Abstract
A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib was conducted in 93 adult patients with relapsed or refractory lymphoma. Patients received tipifarnib 300 mg twice daily on days 1-21 of each 28-day cycle. The median number of prior therapies was 5 (range, 1-17). For the aggressive B-cell, indolent B-cell, and T-cell and Hodgkin lymphoma (HL/T) groups, the response rates were 17% (7/42), 7% (1/15), and 31% (11/36), respectively. Of the 19 responders, 7 were diffuse large B-cell non-Hodgkin lymphoma (NHL), 7 T-cell NHL, 1 follicular grade 2, and 4 HL. The median response duration for the 19 responders was 7.2 months (mean, 15.8 months; range, 1.8-62), and 5 patients in the HL/T group are still receiving treatment at 29-64+ months. The grade 3/4 toxicities observed were fatigue and reversible myelosuppression. Correlative studies suggest that Bim and Bcl-2 should be examined as potential predictors of response in future studies. These results indicate that tipifarnib has activity in lymphoma, particularly in heavily pretreated HL/T types, with little activity in follicular NHL. In view of its excellent toxicity profile and novel mechanism of action, further studies in combination with other agents appear warranted. This trial is registered at www.clinicaltrials.gov as #NCT00082888.
Introduction
Targeting cellular signal transduction pathways that are used by malignant cells for growth and survival is a current focus for the treatment of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL). Gene expression profiling, immunoblotting, and RNA interference technology have identified pathways that are important for lymphoma cell growth and survival. As a result of these investigations, many new agents that target these pathways have been developed and are now in clinical trials. Inhibitors of the phosphoinositide 3-kinase/Akt/mTOR pathway (such as temsirolimus and everolimus),1-5 the B-cell receptor signaling pathway (fostamatinib),6 and protein kinase Cβ (enzastaurin)7,8 have all demonstrated single-agent activity in relapsed NHL. These agents have moved forward into pivotal clinical trials and have provided proof of concept that signal transduction inhibitors are a promising source of new antilymphoma agents.
The present study evaluates the effect of inhibiting the farnesyltransferase (FT) enzyme with tipifarnib (R115777, Zarnestra; Johnson & Johnson Pharmaceutical Research and Development LLC)9,10 in patients with relapsed/refractory NHL or HL. FT is 1 of 3 prenyltransferases used by normal and malignant cells to catalyze covalent attachment of prenyl groups to ∼ 300 polypeptides in the human proteome. In particular, FT transfers the 15-carbon farnesyl group to key cellular polypeptides, including small guanosine triphosphate-binding proteins of the Ras, Rho, and Rheb families; nuclear lamins; the kinetochore proteins CENP-E and CENP-F; and the chaperone protein HDJ-2/Hsp40.11 Agents that inhibit this enzymatic reaction, termed farnesyltransferase inhibitors (FTIs), diminish cell proliferation and induce apoptosis in a variety of preclinical models.11,12 These agents have been tested in phase 1-3 clinical trials in various solid tumors and leukemias.9,13-15
Although FTIs were initially developed to target cancers with Ras mutations, clinical studies have demonstrated activity in neoplasms lacking mutant Ras.16,17 Instead, depending on the model system, FTIs reportedly inhibit prosurvival signaling by Akt18,19 or the Rheb target mTOR.20 Indeed, recent studies in transgenic mice have demonstrated that Rheb overexpression accelerates lymphomagenesis and an experimental FTI kills these cells, highlighting the potential role of Rheb as an FTI target.21 On the other hand, studies described in the accompanying paper22 demonstrate that tipifarnib prominently inhibits Raf/MEK/extracellular signal-regulated kinase (ERK) signaling downstream of H-Ras, leading to Bim up-regulation and Bim-dependent induction of apoptosis in malignant human lymphoid cell lines. These results are consistent with earlier reports that FTIs inhibit signaling by mitogen-activated protein kinases.23-25
The present multi-institution phase 2 study was undertaken to assess the toxicity and single-agent activity of tipifarnib in 3 cohorts of patients with relapsed NHL or HL. We demonstrate that tipifarnib is well tolerated, has modest but definite antilymphoma activity, and can be administered for extended periods of time in this patient population. In addition, correlative studies were undertaken to determine whether FT was inhibited in lymphoma cells in situ and to assess which signal transduction pathways were impacted by the treatment.
Methods
Patient eligibility
Patients were required to have histologically confirmed relapsed or refractory aggressive lymphomas (aggressive B-cell: transformed, diffuse large B-cell lymphoma [DLBCL], mantle cell lymphoma [MCL], follicular lymphoma grade 3 [FL]); indolent B-cell lymphomas (small lymphocytic lymphoma/chronic lymphocytic leukemia, FL grades 1 or 2, extranodal marginal zone B-cell lymphoma of MALT type, nodal marginal zone B-cell lymphoma, splenic marginal zone B-cell lymphoma); or Hodgkin lymphoma/T-cell (HL/T): peripheral T-cell lymphoma, unspecified, anaplastic large cell lymphoma T and null cell type, mycosis fungoides/Sezary syndrome, relapsed HL. Patients were required to have measurable disease (at least 1 lesion with a single diameter of ≥ 2 cm or tumor cells in the blood ≥ 5000 × 106/L) and be previously treated; there was no limitation on the number of prior therapies. Patients were required to be older than 18 years, have an Eastern Cooperative Oncology Group performance status of 0 to 2, absolute neutrophil count (ANC) ≥ 1000 × 106/L, platelet count ≥ 75 000 × 106/L, hemoglobin ≥ 9 g/dL, serum creatinine ≤ 2 × upper limit of normal (ULN), aspartate aminotransferase ≤ 3 × ULN (≤ 5 × ULN if liver involvement was present), and total bilirubin ≤ 2 × UL. If the total bilirubin was > 2 × ULN, then the direct bilirubin was required to be ≤ 1.5 × ULN.
Patients were treated with tipifarnib 300 mg twice daily on days 1-21 every 28 days. Patients remained on this dose if on subsequent cycles the platelet count remained ≥ 50 000 × 106/L and the ANC ≥ 1000 × 106/L. If the blood counts did not achieve these levels, the dose of tipifarnib was sequentially reduced to 200 mg twice daily or 100 mg twice daily. On the other hand, if the platelet count was ≥ 75 000 × 106/L and the ANC was ≥ 1500 × 106/L and the patient was tolerating the treatment well, then the dose could be increased in a stepwise fashion to 400 mg twice daily and 600 mg twice daily per investigator discretion.
Tumor responses were assessed after 2 cycles and then every 3 cycles thereafter. Reponses were categorized using International Workshop Criteria.26 Patients could remain on treatment as long as they were tolerating the tipifarnib and not experiencing tumor progression.
Study design
This study consisted of three 2-stage phase 2 studies conducted by the University of Iowa/Mayo Clinic Lymphoma SPORE, under 1 protocol (LS038B) to assess the efficacy of tipifarnib in 3 different patient population arms, aggressive B NHL, indolent B NHL, or HL/T. The trial was approved by the Institutional Review Board at each treating center. Each of the 3 arms had a 2-stage design with an interim analysis. For populations 1 and 3 (aggressive B and HL/T lymphoma, respectively), an interim response analysis was planned after 12 evaluable patients were enrolled. If no confirmed responses were observed, the regimen would be deemed ineffective and that study arm was to be closed. If at least 1 confirmed response was observed in the first 12 patients, the study would proceed to the second stage. For indolent B, the interim response analysis was planned after 17 patients and required at least 4 responses to proceed to the second stage of accrual. The planned second stage for each study arm was accrual to 37 evaluable patients.
Adverse event stopping rule
Toxicity was defined using the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) Version 3.0 as adverse events that were considered as either possibly, probably, or definitely related to study treatment. The maximum grade for each type of toxicity was recorded for each patient and frequency tables reviewed to determine toxicity results. The protocol stipulated that overall (across all patient studies), if 2 of the first 16, or if at any time 4 or more patients experienced grade 4 nonhematologic toxicities (adverse events that are considered at least possibly related to study treatment), accrual to the study would be suspended to allow for investigation by the study team.
Laboratory correlates
Consenting patients with accessible tumors or circulating tumor cells in the blood were requested to have a repeat fresh tumor sample on day 8 of tipifarnib therapy. These cells were processed in the SPORE Biospecimens Laboratory by Ficoll-Hypaque gradient purification (circulating tumor cells) or passage through a fine screen (lymph node biopsies) followed in both cases by CD19 or CD3 immunomagnetic bead selection. To assess whether FT inhibition had occurred (as manifested by inhibition of substrate farnesylation) and determine what cellular signal pathways had been affected, these paired samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with antibodies to the farnesylated proteins HDJ-2/Hsp40 and prelamin A as well as phosphorylated and total ribosomal S6 protein and ERK and the Bcl-2 family members Bim and Bcl-2 as described.22,27,28 In addition, pretreatment lymphoma samples, as well as CD3+, CD19+, and CD34+ cells isolated from normal marrow, were subjected to immunoblotting with anti-RasGRP1 antibody as described.22
Results
Patient characteristics
Overall, 93 patients (42 aggressive, 15 indolent, and 36 HL/T) were enrolled in this study (Table 1). The median age was 62 years (range, 18-91 years). A total of 71% of patients had stage IV disease. The median number of prior regimens was 5 (range, 1-17). The majority of patients had DLBCL (40%; 37 of 93) or HL (20%; 19 of 93).
Patient characteristics
| . | Aggressive (N = 42) . | Indolent (N = 15) . | HL/T (N = 36) . | Total (N = 93) . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 66.5 | 64.0 | 48.5 | 62.0 |
| Range | 44.0-91.0 | 45.0-83.0 | 18.0-84.0 | 18.0-91.0 |
| Sex, female, no. (%) | 18 (43) | 10 (67) | 14 (39) | 42 (45) |
| Performance score, no. (%) | ||||
| 0 | 12 (29) | 7 (47) | 13 (36) | 32 (34) |
| 1 | 26 (61) | 7 (47) | 14 (39) | 47 (51) |
| 2 | 4 (10) | 1 (6) | 9 (25) | 14 (15) |
| B-symptoms, no. (%) | 4 (10) | 3 (20) | 5 (14) | 12 (13) |
| Stage, no. (%) | ||||
| 1 | 0 (0) | 1 (7) | 2 (6) | 3 (3) |
| 2 | 9 (21) | 0 (0) | 4 (11) | 13 (14) |
| 3 | 7 (17) | 1 (7) | 3 (8) | 11 (12) |
| 4 | 26 (62) | 13 (87) | 27 (75) | 66 (71) |
| Extranodal site involvement, no. (%) | 27 (64) | 13 (87) | 29 (81) | 69 (74) |
| Current disease status, no. (%) | ||||
| Relapse | 34 (81) | 12 (80) | 32 (89) | 78 (84) |
| Refractory | 8 (19) | 3 (20) | 4 (11) | 15 (16) |
| Lymphoma type, no. (%) | ||||
| DLBCL | 37 (88) | — | — | 37 (40) |
| MCL | 4 (10) | — | — | 4 (4) |
| FL III | 1 (2) | — | — | 1 (1) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 5 (33) | — | 5 (5) | |
| Extranodal marginal zone B cell | — | 1 (7) | — | 1 (1) |
| Follicular grade I | — | 3 (20) | — | 3 (3) |
| Follicular grade II | — | 6 (40) | — | 6 (7) |
| HL | — | — | 19 (53) | 19 (20) |
| Mycosis fungoides | — | — | 4 (11) | 4 (4) |
| Peripheral T cell, unspecified | — | — | 8 (22) | 8 (9) |
| Anaplastic large cell, primary systemic | — | — | 2 (6) | 2 (2) |
| Anaplastic large cell, primary cutaneous | — | — | 3 (8) | 3 (3) |
| No. of prior treatments, no. (%) | ||||
| 1 | 2 (5) | 0 (0) | 1 (3) | 3 (3) |
| 2 | 6 (14) | 0 (0) | 2 (6) | 8 (9) |
| 3 | 7 (17) | 2 (13) | 4 (11) | 13 (14) |
| 4 | 4 (10) | 2 (13) | 11 (31) | 17 (18) |
| 5 | 6 (14) | 4 (27) | 4 (11) | 14 (15) |
| > 5 | 17 (40) | 7 (47) | 14 (39) | 41 (44) |
| Prior autologous stem transplant, no. (%) | 17 (41) | 3 (20) | 24 (67) | 44 (47) |
| Previous radiation therapy, no. (%) | 16 (38) | 4 (27) | 20 (56) | 40 (43) |
| . | Aggressive (N = 42) . | Indolent (N = 15) . | HL/T (N = 36) . | Total (N = 93) . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 66.5 | 64.0 | 48.5 | 62.0 |
| Range | 44.0-91.0 | 45.0-83.0 | 18.0-84.0 | 18.0-91.0 |
| Sex, female, no. (%) | 18 (43) | 10 (67) | 14 (39) | 42 (45) |
| Performance score, no. (%) | ||||
| 0 | 12 (29) | 7 (47) | 13 (36) | 32 (34) |
| 1 | 26 (61) | 7 (47) | 14 (39) | 47 (51) |
| 2 | 4 (10) | 1 (6) | 9 (25) | 14 (15) |
| B-symptoms, no. (%) | 4 (10) | 3 (20) | 5 (14) | 12 (13) |
| Stage, no. (%) | ||||
| 1 | 0 (0) | 1 (7) | 2 (6) | 3 (3) |
| 2 | 9 (21) | 0 (0) | 4 (11) | 13 (14) |
| 3 | 7 (17) | 1 (7) | 3 (8) | 11 (12) |
| 4 | 26 (62) | 13 (87) | 27 (75) | 66 (71) |
| Extranodal site involvement, no. (%) | 27 (64) | 13 (87) | 29 (81) | 69 (74) |
| Current disease status, no. (%) | ||||
| Relapse | 34 (81) | 12 (80) | 32 (89) | 78 (84) |
| Refractory | 8 (19) | 3 (20) | 4 (11) | 15 (16) |
| Lymphoma type, no. (%) | ||||
| DLBCL | 37 (88) | — | — | 37 (40) |
| MCL | 4 (10) | — | — | 4 (4) |
| FL III | 1 (2) | — | — | 1 (1) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 5 (33) | — | 5 (5) | |
| Extranodal marginal zone B cell | — | 1 (7) | — | 1 (1) |
| Follicular grade I | — | 3 (20) | — | 3 (3) |
| Follicular grade II | — | 6 (40) | — | 6 (7) |
| HL | — | — | 19 (53) | 19 (20) |
| Mycosis fungoides | — | — | 4 (11) | 4 (4) |
| Peripheral T cell, unspecified | — | — | 8 (22) | 8 (9) |
| Anaplastic large cell, primary systemic | — | — | 2 (6) | 2 (2) |
| Anaplastic large cell, primary cutaneous | — | — | 3 (8) | 3 (3) |
| No. of prior treatments, no. (%) | ||||
| 1 | 2 (5) | 0 (0) | 1 (3) | 3 (3) |
| 2 | 6 (14) | 0 (0) | 2 (6) | 8 (9) |
| 3 | 7 (17) | 2 (13) | 4 (11) | 13 (14) |
| 4 | 4 (10) | 2 (13) | 11 (31) | 17 (18) |
| 5 | 6 (14) | 4 (27) | 4 (11) | 14 (15) |
| > 5 | 17 (40) | 7 (47) | 14 (39) | 41 (44) |
| Prior autologous stem transplant, no. (%) | 17 (41) | 3 (20) | 24 (67) | 44 (47) |
| Previous radiation therapy, no. (%) | 16 (38) | 4 (27) | 20 (56) | 40 (43) |
— indicates not applicable.
Tumor response
The overall response rate (ORR) was 20.4% (19 of 93), with 7% (6 of 93) complete responses (CR) and 14% (13 of 93) partial responses (PR). The median time to best response for all patients was 2.2 months (mean, 6.5 months; range, 2.2-50.6 months). In the groups of aggressive, indolent, and HL/T types of lymphoma, the ORRs were 17%, 7%, and 31%, respectively. In all 19 responders, the median response duration was 7.5 months (95% confidence interval [CI], 4.9-18.5 months) with a mean of 15.8 months (range, 1.8-62 months). The median response duration was 11.3 months (95% CI, 4.9-17.1 months), 2 months, and 7.5 months (95% CI, 3.2-29.8 months) for the groups of aggressive, indolent, and HL/T lymphomas, respectively.
The highest ORR (31%) was demonstrated in the HL/T lymphoma group (Table 2). Within that group, the ORR was 21% (4 of 19) in patients with HL and 50% (6 of 12) in T-cell NHL. Among the aggressive lymphomas, there were responses in 7 of 37 (19%) of patients with DLBCL. There were no responses in 4 patients with MCL. In the indolent lymphoma group, there was 1 response in the 9 patients with FL and no response in 5 cases of chronic lymphocytic leukemia/small lymphocytic lymphoma. Accrual to the indolent group was halted at 15 (instead of the planned 17) because of the low ORR and the impossibility of reaching the statistical goal of 4 responses in the first 17 patients.
Response to tipifarnib in all patients and by disease type
| Disease type . | n (%) . | CR, n (%) . | PR, n (%) . | ORR, (%) (95% CI) . | Median DR (95% CI) . | Median TTP (95% CI) . | Median OS (95% CI) . |
|---|---|---|---|---|---|---|---|
| All patients | 93 | 6 (7) | 13 (14) | 20 (13-30) | 7.5 (4.9-18.5) | 3.6 (2.1-4.5) | 14.8 (7.6-17.8) |
| Aggressive B-cell lymphoma group | 42 | 0 | 7 (17) | 17 (7-31) | 11.3 (4.9-17.1) | 2.8 (1.7-4.2) | 6.4 (4.1-10.7) |
| DLBCL | 37 (88) | 0 | 7 (19) | 19 | — | — | — |
| MCL | 4 (10) | 0 | 0 | 0 | — | — | — |
| FL III | 1 (2) | 0 | 0 | 0 | — | — | — |
| Indolent B-cell lymphoma group | 15 | 0 | 1 (7) | 7 (0.2-32) | 2 (NR) | 5.2 (4-9.2) | 20.6 (NR) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 5 (33) | 0 | 0 | 0 | — | — | — |
| Extranodal marginal zone | 1 (7) | 0 | 0 | 0 | — | — | — |
| FL grade I | 3 (20) | 0 | 0 | 0 | — | — | — |
| FL grade II | 6 (40) | 0 | 1 | 17 | — | — | — |
| HL/T group | 36 | 6 (17) | 5 (14) | 31 (16-48) | 7.5 (3.2-29.8) | 3.2 (1.9-5.8) | 19.7 (9-60) |
| HL | 19 (53) | 2 (11) | 2 (11) | 21 | — | — | — |
| Mycosis fungoides | 4 (11) | 0 | 2 (50) | 50 | — | — | — |
| Peripheral T-cell, unspecified | 8 (22) | 3 (38) | 1 (13) | 50 | — | — | — |
| Anaplastic large cell, cutaneous | 3 (8) | 1 (33) | 0 | 33 | — | — | — |
| Anaplastic large cell, systemic | 2 (6) | 0 | 0 | 0 | — | — | — |
| Disease type . | n (%) . | CR, n (%) . | PR, n (%) . | ORR, (%) (95% CI) . | Median DR (95% CI) . | Median TTP (95% CI) . | Median OS (95% CI) . |
|---|---|---|---|---|---|---|---|
| All patients | 93 | 6 (7) | 13 (14) | 20 (13-30) | 7.5 (4.9-18.5) | 3.6 (2.1-4.5) | 14.8 (7.6-17.8) |
| Aggressive B-cell lymphoma group | 42 | 0 | 7 (17) | 17 (7-31) | 11.3 (4.9-17.1) | 2.8 (1.7-4.2) | 6.4 (4.1-10.7) |
| DLBCL | 37 (88) | 0 | 7 (19) | 19 | — | — | — |
| MCL | 4 (10) | 0 | 0 | 0 | — | — | — |
| FL III | 1 (2) | 0 | 0 | 0 | — | — | — |
| Indolent B-cell lymphoma group | 15 | 0 | 1 (7) | 7 (0.2-32) | 2 (NR) | 5.2 (4-9.2) | 20.6 (NR) |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 5 (33) | 0 | 0 | 0 | — | — | — |
| Extranodal marginal zone | 1 (7) | 0 | 0 | 0 | — | — | — |
| FL grade I | 3 (20) | 0 | 0 | 0 | — | — | — |
| FL grade II | 6 (40) | 0 | 1 | 17 | — | — | — |
| HL/T group | 36 | 6 (17) | 5 (14) | 31 (16-48) | 7.5 (3.2-29.8) | 3.2 (1.9-5.8) | 19.7 (9-60) |
| HL | 19 (53) | 2 (11) | 2 (11) | 21 | — | — | — |
| Mycosis fungoides | 4 (11) | 0 | 2 (50) | 50 | — | — | — |
| Peripheral T-cell, unspecified | 8 (22) | 3 (38) | 1 (13) | 50 | — | — | — |
| Anaplastic large cell, cutaneous | 3 (8) | 1 (33) | 0 | 33 | — | — | — |
| Anaplastic large cell, systemic | 2 (6) | 0 | 0 | 0 | — | — | — |
— indicates not applicable; and NR, not reported.
The median time to progression (TTP) was 3.6 months (95% CI, 2.1-4.5 months) for all patients and 2.8 months (95% CI, 1.7-4.2 months), 5.2 months (95% CI, 4-9.2 months), and 3.2 months (95% CI, 1.9-5.8 months) for the aggressive, indolent, and HL/T lymphoma groups, respectively (P = .36; Figure 1). Five patients in the HL/T group are still receiving treatment at 29 to 64+ months.
Survival parameters in patients treated with tipifarnib. Kaplan-Meier curves for TTP (A) and OS (B) of 93 patients with relapsed lymphoma treated with tipifarnib. The results are shown by aggressive B cell, indolent B cell, and HL/T (uncommon).
Survival parameters in patients treated with tipifarnib. Kaplan-Meier curves for TTP (A) and OS (B) of 93 patients with relapsed lymphoma treated with tipifarnib. The results are shown by aggressive B cell, indolent B cell, and HL/T (uncommon).
The median overall survival (OS) was 14.8 months (95% CI, 7.6-17.8 months) for all patients and 6.4 months (95% CI, 4.1-10.7 months), 20.6 months, and 19.7 months (95% CI, 9-60 months) for the aggressive, indolent, and HL/T groups, respectively (P = .009). Despite the low medians of TTP and OS, the mean TTP was 6.4 ± 1.3 months, 7.8 ± 2.1 months, and 8.2 ± 1.7 months for aggressive, indolent, and HL/T, respectively. The mean OS was 16.6 ± 3.2 months, 22.3 ± 2.7 months, and 29.3 ± 4.6 months for aggressive, indolent, and HL/T, respectively. These results suggest that the distribution of patient survival (OS and TTP) has a positive skew or a long right tail, consistent with the early progression observed in many patients and prolonged TTP and OS observed in a few.
Tipifarnib dosing.
Treatment was initiated with tipifarnib 300 mg twice daily. Thirty-five (38%) patients required a dose reduction. Of these, 28 were reduced to 200 mg, and 7 to 100 mg twice daily. Fourteen patients had dose escalations to 400 mg, and 1 patient to 600 mg twice daily. Five patients in the HL/T group are still receiving treatment with a range of treatment duration of 29 to 64+ months. These 5 patients (2 with HL, 1 anaplastic large cell cutaneous, and 2 peripheral T-cell lymphoma) are currently receiving doses of 400 mg twice daily (1), 200 mg twice daily (2), and 100 mg twice daily (2).
Toxicity.
Tipifarnib was well tolerated on this dose and schedule. Three patients with aggressive lymphoma died on study of progressive disease, but there were no deaths related to tipifarnib treatment. The grade 3 or 4 toxicities at least possibly related to tipifarnib in the 93 patients are summarized in Table 3. These were primarily reversible myelosuppression, with 11% anemia, 37% neutropenia, and 32% thrombocytopenia. Mild (grade 1 or 2) toxicities observed in > 10% of the patients were fatigue (43%, 25% grade 1,18% grade 2), rash (17%; 11% grade 1, 6% grade 2), nausea (27%; 23% grade 1, 4% grade 2), diarrhea (26%; 18% grade 1, 8% grade 2), elevated aspartate aminotransferase (15%), elevated alkaline phosphatase (20%), and sensory peripheral neuropathy (10%; 6% grade 1, 4% grade 2). A total of 10% of patients reported grade 3 fatigue.
Grade 3 or 4 toxicity (adverse events considered at least possibly related to tipifarnib) was observed in 58% (54 of 93) of patients
| Toxicity . | Grade 3 . | Grade 4 . | Total, no. (%) . |
|---|---|---|---|
| General | |||
| Fatigue | 9 | 0 | 9 (10) |
| Hypotension | 0 | 1 | 1 (1) |
| Prolonged PT | 1 | 0 | 1 (1) |
| Dehydration | 2 | 0 | 2 (2) |
| Hematologic | |||
| Anemia | 8 | 2 | 10 (11) |
| Neutropenia | 7 | 27 | 34 (37) |
| Thrombocytopenia | 13 | 17 | 30 (32) |
| Infection | |||
| Febrile neutropenia | 2 | 2 | 4 (4) |
| Bacteremia | 1 | 1 | 2 (2) |
| Pneumonia | 1 | 0 | 1 (1) |
| Respiratory tract | 0 | 1 | 1 (1) |
| Wound/soft tissue | 2 | 0 | 2 (2) |
| Sinus | 1 | 0 | 1 (1) |
| Metabolic | |||
| Hypokalemia | 1 | 0 | 1 (1) |
| Gastrointestinal | |||
| Diarrhea | 1 | 0 | 1 (1) |
| Pulmonary | |||
| Dyspnea | 1 | 0 | 1 (1) |
| Pneumonitis | 1 | 0 | 1 (1) |
| Toxicity . | Grade 3 . | Grade 4 . | Total, no. (%) . |
|---|---|---|---|
| General | |||
| Fatigue | 9 | 0 | 9 (10) |
| Hypotension | 0 | 1 | 1 (1) |
| Prolonged PT | 1 | 0 | 1 (1) |
| Dehydration | 2 | 0 | 2 (2) |
| Hematologic | |||
| Anemia | 8 | 2 | 10 (11) |
| Neutropenia | 7 | 27 | 34 (37) |
| Thrombocytopenia | 13 | 17 | 30 (32) |
| Infection | |||
| Febrile neutropenia | 2 | 2 | 4 (4) |
| Bacteremia | 1 | 1 | 2 (2) |
| Pneumonia | 1 | 0 | 1 (1) |
| Respiratory tract | 0 | 1 | 1 (1) |
| Wound/soft tissue | 2 | 0 | 2 (2) |
| Sinus | 1 | 0 | 1 (1) |
| Metabolic | |||
| Hypokalemia | 1 | 0 | 1 (1) |
| Gastrointestinal | |||
| Diarrhea | 1 | 0 | 1 (1) |
| Pulmonary | |||
| Dyspnea | 1 | 0 | 1 (1) |
| Pneumonitis | 1 | 0 | 1 (1) |
Thirty-six patients (39%) experienced at least one grade 4 toxicity. Regarding nonhematologic toxicity, 18 patients (19%) had at least one grade 3 toxicity, and 3 patients (3%) had one grade 4 toxicity. PT indicates prothrombin time.
Translational research.
Preclinical work examining the mechanism of cytotoxicity of tipifarnib in malignant lymphoid lines22 prompted us to focus the correlative studies on 3 issues: (1) expression of RasGRP1, (2) identification of signal transduction pathways inhibited by tipifarnib in situ, and (3) expression of Bcl-2 family members that might impact response. Accessible lymphoma samples obtained before therapy and, where possible, after tipifarnib treatment were analyzed without knowledge of the lymphoma characteristics or response.
Based on prior studies demonstrating that high levels of RasGRP1 mRNA correlate with response to tipifarnib in 2 acute myelogenous leukemia (AML) trials,29 along with our data showing that siRNA-mediated RasGRP1 down-regulation diminishes tipifarnib-induced lymphoid cell apoptosis,22 we examined pretreatment RasGRP1 protein levels by immunoblotting. Preliminary analysis indicated that RasGRP1 levels were high in normal T cells and lower but detectable in normal B cells (Figure 2A). Samples from 10 enrolled patients could be analyzed and 3 of 10 samples contained readily detectable RasGRP1 (eg, Figure 2B), including samples from a patient with relapsed small lymphocytic NHL who had stable disease for 7 months (patient 4; Figure 2B) and a patient with relapsed DLBCL who achieved a PR lasting 25 months (patient 2; Figures 2B, 3). On the other hand, there were also 2 PRs and 1 prolonged disease stabilization in tumors that were scored RasGRP1 negative.
Evaluation of potential markers of drug effect in samples from this trial. (A) CD19+ cells (lanes 1 and 2) and CD3+ cells from 2 normal (Nl) persons were probed with antibodies to RasGRP1 and, as a loading control, histone H1. (B) Example of RasGRP1 blotting in 4 lymphoma samples. β-actin served as a loading control. Nonadjacent lanes from a single blot have been juxtaposed to assemble this panel. (C) Effect of tipifarnib on signaling and Bim expression in situ. Each blot is a separate patient with pre- and post-tipifarnib results. In patients 5 (MCL) and 11 (small lymphocytic lymphoma), circulating tumor cells were examined; in patients 3 and 6 (both large cell), paired lymph node samples were assayed. The HDJ-2 shift after treatment (patients 3 and 5) confirms tipifarnib-induced inhibition of FT. Effects on ERK phosphorylation were variable, with decreases in patients 5 and 6 but an increase in patient 3. Some or all Bim isoforms increased in patients 5, 6, and 11 with a smaller increase in patient 3. (D) Examples of pretreatment Bcl-2 levels.
Evaluation of potential markers of drug effect in samples from this trial. (A) CD19+ cells (lanes 1 and 2) and CD3+ cells from 2 normal (Nl) persons were probed with antibodies to RasGRP1 and, as a loading control, histone H1. (B) Example of RasGRP1 blotting in 4 lymphoma samples. β-actin served as a loading control. Nonadjacent lanes from a single blot have been juxtaposed to assemble this panel. (C) Effect of tipifarnib on signaling and Bim expression in situ. Each blot is a separate patient with pre- and post-tipifarnib results. In patients 5 (MCL) and 11 (small lymphocytic lymphoma), circulating tumor cells were examined; in patients 3 and 6 (both large cell), paired lymph node samples were assayed. The HDJ-2 shift after treatment (patients 3 and 5) confirms tipifarnib-induced inhibition of FT. Effects on ERK phosphorylation were variable, with decreases in patients 5 and 6 but an increase in patient 3. Some or all Bim isoforms increased in patients 5, 6, and 11 with a smaller increase in patient 3. (D) Examples of pretreatment Bcl-2 levels.
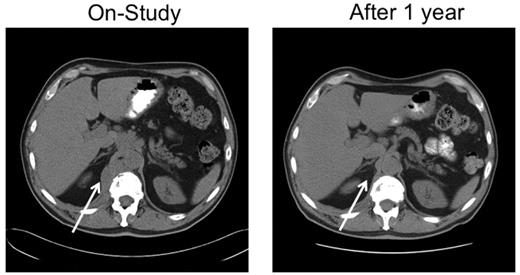
A 67-year-old man with stage IVA diffuse large B-cell lymphoma. He was treated with rituximab, cyclophosphamide, vincristine and prednisone × 6 with an initial complete response, only to relapse 7 months later. He responded to salvage chemotherapy and received an autologous stem cell transplantation with a 6-month TTP. After failing single-agent rituximab, he joined this study. He responded to tipifarnib 300 mg twice daily. Because of myelosuppression, the dose was reduced to 100 mg twice daily. He maintained remission for 25 months before progressing on therapy. Tumor biopsies before and after tipifarnib demonstrated low bcl-2 protein tumor content at baseline with a marked increase in Bim isoforms and decrease in p-ERK after tipifarnib (patient 6; Figure 2C).
A 67-year-old man with stage IVA diffuse large B-cell lymphoma. He was treated with rituximab, cyclophosphamide, vincristine and prednisone × 6 with an initial complete response, only to relapse 7 months later. He responded to salvage chemotherapy and received an autologous stem cell transplantation with a 6-month TTP. After failing single-agent rituximab, he joined this study. He responded to tipifarnib 300 mg twice daily. Because of myelosuppression, the dose was reduced to 100 mg twice daily. He maintained remission for 25 months before progressing on therapy. Tumor biopsies before and after tipifarnib demonstrated low bcl-2 protein tumor content at baseline with a marked increase in Bim isoforms and decrease in p-ERK after tipifarnib (patient 6; Figure 2C).
Further analysis focused on signaling and expression of Bcl-2 family members. Preclinical studies have shown that tipifarnib inhibits mitogen-activated protein kinase signaling, leading to killing that is dependent on Bim up-regulation and is inhibited by high Bcl-2 in malignant human lymphoid lines.22 Analysis of paired samples obtained in this study indicated that the tipifarnib dose of 300 mg twice daily produced inhibition of HDJ-2 farnesylation, documenting FT inhibition (Figure 2C patients 3 and 5). Although effects on S6 phosphorylation, a readout of Rheb inhibition,21,28 were variable in the post-treatment lymphoma samples, tipifarnib strongly inhibited ERK phosphorylation in several lymphomas (eg, Figure 2C patients 5 and 6). Moreover, Bim increased on day 8 in 7 of 10 paired samples examined (Figure 2C; and data not shown), confirming in clinical material the change observed during tipifarnib-induced apoptosis in lymphoid lines.22 Multiple Bim isoforms increased in some cases (Figure 2C patients 5 and 11), whereas one of the shorter, more potent splice variants selectively increased in others (patients 3 and 6). On the other hand, Bcl-2 was also detectable in many of the samples tested (Figure 2D) and, when present at high levels, would be expected to override any impact of Bim up-regulation.22 Consistent with these preclinical findings, we observed 3 PRs (treatment duration 4-25 months) and 1 disease stabilization in 7 lymphoma cases with low/absent Bcl-2 at baseline but no PRs and 1 disease stabilization among 6 lymphoma cases with medium or high Bcl-2 at baseline. This trend, which did not reach statistical significance because of the small sample size, identifies Bcl-2 as a second predictive marker that should be examined further in future clinical trials of tipifarnib in lymphoma.
Discussion
This is the first large phase 2 trial of an FTI in lymphoma. Our results demonstrate that single-agent tipifarnib produced meaningful antitumor responses with manageable toxicity in heavily pretreated patients. Although the ORR for all patients was a modest 20%, there was a higher response rate, including some long-term responses, in patients with T-cell NHL and relapsed/refractory HL. Correlative studies performed in conjunction with this trial, although limited by the number of accessible samples, suggested that induction of Bim expression was a favorable action of tipifarnib, whereas high-level expression of Bcl-2 predicted an unfavorable outcome. Collectively, these results have implications for future studies of FTIs in lymphoma.
The small GTPase H-Ras is a critical signal transduction component that couples receptor tyrosine kinase activation to signaling by the mitogen-activated protein kinase cascade and, in some cells, by the phosphoinositide 3-kinase/Akt pathway as well. FTIs are known to inhibit H-Ras farnesylation, thereby preventing subsequent modifications required for proper H-Ras membrane attachment and function.11,12 Although initial work suggested that cells with H-Ras or N-Ras mutations are particularly sensitive to this class of agents, FTIs have also been shown to be effective when H-Ras is activated without being mutated.12,16,30 These observations led to our interest in exploring the use of tipifarnib in lymphoma.
In this trial, we chose a dose of 300 mg twice daily for 21 days of each 28-day cycle, a schedule and dose suggested by earlier trials in other hematologic malignancies. In particular, Zimmerman et al used the 21 of 28-day schedule in 42 patients with refractory hematologic malignancies and recommended 300 mg tipifarnib twice daily for further study.31 Mesa et al used this dose and schedule in 34 symptomatic patients with myelofibrosis, and 33% of patients benefitted.32 The same schedule was used in a study of 82 patients with myelodysplastic syndrome (MDS); and the ORR was 32% (12% CR), with a median duration of 11.5 months in the CR patients.33 Consistent with previous studies,16,31 we found that this dose inhibits FT (Figure 2C patients 3 and 5) and is tolerated by patients. The major toxicities observed, neutropenia (37%), thrombocytopenia (32%), anemia (11%), and fatigue (10%), were easily managed in our lymphoma patients.
The present study included intrapatient dose escalation as tolerated to approximate other previously reported dosing regimens. In a phase 1 study in poor-risk AML, Karp et al identified dose-limiting toxicity consisting of ataxia, dysarthria, and mental confusion at 1200 mg twice daily and recommended 600 mg tipifarnib twice daily for future single-agent studies.16 In the first trial of tipifarnib for MDS,34 300 to 900 mg twice daily was tested, and 400 mg was determined to be the maximum tolerated dose. When the dose of tipifarnib was increased to 600 mg twice daily for 4 weeks followed by a longer (2-week) rest period, 41% of MDS patients could not tolerate this dose and schedule because of myelosuppression, fatigue, neurotoxicity, or leg pain.35 The present trial allowed escalation to 400 mg or 600 mg twice daily as tolerated, encompassing these previously reported more intensive regimens.
Although the ORR across the 3 cohorts of patients was 20%, differences in response between cohorts provide important clues for potential future development of FTIs in lymphoma. There has been one other study of tipifarnib in lymphoma. Based on in vitro activity of tipifarnib in MCL cell lines,36 Rolland et al treated 11 patients with relapsed MCL with tipifarnib 300 mg twice daily for 21 of 28 days and observed a response in 1 (9%).37 Consistent with that result, none of the 4 patients with MCL in our study responded. This poor response rate in MCL observed in the 2 studies and the equally poor response rate in FL is probably explained by the massive overexpression of Bcl-2 in these neoplasms and the ability of Bcl-2 to neutralize Bim, thereby inhibiting tipifarnib-induced apoptosis.22 Accordingly, further trials of this class of agents in MCL or FL are not recommended.
In contrast, tipifarnib had a 31% response rate (including multiple CRs) in the group of lymphomas that were lumped together in the “HL/T lymphoma” cohort. These included 6 responses (3 CR and 3 PR) in 12 patients with mycosis fungoides and peripheral T-cell lymphoma. Previous results have suggested that high expression of the H-Ras guanine nucleotide exchange factor RasGRP1 is associated with a favorable response in AML29 and with more robust induction of apoptosis in lymphoid cells.22 Because RasGRP1 is constitutively expressed in normal T cells (Figure 2A), it is perhaps reasonable to predict that patients with T-cell neoplasms might respond to this class of agent. Further study in a larger number of patients is required to determine whether RasGRP1 is a bona fide predictor of FTI response in lymphoid malignancies.
In samples from the present trial, we also observed tipifarnib-induced Bim up-regulation in 7 of 10 pairs of lymphoma samples. These results are consistent with our preclinical observation that tipifarnib, by inhibiting a signaling pathway from H-ras through c-Raf and Erk, is able to up-regulate Bim.22 Importantly, these effects on Bcl-2 family members are distinct from the signaling effects observed in AML,28 indicating the potential importance of understanding and monitoring the effects of signal transduction inhibitors in the context of the appropriate cell type. Further analysis suggested a trend toward decreased response in lymphomas with high basal Bcl-2 levels, suggesting that basal Bcl-2 expression should be investigated further as potential marker of response in future trials of FTIs in lymphoid malignancies.
In early tipifarnib studies conducted in AML, phospho-Erk was detected in 36% of AML samples.16 Tipifarnib was shown to dephosphorylate Erk in 4 of 8 samples tested. In a subsequent tipifarnib trial in AML, Erk phosphorylation status was studied but was not found to be useful as a predictive factor for response to tipifarnib.38 In lymphoma, on the other hand, recent studies by Dai et al39 have demonstrated that Erk-1 is present in DLBCL tumors and colocalizes with the MCT-1 (multiple copies of T-cell lymphoma 1) oncogene. The MCT-1 protein, which stimulates proliferation and inhibits apoptosis, is most strongly expressed in DLBCL and T-cell NHL. Dai et al also found that inhibiting Erk-1 resulted in decreased MCT-1 levels and DLBCL cell death.39 These data provide additional support for the role of the MCT-1/Erk pathway in lymphoma and a potential additional explanation for the effectiveness of tipifarnib in Erk-positive lymphomas.
At the present time, tipifarnib is being evaluated in multiagent studies in several diseases. Tipifarnib 200 mg twice daily on days 2 to 7 of each 14-day cycle was combined with doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles as neoadjuvant treatment in 44 patients with previously untreated breast cancer.40 A total of 25% of patients obtained a pathologic CR at surgery, meeting the study's criteria of success. Tissue biopsies demonstrated a reduction in phospho-STAT3 and, like the present study, phospho-Erk. Additional studies in patients with AML and/or MDS have combined tipifarnib 600 mg twice daily on days 6 to 15 with standard daunorubicin 60 mg/m2 per day times 3 and continuous infusion cytosine arabinoside 100 mg/m2 per day times 7 days41 ; with idarubicin and cytosine arabinoside42 ; or with etoposide.28 These studies raise the possibility of future trials of tipifarnib in combination with cytarabine, etoposide, or cyclophosphamide in lymphoma. On the other hand, given the important role of Bcl-2 in modulating the response of lymphoid cells to tipifarnib, the combination of tipifarnib with a Bcl-2 antagonist22 also may be of interest.
In conclusion, the preclinical and clinical data suggest that tipifarnib exerts its antilymphoma effect in vitro and in vivo, in large part by inhibiting farnesylation of H-Ras, which in turn decreases activation of the kinases c-Raf, MEK1/2, and ERK1/2, leading to up-regulation of the BH3-only family member Bim. This mechanism has helped identify potential markers of response (Bcl-2, Bim, ERK1/2, MCT-1, and RasGRP1), which can be examined further in future trials. At a time when there are many signal transduction inhibitors in the pipeline for testing in lymphoma, the criteria for further development include clinical need, biologic rationale, ORR in single-agent trials, convenience of administration, toxicity profile, feasibility of combination therapy, and potential biomarkers that could permit patient selection. Tipifarnib appears to meet these criteria for further development. Tipifarnib targets an enzyme and a pathway not currently being targeted by other available agents. The present trial documents that tipifarnib can be safely administered for prolonged periods and can produce responses as a single agent in relapsed lymphoma at the 20% level in a group of patients who were heavily pretreated with a median of 5 prior therapies. Critical issues for future development will involve the further identification of patients likely to respond and the identification of combinations most appropriate for the lymphoma setting.
Presented in part at the American Society of Hematology Annual Meeting in 2006 and 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants P50 CA97274 and CA127433), the Leukemia & Lymphoma Society (grant 6047-08), and the Henry J. Predolin Foundation.
This study was conducted as a collaborative trial of the University of Iowa/Mayo Clinic Lymphoma SPORE CA97274, the Mayo Clinic Cancer Center P30 CA15083, and the University of Iowa Holden Cancer Center.
National Institutes of Health
Authorship
Contribution: T.E.W. and S.H.K. designed research, performed research, analyzed data, and wrote the paper; H.T., C.A., and M.J.M. analyzed data; and I.N.M.M., S.M.A., B.K.L., D.J.I., L.F.P., P.B.J., J.P.C., S.N.M., G.S.N., C.A.T., M.G., G.W., R.H., P.J.K., H.D., D.L., P.S., K.P., and T.M.H. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas E. Witzig, Mayo Clinic, Stabile 628, 200 First St SW, Rochester, MN 55905; e-mail: witzig@mayo.edu.