Abstract
T-cell large granular lymphocyte leukemia (T-LGLL) is characterized by chronic lymphoproliferation of cytotoxic T lymphocytes (CTLs) and is associated with lineage-restricted cytopenias. Introduction of T-cell receptor (TCR) variable β-chain (Vβ) monoclonal antibodies has facilitated identification and enumeration of clonal CTLs by flow cytometry. A highly skewed TCR Vβ repertoire identified by flow cytometry is strongly associated with monoclonal CDR3 regions by quantitative sequencing and positive TCRγ rearrangement assays. Therefore, Vβ expansions can serve as surrogate markers of CTL clonality to assess clonal kinetics in T-LGLL. We analyzed the TCR repertoire in 143 patients, 71 of which were available for serial measurements over 6 to 96 months. Although the majority (38/71, 54%) maintained a consistent monoclonal expansion, many (26/71, 37%) unexpectedly displayed a change in the dominant clone, whereby the original CTL clone contracted and another emerged as demonstrated by Vβ typing. Our results demonstrate that the T-cell repertoire is more dynamic in T-LGLL than recognized previously, illustrating the heterogeneity of disorders under this categorization.
Introduction
T-cell large granular lymphocyte leukemia (T-LGLL) is characterized by the chronic lymphoproliferation of cytotoxic T lymphocytes (CTL) and is often associated with lineage-restricted cytopenias. Frequently, T-LGLL evolves in the context of autoimmune disease and has a much higher incidence in the elderly.1-4 T-LGLL resembles a reactive rather than totally autonomous malignant process and seems to constitute an extreme pole of normal oligo- or polyclonal immune responses as observed in the context of infection with β-herpes viruses such as EBV and CMV.5-10 Consequently, T-LGLL has been hypothesized to be driven by chronic antigen exposure such as a persistent viral infection, an autoimmune process, or perhaps a malignancy-associated antigen in the context of tumor immune surveillance.11-15
Various clonally restricted signal transduction defects have been described in T-LGLL, indicating some type of deficiency in the normal physiologic apoptosis that leads to termination of an immune response.16-20 However, neither the key molecular defect nor an immunogenetic predisposition has been identified that would explain a propensity toward the marked oligo- or monoclonal dominance within the T-cell repertoire characteristic of T-LGLL. In the absence of an indisputable pathognomonic molecular feature, diagnosis of T-LGLL may be challenging. Current World Health Organization guidelines for diagnosis acknowledge the absence of a recurrent karyotypic abnormality and recognize the small number of chromosomal abnormalities identified.21 Although there is some controversy, many experts in the field agree that a combination of the following characteristics define the disease: increased large granular lymphocyte (LGL) count by peripheral blood smear > 2000 cells/μL (although LGL count between 400 and 2000 cells/μL is sufficient if other criteria are present); abnormal flow cytometry identified by a population of cells expressing CD3+, TCRαβ+, CD4−, CD5dim, CD8+, CD16+, CD27−, CD28−, CD45RO−, and CD57+; TCRγ gene rearrangement by PCR to demonstrate clonality; and persistence of this condition for longer than 6 months. Other factors supporting diagnosis involve the presence of cytopenia in which bone marrow analysis has ruled out myelodysplastic syndrome or aplastic anemia or the presence of autoimmune disease.22
Introduction of T-cell receptor (TCR) variable β-chain (Vβ) monoclonal antibodies has facilitated identification and enumeration of clonal CTLs by flow cytometry.23-25 In the clinical setting, Vβ clonotyping also has found diagnostic utility with the caveat that a significant expansion of a CTL clone characterized by a specific Vβ chain does not strictly prove clonality because various rearrangements within a given Vβ family and combinations with TCRα chains are possible. Consequently, absolute proof of clonality requires molecular testing of TCR rearrangement accomplished by DNA sequencing of the Vβ region and even better by sequencing of both Vβ and Vα chains. Proof of clonality alone is not sufficient for diagnosis, though, because T-cell expansions can occur in reactive conditions as well as in the elderly.25,26 Nevertheless, highly skewed Vβ polarity identified by flow cytometry has been found to be strongly associated with a positive TCRγ rearrangement test and monoclonal CDR3 regions by quantitative sequencing.27 Therefore, flow cytometric Vβ expansions can serve as surrogate markers of CTL clonality in a clinical setting in which application of sequencing is not feasible.28
Vβ typing has been applied largely in cross-sectional studies, and, to date, information is insufficient regarding the dynamics of the clonal process over the extended clinical course of typically chronic T-LGLL patients. Incidental observation of an informative patient whose cytopenia recurred after immunosuppressive therapy revealed that the dominant clonal population expressing a specific Vβ region can contract and be replaced over time by another clonal population of cells expressing a different Vβ region, a process designated as “clonal drift.” Therefore, we hypothesized that the proliferative propensity of T-LGLL CTLs may not be solely intrinsic to the aberrant clone as in the case of a transforming mutation characteristic of other lymphomas or leukemias. We set out to more precisely characterize clonal kinetics during the course of T-LGLL, and we found that clonal drift is commonly observed over the duration of the disease and may or may not be associated with a change in clinical features. The data presented herein are the results of longitudinal analysis of the CTL clonal process in a large cohort of patients with T-LGLL followed for an extended period.
Methods
Patients
Peripheral blood sample collection from patients was performed at clinically indicated testing after informed consent, according to the protocols approved by the Institutional Review Board of the Cleveland Clinic, The Pennsylvania State University, or UCLA and in accordance with the Declaration of Helsinki. A retrospective chart review was carried out under review by the Institutional Review Board of the Cleveland Clinic. Based on World Health Organization guidelines, the following criteria were used to diagnose T-LGLL: monoclonal TCRγ-chain rearrangement, an LGL count by peripheral blood smear of > 2000 cells/μL (not a critical criteria, patients who met all other criteria but with an LGL count < 2000 cells/μL were included); flow cytometric evidence of an abnormal CTL population characterized by expression of CD2, CD3, TCRαβ (or γδ), CD4 (in a few cases), CD5dim, CD8, CD16/56 and CD57 with negativity of CD28, and persistence of this condition for more than 6 months. Each patient must have met at least 3 of these criteria to be included in the study. In addition, TCR Vβ expansions were detected and quantitated according to criteria described previously.29-31 Cytopenias were classified as neutropenia (absolute neutrophil count [ANC], < 1.5 × 103/μL), anemia (hemoglobin, < 13 g/dL), and thrombocytopenia (platelet count, < 150 × 103/μL). Clinical responses were determined according to the modified International Working Group criteria for myelodysplastic syndromes, as reported previously.32,33 Clinical patient characteristics are displayed in Table 1.
Clinical characteristics (n = 143)
| Parameter . | % (proportion) . | Value . |
|---|---|---|
| Age at diagnosis, y | 61 ± 14 | |
| Sex | 79 M:64 F | |
| LGL count, cells/μL | 2491 ± 3465 | |
| Splenomegaly % (proportion) | 38 (54/143) | |
| TCR rearrangement by PCR, % (proportion) | 97 (136/140) | |
| CD57 positive % (proportion) | 88 (126/143) | |
| Hematologic manifestation, % (proportion) | 85 (122/143) | |
| Neutropenia | 50 (72/143) | |
| Anemia | 64 (92/143) | |
| Thrombocytopenia | 33 (47/143) | |
| Multilineage cytopenia | 32 (45/143) | |
| Pancytopenia | 16 (23/143) | |
| Lymphocytosis | 27 (39/143) | |
| Lymphopenia | 19 (27/143) | |
| B-cell or antibody disorder, % (proportion) | 37 (53/143) | |
| MGUS/multiple myeloma | 11 (14/127) | |
| Hypergammaglobinemia | 14 (18/127) | |
| Hypogammaglobinemia | 8 (10/127) | |
| Chronic lymphocytic leukemia | 8 (11/143) | |
| Hairy cell | 1 (1/143) | |
| Non-Hodgkin lymphoma | 1 (2/143) | |
| B-cell lymphoma/LPD | 1 (2/143) | |
| Autoimmune disease, % (proportion) | 20 (29/143) | |
| Rheumatoid arthritis | 13 (18/143) | |
| Type II diabetes | 3 (4/143) | |
| Myasthenia gravis | 1 (2/143) | |
| Pernicious anemia | 1 (2/143) | |
| Sjögren, Crohn | 1 (2/143) | |
| Felty, polymyalgia, psoriasis | 2 (3/143) |
| Parameter . | % (proportion) . | Value . |
|---|---|---|
| Age at diagnosis, y | 61 ± 14 | |
| Sex | 79 M:64 F | |
| LGL count, cells/μL | 2491 ± 3465 | |
| Splenomegaly % (proportion) | 38 (54/143) | |
| TCR rearrangement by PCR, % (proportion) | 97 (136/140) | |
| CD57 positive % (proportion) | 88 (126/143) | |
| Hematologic manifestation, % (proportion) | 85 (122/143) | |
| Neutropenia | 50 (72/143) | |
| Anemia | 64 (92/143) | |
| Thrombocytopenia | 33 (47/143) | |
| Multilineage cytopenia | 32 (45/143) | |
| Pancytopenia | 16 (23/143) | |
| Lymphocytosis | 27 (39/143) | |
| Lymphopenia | 19 (27/143) | |
| B-cell or antibody disorder, % (proportion) | 37 (53/143) | |
| MGUS/multiple myeloma | 11 (14/127) | |
| Hypergammaglobinemia | 14 (18/127) | |
| Hypogammaglobinemia | 8 (10/127) | |
| Chronic lymphocytic leukemia | 8 (11/143) | |
| Hairy cell | 1 (1/143) | |
| Non-Hodgkin lymphoma | 1 (2/143) | |
| B-cell lymphoma/LPD | 1 (2/143) | |
| Autoimmune disease, % (proportion) | 20 (29/143) | |
| Rheumatoid arthritis | 13 (18/143) | |
| Type II diabetes | 3 (4/143) | |
| Myasthenia gravis | 1 (2/143) | |
| Pernicious anemia | 1 (2/143) | |
| Sjögren, Crohn | 1 (2/143) | |
| Felty, polymyalgia, psoriasis | 2 (3/143) |
MGUS indicates monoclonal gammopathy of undetermined significance.
Flow cytometry
Fresh peripheral blood was stained for Vβ flow cytometry analysis to quantitate the percentage of each Vβ family in the CD4 and CD8 lymphocyte populations. The manufacturer's instructions (IOTest Beta Mark kit; Beckman Coulter) were modified as follows: 5 μL of phycoerythrin cyanin (PC) 5-conjugated anti-CD4 (Beckman Coulter) and 5 μL of PC7 anti-CD8 (BD Biosciences) monoclonal antibodies were added. Anti-Vβ 6.7 FITC (Pierce Chemical), anti-CD3 FITC, anti-TCRαβ PC5, and anti-TCRγδ PE (Beckman Coulter) also were included in the panel. A 4-color acquisition protocol was used on an FC500 with CXP Version 2.2 software (Beckman Coulter). FCS Express Version 3.0 (De Novo Software) was used for analysis. The lymphocyte gate was set according to forward and side scatter. For Vβ family T-cell repertoire analysis, gates were set on CD4 and CD8 bright lymphocyte populations and then analyzed for Vβ distribution. Mean and SD values were provided by the manufacturer of the IOTest Beta Mark kit and are based on a control population of 85 volunteers, as described previously.24,27 In addition, a separate Vβ repertoire control group of 69 volunteers was analyzed that did not differ significantly from the Beckman group or previous publications.30 A significant clonal expansion was defined as an expansion that was greater than the mean + 3 SD of healthy controls. Borderline or minor expansions were defined as those within 20% of the mean + 3 SD. In the absence of a detectable CD8 LGL clone by the Vβ panel, indirect evidence of a T-cell expansion was inferred based on a positive monoclonal TCRγ rearrangement test, reversed CD4/CD8 ratio, and the absence of reactivity to antibodies in the Vβ panel, as discussed previously.24,25
PCR
Statistical analysis
JMP 8.0 (SAS Institute) was used for statistical analyses, including linear regression, matched pair analysis, 2-tailed Fisher exact test, Wilcoxon nonparametric test, and survival curves. Because distributions were not normal in most parameters analyzed, nonparametric methods were used whenever possible to compare groups. Kaplan-Meier survival curves were right censored if the subject was alive or lost to follow-up at the time the study was closed.
Results
Patient characteristics
We have collected a large and well annotated cohort (n = 143) of patients with T-LGLL (Table 1). Diagnosis of T-LGLL was established using stringent clinical criteria as described under “Patients.” Clonal TCRγ chain rearrangement as detected by PCR was present in 97% of patients, 4 patients who were negative, and 3 patients for whom the test was not performed at our facility met all other criteria for diagnosis and were included in the cohort. Mean age at diagnosis was 61 ± 14 years. An abnormal T-cell population was present by standard flow cytometry in all patients, and 38% of patients presented with splenomegaly. The primary hematologic manifestation was anemia (64%), followed by neutropenia (50%), multilineage cytopenia (32%), and thrombocytopenia (33%). The most common multilineage cytopenia was anemia with neutropenia (25/45, 55%); 27% patients had lymphocytosis and 19% patients had lymphopenia. Of note is that 15% of patients did not have cytopenia, whereas 14% of patients were pancytopenic.
Flow cytometric Vβ typing
Vβ flow cytometric phenotyping was performed in all patients at the outset and serially at roughly 6-month intervals when possible, with a follow-up of 2.3 ± 1.9 years and with up to 9 measurements per patient in the long-term follow-up group (n = 71). We first studied the role of flow cytometric Vβ typing in identifying the clonal CTL process. A control group of 69 healthy controls (mean age, 32 ± 16 years) including a subgroup of individuals more than 45 years old (n = 28; mean age, 59 ± 10 years) were used to define the normal contribution of 25 Vβ gene products to the overall clonal TCR repertoire (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). There were no differences in the mean or standard deviation Vβ values between younger and older subgroups of healthy controls (data not shown). For patients, a test was considered positive when the percentage of CD8 (or CD4) T cells of a given Vβ family was greater than the mean + 3 SD of controls (supplemental Figure 2).
Reports of clonal skewing in the elderly prompted us to investigate the prevalence of expansions in our control cohort. For this purpose, we also analyzed 4 cord blood samples to explore true baseline levels. Expansions were found in 0/4 cord blood samples, 10/41 (24%) in the younger control group, and 13/28 (46%) in controls > 45 years old (supplemental Table 1). The mean expansion size was 13% ± 6% of CD8 cells, there was no difference in size between younger and older groups, and only 3/23 (13%) controls had an expansion larger than 20% of CD8+ cells. In contrast, almost all the T-LGLL patients demonstrated an expansion (94%), with a mean size of 52% ± 29%, and 112/143 (78%) patients had an expansion larger than 20%. Thus, although the control group may demonstrate occasional T-cell repertoire skewing, the size and prevalence of the expansions are significantly smaller compared with the T-LGLL cohort. Furthermore, with CD4+ TLGLL excluded (n = 9), the CD4/8 ratio was 0.95 ± 0.9 in patients versus 3.1 ± 1.7 in controls. Consequently, when the adjusted CD8 expansion size was calculated by multiplying the expansion percentage by the CD8/4 proportion, an even more drastic difference was observed between patients and controls.
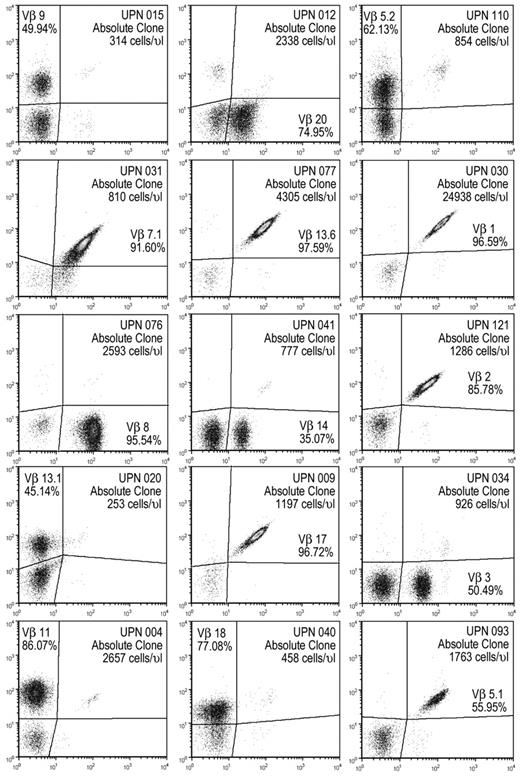
Although a much more imprecise method compared with flow cytometry, LGL count by blood smear has been used to estimate the frequency of abnormal lymphocytes in circulation. We decided to use an analogous flow cytometric estimate termed the absolute clone count (ACC). Absolute clone count by Vβ flow cytometry was calculated by multiplying the absolute white blood cells by the percentage of lymphocytes by the percentage of CD8+ and by the percentage of the specific expanded Vβ family. Vβ-based clonal count correlated reasonably well with LGL count by linear regression (P < .001, R2 = 0.58; supplemental Figure 3A). Because a T cell expresses only 1 TCR type and thus only 1 Vβ chain, flow cytometric analysis of clonal Vβ populations in LGL leukemia yielded precise, unambiguous results (Figure 1). It is possible that T cells expressing the same Vβ could through recombination display a different CDR3 sequence, but sequencing of Vβ CDR3 region has confirmed the clonal nature of the majority of cells within expanded Vβ families detected by flow cytometry (supplemental Figure 3B).
Vβ expansions can dominate the CD8 TCR repertoire and are effectively detected by flow cytometry via fluorescently labeled monoclonal antibodies against specific variable β regions. Representative flow plots of LGL patients gated on lymphocytes that are CD3+CD8+, each panel is 1 patient identified by a unique patient number (UPN). Plots may represent other measurements than baseline for each patient.
Vβ expansions can dominate the CD8 TCR repertoire and are effectively detected by flow cytometry via fluorescently labeled monoclonal antibodies against specific variable β regions. Representative flow plots of LGL patients gated on lymphocytes that are CD3+CD8+, each panel is 1 patient identified by a unique patient number (UPN). Plots may represent other measurements than baseline for each patient.
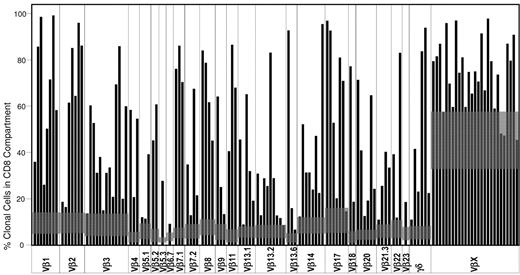
Because the Vβ assay covers ∼ 65% of the healthy control T-cell repertoire, we established mean + 3 SD values for expansions not covered by the panel of monoclonal antibodies but detected by proxy via contractions of Vβ families covered by the antibody panel, as discussed previously,23,25 here designated as VβX. Flow cytometry using cutoff values as described allowed for detection of 23/27 (85%) patients with clonal expansions not directly detected by the panel (Figure 2). Also included in our panel is a measurement of γ/δ CD3+CD8+ positive cells. An additional 6 patients were positive for VβX, but after measurement of the contribution of γ/δ CD8+ T cells, expansions beyond mean + 3 SD were identified (Figure 2).
Vβ expansions across the cohort. Each black bar shows the percentage of the CD8 T-cell population expressing a specific Vβ region. Gray boxes represent the mean + 3 SD of the control population. VβX accounts for Vβ gene products not recognized by the flow cytometry assay and is calculated by subtraction of the sum of all Vβ recognized by the assay from 100.
Vβ expansions across the cohort. Each black bar shows the percentage of the CD8 T-cell population expressing a specific Vβ region. Gray boxes represent the mean + 3 SD of the control population. VβX accounts for Vβ gene products not recognized by the flow cytometry assay and is calculated by subtraction of the sum of all Vβ recognized by the assay from 100.
Vβ use
To study clonal kinetics, we first analyzed clonal repertoire patterns at the outset of the follow-up period. In healthy controls, mean + 3 SD of the number of cells expressing a specific Vβ gene product varies significantly across the Vβ repertoire, with Vβ regions such as Vβ1, Vβ2, Vβ3, Vβ8, Vβ14, and Vβ17 expressed in a higher proportion of cells than other members of the Vβ family (Figure 2 gray boxes). We hypothesized that if the clonal process involves Vβ restriction randomly, then Vβ use will reflect the Vβ family distribution in controls. Largely, this was true because expansions more commonly affected Vβ families occurring at higher proportions in the normal TCR repertoire.
We next asked whether a particular Vβ expansion was associated with specific manifestation of disease, but we did not find a statistically significant relationship (data not shown). Given the heterogeneity of the TCR Vβ repertoire, correlation of cytopenia with a specific Vβ chain may be difficult to establish. However, Vβ1, Vβ4, and Vβ13.6 expansions seemed to be more frequent in patients with neutropenia and anemia but not in patients who are asymptomatic. In our cohort, γ/δ, Vβ9, and Vβ22 expansions were always associated with anemia. Vβ11 and Vβ23 were present in asymptomatic cases only (data not shown).
Clonal kinetics during the course of disease
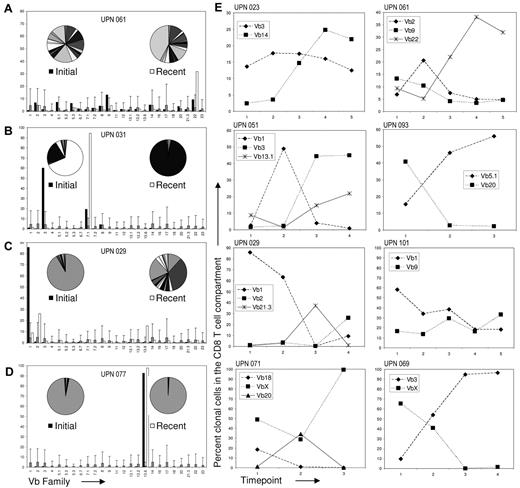
Vβ flow clonotyping allows for the identification of the Vβ restriction of expanded CTL clones and thus Vβ flow can be used to assess the T-cell repertoire during the course of disease. Of the 143 patients in our cohort, 71 were available for serial examinations of Vβ expansions for periods longer than 6 months, for 57 longer than 1 year, and for 27 we had 5 or more serial evaluations (follow-up, >24 months). None of the patients with an initial Vβ expansion returned to a normal Vβ TCR repertoire in the observation period, and 4 patterns of clonal kinetics could be distinguished (Figure 3A-D). Initial baseline and most recent flow cytometric results revealed a proportion of patients (7/71, 10%) who presented with minor expansions on a polyclonal background that over time further expanded with an accompanying relative reduction of other T cells within the TCR repertoire (representative patient shown in Figure 3A). These patients illustrate the typical clinical course of a steady monoclonal expansion that changes from a small to a large immunodominant clone and were not considered representative of clonal drift as the initial expansions were borderline by our criteria. Six of 7 of these patients presented with cytopenia, despite an initially small T-cell expansion.
Analysis of Vβ expansions over time reveals a heterogeneous clonal course in the study population. (A) Representative patient with borderline oligoclonal expansions at the initial observation (left pie representation black bars) that, by the most recent time point, had changed to monoclonal (right pie white bars). (B) Patient with biclonal expansion shown at baseline, then CD8+ T cells expressing Vβ3 decreased, whereas Vβ7.1 became dominant. (C) Patient shown with an initial extreme monoclonal expansion that became biclonal. (D) Patient with unchanging monoclonal expansion. Gray bars represent the mean of the control population and error bars are the mean + 3 SD. Similar results were obtained using absolute counts. All patients shown in this figure were followed for roughly 2 years. (E) Of the 143 patients in our cohort, 71 were available for long term Vβ follow-up beyond 6 months. Twenty-six of 71 patients demonstrated clone switching as detected by flow cytometry; graphs of 8 representative patients are depicted here. The interval between time points varied from 6 months to 1 year.
Analysis of Vβ expansions over time reveals a heterogeneous clonal course in the study population. (A) Representative patient with borderline oligoclonal expansions at the initial observation (left pie representation black bars) that, by the most recent time point, had changed to monoclonal (right pie white bars). (B) Patient with biclonal expansion shown at baseline, then CD8+ T cells expressing Vβ3 decreased, whereas Vβ7.1 became dominant. (C) Patient shown with an initial extreme monoclonal expansion that became biclonal. (D) Patient with unchanging monoclonal expansion. Gray bars represent the mean of the control population and error bars are the mean + 3 SD. Similar results were obtained using absolute counts. All patients shown in this figure were followed for roughly 2 years. (E) Of the 143 patients in our cohort, 71 were available for long term Vβ follow-up beyond 6 months. Twenty-six of 71 patients demonstrated clone switching as detected by flow cytometry; graphs of 8 representative patients are depicted here. The interval between time points varied from 6 months to 1 year.
In contrast, 2 types of changes in the dominant Vβ clone were identified in 26/71 (37%) of patients: some patients demonstrated either a gradual emergence of a dominant clone from an oligoclonal background (14/71, 20%; Figure 3B), or some patients presented with an extremely dominant clone that contracted over the follow-up period while simultaneously another dominant clone evolved (12/71, 17%; Figure 3C). Both of these scenarios represent variants of what we term “clonal drift.” Finally, the last group of patients displayed an extremely dominant clone from the outset of the clinical presentation; this clone remained stable over prolonged periods (in some instances up to 8 years) in 38/71 patients (54%; Figure 3D). Serial analysis of patients in the group characterized by clonal drift revealed a variety of different patterns of the kinetics in the dominant or codominant LGL clone (Figure 3E). Those patients who initially presented with multiple clones were more likely to change clonal dominance (P = .024; data not shown). In addition, several statistically significant differences between the clonal drift group and the stable expansion group were observed (Table 2). Both clone size and absolute clone count were smaller in the clonal drift group (P < .001 and P = .008, respectively). Interestingly, several immunogenetic factors emerged because patients homozygous for HLA A2 (P = .023) were absent from the clonal drift group and HLA A29 (P = .044) was overrepresented in this group. Overall, HLA class I homozygosity was more prevalent in the stable group as well (P = .051). Although not statistically significant at α = .05, patients with lymphocytosis were less likely to exhibit clonal drift (P = .058). Furthermore, trends toward lower incidence of B-cell disorders (P = .081) and decreased number of patients with HLA B7 (P = .095) were observed in the clonal drift group. Subgroup analysis of CD5 expression did not reveal statistical differences (data not shown). Clonal drift was observed both in relapsed and in refractory patients, as well as in patients without symptoms, therapy, or both. Although clonal drift was frequently accompanied by a change in clinical hematologic features, no statistical relationship was found. Twelve of 26 (46%) patients who demonstrated clonal drift had a change in cytopenia (defined as the appearance of a new lineage deficiency not present at the initial evaluation), and 14/45 (31%) patients in the stable or steady expansion group had a cytopenia change (P = .3). This lack of statistical relationship illustrates the heterogeneity of cases. Although in some patients a change in cytopenia may be related to clonal drift as the new clone recognizes a different antigen, this is not universal because the new clone may recognize the same antigen in a different peptide-MHC configuration. In addition, cytopenia changes may be because of disease progression. For example, many T-LGLL patients develop splenomegaly which may, in turn, lead to thrombocytopenia.
Comparison of patients with long-term follow-up (n = 71)
| Parameter . | Stable (n = 45) . | Clonal drift (n = 26) . | P . |
|---|---|---|---|
| Clone size, % | 61 ± 30 | 36 ± 26 | <.001* |
| ACC, cells/μL | 3140 ± 6348 | 794 ± 1457 | .008* |
| Lymphocytosis, proportion (%) | 16/45 (35) | 4/26 (25) | .058 |
| B-cell disorder, proportion (%) | 20/45 (44) | 6/26 (23) | .081 |
| HLA A2 homozygous, proportion (%) | 9/44 (20) | 0/23 (0) | .023* |
| HLA A29, proportion (%) | 1/44 (2) | 4/23 (17) | .044* |
| HLA B7, proportion (%) | 16/41 (39) | 4/23 (17) | .095 |
| HLA class I homozygous, proportion (%) | 16/44 (36) | 3/23 (13) | .051 |
| Asymptomatic, proportion (%) | 5/45 (11) | 4/26 (15) | .715 |
| Parameter . | Stable (n = 45) . | Clonal drift (n = 26) . | P . |
|---|---|---|---|
| Clone size, % | 61 ± 30 | 36 ± 26 | <.001* |
| ACC, cells/μL | 3140 ± 6348 | 794 ± 1457 | .008* |
| Lymphocytosis, proportion (%) | 16/45 (35) | 4/26 (25) | .058 |
| B-cell disorder, proportion (%) | 20/45 (44) | 6/26 (23) | .081 |
| HLA A2 homozygous, proportion (%) | 9/44 (20) | 0/23 (0) | .023* |
| HLA A29, proportion (%) | 1/44 (2) | 4/23 (17) | .044* |
| HLA B7, proportion (%) | 16/41 (39) | 4/23 (17) | .095 |
| HLA class I homozygous, proportion (%) | 16/44 (36) | 3/23 (13) | .051 |
| Asymptomatic, proportion (%) | 5/45 (11) | 4/26 (15) | .715 |
indicates statistical significance at α = 0.05.
We also have used cDNA CDR3 sequencing to see whether there is a structural similarity between TCRs in the clonal drift group. For example, in a representative patient (Figure 4B), the initial biclonal expansion CDR3 AA sequences were ASSPLGAGVYNEQF (Vβ3) and ASSIDRGRRETQ (Vβ17). In contrast, the Vβ12 expansion that began to emerge at time point 6 and then came to dominate the TCR repertoire by the most recent measurement had a CDR3 AA sequence of AISEFPGGQTSDEQFF. Because all 3 of these expansions demonstrate vastly different CDR3 sequences, it seems that clonal drift does not have to be associated with TCR homology.
Long-term TCR repertoire monitoring may have potential as a biomarker for disease progression. Two patients were followed for 8 years. (A) Patient demonstrates an initial biclonal expansion that became monoclonal and then decreased during hematologic remission after therapy. When the clone re-expanded, remission was maintained with the resumption of therapeutic intervention. (B) Patient with initial biclonal expansion that became monoclonal after treatment entered hematologic remission despite the presence of a clone that dominated the TCR repertoire. Emergence of a new clone (Vβ12) preceded relapse and eventually required further intervention. At the most recent measurement, this clone now accounts for > 80% of CD8 T cells. Amino acid sequences of the expanded T-cell populations are shown next to relevant time points.
Long-term TCR repertoire monitoring may have potential as a biomarker for disease progression. Two patients were followed for 8 years. (A) Patient demonstrates an initial biclonal expansion that became monoclonal and then decreased during hematologic remission after therapy. When the clone re-expanded, remission was maintained with the resumption of therapeutic intervention. (B) Patient with initial biclonal expansion that became monoclonal after treatment entered hematologic remission despite the presence of a clone that dominated the TCR repertoire. Emergence of a new clone (Vβ12) preceded relapse and eventually required further intervention. At the most recent measurement, this clone now accounts for > 80% of CD8 T cells. Amino acid sequences of the expanded T-cell populations are shown next to relevant time points.
Correlation with response
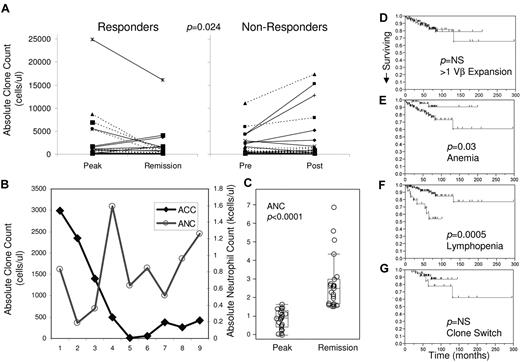
Figure 4 shows 2 examples of clonal kinetics over periods of more than 8 years. The patient depicted in Figure 4A entered hematologic remission paralleled by gradual disappearance of the aberrant Vβ clone. Analysis of the patient in Figure 4B did not reveal a relationship between clone size and remission; instead, the emergence of a new clone (characterized by a different Vβ use and corresponding AA sequence) was associated with relapse. Analysis of absolute clone size at the peak of hematologic disease versus absolute clone size during remission in patients responsive to therapy reveals a trend toward contraction of pathognomonic aberrant clones (Figure 5A). Conversely, a trend toward clonal expansion was noticed in nonresponders (Figure 5B). Matched pairs statistical analysis of the change in ACC demonstrated a significant mean difference between responders and nonresponders to various therapies (matched pairs analysis, P = .024). Long-term analysis of the ACC of the patient in Figure 4A reveals an increase in absolute neutrophil counts paralleling clonal contraction (Figure 5B). In this patient, the average of ANC when ACC was > 1000 is 0.43 (borderline severe to moderate neutropenia), whereas the average when the ACC is < 500 is 0.95 (borderline moderate to mild). When patients with neutropenia who responded to various therapies (cytoxan, cyclosporine/prednisone, or campath) were analyzed at the hematologic peak of disease versus remission, a statistically significant increase in ANC was observed (P < .0001; Figure 5C). Although there may not be an absolute correlation between ACC and ANC, the trend between a decrease in ACC and hematologic improvement suggests that there is a relationship.
Absolute clone count by Vβ flow cytometry can be used to monitor therapy, and survival data suggest possible association with clone switching. Absolute clone size tends to decrease in responders to therapeutic intervention (n = 23) versus nonresponders (n = 26; matched pairs analysis, P = .024). Response was determine by hematologic improvement. (A) Patients with full hematologic remission (left panel broken lines) and patients with partial remission (left panel full lines) tend to demonstrate a decrease in absolute clone size. Patients who do not respond to therapy with no change in hematologic status (right panel broken lines) and patients whose condition deteriorated during treatment (right panel full lines) tend to exhibit an increase in absolute clone size. (B) Patients who displayed an increase in ANC as absolute clone count decreased. Although the proportion of clonal cells increased markedly after time point 6, the ACC increase was more modest and was accompanied by an increase in ANC by the most recent time point. (C) Subgroup analysis of patients with neutropenia who responded to various therapies (n = 23; cytoxan, cyclosporine, campath) demonstrate a significant increase in ANC. Clearly nonsignificant survival data are demonstrated for the presence of > 1 Vβ expansion (D bottom line), whereas patients with anemia (E bottom line) and lymphopenia (F bottom line) tend to do much worse. Clone switching (G bottom line) may have a relationship with survival, but at this analysis the data do not yield statistically significant results.
Absolute clone count by Vβ flow cytometry can be used to monitor therapy, and survival data suggest possible association with clone switching. Absolute clone size tends to decrease in responders to therapeutic intervention (n = 23) versus nonresponders (n = 26; matched pairs analysis, P = .024). Response was determine by hematologic improvement. (A) Patients with full hematologic remission (left panel broken lines) and patients with partial remission (left panel full lines) tend to demonstrate a decrease in absolute clone size. Patients who do not respond to therapy with no change in hematologic status (right panel broken lines) and patients whose condition deteriorated during treatment (right panel full lines) tend to exhibit an increase in absolute clone size. (B) Patients who displayed an increase in ANC as absolute clone count decreased. Although the proportion of clonal cells increased markedly after time point 6, the ACC increase was more modest and was accompanied by an increase in ANC by the most recent time point. (C) Subgroup analysis of patients with neutropenia who responded to various therapies (n = 23; cytoxan, cyclosporine, campath) demonstrate a significant increase in ANC. Clearly nonsignificant survival data are demonstrated for the presence of > 1 Vβ expansion (D bottom line), whereas patients with anemia (E bottom line) and lymphopenia (F bottom line) tend to do much worse. Clone switching (G bottom line) may have a relationship with survival, but at this analysis the data do not yield statistically significant results.
Kaplan–Meier analysis demonstrates a possible trend toward worse survival in patients with a clone switch (Figure 5F blue line), but our cohort was too small or follow-up too short to reach statistically significant results. The presence of > 1 Vβ expansion had no impact on survival, whereas anemia and lymphopenia are strongly associated with poorer survival (Figure 5C-E, respectively). Interestingly, neutropenia, B-cell disorder, or autoimmune disease had no association with survival (data not shown). Although patients went through a variety of therapies during the course of the study, we could not find a relationship between a specific therapy and clone switching.
Discussion
Using Vβ flow cytometry, our data demonstrate for the first time the incidence of clonal drift in a well characterized cohort of patients with T-LGLL. Comparison of stable and clonal drift groups revealed several important differences. Both ACC and clone size were smaller in the clonal drift group, perhaps suggesting observation of the disease in its early manifestation. However, the HLA data, specifically the overrepresentation of stable patients with homozygous HLA A2, suggest immunogenetic factors contribute toward the large, stable monoclonal expansions featured in the majority of T-LGLL patients. Historic research on MHC suggests that homozygosity at an MHC allele would limit the range of potential antigen derived peptides presented and correspondingly, the number of T-cell clones capable of responding to a given peptide-MHC. The statistical relationship between homozygosity and stable monoclonal expansions implies that the lack of immune flexibility is a factor in this T-LGLL phenotype.
Two theories have dominated investigations into the pathogenesis of T-LGLL. First, T-LGLL is a T-cell leukemia characterized by proliferation and accumulation of a transformed T cell because of an acquired intrinsic molecular defect such as a mutation.28 Alternatively, T-LGLL represents global dysregulation of CTL repertoire homeostasis because of persistent antigenic drive in combination with immunogenetic factors promoting extreme T-cell expansions.16 Our results lend support to the latter theory by demonstrating that T-LGLL may involve multiple clones occurring concurrently or even serially. Consequently, it may be that, in a proportion of cases, extreme clonal evolution is the result of a polarized reactive process without a clonally restricted molecular lesion. This mechanism would be in contrast to a traditional malignant evolution and the paucity of molecular lesions such as mutations or recurrent chromosomal abnormalities, normally seen in most leukemias and lymphomas, supports this theory. Clearly, T-LGLL is a heterogeneous group that includes reactive and leukemic cases with a clinically similar phenotype.
It is clinically feasible to use Vβ flow cytometry to monitor clonal kinetics on a routine basis because this technique is capable of identifying specific immunodominant T-cell expansions. As such, our data suggest that there may be a distinction between a monoclonal lymphocytosis and leukemic clonal proliferations. Furthermore, the strong association of T-LGL with various B-cell dyscrasia11,35-40 also argues for a chronic immune process that can engage multiple arms of immunity or suggests some as yet undefined constitutional or acquired dysregulation within T cells that influences the entire lymphoid compartment.
The question as to why some individuals develop various cytopenias but others display T-cell clonopathy of unknown significance is unanswered to date. It is possible that the antigenic target is irrelevant or the effector function of LGLs ineffective in asymptomatic cases. Consistent with this finding, occasional TCR repertoire skewing can be found in seemingly healthy controls, and this phenomenon seems to be more common in the elderly.26 However, clonal expansions in controls may represent an outlier of the normal T-cell response driven by chronic antigenic stimulation more frequently operative in older individuals. In fact, although within expanded Vβ families in T-LGLL one rearranged TCR predominates, in controls the Vβ expansions are more polyclonal (M.W.W., unpublished observations, November 2005). In addition, some patients, in particular the elderly, may in fact have subclinical T-LGLL. Even if the Vβ repertoire may be on occasion skewed in healthy controls, a normal CD4/8 ratio and correspondingly low proportion of CD8 cells shows that, in controls, “normal” expansions are actually much lower than those found in T-LGLL patients when absolute counts are considered. As such, extreme skewing as found in T-LGLL has not been observed in controls; consequently, flow cytometric clonotyping may be useful (particularly with problematic cases) in the management of T-LGLL. Establishment of appropriate cutoff values is matter of some controversy23-25,41 ; however, we believe the strength of the assay lies in the identification of the immunodominant T-cell population and in monitoring potential changes in the TCR repertoire of T-LGLL patients. Although other reports use alternate values as cutoff points,25,41 we argue that the cutoffs used here allow for identification of Vβ expansions in various stages of disease including emergence and transition phases. This assay is not without limitations, because roughly 30% of the T-cell repertoire is not directly recognized by antibodies in the panel. Furthermore, Vβ flow alone is not sufficient for diagnosis of T-LGLL, and many other groups have outlined diagnostic strategies combining molecular, clinical, and flow cytometric criteria.23,25,29
The association of immunosuppression with hematologic recovery in symptomatic cases implies a link between the expanded T-cell population and cytopenia. Although we have shown a statistically significant trend toward clonal contraction in responders and increase in absolute clone size in nonresponders, this relationship is not perfect. Consequently, it is possible that lineage-specific hematopoietic suppression seen in T-LGLL is a by product of the aberrant CTL expansion and that antigens not derived from the hematopoietic compartment drive clonal expansion.
There are several possibilities as to why clonal drift may occur. Cytotoxic or immunosuppressive therapy may result in the contraction of the dominant clone, but because of persistence of the putative autoimmune or viral antigens, another clone is selected as detected by a different Vβ restriction. Our results, however, did not show a relationship between therapy and clone switching. Furthermore, the proportion of asymptomatic untreated patients in the clonal drift and stable groups is roughly equivalent, indicating that this phenomenon occurs in the absence of therapy. In previous studies by our laboratory using molecular clonotyping through sequencing of the CDR3 region, we have shown that alongside the dominant clone, accessory CTL clones, characterized by highly homologous sequences, may be simultaneously present.27,34,42 We also have demonstrated that similar sequences can exist in separate individuals, lending credence to the possibility that T-cell expansions in T-LGLL may be driven by a common antigen.25 Our current work further investigated this issue by comparing the CDR3 sequence of the initial and the emergent clones. These sequences were strikingly different, yet, because of the nature of TCR recognition of peptide-MHC, it remains possible that each clone expanded in response to the same pathogen because the secondary clone could recognize a different peptide from the same pathogen or the same peptide in the context of a different HLA. In this case, LGLs may undergo ontogenetic or functional repertoire change (eg, epitope shift),43 leading to eventual deletion or exhaustion of the dominant clone and emergence of another clone. Finally, it also is possible that generalized dysfunction of homeostatic mechanisms may be a result of genetic polymorphisms44,45 and other immunogenetic elements in combination with environmental factors resulting in the propensity for oligoclonal or clonal dominance of the T-cell repertoire that is in flux.
The possible trend toward poorer survival for patients who undergo a clonal drift may be indicative of the persistence of factors inducing clonal evolution. However, because of the often indolent nature of T-LGLL, longer monitoring of the T-cell repertoire by flow cytometry may be needed to discern the clinical impact of these observations on survival. Because the data presented here only touch on clinical associations, systematic long-term TCR repertoire monitoring is likely to provide clues as to the pathogenesis of LGL leukemia and assist in guiding medical management. Although not sufficient as the sole criteria for diagnosis and proof of clonality, Vβ flow cytometry is a useful ancillary test that is rapidly capable of quantifying the extent and identity of T-cell expansions. The unexpected demonstration of clonal drift in our cohort illustrates the heterogeneity of disorders under this categorization and challenges assumptions about the true leukemic nature of T-LGLL.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants R01 HL082983, U54 RR019391, K24 HL077522, and R01 CA113972 (all J.P.M.)
National Institutes of Health
Authorship
Contribution: J.P.M. is the principal investigator; A.D.V., M.S., N.B., R.L.P., T.P.L., and J.P.M. recruited patients and collected and analyzed clinical data; M.J.C., M.W.W., I.B., and H.M. performed research and analyzed data; M.J.C., M.W.W., A.E.L., E.D.H., R.L.P., T.P.L., and J.P.M. designed the study; and M.J.C. and J.P.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaroslaw P. Maciejewski, Taussig Cancer Center/R40, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: maciejj@ccf.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal