Abstract
Romiplostim, a thrombopoietic agent with demonstrated efficacy against immune thrombocytopenia (ITP) in prospective controlled studies, was recently licensed for adults with chronic ITP. Only France has allowed romiplostim compassionate use since January 2008. ITP patients could receive romiplostim when they failed to respond to successive corticosteroids, intravenous immunoglobulins, rituximab, and splenectomy, or when splenectomy was not indicated. We included the first 80 patients enrolled in this program with at least 2 years of follow-up. Primary platelet response (platelet count ≥ 50 × 109/L and double baseline) was observed in 74% of all patients. Long-term responses (2 years) were observed in 47 (65%) patients, 37 (79%) had sustained platelet responses with a median platelet count of 106 × 109/L (interquartile range, 75-167 × 109/L), and 10 (21%) were still taking romiplostim, despite a median platelet count of 38 × 109/L (interquartile range, 35-44 × 109/L), but with clinical benefit (lower dose and/or fewer concomitant treatment(s) and/or diminished bleeding signs). A high bleeding score and use of concomitant ITP therapy were baseline factors predicting romiplostim failure. The most frequently reported adverse events were: arthralgias (26%), fatigue (13%), and nausea (7%). Our results confirmed that romiplostim use in clinical practice is effective and safe for severe chronic ITP. This trial was registered at www.clinicaltrials.gov as #NCT01013181.
Introduction
Immune thrombocytopenia (ITP) in adults is a chronic autoimmune disorder characterized by low platelet counts and mucocutaneous bleeding.1-5 ITP signs and symptoms vary widely. Some patients have no symptoms or minimal bruising, whereas others experience severe bleeding. Whereas ITP in children is usually a self-limited disease that spontaneously disappears within few weeks, ∼ 70% of adults remain thrombocytopenic after 1 year and, hence, have chronic ITP. The overall prognosis of ITP is good, with < 2% mortality, but the latter can rise to 10% for a subgroup of patients with chronic severe ITP refractory to splenectomy.
ITP has long been considered to be only a matter of accelerated platelet destruction, and most of the currently available therapies increase platelet counts, mainly by slowing the platelet destruction rate. However, some evidence shows that ITP is also a matter of impaired platelet production.6,7 Suboptimal platelet production and relatively low thrombopoietin levels in ITP patients would be consistent with an important role for thrombopoietic agents in ITP management.
Romiplostim is a peptibody that was the first thrombopoietic agent developed for this indication.8 It has been reported to be a highly effective treatment for both splenectomized and nonsplenectomized patients in pivotal clinical studies9-12 and has been accorded Food and Drug Administration approval for the treatment of ITP patients in the United States, after failure of a first-line therapy (either corticosteroids or intravenous immunoglobulins [IVIg]). More recently, romiplostim was licensed in Europe after splenectomy failure or contraindication. Although romiplostim has been available in the United States since January 2009, no data on its efficacy and safety in unselected patients have been reported to date. France has been the only country to allow a romiplostim compassionate-use program (CUP) for ITP patients, since January 2008, before its approval. This report describes our experience with the first 80 ITP patients treated with romiplostim in the French CUP. The study objective was to determine romiplostim efficacy and safety in clinical practice in unselected (no criteria based on comorbidities as for pivotal trials) patients, who had been followed for at least 2 years after their first romiplostim administration.
Methods
Patients
The data from the first 80 consecutive patients with at least 2 years of follow-up after starting romiplostim in this CUP were collected retrospectively and analyzed.
Any hospital physician treating ITP patients in France could include a patient after obtaining authorization of the French Health Authority (Agence Française de Sécurité Sanitaire des Produits de Santé), which allowed us to obtain the list of the patients registered in the CUP. The protocol to conduct the retrospective analysis of the group was approved by the Henri-Mondor Hospital Institutional Review Board and Ethics Committee; all patients received written information before undergoing eligibility screening for consultation of their charts. According to the CUP, inclusion (baseline) criteria were the following: age ≥ 18 years, ITP diagnosis according to the American Society Hematology guidelines,3 failure of successive previous treatment(s) with corticosteroids and/or IVIg, rituximab, splenectomy failure, or if splenectomy was not indicated. According to the CUP criteria, it was possible to administer romiplostim to any patient with persistent ITP (lasting 3-12 months). Exclusion criteria were: other disease(s) known to be associated with ITP (ie, secondary ITP), such as human immunodeficiency or hepatitis C virus infection, lymphoproliferative disorders, thyroid or liver disease, and definite systemic lupus erythematosus (≥ 4 American Rheumatism Association criteria).13,14 Pertinently, no clinical trial criteria were required to be enrolled in this CUP.
Study design
In this retrospective, observational, multicenter study, a patient had to receive romiplostim once weekly by subcutaneous injection, with the first injection administered in the hospital where the patient was followed; thereafter, romiplostim self-administration was allowed. The CUP had no rules dictating hospital or at home, nurse-administered, or self-administered romiplostim injection. Romiplostim was initiated at 1 μg/kg per week; the dose was then adjusted as needed (up to a maximum of 10 μg/kg per week), based on the patient's platelet count, by following the prespecified rules for adjustment described in pivotal trials.11 The target platelet count range was 50-250 × 109/L.
Patients could continue to receive concurrent ITP therapies, including rescue interventions, defined as any agent administered to transiently increase the platelet count (eg, IVIg, corticosteroids, anti-IgD, and/or platelet transfusions). The need to increase the dose of a concurrent ITP medication to levels above those used at baseline was also considered a rescue intervention.
Assessments and outcome measures
The following characteristics were recorded on a standardized form: age, sex, and ITP history; disease duration; previous discontinued medications; results of bone marrow aspirate analysis, if available; associated comorbidities and bleeding assessment within 1 month before romiplostim initiation; and concomitant ongoing medications, if any. Reasons for nonsplenectomy were recorded. Bleeding severity was evaluated at inclusion and during follow-up with a previously reported standardized bleeding score.15 This score quantifies the hemorrhagic syndrome of an ITP patient by adding points associated with the different kinds of clinical bleeding signs seen in ITP patients (eg, generalized petechial purpura = 3 points, hemorrhagic oral bullae = 5 points, etc). Unlike the original score that included age and bleeding to guide treatment, the tool applied herein was modified to measure only bleeding, and age was excluded (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The need for concurrent and/or rescue interventions during follow-up was also recorded. Platelet counts were obtained weekly until the romiplostim dose was stabilized and then at least once monthly.
Romiplostim efficacy was evaluated based on platelet responses and the percentage of patients able to lower or discontinue their doses of concurrent ITP medication(s). A primary platelet response was defined as a platelet count ≥ 50 × 109/L with at least the doubling of the baseline value, without any rescue intervention during the preceding 8 weeks. Long-term responders at 2 years included the patients with sustained platelet responses and those with clinical benefits. A sustained platelet response at 2 years was defined as more than 50 × 109/L and at least two of the three last platelet evaluations at months 22, 23, and 24, independently of continued romiplostim administration. Clinical benefit at 2 years was defined as decreased dose or withdrawal of concomitant treatment(s) and/or diminished bleeding without any rescue treatment for patients who failed to fulfill the sustained platelet-response criteria but were still taking romiplostim. Treatment failure was defined as a platelet count of ≤ 20 × 109/L for 4 consecutive weeks at the highest recommended dose, a major bleeding event, or need to change therapy (including splenectomy and rescue treatment).
Safety was evaluated at least monthly and, when an adverse event occurred, it was graded from 1 to 5. All these data were recorded on a standard case-report form and then analyzed by the same investigator (M.K.).
Statistical analyses
Quantitative data are presented as mean ± SD or median (range) or interquartile range (IQR) as appropriate, and qualitative data as number (%). Quantitative data were compared using the Mann-Whitney nonparametric test and qualitative data with the Fisher exact test. Factors associated with a primary platelet response and long-term responses (as predefined) in the univariate analysis were tested in a logistic-regression model to identify factors independently associated with platelet response to romiplostim. All factors associated with response in univariate analysis at P ≤ .10 were tested in a logistic-regression model. Odds ratios (ORs) estimated by the model are given with their 95% confidence intervals (CIs). Analyses were performed using SPSS Version 18 statistical package. P ≤ .05 was considered significant.
Results
Among the first 80 French patients enrolled in the CUP to receive romiplostim, 8 were excluded from the final analysis because they were not treated for primary ITP (Figure 1). Two patients had underlying features of myelodysplastic syndrome, 2 had secondary ITP (viral hepatitis C-related), and 4 had chronic lymphocytic leukemia. Table 1 summarizes the main characteristics of the 72 patients included. All but 1 patient had previously received corticosteroids and IVIg, and 90% of them had been given rituximab without responding. Their splenectomy rate was 54% (39 of 72). Among the 33 nonsplenectomized patients, 13 had refused to undergo splenectomy, whereas splenectomy was considered contraindicated for a variety of reasons by the treating physicians for the remaining cases (Table 2). Comorbidities and advanced age were the 2 main reasons for contraindicating splenectomy but, notably, 7 coinvestigators did so because of the absence of predominant splenic sequestration observed on 111In-labeled platelet-life span scintigraphs. At the time of the first romiplostim injection, 49 of 72 (68%) patients were receiving concomitant medication(s) for ITP, mainly including corticosteroids (n = 29, 40%), IVIg (n = 29, 40%), danazol or dapsone (n = 5, 7%), or immunosuppressants (n = 8, 11%). Thirty-two (50%) patients had bleeding signs during the month preceding the first romiplostim injection, with a mean bleeding score of 4.7 (range, 0-46). The median platelet count at the time of the first romiplostim administration was 11 × 109/L (IQR, 1-60 × 109/L). The baseline platelet count was more than 30 × 109/L in 6 patients, 3 of whom were receiving concomitant treatment(s) for ITP.
Flow chart of the 2-year follow-up of the 80 severe chronic ITP patients receiving romiplostim in this CUP.
Flow chart of the 2-year follow-up of the 80 severe chronic ITP patients receiving romiplostim in this CUP.
Characteristics of the 72 patients with severe chronic ITP before romiplostim administration
| Characteristic . | Value . |
|---|---|
| Male, n (%) | 28 (39) |
| Median age, y (range) | 63 (20-91) |
| Baseline platelet count, × 109/L, median (range) | 11 (1-60) |
| ITP duration before romiplostim, y, median (range) | 6 (0.1-49) |
| Previous therapies | |
| Median, n (range) | 5 (2-12) |
| Prednisone, n (%) | 72 (100) |
| IVIg, n (%) | 70 (97) |
| Rituximab, n (%) | 65 (90) |
| Splenectomy, n (%) | 39 (54) |
| Azathioprine, n (%) | 21 (29) |
| Intravenous cyclophosphamide pulse(s) | 13 (18) |
| Patients with concomitant therapies at baseline, n (%) | 49 (68) |
| Prednisone | 29 (40) |
| IVIg | 29 (40) |
| Immunosuppressants | 8 (11) |
| Characteristic . | Value . |
|---|---|
| Male, n (%) | 28 (39) |
| Median age, y (range) | 63 (20-91) |
| Baseline platelet count, × 109/L, median (range) | 11 (1-60) |
| ITP duration before romiplostim, y, median (range) | 6 (0.1-49) |
| Previous therapies | |
| Median, n (range) | 5 (2-12) |
| Prednisone, n (%) | 72 (100) |
| IVIg, n (%) | 70 (97) |
| Rituximab, n (%) | 65 (90) |
| Splenectomy, n (%) | 39 (54) |
| Azathioprine, n (%) | 21 (29) |
| Intravenous cyclophosphamide pulse(s) | 13 (18) |
| Patients with concomitant therapies at baseline, n (%) | 49 (68) |
| Prednisone | 29 (40) |
| IVIg | 29 (40) |
| Immunosuppressants | 8 (11) |
Comorbidities and reasons for nonsplenectomy at inclusion of the 72 patients in this CUP
| Specialty . | n . |
|---|---|
| Comorbidity | |
| Cardiology | |
| Anti-platelet–aggregating agent* | 7 |
| Arterial hypertension | 5 |
| Anticoagulant therapy* | 4 |
| Lower limb arteritis | 2 |
| Carotid stenosis | 2 |
| Coronary disease | 2 |
| Previous myocardial infarction | 1 |
| Previous venous thromboembolism | 1 |
| Cardiac arrhythmia | 1 |
| Endocrinology | |
| Diabetes* | 6 |
| Obesity* | 3 |
| Hypothyroidism | 1 |
| Graves disease | 1 |
| Hematology | |
| Monoclonal gammopathy of undetermined significance | 2 |
| Autoimmune hemolytic anemia | 1 |
| Hepatology | |
| Chronic alcoholism | 1 |
| Cirrhosis | 1 |
| Liver allograft | 1 |
| Neurology | |
| Epilepsy | 2 |
| Alzheimer dementia* | 1 |
| Prior stroke | 2 |
| Central demyelinating disease* | 1 |
| Huntington disease* | 1 |
| Intracranial cavernoma | 1 |
| Parkinson disease | 1 |
| Oncology† | |
| Breast cancer | 2 |
| Prostate cancer | 1 |
| Skin cancer | 1 |
| Uterine cancer | 1 |
| Ampullary cancer | 1 |
| Psychiatry | |
| Bipolar disorder | 2 |
| Depression | 1 |
| Miscellaneous | |
| Biermer disease | 1 |
| End-stage renal failure with dialysis* | 1 |
| Previous pulmonary aspergillosis | 1 |
| Sjögren syndrome | 1 |
| Specialty . | n . |
|---|---|
| Comorbidity | |
| Cardiology | |
| Anti-platelet–aggregating agent* | 7 |
| Arterial hypertension | 5 |
| Anticoagulant therapy* | 4 |
| Lower limb arteritis | 2 |
| Carotid stenosis | 2 |
| Coronary disease | 2 |
| Previous myocardial infarction | 1 |
| Previous venous thromboembolism | 1 |
| Cardiac arrhythmia | 1 |
| Endocrinology | |
| Diabetes* | 6 |
| Obesity* | 3 |
| Hypothyroidism | 1 |
| Graves disease | 1 |
| Hematology | |
| Monoclonal gammopathy of undetermined significance | 2 |
| Autoimmune hemolytic anemia | 1 |
| Hepatology | |
| Chronic alcoholism | 1 |
| Cirrhosis | 1 |
| Liver allograft | 1 |
| Neurology | |
| Epilepsy | 2 |
| Alzheimer dementia* | 1 |
| Prior stroke | 2 |
| Central demyelinating disease* | 1 |
| Huntington disease* | 1 |
| Intracranial cavernoma | 1 |
| Parkinson disease | 1 |
| Oncology† | |
| Breast cancer | 2 |
| Prostate cancer | 1 |
| Skin cancer | 1 |
| Uterine cancer | 1 |
| Ampullary cancer | 1 |
| Psychiatry | |
| Bipolar disorder | 2 |
| Depression | 1 |
| Miscellaneous | |
| Biermer disease | 1 |
| End-stage renal failure with dialysis* | 1 |
| Previous pulmonary aspergillosis | 1 |
| Sjögren syndrome | 1 |
A total of 45 of 72 (62.5%) patients with any comorbidity.
Reasons declared as contraindications to splenectomy by treating physicians.
The cancers of all patients included in this CUP were in complete remission.
Efficacy
Primary responders and follow-up.
A primary platelet response to romiplostim was observed at least once in 53 of 72 (74%) patients. Patients who responded at any time during the study had persistent platelet responses for an average 64% (IQR, 37%-100%) of the observation period. Platelet counts rose sharply during the first 4 weeks of administration and then stabilized during the first 4 months at a median of 109 × 109/L (IQR, 48-206 × 109/L; Figure 2A). For 74% of the patients, romiplostim was administered at home (60% by visiting nurses and 14% by the patients themselves); 26% of the patients came to the hospital every week for their injection.
Platelet counts and romiplostim doses throughout the 2 years of follow-up of severe chronic ITP patients responding to romiplostim. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts. (B) Romiplostim dose (μg/kg; mean ± SD).
Platelet counts and romiplostim doses throughout the 2 years of follow-up of severe chronic ITP patients responding to romiplostim. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts. (B) Romiplostim dose (μg/kg; mean ± SD).
The mean (± SD) romiplostim weekly dose for the 52 patients still taking it at 1 year was 4.7 ± 2.1 μg/kg and 5.1 ± 2.8 μg/kg (range, 1-10 μg/kg) for the 45 patients still receiving it at 2 years (Figure 2B), with very similar respective average platelet-count responses of 134 ± 80 × 109/L and 109 ± 31 × 109/L. The mean time to romiplostim-dose stabilization was 2 months. Time to dose stabilization was proportional to the final dose (3-6 months for a dose up to 5.4 μg/kg per week and 7-9 months for a dose up to 6.2 μg/kg per week). After reaching each patient's stable dose, treatment could be pursued at that stable dose ± 2 μg/kg for 82% of the patients.
Among the 52 romiplostim responders at 1 year, 15 of 52 (29%) received only low doses (1-3 μg/kg per week), 27 of 52 (52%) moderate doses (4-6 μg/kg per week), and 10 of 52 (19%) high levels (7-10 μg/kg per week). These subgroups did not differ in terms of baseline characteristics, including age, platelet count, bleeding score, concomitant treatment(s), and splenectomy status (data not shown).
Two patients achieved partial ITP remissions after romiplostim withdrawal at months 14 and 22. Clinicians treating these 2 patients could decrease their romiplostim doses because their platelet counts exceeded 250×109/L with only low-dose (2 or 3 μg/kg per week) and then stopped romiplostim; so far, no relapse has occurred after 9 and 17 months of follow-up.
Fourteen (19%) of the studied patients received rescue interventions during the first year of follow-up; a majority of them (9 of 14, 64%) had not responded to romiplostim. Among the 29 of 49 (59%) receiving concomitant treatment(s) at romiplostim initiation and responding to romiplostim, 86% (25 of 29) could discontinue the other drug(s) and a further 7% (2 of 29) could reduce its/their doses by at least 25%.
Treatment failure and reasons for romiplostim withdrawal.
At 2 years of follow-up, romiplostim was stopped in 2 patients because of partial remission. Romiplostim had been given to 3 patients only transiently in preparation for splenectomy.
Romiplostim was stopped for 22 of 72 (31%) patients because of lack of efficacy (n = 15), deaths (n = 4), intolerance (n = 2), and accepted splenectomy in the second year of follow-up (n = 1) (Figure 1). Causes of death were: cerebromeningeal hemorrhage in a patient, who had not responded to any ITP treatment, including romiplostim at the maximum dose of 10 μg/kg per week; disseminated rectal adenocarcinoma; fatal septic shock related to Staphylococcus aureus infection; and end-stage Alzheimer disease.
In the case of inefficacy, the mean time to romiplostim discontinuation was 5.5 months (range, 1-12 months).
Long-term responders.
At 2 years, 47 of 72 (65%) patients were classified as long-term responders. Thirty-seven (79%) of them had sustained platelet responses with a median platelet count of 106 × 109/L (IQR,75-167 × 109/L). Two of them could stop romiplostim (See “Primary responders and follow-up”). For the 35 patients with sustained platelet responses still receiving romiplostim, the mean plus or minus SD weekly dose was 4.4 ± 2.4 μg/kg. For the 10 patients who did not satisfy the criteria of long-term platelet response but had clinical benefits, 6 were no longer taking concomitant ITP treatment(s) and 8 no longer had bleeding signs with a median platelet count of 38 × 109/L (IQR, 35-44 × 109/L) and a mean ± SD weekly romiplostim dose of 7.6 ± 2.9 μg/kg. Only half of these patients were taking the maximum romiplostim dose (10 μg/kg).
Factors predicting romiplostim efficacy.
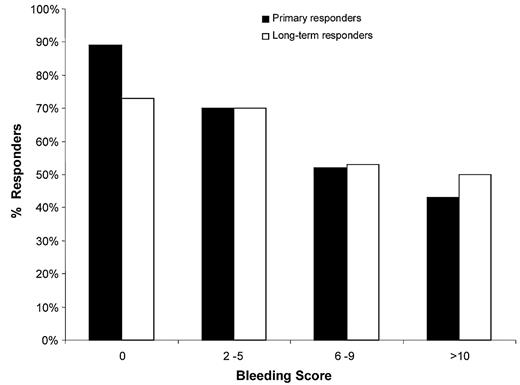
According to our univariate analysis comparing primary responders and nonresponders with romiplostim, baseline factors associated with nonresponse were ongoing concomitant treatment(s), platelet count, and bleeding signs. A linear relationship was observed between the level of these bleeding signs, as assessed by the bleeding score15 and the risk of romiplostim failure (Figure 3). Women had a slight tendency to respond less well (nonresponders to romiplostim: 15 of 44 [33%] vs 4 of 28 [14%] of men, P = .06). Our multivariate analysis (supplemental Table 2) identified failure to respond to romiplostim as being significantly associated with baseline bleeding signs (OR = 1.20; 95% CI, 1.05-1.37, P = .008) and showed a strong trend for initial concomitant ITP treatment(s) (OR = 7.6; 95% CI, 0.9-65.7, P = .06). Notably, each additional point of the inclusion bleeding score was associated with 18% increased likelihood of being a romiplostim nonresponder. By comparing patients defined as long-term responders and those who had stopped romiplostim at 2 years (supplemental Table 3), the bleeding score also appeared to predict efficacy in a multivariate model (OR = 0.89; 95% CI, 0.80-0.99, P = .032).
Rates of primary and long-term romiplostim responders as a function of their baseline bleeding scores.
Rates of primary and long-term romiplostim responders as a function of their baseline bleeding scores.
Nonsplenectomized patients had the same response rate and magnitude as splenectomized patients (respective mean platelet counts of 113 × 109/L vs 99 × 109/L, P = .28) and comparable percentages of long-term responders (54% [21 of 39] vs 45% [15 of 33], P = .48). The mean romiplostim doses for splenectomized and nonsplenectomized patients (respectively: 4.62 vs 4.73 μg/kg per week) were comparable.
Primary platelet responses and long-term response rates were comparable for the 59 patients splenectomized or not and the subgroups of 13 patients who refused splenectomy (respectively, 42 of 59 [71%] vs 11 of 13 [85%] and 38 of 59 [64%] vs 9 of 13 [64%]) or the 7 patients who were not splenectomized because their 111In-labeled platelet-life span scintigraphs did not show predominant splenic platelet destruction (respectively, 5 of 7 [71%] and 6 of 7 [86%]). These 7 patients' baseline characteristics were similar to those of other patients, except for their bleeding score, which was significantly lower (respective median [range] scores: 0 [0-4] vs 2 [0-20], P = .038).
Romiplostim efficacy among patients with comorbidities.
Concerning specific comorbidities (Table 3), patients with a contraindication to splenectomy were significantly older than all the others and had ITP for a shorter duration, whereas those with neurologic disorders were significantly poorer primary responders, and more frequently male.
Comparisons between CUP patients with comorbidities and all cohort patients
| Characteristic . | All patients . | Antiaggregating or anticoagulant therapy . | P . | Splenectomy contraindicated . | P . | Neurologic disorder . | P . | Obesity . | P . |
|---|---|---|---|---|---|---|---|---|---|
| n | 72 | 8 | 20 | 9 | 3 | ||||
| Mean age, y (range) | 63 (20-91) | 71 (63-89) | NS | 69 (29-90) | .021 | 54 (20-80) | NS | 63 (50-74) | NS |
| Male sex, n (%) | 29 (40) | 7 (88) | .003 | 10 (50) | NS | 0 | .01 | 0 | NS |
| ITP duration before romiplostim, y, median (range) | 8.7 (0.1-49) | 14 (2-49) | NS | 5.2 (0-20) | .026 | 14 (0.1-49) | NS | 4 (1-7) | NS |
| Splenectomy, n (%) | 39 (54) | 5 (63) | NS | 0 | NS | 4 (44) | NS | 1 (33) | NS |
| At baseline | |||||||||
| Platelets, × 109/L, mean (range) | 16 (1-60) | 10 (2-20) | NS | 14 (1-60) | NS | 6 (1-15) | .01 | 8 (5-12) | NS |
| Patients with concomitant therapies, n (%) | 48 (67) | 3 (37) | NS | 15 (75) | NS | 7 (78) | NS | 3 (100) | NS |
| Bleeding score, mean (range) | 4.7 (0-46) | 6.4 (0-27) | NS | 2.5 (0-20) | NS | 5.9 (0-15) | NS | 3 (0-7) | NS |
| Primary responders, n (%) | 53 (74) | 6 (75) | NS | 14 (70) | NS | 4 (44) | .048 | 0 | .016 |
| Long-term responders, n (%) | 47 (65) | 6 (75) | NS | 13 (65) | NS | 6 (67) | NS | 1 (33) | NS |
| Characteristic . | All patients . | Antiaggregating or anticoagulant therapy . | P . | Splenectomy contraindicated . | P . | Neurologic disorder . | P . | Obesity . | P . |
|---|---|---|---|---|---|---|---|---|---|
| n | 72 | 8 | 20 | 9 | 3 | ||||
| Mean age, y (range) | 63 (20-91) | 71 (63-89) | NS | 69 (29-90) | .021 | 54 (20-80) | NS | 63 (50-74) | NS |
| Male sex, n (%) | 29 (40) | 7 (88) | .003 | 10 (50) | NS | 0 | .01 | 0 | NS |
| ITP duration before romiplostim, y, median (range) | 8.7 (0.1-49) | 14 (2-49) | NS | 5.2 (0-20) | .026 | 14 (0.1-49) | NS | 4 (1-7) | NS |
| Splenectomy, n (%) | 39 (54) | 5 (63) | NS | 0 | NS | 4 (44) | NS | 1 (33) | NS |
| At baseline | |||||||||
| Platelets, × 109/L, mean (range) | 16 (1-60) | 10 (2-20) | NS | 14 (1-60) | NS | 6 (1-15) | .01 | 8 (5-12) | NS |
| Patients with concomitant therapies, n (%) | 48 (67) | 3 (37) | NS | 15 (75) | NS | 7 (78) | NS | 3 (100) | NS |
| Bleeding score, mean (range) | 4.7 (0-46) | 6.4 (0-27) | NS | 2.5 (0-20) | NS | 5.9 (0-15) | NS | 3 (0-7) | NS |
| Primary responders, n (%) | 53 (74) | 6 (75) | NS | 14 (70) | NS | 4 (44) | .048 | 0 | .016 |
| Long-term responders, n (%) | 47 (65) | 6 (75) | NS | 13 (65) | NS | 6 (67) | NS | 1 (33) | NS |
NS indicates not significant.
We identified 13 of 72 (18%) patients included in the CUP who would not have been eligible for pivotal studies10,11 : patients taking anticoagulants (n = 4), those with ≥ 3 predisposing factors for thromboembolic events (n = 4), history of venous thrombosis (n = 1), cardiac arrhythmia (n = 1), documented arterial thrombosis diagnosed during the preceding year (n = 1), uncontrolled hypertension (n = 1), or serum creatinine concentration > 176.8μM (n = 1). Their respective primary platelet-response and long-term response rates were 9 of 13 (69%) and 10 of 13 (77%).
No difference was found in terms of efficacy (rates of primary or long-term responders) for the different subgroups of patients with comorbidities, except for very few patients with neurologic disorders or obesity.
Safety
Only 2 patients had to stop romiplostim because of persistent intolerance: one for headaches and one for arthralgias. Otherwise, the most frequently reported adverse events were arthralgias (26%), fatigue (13%), and nausea (7%). Transient thrombocytosis (> 400 × 109/L and > 1000 × 109/L) was observed in, respectively, 19% (14 of 72) and 4% (3 of 72) of the patients (Table 4). For the patients with specific comorbidities, those who would have been excluded from the pivotal trials or were not splenectomized because of their lack of splenic sequestration, adverse-event frequencies did not differ compared with the other patients (data not shown). Despite having platelet counts < 100 × 109/L, 2 elderly patients (> 70 years old) with cardiovascular risk factors each experienced a transient ischemic cerebral attack without sequelae. No deep vein thrombosis occurred, and bone-marrow biopsies were not obtained to assess reticulin fibrosis for this population.
Side effects reported with romiplostim
| Side effect . | n (%) . |
|---|---|
| Arthralgias | 18/72 (26) |
| Fatigue | 9/72 (13) |
| Nausea | 5/72 (7) |
| Thrombocytosis | |
| > 400 × 109/L | 14/72 (19) |
| > 1000 × 109/L | 5/72 (7) |
| Transient cerebral ischemic attack | 2/72 (3) |
| Persistent headaches | 1/72 (1) |
| Persistent arthralgias | 1/72 (1) |
| Side effect . | n (%) . |
|---|---|
| Arthralgias | 18/72 (26) |
| Fatigue | 9/72 (13) |
| Nausea | 5/72 (7) |
| Thrombocytosis | |
| > 400 × 109/L | 14/72 (19) |
| > 1000 × 109/L | 5/72 (7) |
| Transient cerebral ischemic attack | 2/72 (3) |
| Persistent headaches | 1/72 (1) |
| Persistent arthralgias | 1/72 (1) |
Discussion
Herein, we described, for the first time, romiplostim efficacy and safety with 2 years of follow-up for a clinical practice group of 72 consecutive adults with severe chronic ITP and with frequent and, sometimes, severe comorbidities, which could be criteria of exclusion in clinical trials. Our results clearly demonstrated that romiplostim in this setting is a highly effective therapy, as overall platelet responses were achieved in more than three-fourths of the patients. Using rather stringent response criteria, more than half of the patients had achieved sustained responses at 2 years. That, after 2 years, > 60% of the patients were still receiving romiplostim further supports its efficacy and safety and emphasizes that some patients, who had not been classified as sustained platelet responders, had indeed achieved clinical benefit. Moreover, almost all of the patients who had been taking concomitant medications when romiplostim was first administered achieved responses enabling them to either discontinue or at least lower their concurrent medication doses.
The retrospective design of this study may have induced some bias. However, the risk of missing data seems limited because, according to the official rules of the French CUP, all physicians had to monitor platelet counts at least once monthly and report romiplostim efficacy and safety data to healthcare authorities. Moreover, no patients were lost to follow-up. So, despite being retrospective, our data seem to be valid for assessing this peptibody's efficacy.
Notably, our overall response rate was comparable with those obtained in the pivotal studies,10,11 which led to romiplostim approval, with similar percentages of immediate- and long-term responses and a comparable percentage of patients who required rescue interventions at some time during the observation period (supplemental Table 4). Importantly, these response rates were obtained in a group of patients with chronic severe ITP often complicated by comorbidities. Moreover, almost all the patients had previously failed to respond to rituximab, and more than one-third of them had previously received cytotoxic or immunosuppressive agents. Furthermore, more than half of the patients were on treatment when romiplostim was started, and half of them had bleeding signs during the month preceding the first romiplostim administration.
In contrast to previous findings, romiplostim efficacy did not seem to be influenced by splenectomy status. Nonresponse to romiplostim was predicted only by baseline ITP severity, attested by the use of concomitant therapies, or bleeding signs. The significant association between a high bleeding score and failure to respond to romiplostim could reflect the ITP mechanism, and the balance between platelet destruction and impaired platelet production. We previously demonstrated that, in chronic ITP, the bleeding score was closely associated with antiplatelet antibodies detected by monoclonal antibody-specific immobilization of platelet antigens.16 One hypothesis is that antiplatelet antibodies might be a marker of marked platelet destruction that could explain the poor response to thrombopoietin-mimetics.
Our mean weekly romiplostim dose of 4.7 μg/kg was higher than the mean dose reported for nonsplenectomized patients in the first pivotal study11 but was comparable with that observed by Bussel et al10 in the open-label, long-term, extension study and the latest study by Kuter et al17 comparing romiplostim with standard of care for ITP. Our higher dose could be explained by the ITP severity of our patients because more than two-thirds of them had concomitant treatment(s) for ITP at baseline versus only 28% and 32%, respectively, for those included in the pivotal studies by Kuter et al11 and Bussel et al.10 Interestingly, the mean weekly romiplostim dose for the patients with a clinical benefit did not reach the maximum level of 10 μg/kg, meaning that some clinicians prefer a clinical response over a platelet response.
The observed variability of responses, with week-to-week fluctuations of platelet counts, has been reported as a potential limitation for long-term romiplostim use.10 However, these fluctuations were minor and seldom clinically relevant, as the mean weekly romiplostim dose almost always remained within ± 2 μg/kg of our patients' most frequently administered dose.
In international guidelines, splenectomy has long been considered the “gold standard” and the main second-line therapy for ITP patients who fail to respond durably to first-line therapy.3 However, increasing numbers of patients are reluctant to undergo splenectomy, and many physicians have also become less enthusiastic to recommend it. The United States Food and Drug Administration approved romiplostim as a long-term treatment for chronic ITP in adults who did not respond to standard medical treatments, regardless of their splenectomy status. In Europe, the drug has been licensed only for adults with truly chronic refractory ITP, namely, those who failed to respond to splenectomy or for whom splenectomy is contraindicated. Notably, in this CUP, only half of the patients included were splenectomized, despite the severe evolution of their disease. This finding underscores the need for an effective and safe alternative to splenectomy. The reported reasons for not undergoing splenectomy were either patient refusal for approximately one-third or its contraindication for two-thirds. The main contraindications of advanced age and/or comorbidities often failed to hold up to our scrutiny, thereby confirming that physicians now view splenectomy less favorably.
Hence, the choice of romiplostim versus splenectomy is a crucial issue. Notably, 7 physicians preferred treating patients with romiplostim than recommending splenectomy because 111In-labeled platelet-life span scintigraphy showed no predominant splenic sequestration. However, conflicting results have been reported regarding the value of this complementary investigation to predict splenectomy outcome.18,19 Future studies focusing on the predictive value of platelet-longevity scintigraphy may help determine better whether a patient could benefit from romiplostim before undergoing splenectomy.
In clinical practice, it is important to assure the good safety of a newly introduced agent that has so far been tested only on selected patients through clinical trials. Romiplostim safety seemed to be very good in this study, and both the frequency and severity of adverse events were comparable with those observed in the pivotal studies,10,11 except for headaches, which were less frequent. The latter could be explained by underreporting of mild side effects because of the retrospective design of the study and the difficulty of recording adverse events extensively on a daily basis outside clinical trials. In contrast, that a severe adverse event could have been missed and not reported seems unlikely, as no patient was lost-to-follow-up, and only 2 patients had to stop romiplostim for intolerance: one complained of persistent headaches and the other of arthralgias. Transient thrombocytosis was rare and not complicated by thrombotic events. Two elderly patients with cardiovascular risk factors had transient ischemic attacks without sequelae. This potentially severe side effect was previously reported in a pivotal study,10 but their platelet counts were still low at the time of these events and, in light of the patients' ages and their cardiovascular risk factors, a romiplostim role seems unlikely. Although all our patients had 2 years of follow-up, only more prolonged observation will be able to affirm the low romiplostim toxicity over the very long term. However, because reticulin deposition was previously observed with thrombopoietin-receptor agonists,20 we cannot determine the magnitude of this risk at this time in the absence of bone-marrow biopsies, which were not done for our patients.
In conclusion, romiplostim unequivocally appeared to be highly effective in unselected patients with severe chronic refractory ITP. Moreover, even though additional long-term postmarketing data are warranted, the romiplostim safety profile after 2 years of follow-up is also reassuring. However, because romiplostim has been licensed in Europe only for splenectomized patients and increasing numbers of patients and physicians have become reluctant to consider splenectomy, new therapeutic approaches are still needed for a better management of adult ITP in the persistent or chronic phase before splenectomy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Janet Jacobson for editorial assistance and all the others physicians who had included one patient in this study: Didier Albert, Olivier Allangba, Sergio Altamiranda, Radu Antal, Jean-Noël Bastie, Pauline Brice, Bernard Christian, Jean-François Claisse, Laurent Garderet, Stéphane Girault, Pascal Godmer, Marie Gourin-Chaury, Jean-François Guichard, Arnaud Jaccard, Fabrice Jardin, Sophie Lefort, François Lifermann, Élena Loppinet, Margaret Macro, Michel Maigre, Nathalie Marchay, Véronique Morel, Eric Oksenhendler, Brigitte Pegourie, Jana Ranta, Frédérique Roy-Péaud, Anne-Laure Taksin, Alexia Thannberger, Xavier Thomas, Madalina Uzunov, and Philippe Weber.
This work was supported in part by Amgen France for data collection. Amgen played no role whatsoever in designing the study, collecting and analyzing the data, writing the manuscript, or deciding to submit it for publication.
Authorship
Contribution: B.G. and M.K. designed the study and initiated this work; M.K. and L.L. collected data; M.K., B.G., M. Michel, and P.B. wrote the report; and F.R.-T. performed all statistical analyses. P.Q., J.F.V., M.A., S.C., J.M.-D., F.L., L.G., O.L., G.P., B.S., G.D., G.S., E.G., X.D., N.D., B.R., N.S., J.F.-R and M. Mahévas participated to this study by sending follow-up data of their included patients in this CUP.
Conflict-of-interest disclosure: M.K. and M. Michel served on Scientific Advisory Boards for Roche, GSK and Amgen Laboratories. B.G. is a consultant for the Laboratoire de Fractionnement et de Biotechnologies (Les Ulis, France) and Amgen France; he received research funds from Roche France and serves on the Advisory Board for GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Bertrand Godeau, Department of Internal Medicine, Centre Hospitalier Universitaire Henri-Mondor, 51, avenue du Maréchal-de-Lattre-de-Tassigny, 94010 Créteil Cedex, France; e-mail: bertrand.godeau@hmn.aphp.fr.

![Figure 2. Platelet counts and romiplostim doses throughout the 2 years of follow-up of severe chronic ITP patients responding to romiplostim. (A) Median (25th [Q1] and 75th [Q3] percentiles) platelet counts. (B) Romiplostim dose (μg/kg; mean ± SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/16/10.1182_blood-2011-03-340166/4/m_zh89991179180002.jpeg?Expires=1769178494&Signature=KJmaVcxzMKKtbfmoswMhV0kFfqFAQu0xkpRyLEEgL~G8JMyE95Zg~aPOUwTzwsONcIvmHAYtk3VcI5wYebtoKHkLSri8KkoqqtuZYMbUX1UiBgvDM01y2m0gPEBRWB-IqiWOfOuxjq6~EBfimSbmQeMh7NPpEm~Z6Qi~6bXU-A0btUSYZl1whpaY7GbhEOlQMzXp9vfzWE1N-3Ev-7PJFoNTjZuwisXSqvCgCIryQZJ7lcaH33vYa7gQ-SjKEbB1WAK8UJ0qGkiJIbwtxUI1leFqTGV5J1fEL7sm14zHEwMf26-X6MyB7WX~s-MB6NDnWDbmEKOpm08PBmrzHWHmqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal