Abstract
Bone morphogenetic protein (BMP) signaling induces hepatic expression of the peptide hormone hepcidin. Hepcidin reduces serum iron levels by promoting degradation of the iron exporter ferroportin. A relative deficiency of hepcidin underlies the pathophysiology of many of the genetically distinct iron overload disorders, collectively termed hereditary hemochromatosis. Conversely, chronic inflammatory conditions and neoplastic diseases can induce high hepcidin levels, leading to impaired mobilization of iron stores and the anemia of chronic disease. Two BMP type I receptors, Alk2 (Acvr1) and Alk3 (Bmpr1a), are expressed in murine hepatocytes. We report that liver-specific deletion of either Alk2 or Alk3 causes iron overload in mice. The iron overload phenotype was more marked in Alk3- than in Alk2-deficient mice, and Alk3 deficiency was associated with a nearly complete ablation of basal BMP signaling and hepcidin expression. Both Alk2 and Alk3 were required for induction of hepcidin gene expression by BMP2 in cultured hepatocytes or by iron challenge in vivo. These observations demonstrate that one type I BMP receptor, Alk3, is critically responsible for basal hepcidin expression, whereas 2 type I BMP receptors, Alk2 and Alk3, are required for regulation of hepcidin gene expression in response to iron and BMP signaling.
Introduction
The hepatic hormone hepcidin regulates serum iron levels in mice and humans by inducing degradation of the iron exporter, ferroportin.1,2 Low ferroportin levels reduce intestinal iron absorption and the release of iron from macrophage stores. Human hereditary hemochromatosis is characterized by low hepcidin levels, leading to iron accumulation in liver, heart, and endocrine organs.1,3 Similarly, hepcidin deficiency causes hepatic iron overload in mice.2,4 In contrast, high hepcidin levels contribute to the anemia of chronic disease (ACD) by reducing iron bioavailability for erythropoiesis.5,6
Recent studies have demonstrated a critical role for bone morphogenetic protein (BMP) signaling in the regulation of hepcidin expression by iron.7-11 Binding of BMP ligands to type I and type II BMP receptors induces the type II receptor to phosphorylate and activate the type I receptor. The activated type I receptor, in turn, phosphorylates intracellular signaling molecules, including SMADs 1, 5, and 8. Phosphorylated SMADs 1, 5, and 8 bind SMAD4 and together translocate to the nucleus, where they activate the expression of genes, including hepcidin and the Id family of transcription factors.12 Deficiency of Smad4,10 the BMP coreceptor hemojuvelin,13,14 or BMP615,16 in hepatocytes reduces expression of hepcidin17,18 and induces iron overload. In addition, BMP signaling appears to have an important role in the induction of hepcidin expression by inflammatory mediators that are involved in ACD.11,19,20
There are 4 type I BMP receptors: Alk1, Alk2, Alk3, and Alk6. The identity of the type I BMP receptor(s) responsible for iron-dependent signaling and the regulation of hepcidin expression in hepatocytes are unknown. Alk1 is predominantly expressed in the endothelium. Alk6 is expressed at low levels in murine liver,21 and global Alk6 deficiency does not induce iron overload in mice (D. R. Campagna, P. J. Schmidt, and M.D.F., unpublished observations, January 2011). In contrast, Alk2 and Alk3 are abundantly expressed in hepatocytes.21 To identify the type I BMP receptor required for the BMP-mediated regulation of hepatic hepcidin expression and iron homeostasis, we characterized the phenotypes of mice with liver-specific deletion of either the Alk2 or Alk3 receptor. In addition, we studied the impact of Alk2 or Alk3 deficiency on BMP signaling in isolated hepatocytes and on the response to iron challenge in mice.
Methods
Animals
All mouse experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Alk2+/fl mice on a mixed C57BL/6; SV129 background or Alk3+/fl mice on a C57BL/6 background22,23 were bred to B6.Cg-Tg(Alb-Cre)21Mgn/J mice (The Jackson Laboratory) to generate compound heterozygous animals that were intercrossed to obtain second-generation (F2) animals homozygous for the Alk2fl or Alk3fl alleles with or without the Alb-Cre transgene. Mice were fed a standard, iron-replete diet (Prolab 5P75 Isopro 3000, 380 ppm iron). Twelve-week- old female mice were used for phenotypic characterization, and 8- to 12-week-old female mice were used in iron challenge experiments.
For studies of the impact of iron challenge on hepcidin gene expression, mice were injected with 0.2 g/kg dextran or iron dextran (Sigma-Aldrich) via a tail vein, as described previously,11 and blood and tissue samples were harvested 2 hours after injection.
Hematologic and iron parameters
Blood for serum iron parameters and complete blood count was obtained by retro-orbital puncture. Serum iron concentrations and transferrin saturations were determined using Iron/UIBC Kit (Thermo Scientific). Complete blood counts were measured using a HemaVet Veterinary Analyzer (Heska). After mice were killed, liver, kidney, and spleen tissues were harvested, and non-heme tissue iron levels were determined, as previously described.13
Hepatic mRNA levels
Total RNA was extracted from mouse hepatocytes and liver tissues using Trizol (Invitrogen). Reverse RNA transcription was accomplished using MMLV-RT (Promega). Quantitative RT-PCR was performed using SYBR Green (Thermo Fisher Scientific) or Kapa Probe (for TaqMan primers) on a Mastercycler Realplex2 (Eppendorf) using primers listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The level of target transcripts was normalized to the level of 18S rRNA using the relative CT method.
Prussian blue staining and diaminobenzidine enhancement
Prussian blue staining was performed on paraffin-embedded sections, as previously described.13 To enhance iron staining, paraffin-embedded hepatic sections from Alk2fl/fl and Alk2fl/fl; Alb-Cre mice were stained with Prussian blue and then incubated with diaminobenzidine for 2 minutes at room temperature, yielding a brown reaction product. Sections were analyzed by light microscopy with a Nikon Eclipse 80i microscope (Nikon Instruments).
Primary hepatocyte cultures
Hepatocytes were isolated and cultured for 2 days using a bottom and top layer of collagen, called a “collagen sandwich,” as described previously.24 After serum starvation for 6 hours in 0.1% FBS, hepatocytes were stimulated with and without BMP2 (100 ng/mL), and RNA was harvested 2 hours later.
Statistics
All values were expressed as mean ± SEM. Data were analyzed using the Student t test and 1-way ANOVA with Bonferroni posthoc tests, when applicable. Statistical significance was considered for P values < .05.
Results
Liver-specific deletion of Alk2 and Alk3 leads to iron overload in mice
Mice that are globally deficient in either Alk2 or Alk3 are not viable.25-28 Therefore, to characterize the potential roles of each BMP type I receptor in the regulation of hepatocellular hepcidin expression and systemic iron metabolism, we studied mice with liver-specific deletion of Alk2 or Alk3. To delete Alk2 or Alk3 selectively in hepatocytes, we generated mice homozygous for conditionally targeted (“floxed”) Alk2 (Alk2fl/fl) or Alk3 (Alk3fl/fl) alleles that also carried a Cre recombinase transgene under the control of the albumin gene promoter (Alb-Cre).29
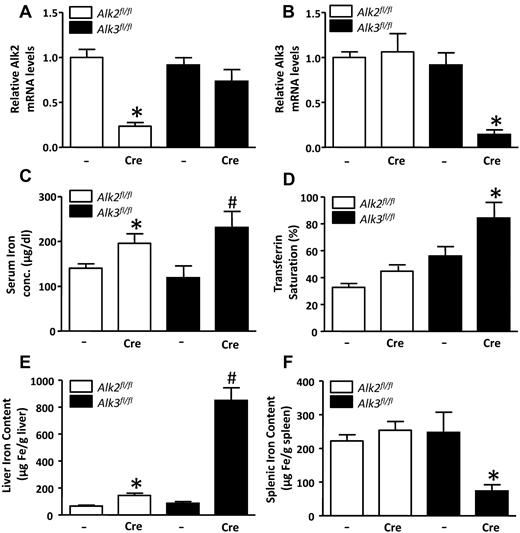
Alk2 and Alk3 mRNA levels were markedly reduced in the livers of Alk2fl/fl; Alb-Cre mice and Alk3fl/fl; Alb-Cre mice, respectively (Figure 1A-B). Serum iron levels were higher in Alk2fl/fl; Alb-Cre mice than in Alk2fl/fl control mice without the Cre transgene, but transferrin saturation did not differ (Figure 1C-D). In contrast, both serum iron level and transferrin saturation were higher in Alk3fl/fl; Alb-Cre mice than in Alk3fl/fl controls (Figure 1C-D). Liver iron content was modestly greater in Alk2fl/fl; Alb-Cre mice than in Alk2fl/fl mice. In contrast, liver iron content was dramatically greater in Alk3fl/fl; Alb-Cre mice than in Alk3fl/fl controls (Figure 1E). Splenic iron content did not differ in Alk2fl/fl; Alb-Cre and Alk2fl/fl; mice but was markedly less in Alk3fl/fl; Alb-Cre than in Alk3fl/fl mice (Figure 1F). Of note, the ratio of liver iron content to splenic iron content was increased in both Alk2fl/fl; Alb-Cre mice and Alk3fl/fl; Alb-Cre mice (supplemental Figure 1). Kidney tissue iron content was also greater in Alk3fl/fl; Alb-Cre mice than in Alk3fl/fl mice (supplemental Figure 2A). There were no hematologic abnormalities suggestive of the presence of a secondary, anemia-induced iron overload phenotype in mice with liver-specific Alk2 or Alk3 deficiency (supplemental Figure 3). Moreover, hepatic expression of hereditary hemochromatosis disease-causing genes, including transferrin receptor-2 (Tfr2),30 Hfe,31,32 hemojuvelin (Hjv),9,13,14,33 and ferroportin 1 (Fpn1),34 did not differ between genotypes (supplemental Figure 4). These results demonstrate a mild iron overload phenotype in Alk2fl/fl; Alb-Cre mice and a severe iron overload phenotype in Alk3fl/fl; Alb-Cre mice and indicate that both Alk2 and Alk3 have roles in the regulation of systemic iron homeostasis.
Serum and tissue iron levels are increased in Alk2fl/fl; Alb-Cre and Alk3fl/fl; Alb-Cre mice. (A) Hepatic Alk2 mRNA levels were measured in Alk2fl/fl and Alk2fl/fl; Alb-Cre mice, and Alk3fl/fl and Alk3fl/fl; Alb-Cre mice. Mice carrying the Cre recombinase transgene are indicated by “Cre.” *P < .0001, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. (B) Hepatic Alk3 mRNA levels were measured in Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl and Alk3fl/fl; Alb-Cre mice. *P = .001, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (C) Serum iron concentration measured in all 4 genotypes (n ≥ 8 per group). *P = .04, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. #P = .04, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (D) Transferrin saturation measured in all 4 genotypes (n ≥ 8 per group). P = .054, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. *P = .049, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (E) Liver iron content measured in all 4 genotypes (n ≥ 8 per group). *P = .0025, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. #P = .0005, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (F) Spleen iron content measured in all 4 genotypes (n ≥ 8 per group). *P = .04: Alk3fl/fl; Alb-Cre versus Alk3fl/fl.
Serum and tissue iron levels are increased in Alk2fl/fl; Alb-Cre and Alk3fl/fl; Alb-Cre mice. (A) Hepatic Alk2 mRNA levels were measured in Alk2fl/fl and Alk2fl/fl; Alb-Cre mice, and Alk3fl/fl and Alk3fl/fl; Alb-Cre mice. Mice carrying the Cre recombinase transgene are indicated by “Cre.” *P < .0001, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. (B) Hepatic Alk3 mRNA levels were measured in Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl and Alk3fl/fl; Alb-Cre mice. *P = .001, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (C) Serum iron concentration measured in all 4 genotypes (n ≥ 8 per group). *P = .04, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. #P = .04, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (D) Transferrin saturation measured in all 4 genotypes (n ≥ 8 per group). P = .054, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. *P = .049, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (E) Liver iron content measured in all 4 genotypes (n ≥ 8 per group). *P = .0025, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. #P = .0005, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (F) Spleen iron content measured in all 4 genotypes (n ≥ 8 per group). *P = .04: Alk3fl/fl; Alb-Cre versus Alk3fl/fl.
Histologic staining reveals periportal hepatocellular iron accumulation in Alk2fl/fl; Alb-Cre mice, but centrilobular hepatocellular iron accumulation in Alk3fl/fl; Alb-Cre mice
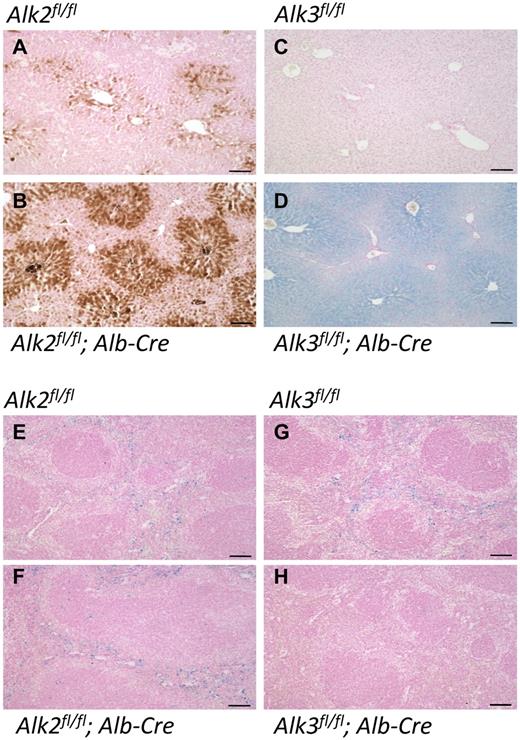
In recessive hemochromatosis syndromes, iron is deposited preferentially in periportal hepatocytes. In the livers of Alk2fl/fl; Alb-Cre mice, we observed low levels of iron accumulation with the typical periportal pattern (Prussian blue staining with diaminobenzidine enhancement; Figure 2A-B). In contrast, there was abundant iron accumulation in the livers of Alk3fl/fl; Alb-Cre mice (Prussian blue staining alone), and the iron deposition was most prominent in the centrilobular region (Figure 2C-D). In neither Alk2fl/fl; Alb-Cre nor Alk3fl/fl; Alb-Cre animals was there substantial iron present in Kupffer cells (Figure 2B,D), the resident macrophages of the liver that usually do not become overloaded with iron until the late stages of hemochromatosis disease progression.1 Iron storage in splenic macrophages was reduced in Alk3fl/fl; Alb-Cre mice but not Alk2fl/fl; Alb-Cre mice (Figure 2F,H), consistent with the reduced splenic iron content that we observed in the former. Prussian blue staining also detected iron accumulation in the proximal renal tubules, as well as in the heart and pancreas of Alk3fl/fl; Alb-Cre mice (supplemental Figure 2B), but not in the tissues of Alk2fl/fl; Alb-Cre mice (data not shown).
Iron deposition in liver and spleen in Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl and Alk3fl/fl; Alb-Cre mice. Formalin-fixed, paraffin-embedded liver (A-D) and spleen (E-H) sections prepared from Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl, and Alk3fl/fl; Alb-Cre mice were stained with Prussian blue and diaminobenzidine (A-B) or Prussian blue alone (C-H). Sections were prepared from at least 4 animals in each group; representative photomicrographs are shown. Scale bars represent 100 μm.
Iron deposition in liver and spleen in Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl and Alk3fl/fl; Alb-Cre mice. Formalin-fixed, paraffin-embedded liver (A-D) and spleen (E-H) sections prepared from Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl, and Alk3fl/fl; Alb-Cre mice were stained with Prussian blue and diaminobenzidine (A-B) or Prussian blue alone (C-H). Sections were prepared from at least 4 animals in each group; representative photomicrographs are shown. Scale bars represent 100 μm.
Impact of BMP type I receptor deletion on hepcidin, transferrin receptor 1 (Tfrc), BMP6, and Id1 mRNA expression levels
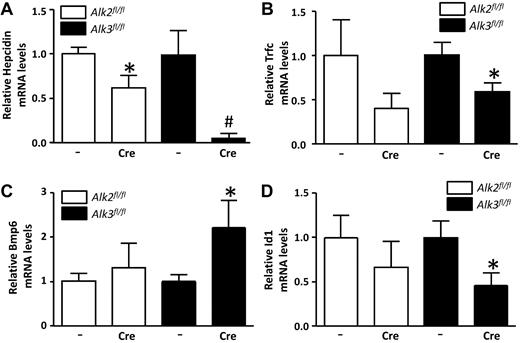
Hepatocellular iron accumulation and macrophage iron depletion are the cellular hallmarks of systemic hepcidin deficiency. A mild reduction in hepatic hepcidin expression was observed in Alk2fl/fl; Alb-Cre mice, and a more marked reduction was seen in Alk3fl/fl; Alb-Cre mice (Figure 3A). As is typical in hepatic iron overload states,35,36 we found reduced Tfrc and increased Bmp6 mRNA levels in the livers of Alk3fl/fl; Alb-Cre mice with similar trends seen in Alk2fl/fl; Alb-Cre mice (Figure 3B-C). The expression of Id1 was also reduced in Alk3fl/fl; Alb-Cre animals (Figure 3D). Taken together, the observations that mice with liver-specific deficiency of BMP type I receptors have reduced hepcidin expression and iron overload in the context of increased Bmp6 gene expression suggest that the ability to respond to BMP signaling is impaired in mice with hepatic Alk2 or Alk3 deficiency.
Hepatic hepcidin, transferrin receptor 1 (Tfrc), Bmp6, and Id1 mRNA levels are decreased in Alk3fl/fl; Alb-Cre mice at the age of 12 weeks. (A) Hepatic hepcidin mRNA levels were determined by quantitative RT-PCR in Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl, and Alk3fl/fl; Alb-Cre mice (n ≥ 7 per group). *P = .03, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. #P = .02, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (B) Hepatic Tfrc (n ≥ 7 per group). *P = .04, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (C) BMP6 (n ≥ 7 per group). *P = .049, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (D) Id1 (n ≥ 7 per group). *P = .04, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. mRNA levels were also measured.
Hepatic hepcidin, transferrin receptor 1 (Tfrc), Bmp6, and Id1 mRNA levels are decreased in Alk3fl/fl; Alb-Cre mice at the age of 12 weeks. (A) Hepatic hepcidin mRNA levels were determined by quantitative RT-PCR in Alk2fl/fl, Alk2fl/fl; Alb-Cre, Alk3fl/fl, and Alk3fl/fl; Alb-Cre mice (n ≥ 7 per group). *P = .03, Alk2fl/fl; Alb-Cre versus Alk2fl/fl. #P = .02, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (B) Hepatic Tfrc (n ≥ 7 per group). *P = .04, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (C) BMP6 (n ≥ 7 per group). *P = .049, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. (D) Id1 (n ≥ 7 per group). *P = .04, Alk3fl/fl; Alb-Cre versus Alk3fl/fl. mRNA levels were also measured.
Impact of BMP type 1 receptor deficiency on the ability of BMP signaling to modulate hepatic hepcidin gene expression
To further evaluate the impact of deleting Alk2 or Alk3 on the regulation of hepcidin gene expression, we measured basal hepcidin expression, as well as the ability of BMP ligands to induce hepcidin gene expression, in primary hepatocytes isolated from animals of each of the 4 genotypes. In the absence of exogenous BMP ligand, hepcidin mRNA levels did not differ in Alk2fl/fl; Alb-Cre and Alk2fl/fl hepatocytes (Figure 4A). However, basal hepcidin expression was substantially reduced in Alk3fl/fl; Alb-Cre hepatocytes compared with Alk3fl/fl control hepatocytes (Figure 4B). Taken together with our in vivo data, these results suggest that Alk3 is the type I BMP receptor responsible for basal hepcidin expression in hepatocytes.
Alk2 and Alk3 are required for BMP2-mediated induction of hepcidin gene expression and loss of Alk3 suppresses basal hepcidin mRNA levels in isolated hepatocytes. Isolated hepatocytes were treated with and without BMP2 (100 ng/mL) for 2 hours (n = 5 hepatocyte isolations from Alk2fl/fl mice; n = 3 hepatocyte isolations from Alk2fl/fl; Alb-Cre, Alk3fl/fl and Alk3fl/fl; Alb-Cre mice, each). (A) Hepcidin mRNA levels in Alk2fl/fl and Alk2fl/fl; Alb-Cre hepatocytes. *P = .0075, Alk2fl/fl without versus with BMP2. (B) Hepcidin mRNA levels in similarly treated Alk3fl/fl and Alk3fl/fl; Alb-Cre hepatocytes. *P < .05, Alk3fl/fl without versus with BMP2. #P < .0001 Alk3fl/fl; Alb-Cre without BMP2 versus Alk3fl/fl without BMP2.
Alk2 and Alk3 are required for BMP2-mediated induction of hepcidin gene expression and loss of Alk3 suppresses basal hepcidin mRNA levels in isolated hepatocytes. Isolated hepatocytes were treated with and without BMP2 (100 ng/mL) for 2 hours (n = 5 hepatocyte isolations from Alk2fl/fl mice; n = 3 hepatocyte isolations from Alk2fl/fl; Alb-Cre, Alk3fl/fl and Alk3fl/fl; Alb-Cre mice, each). (A) Hepcidin mRNA levels in Alk2fl/fl and Alk2fl/fl; Alb-Cre hepatocytes. *P = .0075, Alk2fl/fl without versus with BMP2. (B) Hepcidin mRNA levels in similarly treated Alk3fl/fl and Alk3fl/fl; Alb-Cre hepatocytes. *P < .05, Alk3fl/fl without versus with BMP2. #P < .0001 Alk3fl/fl; Alb-Cre without BMP2 versus Alk3fl/fl without BMP2.
In isolated control hepatocytes, BMP2 more robustly increased hepcidin mRNA levels than did BMP6 (data not shown). We therefore used BMP2 to investigate the effect of hepatic Alk2 and Alk3 deficiency on the regulation of hepcidin gene expression by BMP signaling. BMP2 stimulated hepcidin expression in Alk2fl/fl and Alk3fl/fl control hepatocytes, but not in hepatocytes of either Alk2fl/fl; Alb-Cre or Alk3fl/fl; Alb-Cre mice. These results suggest that the presence of both Alk2 and Alk3 is required for the induction of hepcidin gene expression by BMP2.
Influence of BMP type I receptor deficiency on the ability of iron challenge to induce hepatic hepcidin gene expression
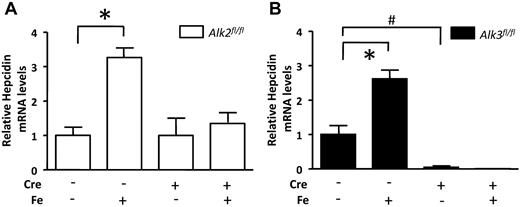
We11 and others8-10,15 have previously reported that iron challenge induces hepcidin gene expression in a BMP-dependent manner. To test whether iron requires Alk2, Alk3, or both to increase hepcidin expression, we measured hepatic hepcidin mRNA levels in mice challenged with iron dextran (Figure 5). After iron administration, serum iron levels and transferrin saturations were similar in mice of all 4 genotypes (supplemental Figure 5A-D), and iron challenge did not alter hepatic Alk2 or Alk3 mRNA levels (supplemental Figure 5E-H). In both Alk2fl/fl and Alk3fl/fl animals, iron challenge increased hepcidin mRNA levels ∼ 3-fold (Figure 5). Similarly, we observed that iron challenge induced Id1 gene expression (supplemental Figure 5I-J). Iron challenge did not increase hepcidin or Id1 mRNA levels in mice with hepatocyte-specific Alk2 or Alk3 deficiency. These studies of iron-challenged mice, like our studies of BMP2-stimulated hepatocytes, suggest that both of these BMP type I receptors are required for induction of hepcidin gene expression in response to BMP signaling.
Alk2 and Alk3 are essential for iron-dependent hepcidin regulation in vivo. Eight-week-old mice were challenged intravenously with dextran (as a control) or iron-dextran (Fe; 0.2 mg/kg). (A) Hepatic hepcidin mRNA levels in Alk2fl/fl and Alk2fl/fl; Alb-Cre mice (n ≥ 6 per group). *P < .0001, Alk2fl/fl mice challenged with dextran versus Alk2fl/fl mice challenged with iron-dextran. (B) Hepatic hepcidin mRNA levels in similarly challenged Alk3fl/fl and Alk3fl/fl; Alb-Cre mice (n ≥ 3 per group). *P < .0045, versus Alk3fl/fl mice challenged with dextran versus Alk3fl/fl mice challenged with iron-dextran. #P < .005, Alk2fl/fl; Alb-Cre mice challenged with dextran versus Alk3fl/fl mice challenged with dextran.
Alk2 and Alk3 are essential for iron-dependent hepcidin regulation in vivo. Eight-week-old mice were challenged intravenously with dextran (as a control) or iron-dextran (Fe; 0.2 mg/kg). (A) Hepatic hepcidin mRNA levels in Alk2fl/fl and Alk2fl/fl; Alb-Cre mice (n ≥ 6 per group). *P < .0001, Alk2fl/fl mice challenged with dextran versus Alk2fl/fl mice challenged with iron-dextran. (B) Hepatic hepcidin mRNA levels in similarly challenged Alk3fl/fl and Alk3fl/fl; Alb-Cre mice (n ≥ 3 per group). *P < .0045, versus Alk3fl/fl mice challenged with dextran versus Alk3fl/fl mice challenged with iron-dextran. #P < .005, Alk2fl/fl; Alb-Cre mice challenged with dextran versus Alk3fl/fl mice challenged with dextran.
Discussion
The study of hereditary hemochromatosis and iron overload disorders in humans and mice has identified a critical role for BMP signaling in the regulation of systemic iron homeostasis. However, the specific type I BMP receptors required for iron homeostasis were unknown. In the present study, we report that hepatocyte-specific deficiency of either Alk2 or Alk3 causes systemic iron overload and blocks the ability of BMP ligands and iron to induce hepatic hepcidin expression. The iron overload phenotype was more severe in Alk3fl/fl; Alb-Cre than in Alk2fl/fl; Alb-Cre mice, probably because of a marked reduction in basal BMP signaling and hepcidin expression in Alk3fl/fl; Alb-Cre mice. These findings suggest that Alk3 is the type I BMP receptor responsible for basal hepcidin expression in hepatocytes. In contrast, both Alk2 and Alk3 were required for the induction of hepcidin by BMP and iron. These results demonstrate that Alk2 and Alk3 have nonredundant roles in iron homeostasis.
The iron overload phenotype and reduction in basal hepcidin mRNA levels were more marked in Alk3fl/fl; Alb-Cre mice than in Alk2fl/fl; Alb-Cre mice. An increase in hepatic BMP6 mRNA levels was detected in mice with hepatic-specific Alk3 deficiency, but not in mice with hepatocyte-specific Alk2 deficiency. Potential explanations for the difference in severity of iron overload in Alk2- versus Alk3-deficient livers include the possibility that Alk3 is more highly expressed in hepatocytes than is Alk2 or that Alk3 is more sensitive to the BMPs present in the hepatic milieu.
Experiments in isolated hepatocytes and in intact mice revealed that both BMP type I receptors, Alk2 and Alk3, are required for the induction of hepatic hepcidin gene expression by BMP2 or iron. Intriguingly, Little and Mullins37 have recently demonstrated that the zebrafish orthologs of the Alk2 and Alk3 receptors have nonredundant roles in dorsal-ventral axis specification. Moreover, the 2 BMP type I receptors in zebrafish assemble into heteromeric BMP receptor complexes, which appear to be more sensitive to BMP ligand heterodimers. It is conceivable that similar heteromeric receptor complexes containing both Alk2 and Alk3 are required for BMP ligands and iron to induce hepcidin gene expression in hepatocytes. Additional studies will be necessary to investigate the possibility that Alk2 and Alk3 can form heteromeric complexes in mammalian hepatocytes.
The pattern of iron deposition varies in the different types of hemochromatosis and experimental iron overload models. For example, in hemochromatosis resulting from autosomal dominant mutations in the gene encoding Fpn1, iron is preferentially deposited in Kupffer cells. In contrast, in the more common autosomal recessive forms of hemochromatosis, including those attributable to mutations in the Hfe and Tfr2 genes, iron is preferentially deposited in periportal hepatocytes.1,38,39 It has been hypothesized that the periportal distribution of iron seen in most autosomal recessive forms of hemochromatosis is attributable to a first pass effect of intestinally-absorbed iron carried by the portal venous system.40 In contrast, in hepcidin4 - and BMP616 -deficient mice, iron deposition is predominantly centrilobular. Moreover, Kautz et al41,42 reported that BMP6 immunoreactivity was abundant in centrilobular hepatocytes of mice with iron overload induced by an iron-rich diet or Hfe deficiency, both of which are characterized by periportal iron deposition. We observed modest iron deposition in the periportal region of mice with liver-specific Alk2 deficiency. In contrast, abundant centrilobular iron deposition was found in mice with liver-specific Alk3 deficiency. Our findings of centrilobular iron deposition in mice with hepatocyte-specific Alk3 deficiency, together with those made in hepcidin4 - and BMP616 -deficient mice, strongly suggest that the distribution of hepatic iron in overload states is not the result of a “passive” first-pass effect. Instead, it appears that hepatic iron deposition may be an active process that is regulated, at least in part, by BMP signaling.
Under physiologic conditions, hepcidin degrades Fpn1, the sole iron exporter in the body. Interestingly, Zhang et al43 and Ramey et al44 showed that Fpn1 is predominantly localized in the periportal region. Ramey et al hypothesized that in iron overload models associated with very low or absent hepcidin levels, high periportal Fpn1 expression protects the periportal region from iron overload, resulting in a centrilobular distribution of iron deposition.44 In contrast, in iron overload states associated with relatively preserved hepcidin expression, lower Fpn1 levels are less able to protect the periportal region from iron deposition.
Our observations appear to be consistent with the hypothesis put forward by Ramey et al.44 Hepcidin levels are very low at baseline in Alk3-deficient hepatocytes, probably leading to abundant periportal Fpn1 levels and relative sparing of the periportal region and accumulation of iron in the centrilobular region. In contrast, hepcidin levels are normal or only modestly reduced in Alk2-deficient hepatocytes, probably reducing periportal Fpn1 levels and leading to periportal iron accumulation.
Our findings have several therapeutic implications. First, BMP signaling inhibitors that can increase serum iron levels represent an attractive therapeutic strategy for the treatment of ACD.11,20,45 The observation that Alk3 plays a critical role in basal hepcidin gene expression suggests that strategies that target Alk3 will reduce hepcidin expression and increase serum iron levels. Alk3-specific inhibitors may be superior to nonselective BMP signaling inhibitors for treating ACD because the former would not be expected to inhibit the diverse effects mediated by other type I BMP receptors.
In conclusion, we report that hepatocyte-specific deficiency of either Alk2 or Alk3 caused systemic iron overload and blocked the ability of iron and BMP ligands to induce hepatic hepcidin expression in mice. The iron overload phenotype was more severe in Alk3fl/fl; Alb-Cre than in Alk2fl/fl; Alb-Cre mice, probably because of a marked reduction in basal BMP signaling and hepcidin gene expression in the former. In contrast, both Alk2 and Alk3 were required for the induction of hepcidin by BMP and iron. Taken together, these data indicate partially overlapping but nonredundant functions for these BMP type I receptors in hepatocellular regulation of systemic iron metabolism.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yuji Mishina and Vesa Kaartinen for providing Alk2+/fl and Alk3+/fl mice, respectively; Martin Dib, Seth Karp, Sharon M. Cawley, Rita Nobre, and Monica Calicchio for technical advice and support; and Dean Campagna and Paul Schmidt for providing unpublished data from their characterization of mice globally deficient in Alk6.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG SW 119/3-1, A.U.S.), the National Institute of Diabetes and Digestive and Kidney Diseases (K99DK084122 to T.B.B., R01DK082971 to M.D.F., and R01DK082971 to R.T.P. and K.D.B.), and the Fondation LeDucq (P.B.Y. and K.D.B).
National Institutes of Health
Authorship
Contribution: A.U.S., L.K.L., P.L., C.M., S.M.K., and A.E.P. performed experiments; A.U.S., T.B.B., L.K.L., P.L., C.M., S.M.K., A.E.P., M.D.F., and K.D.B. analyzed data; A.U.S., T.B.B., R.T.P., P.B.Y., D.B.B., M.D.F., and K.D.B. supervised the experimental design; and A.U.S., T.B.B., M.D.F., D.B.B., and K.D.B. wrote the manuscript.
Conflict-of-interest disclosure: The Massachusetts General Hospital has filed patents related to the use of small molecule inhibitors of BMP signaling to modulate iron metabolism, and P.B.Y., R.T.P., and K.D.B. may be eligible to receive royalties. The remaining authors declare no competing financial interests.
Correspondence: Andrea U. Steinbicker, Department of Anesthesia and Intensive Care Medicine, University Hospital Muenster, University of Muenster, Albert-Schweitzer-Campus 1, 48149 Muenster, Germany; e-mail: andrea.steinbicker@gmail.com or andrea.steinbicker@ukmuenster.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal