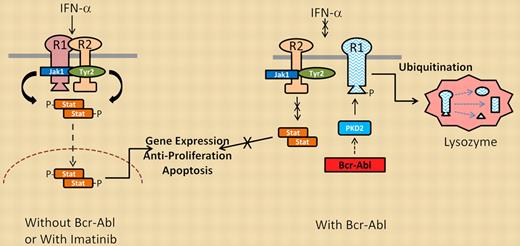
Interferon signaling in the absence (left) or presence (right) of Bcr-Abl kinase activity.
Interferon signaling in the absence (left) or presence (right) of Bcr-Abl kinase activity.
Bhattacharya and colleagues used 2 imatinib/IFN-α–responsive CML cell lines to initially demonstrate that the combination of these 2 agents was better in reducing cell viability than either agent alone. They further showed that imatinib potentiated IFN-α signal transduction through effects on one of the IFN-α receptor proteins (IFNAR1). IFNAR1 undergoes phosphorylation by receptor complex–associated kinases (Jak1, Tyr2) as well as downstream serine/threonine kinases (PKD2, CK1a) that phosphorylate IFNAR1 at sites essential for its proteolytic destruction. This phosphorylation sequence may be essential in attenuating the duration of IFN-α signaling under normal conditions. However, cells expressing Bcr-Abl (even cells transformed by mechanisms independent of Bcr-Abl) activate PKD2 through its tyrosine phosphorylation (Y438), resulting in serine phosphorylation (S535) of IFNAR1. Phosphorylated IFNAR1 can then be recognized by ubiquitin ligases, which promote its ubiquitination, endocytosis, and lysosomal degradation. Therefore, Bcr-Abl kinase activity reduces surface expression of functional IFN-α receptors, which in turn attenuates IFN-α signal transduction, as outlined in the figure. Treatment with imatinib blocks PKD2 activation and suppresses S535 IFNAR1 phosphorylation to significantly increase the stability and signaling activity of the IFN-α receptor complex. This effect may explain the clinical benefit of adding IFN-α to CML patients on imatinib therapy.
The Bcr-Abl/IFNAR1 signaling framework provided by Bhattacharya et al provides a roadmap for additional analyses. Individual phosphoproteins in this cascade can be measured to assess their role in IFN-α activity. For example, does basal PKD2 activity in CML cells correlate with intrinsic sensitivity to IFN-α or predict IFN-α impact in imatinib-treated patients? Does PKD2 protect the CML cells that do not undergo apoptosis in response to Bcr-Abl inhibition even though they have activated Bcr-Abl (ie, CML stem cells or niche-protected cells)? Can these observations help explain the clinical activity of IFN-α before the introduction of imatinib, or does the IFN-α response have little to do with direct activity against CML cells and instead function through other effector cells? As suggested by early studies and supported by recent animal models, IFN-α may directly target CML stem cells.9 Conversely, how do effector cells contribute to durable remissions in IFN-α–treated CML patients (as an association has been described)1,10 ? The precise answers may still be years away, but the important observations of Bhattacharya and colleagues provide important leads that can now begin to address the complexity of IFN-α responsiveness in CML, from early observations of its clinical activity to its role in the era of targeted therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■