Abstract
TET2 is mutated/deleted with high frequencies in multiple forms of myeloid malignancies including MDS, CMML, MPN, and AML. However, little is known regarding the biological function of TET2 and its role in the pathogenesis of myeloid malignancies. To study the function of TET2 in vivo, we generated a Tet2 knock out mouse model. Deletion of Tet2 in mice led to dramatic reduction in the 5-hydroxymethylcytosine levels and concomitant increase in the 5-methylcytosine levels in the genomic DNA of BM cells. The Tet2−/− mice contained an increased Lin−Sca-1+c-Kit+ (LSK) cell pool before the development of myeloid malignancies. A competitive reconstitution assay revealed that Tet2−/− LSK cells had an increased hematopoietic repopulating capacity with an altered cell differentiation skewing toward monocytic/granulocytic lineages. Approximately 1/3 of Tet2−/− and 8% of Tet2+/− mice died within 1 year of age because of the development of myeloid malignancies resembling characteristics of CMML, MPD-like myeloid leukemia, and MDS. Furthermore, transplantation of Tet2−/−, but not wild-type (WT) or Tet2+/− BM cells, led to increased WBC counts, monocytosis, and splenomegaly in WT recipient mice. These data indicate that Tet2-deficient mice recapitulate patients with myeloid malignancies, implying that Tet2 functions as a tumor suppressor to maintain hematopoietic cell homeostasis.
Introduction
Several groups have reported alterations of a novel gene, Ten-Eleven-Translocation-2 (TET2), in a variety of myeloid malignancies including myelodysplastic syndrome (MDS, up to 30% of cases), myeloproliferative neoplasms (MPN, up to 20%), chronic myelomonocytic leukemia (CMML, up to 42%; classified by the WHO as a myelodysplastic/myeloproliferative neoplasm), and acute myeloid leukemia (AML, up to 20%).1-7 The majority of the TET2 alterations includes single-copy defects, double-copy defects, or nonsense/frameshift mutations, suggesting that the TET2 lesions likely result in loss of function.1-7 Regions of uniparental disomy (usually harbor homozygous mutations) on chromosome 4 involving TET2 have been found in patients with these myeloid malignancies.3-5 Several investigators, therefore, have speculated that TET2 is a putative tumor suppressor gene of myelopoiesis that is strongly implicated in the pathogenesis of myeloid malignancies.
TET2 belongs to a 3-member family that also includes TET1 and TET3; TET1 was originally identified as a partner for the MLL gene within t(10;11)(p12;q23) translocations in AML.8,9 All 3 paralogs share a highly homologous catalytic domain catalyzes the conversion of 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC), which could epigenetically regulate gene expression by altering methylation-driven gene silencing.10-14 Therefore, TET2 might act as a tumor suppressor gene by regulating DNA methylation and epigenetic control of gene expression at critical loci important for myelopoiesis and leukemogenesis. The ability to inactivate (“knock out”) candidate tumor suppressor genes in the mouse germ line provides a powerful tool for validating candidate tumor suppressor genes.15 Almost all of the well studied tumor suppressor genes have been knocked out in the germ line of an inbred mouse strain, such as p53, NF1, AML1, APC, RB, and VHL.16-21 The development of any type of tumor at an elevated frequency in such genetically altered mice adds persuasive evidence to support the candidacy of a gene as a tumor suppressor gene.
Delhommeau et al have shown that CD34+ hematopoietic stem cells (HSC) from 2 MPN/MDS patients with TET2 defects exhibited enhanced repopulating capacities compared with that of patients without TET2 defects in a NOD/SCID murine system.4 Several reports have recently shown that small hairpin RNA-mediated depletion of Tet2 in murine hematopoietic precursors alters their cell differentiation toward monocyte/macrophage lineages in vitro.12,22 These results suggest that Tet2 is important for the regulation of normal hematopoiesis. However, the physiologic function of Tet2 in vivo has not been defined to date, and the role of Tet2 in the development of myeloid malignancies remains to be elucidated.
To challenge these critical scientific questions, we generated a Tet2-null murine model and analyzed the hematologic phenotype associated with the loss of Tet2 function in mice. Our results demonstrate that deletion of Tet2 is sufficient to cause myeloid malignancies in mice and imply that TET2 functions as a tumor suppressor in myelopoiesis.
Methods
Construction of the Tet2-targeting vector and generation of Tet2-null mice
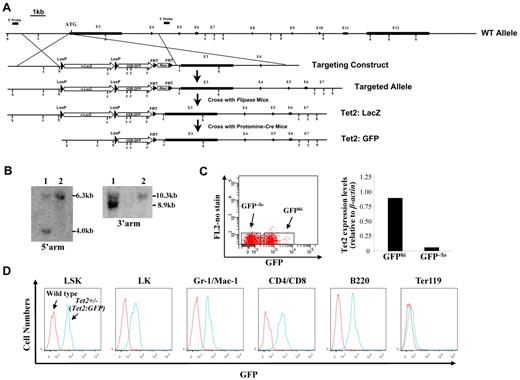
A nuclear ß-galactosidase (nlacZ) flanked by 2 loxP sites followed by a nuclear H2B-GFP (nGFP) and neomycin (Neo) was inserted 6 bp upstream of Tet2 start codon (cassette map: loxP-lacZ-polyA-loxP-H2B-GFP-polyA-FRT-Neo-FRT; endogenous ATG was disrupted; Figure 1A). The 5′ and 3′ arms of the targeting vector were amplified on 129/sv mouse genomic DNA. The nlacZ/nGFP is expressed under the control of the endogenous Tet2 promoter. Because the endogenous ATG was disrupted, the Tet2:nlacZ/nGFP is also a heterozygous null for Tet2 (Tet2+/−) after the recombination. The targeting vector was linearized and electroporated into 129/sv mouse ES cells, and subsequently screened by Southern blot (Figure 1B). Two positive clones were selected for the blastocyst (C57BL/6) injection. Male chimeric mice were crossed to C57BL/6 females to screen for germ line transmission of Tet2:nLacZ/nGFP knock-in allele. Tet2:nlacZ/nGFP mice were then crossed to Flippase deleter mice to remove the Neo cassette.23,24 Tet2:nGFP mice were generated by crossing Tet2:nlacZ/nGFP to Protomine:Cre mice.23,24 Mice harboring the Tet2:nlacZ/nGFP allele were routinely genotyped by PCR using primers that discriminated between the WT Tet2 and Tet2:nlacZ/nGFP alleles. Heterozygous Tet2:nlacZ/nGFP (Tet2+/−) mice were interbred to obtain WT, Tet2+/− and homozygous Tet2:nlacZ/nGFP (Tet2−/−) mice, which were used for all of the experiments except the analysis of GFP expression. All of the animals are bred on a mixed genetic background (129/sv & C57BL/6). All mice studies were approved by the MSSM Animal Care and Use Committee.
Generation of Tet2:nlacZ/nGFP knock-in mice and evaluation of the levels of GFP (Tet2) expression in different hematopoietic cell populations. (A) A nlacZ/nGFP-FRTNeoFRT cassette was introduced into 6bp upstream of Tet2 start codon (exon 3). (B) Southern blot of ES cell DNA digested with ScaI (S) and hybridized with a genomic fragment external to the 5′ arm displayed a wild-type (WT) band of 6.3 kb and a recombinant band of 4.0 kb, and ES cell DNA digested with EcoRI (E) and hybridized with a probe external to the 3′ arm displayed a WT band of 10.3 kb and a recombinant band of 8.9 kb. Square bars indicate exons. (C) BM cells from 6- to 8-week-old heterozygous Tet2:nGFP mice were separated into GFPhi and GFPlo/− cells and Tet2 expression levels were measured by quantitative real-time PCR. (D) GFP (Tet2) expression levels in various hematopoietic cell populations of BM cells from a representative 7-week-old heterozygous Tet2:GFP mice.
Generation of Tet2:nlacZ/nGFP knock-in mice and evaluation of the levels of GFP (Tet2) expression in different hematopoietic cell populations. (A) A nlacZ/nGFP-FRTNeoFRT cassette was introduced into 6bp upstream of Tet2 start codon (exon 3). (B) Southern blot of ES cell DNA digested with ScaI (S) and hybridized with a genomic fragment external to the 5′ arm displayed a wild-type (WT) band of 6.3 kb and a recombinant band of 4.0 kb, and ES cell DNA digested with EcoRI (E) and hybridized with a probe external to the 3′ arm displayed a WT band of 10.3 kb and a recombinant band of 8.9 kb. Square bars indicate exons. (C) BM cells from 6- to 8-week-old heterozygous Tet2:nGFP mice were separated into GFPhi and GFPlo/− cells and Tet2 expression levels were measured by quantitative real-time PCR. (D) GFP (Tet2) expression levels in various hematopoietic cell populations of BM cells from a representative 7-week-old heterozygous Tet2:GFP mice.
Analysis of 5-hmC and 5-mC levels using dot blot
Levels of 5-hmC and 5-mC in BM cells were detected using dot blot as described previously.12 Genomic DNA was isolated from BM cells of WT, Tet2+/− or Tet2−/− mice. DNA samples were denatured and 2-fold serial dilutions were spotted on nitrocellulose membranes in an assembled Bio-Dot apparatus according to manufacturer's instructions (Bio-Rad). The blotted membranes was washed with 2× SSC buffer, air-dried, vacuum-baked at 80°C for 2 hours, blocked and incubated with anti–5-hmC or anti–5-mC antibody (1:1000) overnight at 4°C. After incubating with HRP-conjugated secondary antibody, the membrane was visualized by enhanced chemiluminescence. Signal density was then quantified using FluorChem HD2 (Alpha Innotech). To ensure equal spotting of total DNA on the membranes, the same blot was stained with 0.02% methylene blue in 0.3M sodium acetate.
Analyses of mice
PB was collected by retro-orbital bleeding of WT, Tet2+/− and Tet2−/− mice and was smeared for May-Grunwald-Giemsa staining, and/or subjected to an automated blood count (Hemavet System 950FS). Morphologic analysis and cell differential of BM, spleen, and liver samples were performed on cytospins (5 × 105 total cells/sample) followed by May-Grunwald-Giemsa staining. For histopathology analyses, femurs were fixed in formaldehyde, decalcified, and paraffin embedded. Spleens and livers were treated similarly except for the step of decalcification. Sections (4.5 μm) were stained with H&E.
Flow cytometry analysis and hematopoietic progenitor cell (HPC) assay
Total white blood cells were obtained after lysis of PB with a red cell lysis buffer.25 Single-cell suspensions from BM, spleen, liver, and PB were stained with panels of fluorochrome-conjugated antibodies. Flow cytometric analysis of HSCs and HPCs were performed as previously described.26,27 Dead cells were excluded by DAPI staining. The analyses were performed using a BD FACSCantoII or LSRII flow cytometer. All data were analyzed by BD FACSDiva 6.0 and/or FlowJo 7.6 software. For colony-forming unit (CFU) assays, total BM or spleen cells isolated from each genotype of mice were plated in duplicate in methylcellulose medium (Methocult M3231; StemCell Technologies) supplemented with mIL-3, hIL-6, hEpo and mSCF, and scored in 8-10 days, whereas the CFU-erythroid (CFU-E) colonies were scored in 2-3 days.28
Real-time PCR analysis
Total RNA was isolated from BM cells of each mouse genotype and treated with RNase-free DNase to remove contaminating genomic DNA. First-strand cDNA was synthesized. Real-time PCR was performed using QuantiTect SYBR Green PCR Kit. PCR amplifications were performed in triplicate for Tet2, Tet1, and Tet3 along with parallel measurements of β-actin cDNA (an internal control). To confirm specific amplification of the desired PCR product, melting curves were analyzed and PCR products were separated on a 3% agarose gel. The primers used for the amplification of each gene are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Transplantation and competitive repopulation assay
The transplantability of tumors was determined by injecting 1 × 106 spleen cells from the deceased/moribund Tet2−/− or Tet2+/− mice into sublethally irradiated (600 cGy) C57BL/6 secondary recipient mice through tail veins. The competitive repopulation assay was performed by transplanting BM cells (1 × 106, CD45.2+) from WT, Tet2+/− or Tet2−/− mice plus the competitor BM cells (1 × 106, CD45.1+) from B6.SJL mice, into lethally irradiated (10.5 Gy) B6.SJL mice by tail vein injection. Mice were monitored daily for signs of pathology.
Statistical analysis
Statistical analyses were performed using the Excel program. Differences between 2 groups of samples were accessed using t tests. P < .05 (2-tailed) are considered significantly different.
Results
Generation of Tet2:nlacZ/nGFP knock-in and Tet2-null mouse models
We produced targeted Tet2 reporter mice by replacing part of exon 3 sequences of the Tet2 gene with nlacZ/nGFP (inserted 6 bp upstream of start codon, Figure 1A). The targeted allele results in transcription of nlacZ (Tet2:nlacZ/nGFP) or nGFP (Tet2:nGFP) mRNA instead of Tet2 (endogenous ATG was disrupted). Heterozygous Tet2:nlacZ/nGFP (Tet2+/−) mice were interbred to obtain homozygous Tet2:nlacZ/nGFP (Tet2−/−) mice. The mean litter size was normal (9.5 ± 1.2 pups/litter); each of the genotypes was born at the expected Mendelian frequencies. Crossing between Tet2−/− mice indicated that the Tet2−/− mice were fertile. Up to at least 4 months of age, Tet2+/− mice were phenotypically indistinguishable with respect to survival, hematopoietic cellularity and lineage composition from WT littermates (data not shown). To determine whether GFP expression level correlated with Tet2 expression in 6-8 week old heterozygous Tet2:nGFP mice, BM cells were separated into GFPhi and GFP−/lo populations by FACS sorting and Tet2 expression levels were measured by quantitative real time PCR. Tet2 expression level in the GFPhi population was ∼ 15-fold higher than that of the GFP−/lo population (Figure 1C), indicating that the GFP expression level correlated well with the Tet2 expression.
We then examined the GFP (Tet2) expression in hematopoietic cell populations from BM of 6- to 7-week-old heterozygous Tet2:nGFP mice flow cytometrically. GFP was expressed at a high level in all of the LSK cells, a HSC-enriched cell population. The overwhelming majority of Lin−Sca-1−c-kit+ (LK) progenitor cells, Gr-1+/CD11b+ granulocytes, CD4+/CD8+ T cells and B220+ B cells also expressed high levels of GFP, while, only a small proportion of Ter119+ erythroid cells expressed GFP at a relatively high levels (Figure 1D). When GFP expression was analyzed in the total cell preparation of various organs/tissues, the majority of the cells in BM, spleen, thymus, liver, blood vessel, brain, sciatic nerve, lung, kidney, pancreas, intestine, and skin expressed high levels of GFP; while a small proportion of the heart and bone cells exhibited high GFP expression (supplemental Figure 1). These results indicate that Tet2 is ubiquitously expressed in various organs/tissues and all of the HSCs express high levels of Tet2.
Analyses of Tet gene expression and levels of 5-hmC in BM cells
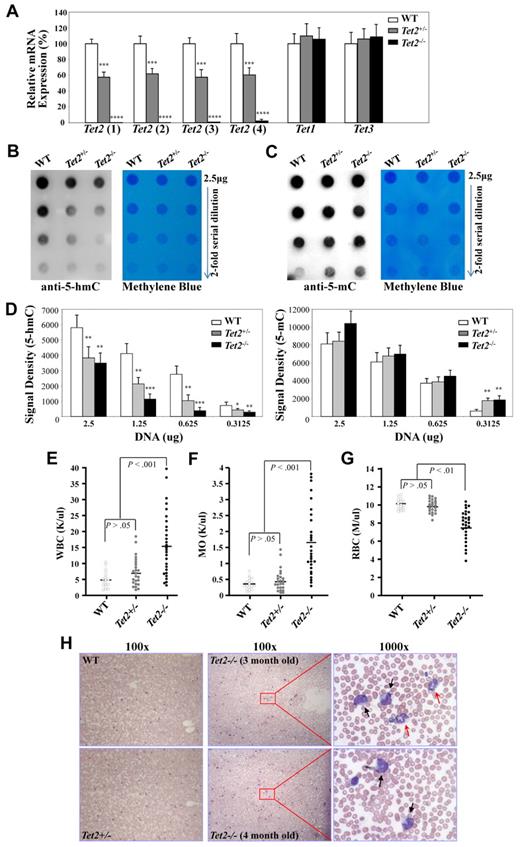
We then examined the mRNA expression levels of Tet2 and the other 2 members of the Tet family, Tet1 and Tet3 in BM cells of 6- to 7-week-old WT, Tet2+/− and Tet2−/− mice by quantitative real-time PCR. Single-strand cDNA was synthesized from total RNA of BM cells. The relative expression levels of Tet2 mRNA in each genotype were analyzed with 4 different sets of primers (supplemental Table 1 and supplemental Figure 2). The Tet2 mRNA expression in the BM cells of Tet2−/− mice was not detectable using primer sets 1-3; while a very low level of Tet2 mRNA was detected using primer set 4 with the Tet2 products appearing at least 6 cycles behind that of WT BM cDNA (Figure 2A). Cloning of the PCR products from Tet2−/− BM cell cDNA with primer set #4 and subsequent sequencing of 10 clones revealed that the PCR products were not derived from Tet2 transcripts, but represented none-specific PCR products (data not shown). These data indicate that the Tet2 knock-out in these mice resulted in a complete deletion of Tet2 transcripts. The Tet2 mRNA expression in Tet2+/− BM cells was decreased by 40%-50% compared with WT BM cells (Figure 2A). The levels of Tet1 and Tet3 mRNA expression were similar in the BM cells from each genotype of mice (Figure 2A), indicating that there was no compensatory up-regulation of other Tet family genes in the Tet2-null mice.
Analyses of Tet gene expression and 5-hmC levels in BM cells of WT, Tet2+/−, and Tet2−/− mice. (A) Analysis of the mRNA expression levels of each Tet genes in BM cells of 6- to 7-week-old WT (n = 4), Tet2+/− (n = 6) and Tet2−/− (n = 6) mice by quantitative real-time PCR. The relative mRNA expression of Tet2, Tet1, and Tet3 was determined using ß-actin as internal calibrator. The mRNA expression levels are reported as relative expression units to the respective Tet expression in WT mice. (B-D) Genomic DNA was extracted from BM cells of 6- to 7-week-old WT (n = 3), Tet2+/− (n = 4), and Tet2−/− (n = 4) mice and blotted onto nitrocellulose membrane after 2-fold serial dilution. 5-hmC (B) and 5-mC (C) levels were detected with an anti–5-hmC (ActiveMotif, #39791) or 5-mC (Calbiochem; NA#81) antibody. Methylene blue staining was performed to ensure equal spotting of total DNA on the membranes. Quantification of the signal density (D) was shown. *P < .05, **P < .01 ***P < .001, ****P < .0001 (E-H) Tet2−/− mice developed a phenotype resembling characteristics of CMML as early as 2-4 months of age. WT (n = 18), Tet2+/− (n = 24), and Tet2−/− (n = 28) mice were killed at 2-4 months of age and were analyzed for PB WBC (E), monocyte (MO, F), and RBC (G) counts. (H) Representative May-Giemsa stained PB smears prepared from WT, Tet2+/−, and Tet2−/− mice at 3-4 months of age. Black arrows indicate monocytes, and red arrows, indicate neutrophils.
Analyses of Tet gene expression and 5-hmC levels in BM cells of WT, Tet2+/−, and Tet2−/− mice. (A) Analysis of the mRNA expression levels of each Tet genes in BM cells of 6- to 7-week-old WT (n = 4), Tet2+/− (n = 6) and Tet2−/− (n = 6) mice by quantitative real-time PCR. The relative mRNA expression of Tet2, Tet1, and Tet3 was determined using ß-actin as internal calibrator. The mRNA expression levels are reported as relative expression units to the respective Tet expression in WT mice. (B-D) Genomic DNA was extracted from BM cells of 6- to 7-week-old WT (n = 3), Tet2+/− (n = 4), and Tet2−/− (n = 4) mice and blotted onto nitrocellulose membrane after 2-fold serial dilution. 5-hmC (B) and 5-mC (C) levels were detected with an anti–5-hmC (ActiveMotif, #39791) or 5-mC (Calbiochem; NA#81) antibody. Methylene blue staining was performed to ensure equal spotting of total DNA on the membranes. Quantification of the signal density (D) was shown. *P < .05, **P < .01 ***P < .001, ****P < .0001 (E-H) Tet2−/− mice developed a phenotype resembling characteristics of CMML as early as 2-4 months of age. WT (n = 18), Tet2+/− (n = 24), and Tet2−/− (n = 28) mice were killed at 2-4 months of age and were analyzed for PB WBC (E), monocyte (MO, F), and RBC (G) counts. (H) Representative May-Giemsa stained PB smears prepared from WT, Tet2+/−, and Tet2−/− mice at 3-4 months of age. Black arrows indicate monocytes, and red arrows, indicate neutrophils.
We next examined the levels of 5-hmC and 5-mC in the genomic DNA of BM cells from 6- to 7-week-old WT, Tet2+/− or Tet2−/− mice using a dot blot assay as described previously.12 Dot blot assays were performed to plot the signal obtained with 2-fold dilutions of genomic DNA with an anti–5-hmC (Figure 2B) or anti–5-mC (Figure 2C) antibody. Analysis of BM DNA from 3 WT, 4 Tet2+/− and 4 Tet2−/− mice revealed dramatic lower genomic 5-hmC levels and concomitant higher 5-mC levels in Tet2−/− BM cells compared with WT and Tet2+/− BM cells; while, a significant lower genomic 5-hmC level was also documented in Tet2+/− BM cells compared with WT BM cells (Figure 2B-D). These data indicate that deletion of Tet2 in mice resulted in a dramatically decreased level of 5-hmC in their genomic DNA in vivo.
Tet2−/− mice developed a phenotype resembling characteristics of CMML as early as 2-4 months of age
The hematologic parameters were examined in a cohort of 2-4 month old WT (n = 18), Tet2+/− (n = 24) and Tet2−/− (n = 28) mice. While there were no significant differences in blood counts between Tet2+/− and WT groups of mice up to 4-month of age, 2 of the 24 Tet2+/− mice exhibited moderately elevated WBC and monocyte counts (Figure 2E-F). By contrast, the majority of these 2- to 4-month-old Tet2−/− mice exhibited increased WBC counts with disproportionate numbers of monocytes and neutrophils (neutrophilia and monocytosis), moderate yet significantly lower red blood cell counts and hemoglobin levels compared with WT and Tet2+/− littermates (Figure 2E-G and data not shown). The platelet counts, mean red cell volume and red cell distribution width were comparable in each genotype of mice (data not shown). Consistent with the complete blood counts, morphologic analysis of PB smears from the Tet2−/− mice showed dramatically increased leukocytes compared with WT and Tet2+/− mice; with increased monocytes and/or neutrophils seen in the majority (Figure 2H), suggesting monocytosis and/or neutrophilia in the Tet2−/− cohort of mice. No blasts, immature myeloblasts or erythroid precursors were noted in the PB of each of the Tet2−/− mice examined. In addition, these 2- to 4-month-old Tet2−/− mice also had increased BM cellularity, splenomegaly and moderately enlarged liver compared with Tet2+/− and WT littermates (data not shown). A marked increase in the numbers of CFU-GM in both BM and spleen of these Tet2−/− mice was documented (Table 1). There was no difference in each of these parameters analyzed between Tet2+/− and WT groups of mice. Therefore, 2- to 4-month-old Tet2−/− mice phenotypically show multiple characteristics of CMML.
Increased colony forming capacity of BM or spleen cells from Tet2−/− mice
| Genotype . | Cells . | CFU-GM . | BFU-E . | CFU-GEMM . | Total . |
|---|---|---|---|---|---|
| WT Control (n = 6) | BM | 32 ± 2 | 2 ± 1 | 1 ± 1 | 35 ± 5 |
| SP | 10 ± 2 | 1 ± 1 | 0 | 11 ± 3 | |
| Tet2−/− (2-4 months; n = 8) | BM | 52 ± 12* | 3 ± 2 | 3 ± 1 | 58 ± 13* |
| SP | 64 ± 8** | 4 ± 2* | 0 ± 1 | 68 ± 10** | |
| Tet2−/− (erythroid infiltration; n = 6) | BM | 1 ± 1** | 12 ± 8** | 0 | 13 ± 7 |
| SP | 6 ± 2 | 22 ± 10** | 0 | 28 ± 14 | |
| Tet2−/− or Tet2−/− myeloid infiltration (n = 5) | BM | 48 ± 10** | 0 | 2 ± 1 | 50 ± 11** |
| SP | 26 ± 7** | 0 ± 1 | 0 | 27 ± 8** |
| Genotype . | Cells . | CFU-GM . | BFU-E . | CFU-GEMM . | Total . |
|---|---|---|---|---|---|
| WT Control (n = 6) | BM | 32 ± 2 | 2 ± 1 | 1 ± 1 | 35 ± 5 |
| SP | 10 ± 2 | 1 ± 1 | 0 | 11 ± 3 | |
| Tet2−/− (2-4 months; n = 8) | BM | 52 ± 12* | 3 ± 2 | 3 ± 1 | 58 ± 13* |
| SP | 64 ± 8** | 4 ± 2* | 0 ± 1 | 68 ± 10** | |
| Tet2−/− (erythroid infiltration; n = 6) | BM | 1 ± 1** | 12 ± 8** | 0 | 13 ± 7 |
| SP | 6 ± 2 | 22 ± 10** | 0 | 28 ± 14 | |
| Tet2−/− or Tet2−/− myeloid infiltration (n = 5) | BM | 48 ± 10** | 0 | 2 ± 1 | 50 ± 11** |
| SP | 26 ± 7** | 0 ± 1 | 0 | 27 ± 8** |
In vitro hematopoietic colony-forming assays were performed with BM (1 × 104) and spleen (SP; 1 × 105) cells from indicated genotypes of mice in the presence of mSCF, mIL-3, IL-6, and EPO. Colonies were enumerated on day 8 of culture. Data are presented as mean ± SEM.
P < .05,
P < .01.
Tet2−/−mice evolved to a wide spectrum of lethal myeloid malignancies
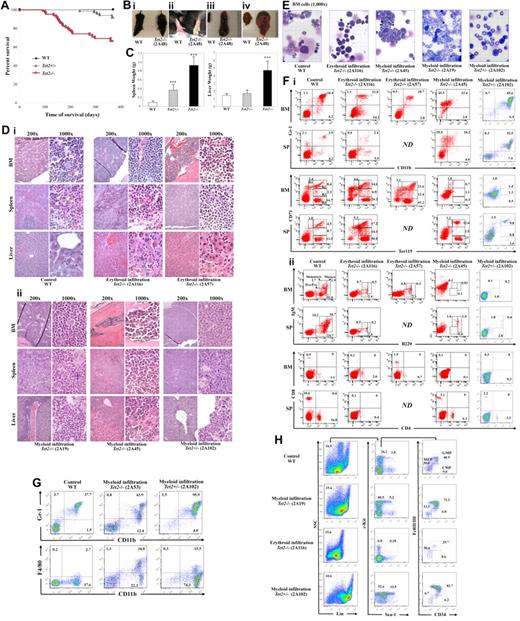
Intriguingly, approximately one-third (21 of 62) of the Tet2−/− mice and 8% (5 of 66) of the Tet2+/− mice died or were killed because of a moribund condition (Figure 3Bi) within 1 year of their life, whereas none of the WT mice (n = 48) died at the end point of our evaluation (Figure 3A). Necropsy of the moribund/deceased Tet2−/− or Tet2+/− mice revealed pronounced splenomegaly, while Tet2−/− mice also had profound hepatomegaly and 1/3 of these mice exhibited pale footpads suggesting anemia (Figure 3B). Splenomegaly and hepatomegaly were more striking in the moribund/deceased Tet2−/− mice, which were 8-15 times and 2-5 times larger than that of age-matched WT mice, respectively (Figure 3C).
Tet2−/−mice evolved to lethal myeloid malignancies. (A) Kaplan-Meier survival curve of WT (n = 48), Tet2+/− (n = 66), and Tet2−/− (n = 62) mice up to 1 year of age. (B) Appearance of a representative moribund Tet2−/− mouse (2A48) with distended abdomens (i) and pale footpads (ii), the gross morphology of spleen (iii), and liver (iv) of this moribund Tet2−/− mice are drastically different from WT control. (C) Spleen and liver weights of moribund/deceased Tet2−/− (n = 18) and Tet2+/− (n = 5) mice as well as age matched WT controls. (D) H&E staining of paraffin-embedded sections of spleen, liver, and femurs from representative deceased/moribund Tet2−/− (2A116, 2A57, 2A45, 2A19) and Tet2+/− (2A102) mice. (E) May-Giemsa–stained BM cytospin-preparations from representative deceased/moribund Tet2−/− (2A116, 2A45, 2A19) or Tet2+/− (2A102) mice. (F) Lineage distribution of BM and spleen cells of representative deceased/moribund Tet2−/− (2A116, 2A57, 2A45) and Tet2+/− (2A102) mice. (G) Flow cytometric analysis of monocytic lineages in representative deceased/moribund Tet2−/− (3G53) and Tet2+/− (2A102) mice. (H) Flow cytometric analysis of LSK (Lin–Sca-1+Kit+) and LK (Lin–Sca-1−Kit+) cell population in representative deceased/moribund Tet2−/− (2A19 and 2A116) and Tet2−/− (2A102) mice. The numbers indicate the percentages of cells in each cell population. ***P < .001.
Tet2−/−mice evolved to lethal myeloid malignancies. (A) Kaplan-Meier survival curve of WT (n = 48), Tet2+/− (n = 66), and Tet2−/− (n = 62) mice up to 1 year of age. (B) Appearance of a representative moribund Tet2−/− mouse (2A48) with distended abdomens (i) and pale footpads (ii), the gross morphology of spleen (iii), and liver (iv) of this moribund Tet2−/− mice are drastically different from WT control. (C) Spleen and liver weights of moribund/deceased Tet2−/− (n = 18) and Tet2+/− (n = 5) mice as well as age matched WT controls. (D) H&E staining of paraffin-embedded sections of spleen, liver, and femurs from representative deceased/moribund Tet2−/− (2A116, 2A57, 2A45, 2A19) and Tet2+/− (2A102) mice. (E) May-Giemsa–stained BM cytospin-preparations from representative deceased/moribund Tet2−/− (2A116, 2A45, 2A19) or Tet2+/− (2A102) mice. (F) Lineage distribution of BM and spleen cells of representative deceased/moribund Tet2−/− (2A116, 2A57, 2A45) and Tet2+/− (2A102) mice. (G) Flow cytometric analysis of monocytic lineages in representative deceased/moribund Tet2−/− (3G53) and Tet2+/− (2A102) mice. (H) Flow cytometric analysis of LSK (Lin–Sca-1+Kit+) and LK (Lin–Sca-1−Kit+) cell population in representative deceased/moribund Tet2−/− (2A19 and 2A116) and Tet2−/− (2A102) mice. The numbers indicate the percentages of cells in each cell population. ***P < .001.
Histologic examination of H&E stained BM, spleen, and liver sections of these moribund/deceased Tet2−/− or Tet2+/− mice revealed extensive infiltration of 2 types of cell populations: (1) morphologically recognizable erythroblasts (12 of 21 Tet2−/− mice), and (2) various mature myeloid cells (9 of 21 Tet2−/− mice and all 5 Tet2+/− mice). Massive hematopoietic cell infiltration disrupted the splenic and hepatic architecture in each of the cases (Figure 3D). The infiltration patterns of erythroblasts and mature myeloid cells in the liver were different. The myeloid cell infiltration shows a perivascular pattern; by contrast, the erythroblast infiltration was diffuse (Figure 3D). Histopathology of the BM of moribund/deceased Tet2−/− or Tet2+/− mice showed a severely skewed erythroid/myeloid ratio (WT = 0.38 ± 0.06, n = 5; Tet2−/− with erythroid infiltration = 2.8 ± 1.1, n = 8; Tet2−/− or Tet2+/− with myeloid infiltration = 0.08 ± 0.10, n = 5). Cytospin preparations of BM cells from these Tet2−/− or Tet2+/− mice confirmed that the cells were predominantly erythroblasts or various myeloid cells such as myeloblasts, monocytes/macrophages and neutrophils, in contrast to the normal trilineage hematopoiesis seen in the WT controls (Figure 3E). Thymus and lymph nodes displayed a similar histologic feature in the moribund/deceased animals compared with WT control mice (data not shown).
Flow cytometric analyses were then performed with spleen and BM cell preparations to characterize the cell surface epitopes on the abnormally infiltrated neoplastic cells in moribund/deceased Tet2−/− or Tet2+/− mice, compared with age matched WT control mice. Consistent with the histologic analyses, in 12 Tet2−/− mice (with erythroblasts infiltration), the BM and spleen contained predominant proportions of erythroblasts at various stages (CD71hiTer119low, R1, proerythroblasts; Ter119hiCD71hi, R2, basophilic erythroblast; Ter119hiCD71med, R3, late basophilic and polychromatophilic erythroblast; and Ter119hiCD71low, R4, orthochromatophilic erythroblast; Figure 3F). In 9 Tet2−/− and 5 Tet2+/− moribund/deceased animals (with myeloid cell infiltration), the BM and SP cells contained predominant proportions of various myeloid cell populations (such as Gr-1+CD11b+, Gr-1lowCD11b+ and Gr-1lowCD11blow cells; Figure 3F); while in 4 of the 9 Tet2−/− and 3 of the 5 Tet2+/− moribund/deceased animals, there were predominant proportions of CD11b+F4/80+Gr-1low monocytic cell populations in the BM and spleen (Figure 3G). In 1 of the moribund Tet2+/− mice (2A102) with Gr-1+CD11b+ cell infiltration, a large peritoneal mass was developed and the majority of cells in this mass was Gr-1+CD11b+ cells, suggesting myeloid sarcoma (supplemental Figure 3A). In one of the moribund Tet2−/− mice with Gr-1+CD11b+ cell infiltration (2A19), multiple white nodules were found in the liver, which also contained predominantly Gr-1+CD11b+ cells (supplemental Figure 3B). Interestingly, c-Kit expression was only observed on the infiltrated myeloid population in 1 animal (2A45, data not shown). In each of these moribund/deceased Tet2−/− or Tet2+/− mice, the proportion of B and T cells were comparable or decreased in the BM and spleen compared with WT control mice (Figure 3F). Given the fact that thymus and lymph node histology were normal in these Tet2−/− or Tet2−/− mice, the reduced B- and T-cell population is likely relative to the predominance of erythroid or myeloid cell populations rather than a primary defect.
To examine if the HSC/HPC cell population were altered in these moribund/deceased Tet2−/− or Tet2+/− mice, BM cells were subject to flow cytometric analysis with HSC/HPC cell markers as described previously.26,27 The proportion of LK cell population was dramatically increased and the LSK cell population was moderately increased in the moribund/deceased Tet2−/− or Tet2+/− mice with myeloid infiltration compared with WT mice (Figure 3H). Further analysis showed that the LK cell population were predominantly granulocyte-monocyte progenitors (GMP), indicating a preferential expansion of GMP in these mice. By contrast, the proportions of both LSK and LK cell populations were dramatically decreased in the moribund/deceased Tet2−/− mice with erythroid infiltration, suggesting exhaustion and/or depletion of HSC/HPC in these Tet2−/− mice. Consistent with the phenotypic analysis, CFU assays showed that the BM and spleen cells of Tet2−/− mice with erythroid infiltration contained significantly increased CFU-E and BFU-E, but decreased CFU-GM (Table 1); while BM and spleen cells from Tet2−/− or Tet2+/− mice with myeloid infiltration contained increased CFU-GM, but decreased BFU-E and CFU-E (Table 1).
To evaluate the malignant nature of the abnormally infiltrated hematopoietic cells in the moribund/deceased Tet2−/− or Tet2+/− mice, spleen cells from 5 primary recipient mice were transplanted into sublethally irradiated secondary WT recipients (4 recipients per primary donor). Transplantation of WT spleen cells failed to develop disease in recipient mice. Interestingly, 11 of the 12 animals receiving spleen cells from moribund/deceased mice with myeloid cell infiltration developed diseases with similar characteristics as those observed in the primary animals including elevated WBC counts, monocytosis and/or neutrophilia, splenomegaly and death (supplemental Table 2). The latency of disease ranged from 47 to 117 days (supplemental Table 2). Flow cytometric analysis of spleen and BM cells of recipient mice revealed increased proportion of Gr-1+/CD11b+ monocytic/granulocytic cells similar tothat observed in primary Tet2−/− and Tet2+/− mice (data not shown). By contrast, none of the recipients receiving spleen cells from moribund/deceased mice with erythroid infiltration developed pathology or gross evidence of disease within 4 months of transplantation (supplemental Table 2). These data suggest that the Tet2-deletion induced myeloid malignancies with myeloid cell infiltration, but not erythroblast infiltration, are transplantable, indicating that these infiltrated myeloid cells are malignant in nature.
Based on the criteria of Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice,29 these moribund/deceased Tet2−/− mice developed a wide spectrum of myeloid malignancies, including MDS with erythroid predominance, CMML, MPD-like myeloid leukemia and myeloid leukemia with maturation (Table 2). These data demonstrate that deletion of Tet2 alone is sufficient to cause myeloid malignancies in mice. The development of myeloid malignancies in a significant percentage of Tet2+/− mice indicates a haploinsufficiency effect of Tet2 on the pathogenesis of myeloid malignancies.
Diagnosis and subclassification of the myeloid malignancies in moribund/deceased Tet2−/− and Tet2+/− mice up to 1 year of age
| Genotype . | Survival, d . | Frequency . | Necropsy and other findings . | Blasts*, % . | Transplantability . | Diagnosis and subclasification . |
|---|---|---|---|---|---|---|
| Tet2−/− | 92-196 | 12/21 | Hepatosplenomegaly anemia | < 20% | No | MDS with erythroid predominance |
| Tet2−/− | 208-298 | 4/21 | Hepatosplenomegaly monocytosis | < 20% | ND | CMML |
| Tet2−/− | 378-360 | 4/21 | Hepatosplenomegaly neutrophilia | < 20% | Yes | MPD-like Myeloid leukemia |
| Tet2−/− | 206 | 1/21 | Hepatosplenomegaly neutrophilia | > 20% | Yes | Myeloid leukemia with maturation |
| Tet2+/− | 316-365 | 2/5 | Hepatosplenomegaly neutrophilia (1 had myeloid sarcoma) | < 20% | Yes | MPD-like Myeloid leukemia |
| Tet2+/− | 238-365 | 3/5 | Splenomegaly monocytosis | < 20% | ND | CMML |
| Genotype . | Survival, d . | Frequency . | Necropsy and other findings . | Blasts*, % . | Transplantability . | Diagnosis and subclasification . |
|---|---|---|---|---|---|---|
| Tet2−/− | 92-196 | 12/21 | Hepatosplenomegaly anemia | < 20% | No | MDS with erythroid predominance |
| Tet2−/− | 208-298 | 4/21 | Hepatosplenomegaly monocytosis | < 20% | ND | CMML |
| Tet2−/− | 378-360 | 4/21 | Hepatosplenomegaly neutrophilia | < 20% | Yes | MPD-like Myeloid leukemia |
| Tet2−/− | 206 | 1/21 | Hepatosplenomegaly neutrophilia | > 20% | Yes | Myeloid leukemia with maturation |
| Tet2+/− | 316-365 | 2/5 | Hepatosplenomegaly neutrophilia (1 had myeloid sarcoma) | < 20% | Yes | MPD-like Myeloid leukemia |
| Tet2+/− | 238-365 | 3/5 | Splenomegaly monocytosis | < 20% | ND | CMML |
ND indicates not done.
Percentage of blasts in the BM.
Deletion of Tet2 alters HSC compartment in mice as well as HSC proliferation and differentiation potential in vitro
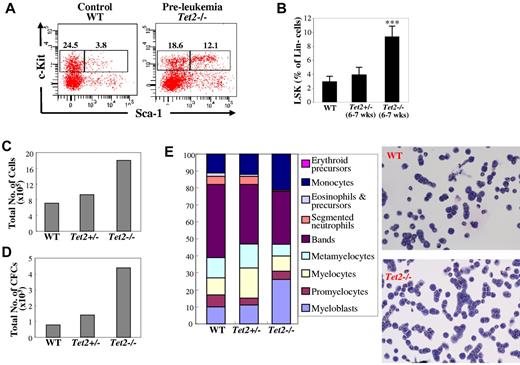
To determine whether deletion of Tet2 affects the HSC pool in vivo before the development of myeloid malignancies, we analyzed LSK and LK cell population in the BM of 6- to 7 week-old Tet2−/− mice. The proportion of LSK cells was significantly increased in the 6- to 7-week-old Tet2−/− mice without hematologic malignancies compared with that of WT mice, indicating the presence of a dysregulated HSC population before the development of myeloid malignancies (Figure 4A-B). The LK cell populations were comparable in the 6- to 7-week-old Tet2−/− and WT mice.
Deletion of Tet2 alters HSC compartments in mice before their development of myeloid malignancies as well as HSC proliferation and differentiation potential in vitro. (A) Flow cytometric analysis of LSK (Lin–Sca-1+Kit+) HSC and LK (Lin–Sca-1−Kit+) progenitor cell population in a representative preleukemic 6- to 7-week-old Tet2−/− mice. (B) The percentage of LSK cells within the Lin− cell populations in the BM of preleukemic Tet2−/−, Tet2+/−, and WT control mice (average ± SD of 4-8 animals). (C-E) The proliferation and differentiation potential of various genotypes of LSK cells were examined by culturing 500 LSK cells in the presence of 4 growth factors and assaying their ability to generate total cells (C) and CFCs (D) after 7 days of culture. CFCs in each culture were evaluated by colony assay of a fraction of the cultures. Cytospin preparations of the progenies generated from each genotype of LSK cells after 7 days of culture were stained with May-Giemsa, a 200-cell differential was performed (E). Representative data from 2 separate experiments are shown. ***P < .001.
Deletion of Tet2 alters HSC compartments in mice before their development of myeloid malignancies as well as HSC proliferation and differentiation potential in vitro. (A) Flow cytometric analysis of LSK (Lin–Sca-1+Kit+) HSC and LK (Lin–Sca-1−Kit+) progenitor cell population in a representative preleukemic 6- to 7-week-old Tet2−/− mice. (B) The percentage of LSK cells within the Lin− cell populations in the BM of preleukemic Tet2−/−, Tet2+/−, and WT control mice (average ± SD of 4-8 animals). (C-E) The proliferation and differentiation potential of various genotypes of LSK cells were examined by culturing 500 LSK cells in the presence of 4 growth factors and assaying their ability to generate total cells (C) and CFCs (D) after 7 days of culture. CFCs in each culture were evaluated by colony assay of a fraction of the cultures. Cytospin preparations of the progenies generated from each genotype of LSK cells after 7 days of culture were stained with May-Giemsa, a 200-cell differential was performed (E). Representative data from 2 separate experiments are shown. ***P < .001.
To examine the effects of Tet2 deletion on the proliferation and differentiation potential of LSK cells, single-cell clonogenic assays were performed with LSK cells sorted from the BM of 6- to 7-week-old WT, Tet2+/− or Tet2−/− mice in the presence of mSCF, mIL-3, IL-6 and EPO. After 7 days of single cell culture, Tet2−/− LSK cells exhibited a greater cloning efficiency (71%) than that of Tet2+/− (48%) and WT (40%) cells (supplemental Table 3). The colonies formed by Tet2−/− LSK cells were predominantly high-proliferative potential-CFU-GM (supplemental Table 3). LSK cells of various genotypes were also subjected to suspension cultures in 12-well plates with the same cytokine combinations. After 7 days of culture, the cells in each well were split into 2 tubes for either semisolid methycellulose cultures or cytospins. Tet2−/− LSK cells generated more total cells and significantly greater numbers of total colonies-forming cells (CFCs) compared with Tet2+/− and WT LSK cells (Figure 4C-D). In addition, cytospins of the cultured cells followed by May-Grünwald-Giemsa staining revealed that the progenies in the Tet2−/− LSK cell culture contained a significantly higher percentage of myeloblasts and monocytes compared with that in the Tet2+/− and WT LSK cell cultures (Figure 4E).
These data indicate that deletion of Tet2 in HSCs increases proliferative capacity and alters differentiation program which is skewed toward the monocytic lineage. The increased HSC pool and abnormal proliferation/differentiation of HSC/HPC in Tet2−/− mice might facilitate the development of myeloid malignancies.
Cell autonomous defects of Tet2−/− HSCs
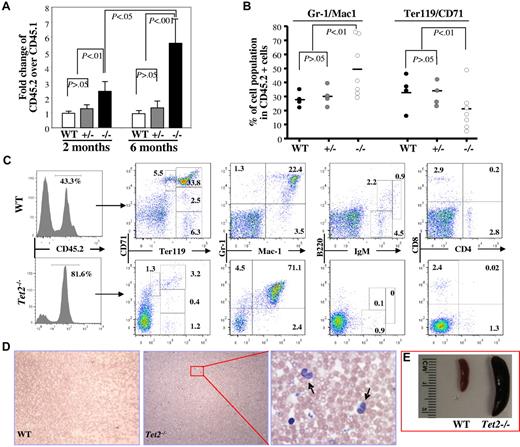
To determine whether deletion of Tet2 affects the function of HSCs in vivo, we performed a competitive repopulation assay by transplanting total CD45.2+-nucleated BM cells (1 × 106) from 6- to 7-week-old WT, Tet2+/− or Tet2−/− mice along with an equal number of WT CD45.1+ cells, into lethally irradiated CD45.1+ congenic recipient mice. The recipient mice were killed 2 (3-5 mice/genotype) or 6 months (3-7 mice/genotype) after transplantation; the phenotype, CD45.2/CD45.1 chimeras and multilineage contribution of CD45.2+ cells in the BM were analyzed. Two months after transplantation, Tet2−/− donor cells (CD45.2/CD45.1 ratio = ∼ 2.5) generated higher proportions of hematopoietic cells in the BM of the recipient mice than Tet2+/− and WT donor cells (CD45.2/CD45.1 ratio = ∼ 1.0; Figure 5A), which due likely to the greater percentage of LSK cells in the Tet2−/− BM cells. Interestingly, 6 months after transplantation, the CD45.2/CD45.1 ratio in the mice receiving Tet2−/− BM cells increased to ∼ 5.6, while the ratio remained the same in mice receiving WT or Tet2+/− BM cells (Figure 5A-C). Furthermore, Tet2−/− BM cells contributed to a greater proportion of Gr-1+/CD11b+ granulocytic/monocytic cells and a smaller proportion of Ter119+ erythroid cells within the CD45.2+ BM cell populations compared with Tet2+/− or WT BM cells (Figure 5B-C). In addition, 6 months after transplantation, 2 of the 7 mice receiving Tet2−/− BM cells, but none of the mice receiving WT or Tet2+/− BM cells, exhibited elevated PB WBC counts, monocytosis (Figure 5D-F) and splenomegaly (Figure 5G), reminiscent of the phenotype displayed in 2- to 4-month-old Tet2−/− mice. These data indicate that the Tet2-deletion induced-phenotype is cell autonomous and Tet2−/− BM cells have advantageous proliferative capacity over WT BM cells in vivo.
Deletion of Tet2 promoted the proliferative capacity of BM cells in vivo and Tet2 deletion induced-phenotype is cell-autonomous. Recipient mice receiving 1 × 106 BM cells each from B6SJL mice (CD45.1) and 6- to 7-week-old WT, Tet2+/− or Tet2−/−, mice (CD45.2) were killed 2 or 6 months after transplantation. (A) The ratio of CD45.2+ versus CD45.1+ BM cells in each recipient mice were examined at 2 or 6 months after transplantation (mean ± SD of 3-7 animals). (B-C) The lineage distribution within the CD45.2+ cells in the BM of the recipient mice 6 months after receiving WT, Tet2+/−, or Tet2−/− BM cells (B). Flow cytometric analysis of representative recipient mice 6 months after receiving WT or Tet2−/− BM cells (C). Histogram shows the percentage of CD45.2+ cells in the BM. (D-E) blood smear (May-Giemsa staining, D) and spleen weight/size (E) of representative mice receiving WT or Tet2−/− (arrows indicate monocytes). BM cells are shown.
Deletion of Tet2 promoted the proliferative capacity of BM cells in vivo and Tet2 deletion induced-phenotype is cell-autonomous. Recipient mice receiving 1 × 106 BM cells each from B6SJL mice (CD45.1) and 6- to 7-week-old WT, Tet2+/− or Tet2−/−, mice (CD45.2) were killed 2 or 6 months after transplantation. (A) The ratio of CD45.2+ versus CD45.1+ BM cells in each recipient mice were examined at 2 or 6 months after transplantation (mean ± SD of 3-7 animals). (B-C) The lineage distribution within the CD45.2+ cells in the BM of the recipient mice 6 months after receiving WT, Tet2+/−, or Tet2−/− BM cells (B). Flow cytometric analysis of representative recipient mice 6 months after receiving WT or Tet2−/− BM cells (C). Histogram shows the percentage of CD45.2+ cells in the BM. (D-E) blood smear (May-Giemsa staining, D) and spleen weight/size (E) of representative mice receiving WT or Tet2−/− (arrows indicate monocytes). BM cells are shown.
Discussion
Several reports have shown that TET2 gene alterations are involved by patients with a variety of myeloid malignancies including MDS, CMML, MPN and AML.1-7 TET2 mutations were identified in up to 30% of MDS and 42% of CMML patients, making it one of the most frequently mutated genes in these myeloid neoplasms.1-7 However, it remains unknown whether TET2 mutation/deletion can serve a primary event leading to clinical manifestations of these myeloid malignancies.30 In the present study, we generated a Tet2:nlacZ/nGFP knock-in mouse model with abrogation of Tet2 gene expression and concomitant expression of the LacZ/GFP protein. We aimed to determine the pathologic consequences of loss of Tet2 in vivo. As early as 2-4 months of age, Tet2−/− mice displayed a disordered hematopoiesis and a phenotype resembling many characteristics of CMML, including hepatomegaly, splenomegaly, increased WBC counts with a disproportionate number of monocytes, and increased cellularity in BM. The Tet2−/− mice and a significant percentage of the Tet2+/− mice progressed to lethal myeloid malignancies (such CMML, MPD-like myeloid leukemia, myeloid leukemia with maturation and MDS) characterized by pronounced hepatomegaly and splenomegaly with extensive infiltration of liver, spleen, and BM by erythroblasts or various mature myeloid cells. These results indicate that deletion of Tet2 in mice is sufficient to recapitulate disease manifestations similar to patients with myeloid malignancies and imply that TET2 functions as a tumor suppressor in myelopoiesis. Loss of TET2 function gene alterations, therefore, likely represents a primary event in patients with myeloid malignancies. The novel Tet2-targeted mouse mice allow us to model patients with myeloid malignancy.
Tet2 is expressed in various populations of murine and human hematopoietic cells as analyzed by semiquantitative and quantitative RT-PCR using purified cell populations.5,12 However, it remains unclear whether all cells within the population express moderate levels of Tet2 or whether rare cells within the population express high levels of Tet2. The Tet2:GFP knock-in mice allowed us to adequately address this question. Using these targeted reporter (heterozygous Tet2:nGFP) murine system, we showed that Tet2 is expressed in all HSCs at high levels and the majority of the LK progenitor cells, Gr-1+/CD11b+ granulocytes, CD4+/CD8+ T cells and B220+ B cells also express comparable levels of Tet2 as LSK cells; while only a small proportion of Ter119+ erythroid cells expressed Tet2 at a relatively high levels. In addition, Tet2 is ubiquitously expressed in various organs/tissues. Analyses of the Tet2−/− mice before the development of myeloid malignancies revealed a disordered hematopoiesis with expansion of HSCs. Single cell culture of individual LSK cells revealed an increased cloning efficiency and proliferation of the homozygous knockouts. In addition, Tet2−/− HSCs exhibited an increased ability to reconstitute hematopoiesis in vivo. Furthermore, both in vitro and in vivo studies demonstrated an altered differentiation of Tet2−/− LSKs, which is skewed to granulocytic/monocytic lineage. These data are consistent with recent reports by Ko et al and Figueroa et al that small hairpin RNA-mediated depletion of Tet2 in mouse HSC/HPCs leads to increased monocyte/macrophage lineage differentiation12 and proportion of cells expressing HSC/HPC surface markers22 in in vitro culture. The phenotype displayed in Tet2−/− cells are likely cell-autonomous to HSC/HPC, becauseWT recipient mice receiving Tet2−/− BM cells developed a CMML phenotype similar to 2- to 4-month-old Tet2−/− mice. Collectively, deletion of Tet2 is likely targeting HSC and primitive HPCs with a greater proliferative capacity and an altered differentiation program, which might facilitate the development of myeloid malignancies.
Approximately one-third of the Tet2−/− mice died of myeloid malignancies including MDS, CMML, and MPD-like myeloid leukemia within first year of life. The Tet2−/− mice that died from MDS with erythroid predominance had a shorter latency time (92-196 days) compared with the Tet2−/− mice suffered from myeloid leukemia (≥ 206 days). Deletion of Tet2 seems to cause an impaired erythroid differentiation leading to insufficient erythropoiesis and accumulation of erythroblasts in hematopoietic organs of the Tet2−/− mice. Deletion of Tet2 also promoted the proliferation and differentiation potential of HSC/HPC toward GMP and monocytic/granulocytic lineages, leading to infiltration of various mature myeloid cells in hematopoietic organs. The diverse types of myeloid malignancies and varying latency times in Tet2−/− mice could because of: (1) the degree of severity of impaired erythroid and increased monocytic/granulocytic differentiation, Tet2−/− mice with severely impaired erythroid differentiation would develop MDS with erythroid predominance; (2) the mixed genetic background of the Tet2 knock-out mice, in which an unknown stain specific compensatory machinery might contribute to diverse malignant manifestations; and (3) Tet2-deficient mice predispose to myeloid malignancies, the development of which requires the acquisition of a mutation (so-called second hit) in a HSC/HPC.
Approximately 8% of the Tet2+/− mice also developed lethal myeloid malignancies in first year of life, suggesting that loss of one copy of Tet2 gene have already provided haploinsufficiency effects to HSC/HPC. These findings are of clinical significant because the majority of the TET2 gene alterations in patients with myeloid malignancies are heterozygous TET2 mutations/deletions.1-7 The disease latency in Tet2+/− mice were much longer that that in Tet2−/− mice. In addition, Tet2+/− mice seemed to have much milder anemia and do not develop erythroblast infiltration/accumulation. These results indicate that the Tet2 inactivation induced phenotype is gene dosage dependent. The specific biochemical and biologic actions of Tet2 in preventing runaway proliferation in myelopoiesis remains quite obscure. The TET family of proteins was recently implicated in the conversion of 5-mC to 5-hmC thereby influencing DNA-methylation processes.10-14 Indeed, we found that deletion of Tet2 in mice led to a decreased 5-hmC level and a concomitant increased 5-mC level in genomic DNA of BM cells. Therefore, TET2 is likely act as a tumor suppressor gene by regulating DNA methylation and subsequent epigenetic control of gene expression at critical loci important for myelopoiesis and leukemogenesis. Identification of Tet2-targeted genes in myelopoiesis is critical to uncover the molecular mechanisms by which Tet2 regulates hematopoiesis and serve as a tumor suppressor gene in myelopoiesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kevin Kelly of MSSM Mouse Genetics Core for ES cell injection.
This study was supported in part by a grant from the Leukemia and Lymphoma Society of America (to M.X.) and a Developmental Grant from the Tisch Cancer Institute of MSSM (to M.X.).
Authorship
Contribution: Z.L. performed the experiments and analyzed the data; W.Z. and B.E.P. reviewed the blood smears and histopathologic sections, and revised the manuscript; J.W. and X.C. assisted with the experiments; C.-L.C. and F.-C.Y. provided assistance in designing the study and revised the manuscript, and M.X. designed and supervised the studies, performed the experiments, analyzed data, wrote the manuscript and is responsible for its final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mingjiang Xu, MD, PhD, Division of Hematology/Oncology, Tisch Cancer Institute and Department of Medicine, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1079, New York, NY 10029-6574; e-mail: mingjiang.xu@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal