Abstract
Th17 cells, in addition to their proinflammatory functions, have been recognized as potent inducers of angiogenesis in autoimmune diseases and malignancies. In the present study, we demonstrate distinct mechanisms by which IL-17 induces lymphangiogenesis. Using the mouse cornea micropocket and cell culture assays, our data demonstrate that IL-17 directly promotes growth of lymphatic vessels by inducing increased expression of prolymphangiogenic VEGF-D and proliferation of lymphatic endothelial cells. However, IL-17–induced growth of blood vessels is primarily mediated through IL-1β secretion by IL-17–responsive cells. Furthermore, in vivo blockade of IL-17 in a preclinical model of Th17-dominant autoimmune ocular disease demonstrates a significant reduction in the corneal lymphangiogenesis and in the progression of clinical disease. Taken together, our findings demonstrate a novel prolymphangiogenic function for Th17/IL-17, indicating that IL-17 can promote the progression and amplification of immunity in part through its induction of lymphangiogenesis.
Introduction
CD4+T helper-17 (Th17) cell-secreted IL-17A (also known as IL-17) is a potent proinflammatory cytokine that plays a critical role in the pathogenesis of multiple autoimmune diseases,1,2 malignancies,3 and transplant rejections.4 Although IL-17 is expressed only by Th17 cells, its receptors are ubiquitously expressed in epithelial cells, endothelial cells, hematopoietic cells, fibroblasts, and osteoblasts.5 Recently, accumulating evidence has demonstrated that, in addition to its proinflammatory activity, IL-17 is a potent angiogenic factor and induces pathologic hemangiogenesis (blood vessel growth) in tumors6 and in rheumatoid arthritis.7 However, the effects of IL-17 on lymphangiogenesis are still not well understood. In the context of immunity, lymphatics serve as chief conduits for the migration of antigen-presenting cells from the peripheral tissues to draining lymphoid tissues where they prime naive T cells to alloantigens or autoantigens.8,9 Lymphangiogenesis is also thought to play a critical role in cancer metastases to the lymph nodes.10,11 Furthermore, it is not clear whether IL-17 alone can directly induce angiogenesis or whether this is secondary to its capacity to induce IL-17–responsive cells to express the inflammatory cytokine, IL-1β,12,13 which also possesses potent angiogenic activity.14-16 In the present study, we attempted to dissect the hemangiogenic and lymphangiogenic functions of IL-17 using a cornea model of neovascularization and in vitro assays in the presence of IL-1β blockade. In addition, we demonstrated the pathologic relevance of IL-17–induced lymphangiogenesis in a preclinical model of Th17-dominant autoimmune ocular surface disease.
Methods
Corneal micropocket assay
The corneal micropocket assay was performed as described previously.17 Briefly, hydron pellets containing 100 ng of murine IL-1β, IL-17 (PeproTech), or BSA were implanted into the cornea of BALB/c mice (n = 6/group). Some of the mice with IL-17 pellet received intraperitoneal injections of 200 μg/day either anti–IL-1β antibodies (eBioscience) or soluble-VEGFR3-Fc chimera (R&D Systems). All animal studies described herein were conducted under Schepens Eye Research Institute's Animal Care and Use Committee approval.
Preclinical model of autoimmune DED
Dry eye disease (DED) was induced in C57BL6 mice as described previously.2 Clinical disease was scored by fluorescein staining using the National Eye Institute grading system.18 After DED induction, mice were divided into 3 groups at day 5 and received twice-daily topical 3 μL/eye of 1% anti–IL-17 or isotype antibodies (R&D Systems) or remained untreated up to day 12.
Cell culture assays
Primary human corneal epithelial cells (CECs) were isolated by corneal limbal explant culture method, as described previously.19 Human dermal primary lymphatic microvascular endothelial cells (LECs) were cultured in EGM-2MV-BulletKit medium (Lonza). Both cell types express IL-17 receptors (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cells were stimulated with 10 ng/mL of human IL-17, IL-1β (PeproTech), or IL-17 along with neutralizing anti–IL-1β antibodies (10 μg/mL; eBioscience) for 24 hours. LEC proliferation was measured using the BrdU proliferation kit (Millipore). CEC and culture supernatants were analyzed for the expression of different VEGF species by real-time PCR and ELISA kits (R&D Systems).
Tube formation assay
A transwell Matrigel assay was set up in a 24-well plate in triplicates. A total of 2 × 105 Th17 cells (expressing IL-17, but not VEGFs; supplemental Figures 1B and 2) were added to the upper wells of the transwell along with CD3 antibody (0.5 μg/mL; eBioscience) to activate Th17 cells to secrete IL-17, and 1 × 105 primary corneal epithelial cells to secrete different VEGFs in response to IL-17–IL-17 receptor interaction. Primary LECs (5 × 104) were added in the lower well on Matrigel (Geltrex; Invitrogen). In some wells of Th17-LEC coculture, soluble IL-17 receptor-Fc or soluble-VEGFR3-Fc (R&D Systems) was added in media.
Immunohistochemistry
Corneal mounts were immunostained with FITC-conjugated CD31 (Santa Cruz Biotechnology) and LYVE-1 (Abcam), and rhodamine-conjugated secondary antibody to LYVE-1 for epifluorescent microscopy.17 The area covered by blood (CD31hi/LYVE1−) and lymph (CD31low/LYVE1hi) vessels was calculated using ImageJ 1.34s software.17
Real-time PCR
RNA was isolated from cells or corneas using RNeasy kit (QIAGEN), and reverse transcribed using Superscript-III Kit (Invitrogen). PCR was performed using TaqMan mastermix and preformulated primers for murine and human VEGF-A, VEGF-C, VEGF-D, and GAPDH (Applied Biosystems).
Results and discussion
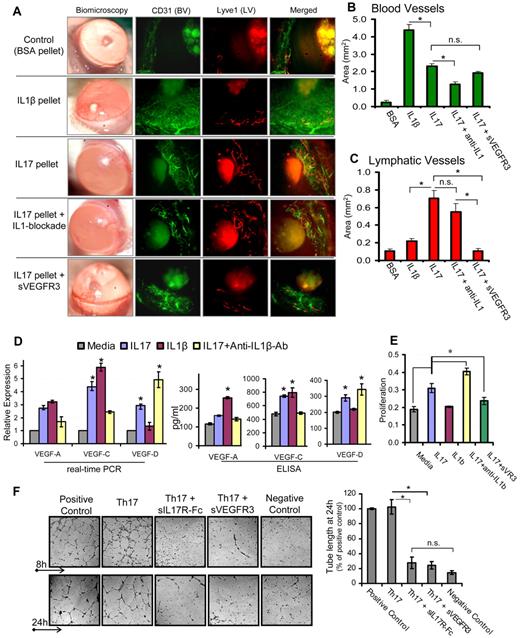
The cornea serves as an ideal site for angiogenic studies because of its accessible location, transparent nature, and blood and lymphatic vessel-free character. Vessels are restricted only to the limbal area (periphery of the cornea), which grows toward the central cornea in response to an angiogenic trigger.17,20 Thus, we used a mouse corneal micropocket model of neovascularization17 to characterize hemangiogenic and lymphangiogenic activities of IL-17. Pellets containing IL-1β, IL-17, or IL-17 along with systemic blockade of IL-1β or VEGFR3 were placed in cornea to induce neovascularization. Quantification of CD31 and Lyve-1–stained corneas (Figure 1A-B) showed maximum growth of blood vessels with IL-1β pellet, which was ∼ 2-fold higher than that observed with the IL-17 pellet (P < .001). Moreover, IL-17 pellet insertion with concurrent IL-1β blockade showed a significant 50% reduction (P < .002) in blood vessels compared with IL-17 pellet alone, suggesting that the hemangiogenic property of IL-17 is primarily via IL-1β secretion. Quantification of Lyve-1–stained lymphatics (Figure 1A,C) showed significantly higher (3-fold) lymphangiogenesis with the IL-17 pellet compared with the IL-1β pellet (P < .002). Moreover, the IL-17 pellet with IL-1β blockade did not show a significant reduction in lymphangiogenesis compared with the IL-17 pellet alone (P = .3), clearly indicating that IL-17 primarily has prolymphangiogenic activity independent of the proangiogenic activity of IL-1β. In addition, IL-17 pellet with concurrent VEGFR3 blockade showed a significant inhibition in IL-17–induced lymphangiogenesis (P < .005), but not hemangiogenesis, suggesting that IL-17 induces lymphangiogenesis via a VEGFR3-dependent pathway.
IL-17 promotes lymphangiogenesis by inducing VEGF-D secretion, and proliferation of and tube formation by LECs. (A) Pellets containing 100 ng of IL-1β, IL-17, or IL-17 along with systemic blockade of IL-1β or VEGFR3, were placed in corneal micropockets (n = 6 mice/group) to induce angiogenesis.17 After 7 days, corneas were evaluated biomicroscopically and then harvested for immunostaining with CD31 (green) and Lyve1 (red). Digital micrographs using epifluorescence microscopy were captured, and ImageJ 1.34s software was used to quantify the growth of (B) blood (CD31hiLyve1−) and (C) lymphatic (CD31loLyve1+) vessels. (D) Primary human corneal epithelial cells were cultured with 10 ng/mL concentration of IL-1β, IL-17, and IL-17 with IL-1β–blocking antibodies for 24 hours, and expression levels of VEGF-A, VEGF-C, and VEGF-D mRNA in cells, and protein in culture supernatants, were measured by real-time PCR and ELISA, respectively. (E) Primary human LECs were cultured with 10 ng/mL concentrations of IL-1β, IL-17, and IL-17 with blockade of IL-1β or VEGFR-3 for 24 hours, and then proliferation was measured using BrdU incorporation assay. (F) In a transwell Matrigel assay, in vitro polarized 2 × 105 Th17 cells (in transwell) were cocultured with 5 × 104 LECs (on Matrigel) in basal MEM with reduced serum (2% FBS). Positive controls consisted of LEC cultures on Matrigel in MEM supplemented with growth factors (5% FBS, VEGF, FGF, EGF). Negative controls consisted of LEC cultures on Matrigel in basal MEM only with reduced serum (2% FBS). In some wells of Th17-LEC coculture, soluble-IL-17receptor-Fc or soluble-VEGFR3-Fc was added in media. After 8- and 24-hour incubation at 37°C, wells were visualized under bright-field inverted microscope to study the LEC tube formations on Matrigel, and digital micrographs were then captured for quantitative analysis of tube length. Data are mean ± SEM values of 3 independent experiments. *P < .05, as determined by Student t test. n.s. indicates not significant.
IL-17 promotes lymphangiogenesis by inducing VEGF-D secretion, and proliferation of and tube formation by LECs. (A) Pellets containing 100 ng of IL-1β, IL-17, or IL-17 along with systemic blockade of IL-1β or VEGFR3, were placed in corneal micropockets (n = 6 mice/group) to induce angiogenesis.17 After 7 days, corneas were evaluated biomicroscopically and then harvested for immunostaining with CD31 (green) and Lyve1 (red). Digital micrographs using epifluorescence microscopy were captured, and ImageJ 1.34s software was used to quantify the growth of (B) blood (CD31hiLyve1−) and (C) lymphatic (CD31loLyve1+) vessels. (D) Primary human corneal epithelial cells were cultured with 10 ng/mL concentration of IL-1β, IL-17, and IL-17 with IL-1β–blocking antibodies for 24 hours, and expression levels of VEGF-A, VEGF-C, and VEGF-D mRNA in cells, and protein in culture supernatants, were measured by real-time PCR and ELISA, respectively. (E) Primary human LECs were cultured with 10 ng/mL concentrations of IL-1β, IL-17, and IL-17 with blockade of IL-1β or VEGFR-3 for 24 hours, and then proliferation was measured using BrdU incorporation assay. (F) In a transwell Matrigel assay, in vitro polarized 2 × 105 Th17 cells (in transwell) were cocultured with 5 × 104 LECs (on Matrigel) in basal MEM with reduced serum (2% FBS). Positive controls consisted of LEC cultures on Matrigel in MEM supplemented with growth factors (5% FBS, VEGF, FGF, EGF). Negative controls consisted of LEC cultures on Matrigel in basal MEM only with reduced serum (2% FBS). In some wells of Th17-LEC coculture, soluble-IL-17receptor-Fc or soluble-VEGFR3-Fc was added in media. After 8- and 24-hour incubation at 37°C, wells were visualized under bright-field inverted microscope to study the LEC tube formations on Matrigel, and digital micrographs were then captured for quantitative analysis of tube length. Data are mean ± SEM values of 3 independent experiments. *P < .05, as determined by Student t test. n.s. indicates not significant.
Furthermore, real-time PCR and ELISA analyses (Figure 1D) of differently stimulated CECs and culture supernatants showed that IL-17 induced significant increase in the expression of VEGF-A, VEGF-C, and VEGF-D (P < .05). In contrast, IL-1β primarily induced increased expression of VEGF-A and VEGF-C, but not of VEGF-D. Interestingly, CEC stimulated with IL-17 in the presence of IL-1β–blocking antibody showed a significant decrease in the expression of VEGF-C and VEGF-A, and an increase in VEGF-D expression compared with those cells stimulated by IL-17 alone (P < .05; Figure 1D). These results clearly indicate that IL-17–induced expression of VEGF-A and VEGF-C was primarily mediated by IL-1β production. However, increased expression of VEGF-D by IL-17 (even in the presence of IL-1β blockade) suggests that IL-17 directly up-regulates the VEGF-D expression. Whereas IL-1β has an inhibitory effect on VEGF-D expression. Similar to the VEGF-D expression profile, LECs showed an increase in proliferation when stimulated with IL-17, but not with IL-1β, which was further enhanced in the presence of IL-1β blockade (P < .05; Figure 1E). However, IL-17–mediated LEC proliferation was significantly inhibited in the presence of sVEGFR-3 (P < .05), suggesting that IL-17 mediates LEC proliferation indirectly via the VEGFR-3 pathway. Corroborating our findings on the suppressive effects of IL-1β on VEGF-D, a previous report has shown that IL-1β down-regulates VEGF-D expression in cardiac microvascular endothelial cells via the involvement of ERK1/2, JNKs, and PKCα/β pathways.21
Next, to establish a direct link between Th17 cells, IL-17, and lymphangiogenesis, we conducted an ex vivo experiment coculturing Th17 cells and LECs, and measured LEC tube formation (Figure 1F). LECs cocultured with Th17 cells showed significantly higher numbers of tube formations, which were comparable with positive control. However, LEC tube formation was significantly inhibited when either IL-17 or VEGFR3 was blocked in the Th17-LEC cocultures (P < .025). These findings clearly demonstrate and confirm that Th17-secreted IL-17 induces lymphangiogenesis via a VEGFD/C–VEGFR3 signaling pathway, corroborating the data of previous experiments showing increased expression of VEGF-D by IL-17–responsive cells (Figure 1D).
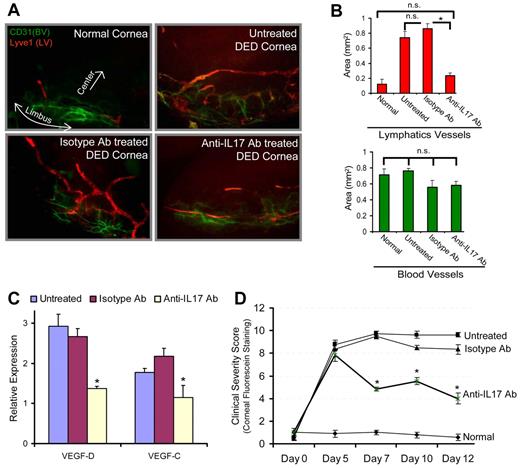
Lymphangiogenesis plays a critical role in tumor metastasis and in the inflammatory diseases by facilitating immune cell trafficking.8-11 A recent study on lung cancer showed an association between intratumoral IL-17–postitive cell frequency and lymphatics density, suggesting that IL-17 may play a role in the metastasis of lung cancer by promoting lymphangiogenesis.22 In addition, in a preclinical model of Th17-dominant autoimmune DED,2,23 we have recently reported the occurrence of corneal lymphangiogenesis,24 and significantly elevated homing of MHC IIhiCD11b+ antigen-presenting cells to the lymphoid tissues24 where they induce autoreactive T-cell responses.2 We therefore next tested the relevance of IL-17–mediated lymphangiogenesis using a mouse model of autoimmune DED2,23 by investigating whether in vivo IL-17 blockade could inhibit corneal lymphangiogenesis and progression of the disease. Our results demonstrate that IL-17 blockade significantly reduced the lymphangiogenesis compared with untreated and isotype Ab-treated DED corneas (P < .0016; Figure 2A-B). Similarly, harvested corneas showed significantly low expression of prolymphangiogenic VEGF-D and VEGF-C in anti–IL-17 Ab-treated DED corneas compared with untreated and isotype Ab-treated DED corneas (P < .05; Figure 2C). Moreover, corneal disease scores showed significant reduction in the progression of clinical disease in the anti–IL-17 Ab-treated group compared with untreated and isotype Ab-treated DED groups (P < .004; Figure 2D). Taken together, these data suggest that, in addition to causing corneal damage, Th17 cell-secreted IL-17 may promote the growth of corneal lymphatic vessels in autoimmune disease. Although it is generally recognized that innate immunity plays a crucial role in the induction of lymphangiogenesis,25 our data indicate a new adaptive immune Th17/IL-17–mediated mechanism in inducing lymphangiogenesis.
In vivo blockade of IL-17 inhibits inflammatory lymphangiogenesis in Th17-dominant autoimmune DED. DED was induced in wild-type C57BL6 mice by exposing them continuously to a dry air-controlled environment,2 which induces an autoimmune ocular surface disease.4 After 4 days of disease induction, mice were randomized into 3 groups receiving topically anti–IL-17 antibody, control isotype antibody, or left untreated. Progression of clinical disease was monitored by corneal fluorescein staining,18 a readout for clinical signs of dry eye inflammation, from day 0 to day 12, at which time corneas were harvested for immunohistochemical and real-time PCR analyses. (A) Corneas were immunostained with CD31 (green) and Lyve1 (red) antibodies. Digital micrographs using epifluorescence microscopy were captured and ImageJ software was used to (B) quantify the growth of blood (CD31hiLyve1−) and lymphatic (CD31loLyve1+) vessels. (C) Real-time PCR analysis was performed to measure the expression levels of lymphangiogenic-specific VEGF-D and VEGF-C in untreated, isotype Ab-treated, and anti–IL-17 Ab-treated DED corneas. Expression levels of VEGF-D and VEGF-C were normalized to GAPDH levels as an internal control and then with their levels in normal corneas. (D) Corneal fluorescein staining scores showing severity of clinical disease in untreated, isotype, and anti–IL-17 Ab-treated groups. Each group consists of 6 mice. Data are mean ± SEM. *P < .05, by Student t test. n.s. indicates not significant.
In vivo blockade of IL-17 inhibits inflammatory lymphangiogenesis in Th17-dominant autoimmune DED. DED was induced in wild-type C57BL6 mice by exposing them continuously to a dry air-controlled environment,2 which induces an autoimmune ocular surface disease.4 After 4 days of disease induction, mice were randomized into 3 groups receiving topically anti–IL-17 antibody, control isotype antibody, or left untreated. Progression of clinical disease was monitored by corneal fluorescein staining,18 a readout for clinical signs of dry eye inflammation, from day 0 to day 12, at which time corneas were harvested for immunohistochemical and real-time PCR analyses. (A) Corneas were immunostained with CD31 (green) and Lyve1 (red) antibodies. Digital micrographs using epifluorescence microscopy were captured and ImageJ software was used to (B) quantify the growth of blood (CD31hiLyve1−) and lymphatic (CD31loLyve1+) vessels. (C) Real-time PCR analysis was performed to measure the expression levels of lymphangiogenic-specific VEGF-D and VEGF-C in untreated, isotype Ab-treated, and anti–IL-17 Ab-treated DED corneas. Expression levels of VEGF-D and VEGF-C were normalized to GAPDH levels as an internal control and then with their levels in normal corneas. (D) Corneal fluorescein staining scores showing severity of clinical disease in untreated, isotype, and anti–IL-17 Ab-treated groups. Each group consists of 6 mice. Data are mean ± SEM. *P < .05, by Student t test. n.s. indicates not significant.
In conclusion, the present study provides compelling novel evidence that Th17/IL-17 has a prolymphangiogenic function that can further promote the progression and amplification of immunity, in part through its induction of lymphangiogenesis. Because Th17 cell dysfunctions and lymphangiogenesis are involved in a diverse array of immunoinflammatory and malignancy disorders, our findings of inhibiting lymphangiogenesis by targeting Th17/IL-17 may have broader clinical implications beyond the treatment of ocular immune diseases alone.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Daniel Saban and Sharmila Masli, Schepens Eye Research Institute, Boston, MA, for helpful discussion, and Dr Lu Chen, University of California, Berkeley, CA, for providing human primary dermal microvascular lymphatic endothelial cells.
This work was supported in part by the National Institutes of Health (grant EY20889, R.D.) and the Sjögren's Syndrome Foundation (S.K.C.).
National Institutes of Health
Authorship
Contribution: S.K.C., Y.J., S.G., H.S.L., T.A.F., and H.K.L. performed experiments and assisted in data analysis; and S.K.C. and R.D. contributed to the underlying hypothesis, designed the experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: Schepens Eye Research Institute has filed for intellectual property rights to technologies derived from this study. The authors declare no competing financial interests.
Correspondence: Sunil K. Chauhan, Department of Ophthalmology, Harvard Medical School, Schepens Eye Research Institute, 20 Staniford St, Boston, MA 02114; e-mail: sunil.chauhan@schepens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal