Abstract
Sequestration of parasitized erythrocytes and dysregulation of the coagulation and complement system are hallmarks of severe Plasmodium falciparum malaria. A link between these events emerged through the discovery that the parasite digestive vacuole (DV), which is released together with infective merozoites into the bloodstream, dually activates the intrinsic clotting and alternative complement pathway. Complement attack occurs exclusively on the membrane of the DVs, and the question followed whether DVs might be marked for uptake by polymorphonuclear granulocytes (PMNs). We report that DVs are indeed rapidly phagocytosed by PMNs after schizont rupture in active human serum. Uptake of malaria pigment requires an intact DV membrane and does not occur when the pigment is extracted from the organelle. Merozoites are not opsonized and escape phagocytosis in nonimmune serum. Antimalarial Abs mediate some uptake of the parasites, but to an extent that is not sufficient to markedly reduce reinvasion rates. Phagocytosis of DVs induces a vigorous respiratory burst that drives the cells into a state of functional exhaustion, blunting the production of reactive oxygen species (ROS) and microbicidal activity upon challenge with bacterial pathogens. Systemic overloading of PMNs with DVs may contribute to the enhanced susceptibility of patients with severe malaria toward invasive bacterial infections.
Introduction
Severe malaria develops as a consequence of capillaric sequestration of parasitized RBCs (pRBCs),1-3 activation of complement4-6 and coagulation,7-9 and increases in vascular permeability, which together can lead to microcirculatory disturbances with comatous death as the ultimate outcome.1,7,10,11 One unresolved puzzle relates to the fact that children with severe malaria frequently suffer from septicemia due to bacteria that otherwise play no major role in this potentially fatal affliction.12-14 Approximately 50% of these infections are caused by nontyphoidal Salmonellae and other Enterobacteriae, indicating a gut origin. Current hypotheses regarding the causes underlying this general state of immune compromise focus mainly on disturbances in the function of macrophages, dendritic cells, and the adaptive immune system.15
During its developmental cycle in the RBC, the malaria parasite ingests hemoglobin and packages the waste product hemozoin, which is also known as malaria pigment, in an organelle designated the digestive vacuole (DV).16 Copious numbers of DVs are released into the bloodstream in patients with severe Plasmodium falciparum malaria, yet their biologic properties and fates have been only minimally studied. Hematin is widely regarded to be equivalent to hemozoin,17,18 and most investigators have focused on the effects of this artificial molecule on the immune system.19-25 However, hematin is not packaged within a membrane-bound organelle and any biologic activity deriving from the DV membrane would therefore be missed. In a different study, we have shown that the DV membrane, but not hemozoin itself, is endowed with the unique capacity to dually activate the alternative complement and the intrinsic clotting pathway (P.D., S. Heber, S. Baumeister, K.L., K.R., S.C.B., and S. Bhakdi, unpublished data, September 2011). Overactivation of these ancient enzyme cascades would be expected to trigger and sustain pathobiological events that coincide with severe malaria. The discovery that DVs activate complement prompted investigations into the fate of this organelle. In particular, we strove to determine whether complement would mark the DV for uptake by polymorphonuclear granulocytes (PMNs). If so, this would satisfactorily account for the long-known fact that malaria pigment can regularly be observed in circulating PMNs of patients with severe malaria.26,27 By extrapolation, it would also provide a further explanation as to why hemozoin is found in tissue macrophages of animals after infection.28
Several novel observations were made in the present study. First, DVs are opsonized and rapidly phagocytosed by PMNs after schizont rupture in active human serum. Second, the uptake of DVs induces a respiratory burst in PMNs, but the generated reactive oxygen species (ROS) fail to suppress the infective capacity of invading merozoites. Third, firing of ROS on ingested DVs drives PMNs into a state of functional exhaustion. Their ability to phagocytose bacteria prevails, but their capacity to mount a respiratory burst is reduced and microbicidal activity is compromised. It is proposed that these events might be linked to the development of septicemic episodes in patients with severe malaria.
Methods
Cell preparation
P falciparum culture, isolation of digestive vacuoles and hemozoin, and staining procedures for DNA and C3 were undertaken as described previously (P.D., S.H., S. Baumeister, K.L., K.R., S.C.B., and S. Bhakdi, unpublished data, September 2011) and as detailed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Human PMNs were isolated from heparinized blood of healthy volunteers using conventional procedures29 and were kept in PBS on ice until use. Surface labeling of PMNs was performed with PE-conjugated polyclonal rabbit anti–human CD16 IgG (BD Biosciences) following the manufacturer's recommendation. After labeling, 50 μL of Ab solution was added to 0.5 mL of cells (107 PMNs/mL) for 20 minutes at room temperature, and the cells were subsequently washed twice with PBS. By microscopic assessment, it was ascertained that surface labeling had no effect on phagocytic function. Erythrocytes, banked human blood, and human sera, all group O, Rh+, were kindly provided by Roland Conradi and Walter Hitzler (Blood Transfusion Center of the University of Mainz, Mainz, Germany). C8-deficient human serum was prepared as described previously.30 Polyclonal rabbit anti–human C3 IgG was obtained from Dakocytomation.
PMNs phagocytosis of DVs
PMNs suspended at 5 × 106 cells/mL in 10% active or heat-inactivated serum were challenged with 3-5 DVs/cell and phagocytosis was analyzed by microscopy of Giemsa-stained smears. To obtain quantitative data, DVs were fluorescently labeled by 20 minutes of incubation with 7.5 μg/mL CellMask Deep Red plasma membrane stain (Invitrogen), washed twice, and added to the PMNs. After the given times at 37°C, 50 μL of the reaction mixture was removed, diluted in 500 μL of veronal buffered saline (VBS), and analyzed in a FACSScan flow cytometer (BD Biosciences).
Detection of ROS generation
Single-cell observations were conducted using PMNs loaded with 0.1μM 2′,7′-dichlorodihydrofluorescein diacetate (Sigma-Aldrich) for 30 minutes at 37°C, and washed 4 times with PBS. Next, 0.2 mL of cell suspensions (3 × 106/mL in VBS with 10% active serum) were transferred to Eppendorf tubes and DVs/PMNs at a ratio of 2:5 were added. Incubation was conducted at 37°C. Smears were prepared every 2 minutes and viewed in an Axioskop 2 microscope (Carl Zeiss) at a 1000× magnification. Images were obtained using AxioVision software.
Luminol-based quantification of ROS
Experiments were performed in microtiter plates using a thermostated luminometer (MicroLumat LB96P; Berthold Technologies). Each well contained 0.2 mL of PMNs (3 × 106/mL in VBS) with 10% active or inactive serum. Luminol (Sigma-Aldrich) was added to a final concentration of 125μM to each well. DVs were applied at a ratio of 3:5 DVs/PMNs and chemiluminescence was measured every 4 minutes. In bacterial challenge experiments, luminol was re-added after 60 minutes, a ratio of 10:15 Staphylococcus aureus/PMNs was applied, and measurements were continued for another 60 minutes.
Assessment of uptake of DVs and merozoites by PMNs after rupture of pRBCs
Synchronized enriched cultures containing 60%-70% late-stage pRBCs were diluted to 0.2% hematocrit in RPMI 1640 medium and maintained at 37°C. Giemsa-stained smears were prepared every 30 minutes. When the first schizont rupture was observed, cultures were continued for another 30 minutes, during which time > 75% of the parasitized cells lysed. The culture was then centrifuged in a Beckman Coulter Allegra 6KR centrifuge at 200g for 5 minutes to sediment unlysed cells. The supernatant was centrifuged at 3000g for 10 minutes in a Sorvall RC2B centrifuge to pellet the merozoites and DVs. The pellet was resuspended in RPMI and centrifuged once again at low speed (400g) for 1 minute to remove any residual unlysed cells. The supernatant was then centrifuged at 17 000g for 2 minutes, washed 3 times, and stained with Hoechst 33342. Isolated DVs and merozoites were added to Eppendorf tubes, each containing 3 × 106/mL of PMNs (prelabeled with PE-conjugated polyclonal rabbit anti–human CD16 IgG) in 0.2 mL of VBS with 10% active serum (3-5 DVs and 50-100 merozoites/PMNs). After 30 minutes at 37°C, thin smears were prepared and air dried, and Z-stacked images were taken in a fluorescence microscope (Axiovert 200M; Carl Zeiss) using AxioVision Version 4.7 software.
Infection experiments
Synchronized late-stage pRBCs were enriched to 55%-70% and suspended at 0.4% hematocrit in RPMI with 20% active human serum; 0.5 mL was applied per well in a 24-well cell culture plate (Greiner Bio-One). To this, 0.1 mL of 10% noninfected RBCs ± 106 PMNs were added, and the total volume brought to 1 mL with RPMI to give a final ratio of PMNs:pRBCs:RBCs of ∼ 1:10:100. After 24 hours, 0.2 mL of the culture was stained with 100μM hydroethidine for 30 minutes at 37°C, and infection rates were quantified by flow cytometry.31 Slides were prepared and stained with Giemsa for microscopy.
Ab protection experiments
Assessment of Ab protection was undertaken using a 10-fold higher number of PMNs and the 3H-hypoxanthine incorporation assay.32 For this assay, 5 × 106 schizonts and 5 × 107 noninfected RBCs were suspended in 0.2 mL total volume in 96-well flat-bottom microtiter suspense culture plates (Greiner Bio-One) and supplemented as follows: (1) 20% active serum, (2) 20% active serum + 107 PMNs, (3) 20% active serum + 0.2 mg of IgG, and (4) 20% active serum + 0.2 mg of IgG and 107 PMNs. Experiments were performed in duplicate. Plates were incubated for 10-12 hours at 37°C, after which time the medium was exchanged and 1μCi 3H-hypoxanthine (Perkin Elmer) was added per well. After another 12 hours, genomic DNA was isolated using the DNeasy Blood and Tissue Kit (QIAGEN) and hypoxanthine incorporation was measured in a liquid scintillation counter (LS 6000 TA; Beckman Coulter).
Abs
Sera were obtained from Thai patients with acute P falciparum malaria, which was confirmed by microscopic examination of Giemsa-stained blood films on admission. The sera were tested for Abs to malarial antigens with the indirect fluorescent Ab test using methanol-fixed films of P falciparum–infected RBCs. Five patients with high titers (> 1:625) had parasitemia ranging from 4.5%-21%; 4 patients had low indirect fluorescent Ab titers (< 1:25) and parasitemia ranging from 0.5%-1.1%. Pools of the high- and low-titered sera were prepared. Experiments were performed with both the individual sera and the 2 serum pools. IgG was purified using Spintrap columns (GE Healthcare Life Sciences) and concentrated using Vivaspin 6 Centricon tubes (Sartorius) to their original volume. The IgG samples were dialyzed against RPMI 1640, the protein concentrations were measured using Bradford reagent and the Bio-Rad protein assay, and stored at −20°C until use.
Phagocytosis and bacterial killing assays
Experiments with S aureus were performed with cell-rich plasma obtained after dextran sedimentation of heparinized blood samples from healthy volunteers.33 DVs were added to 0.9 mL of cell-rich plasma to give a ratio of 3:5 DVs/PMNs and incubated at 37°C for 1 hour. Thereafter, 0.1 mL of S aureus in PBS was added to give a ratio of 10:20 bacteria/PMNs. At given time points, 0.1-mL samples were withdrawn and admixed with 0.1 mL of a 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) solution. After 5 minutes at 37°C, 0.8 mL of water was added and incubation continued for 3 minutes at 37°C. This treatment ensured complete liberation of bacteria from the cells under retention of their viability.33 Finally, 20-μL samples were diluted 1:200 in saline and 20 μL of this solution was plated in duplicate for colony counting.
Experiments conducted with a clinical isolate of Salmonella typhimurium (obtained from the Diagnostics Laboratory of the Department of Medical Microbiology and Hygiene, University of Mainz, Mainz, Germany) were performed using isolated PMNs and C8-deficient serum that had been prepared as described previously.30 PMNs were suspended at 2 × 106 cells/mL in 50% C8-deficient serum/VBS and experiments were conducted as described above.
Transmission electron microscopy
DVs were centrifuged, fixed with 2.5% glutaraldehyde in PBS at 4°C overnight, washed in PBS, post-fixed in 2% OsO4, dehydrated in ethanol, and embedded in Araldite (Sigma-Aldrich). Ultrathin sections were mounted on Formvar-coated grids and double stained with a saturated solution of uranyl acetate in 70% methanol and lead citrate. The grids were examined with a Zeiss EM 900 transmission electron microscope equipped with a digital camera system.
Statistical analysis
The assumptions for normality and equal variance were verified with SigmaStat 3.1 software (Erkrath; SYSSTAT). The Holm-Sidak test was used for comparisons against a control group. Results represent means ± SEM of at least 3 independent experiments or the means of at least triplicates of 1 experiment ± SD as indicated. P < .05 was considered statistically significant.
Results
Rapid complement–dependent phagocytosis of intact DVs by PMNs
DVs were isolated from supernatants of hemolyzed pRBCs and, in accordance with descriptions in the literature,34,35 were found to comprise a population of dispersed, 1- to 2-μM sized vesicles with various contents of hemozoin crystals and amorphous material (Figure 1A). No merozoites or RBCs membrane debris could be discerned in the electron micrographs.
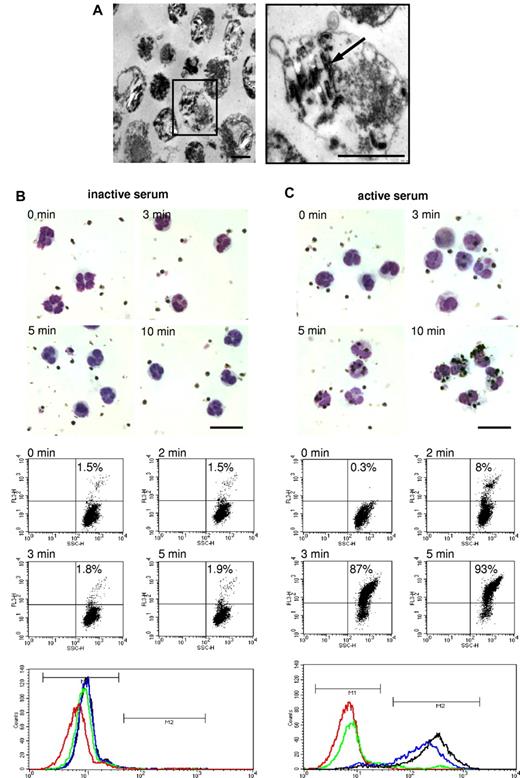
Phagocytosis of isolated DVs by PMNs. (A) Electron microscopic appearance of DVs naturally liberated from late-stage parasitized RBCs showing ∼ 1- to 2-μM vesicles with varying content of hemozoin (arrow) bound by a limiting membrane. Scale bars indicate 1 μm. (B) DVs were incubated with PMNs prelabeled with PE-conjugated anti-CD16 IgG in 10% inactive serum. Microscopic Giemsa smears were prepared at the depicted times and flow cytometric analyses of phagocytosis were performed by gating PMNs and assessing the number of red fluorescent cells. Histograms of the corresponding dot plots are shown in the bottom panel (red: 0 minutes; green: 2 minutes; blue: 3 minutes; and black: 5 minutes). M1 is the region set for nonfluorescent cells and M2 for fluorescent cells. The numbers counted in the M2 region are shown in the dot plots. (C) Same experiment performed in active serum. The results of flow cytometric analyses are shown at 2 and 3 minutes to illustrate the abrupt onset of phagocytosis occurring in active serum. The findings in inactive serum remained stationary over the period of observation of 30 minutes. One representative experiment of 3 independent experiments is shown. Scale bars indicate 20 μm.
Phagocytosis of isolated DVs by PMNs. (A) Electron microscopic appearance of DVs naturally liberated from late-stage parasitized RBCs showing ∼ 1- to 2-μM vesicles with varying content of hemozoin (arrow) bound by a limiting membrane. Scale bars indicate 1 μm. (B) DVs were incubated with PMNs prelabeled with PE-conjugated anti-CD16 IgG in 10% inactive serum. Microscopic Giemsa smears were prepared at the depicted times and flow cytometric analyses of phagocytosis were performed by gating PMNs and assessing the number of red fluorescent cells. Histograms of the corresponding dot plots are shown in the bottom panel (red: 0 minutes; green: 2 minutes; blue: 3 minutes; and black: 5 minutes). M1 is the region set for nonfluorescent cells and M2 for fluorescent cells. The numbers counted in the M2 region are shown in the dot plots. (C) Same experiment performed in active serum. The results of flow cytometric analyses are shown at 2 and 3 minutes to illustrate the abrupt onset of phagocytosis occurring in active serum. The findings in inactive serum remained stationary over the period of observation of 30 minutes. One representative experiment of 3 independent experiments is shown. Scale bars indicate 20 μm.
DVs and PMNs were admixed at a ratio of 5:1 in 10% active or 10% inactive human serum and phagocytosis was followed microscopically and by flow cytometry. For the latter analyses, DVs were fluorescently labeled, the PMNs were gated based on side and forward scatter and the uptake of fluorescently labeled DVs was assessed. No phagocytosis of the DVs by PMNs occurred in inactive serum within the 30-minute period of observation, as was apparent from the Giemsa-stained smears and from the corresponding absence of a red fluorescent shift of the cells (Figure 1B). In contrast, phagocytosis in active serum could be readily observed microscopically, commencing within 3 minutes and essentially reaching completion at 5-8 minutes. Correspondingly, PMNs abruptly started to assume red fluorescence within 2-3 minutes and, in agreement with the microscopic observations, > 90% of the cells were laden with DVs after just 5 minutes (Figure 1C).
PMNs were loaded with dichlorofluorescein, which allowed visualization of ROS production in single cells. In agreement with the classic response during phagocytosis, a burst of ROS could always be observed surrounding a DV during and immediately after its uptake (Figure 2A). The single-cell observations were borne out by luminol-based quantification of ROS generation, which revealed a bell-shaped chemiluminescence response covering a time span of ∼ 40 minutes that was not seen when heat-inactivated serum was used (Figure 2B).
Phagocytosis of DVs induces ROS production in PMNs. (A) PMNs were laden with dichlorol-fluorescein to visualize ROS generation at the single-cell level. Phagocytosis of DVs was accompanied by triggering of the respiratory burst, and ROS generation was seen to surround intracellular DVs. ROS generation was not observed when DVs were just attached to the cells (arrows). Scale bar indicates 10 μm. (B) Luminol-based chemiluminescence assay for ROS generation in PMNs during phagocytosis of DVs revealed a bell-shaped response curve covering a time span of 30-40 minutes. PMNs were challenged with 3-5 DVs/cell and the chemiluminescence response was recorded in the presence of 10% active or heat-inactivated human serum. One representative experiment of 3 independent experiments is shown.
Phagocytosis of DVs induces ROS production in PMNs. (A) PMNs were laden with dichlorol-fluorescein to visualize ROS generation at the single-cell level. Phagocytosis of DVs was accompanied by triggering of the respiratory burst, and ROS generation was seen to surround intracellular DVs. ROS generation was not observed when DVs were just attached to the cells (arrows). Scale bar indicates 10 μm. (B) Luminol-based chemiluminescence assay for ROS generation in PMNs during phagocytosis of DVs revealed a bell-shaped response curve covering a time span of 30-40 minutes. PMNs were challenged with 3-5 DVs/cell and the chemiluminescence response was recorded in the presence of 10% active or heat-inactivated human serum. One representative experiment of 3 independent experiments is shown.
Merozoites escape phagocytosis by PMNs in nonimmune serum
Late-stage synchronized pRBCs were cultured at 0.2% hematocrit in medium containing 10% active, nonimmune human serum until schizont rupture occurred, and merozoites and DVs were harvested and stained for DNA and bound C3 (Figure 3A). The polyclonal Abs used recognized native and activated C3 (C3b). Hemozoin contained within the DVs was visualized in the phase-contrast image. Merozoites were not seen here, but showed up in the blue fluorescence image generated by the DNA stain. Immunofluorescence staining revealed the presence of C3 exclusively on the DVs, as was strikingly apparent in merged images, in which the red C3 fluorescent stain is seen colocalizing with DVs, but was invariably absent on merozoites. Weaker stainings were also obtained using an Ab against C3d, which reacts with the remnant molecule after C3b is cleaved and removed (data not shown). This experiment was performed 3 times with serum from different donors. Merged images were randomly taken and 100 hemozoin particles were evaluated for the presence of C3. In all 3 experiments, C3 colocalized with > 85% of the DVs. DVs liberated just before harvest would have had insufficient time to become sufficiently coated with C3b. In striking contrast to these findings, not a single merozoite was ever observed to carry C3 deposits.
Selective opsonization and phagocytosis of DVs after pRBC rupture. (A) Immediately after lysis of late-stage pRBCs, merozoites and DVs were harvested and stained with polyclonal Abs directed against native and activated C3 (C3b) and DNA. C3 immunoreactivity was exclusively restricted to DV membranes. Scale bar indicates 20 μm. (B) DNA staining was used to identify merozoites and PE-surface-labeled PMNs were added to the mixture with DVs. Stacked images (i-vi: top to bottom) were prepared that revealed extracellular localization of merozoites. Left rows: fluorescence; right rows: merged fluorescence and phase-contrast images. The micrographs shown are representative of 3 independent experiments. Scale bar indicates 20 μm.
Selective opsonization and phagocytosis of DVs after pRBC rupture. (A) Immediately after lysis of late-stage pRBCs, merozoites and DVs were harvested and stained with polyclonal Abs directed against native and activated C3 (C3b) and DNA. C3 immunoreactivity was exclusively restricted to DV membranes. Scale bar indicates 20 μm. (B) DNA staining was used to identify merozoites and PE-surface-labeled PMNs were added to the mixture with DVs. Stacked images (i-vi: top to bottom) were prepared that revealed extracellular localization of merozoites. Left rows: fluorescence; right rows: merged fluorescence and phase-contrast images. The micrographs shown are representative of 3 independent experiments. Scale bar indicates 20 μm.
It followed that selective phagocytosis might occur as a natural consequence of intravascular schizont rupture. Therefore, in the next experiment, merozoites and DVs were isolated as above and DNA staining was undertaken to stain the parasites. PE-surface-labeled PMNs (1 PMN:3-5 DVs: 50-100 merozoites) were then added together with 10% NHS, and stacked fluorescence microscopic images were prepared after 30 minutes. These revealed virtually exclusive uptake of DVs, and merozoites were rarely seen to colocalize with the DVs in the central plane of the cells (Figure 3Biii and 3Biv).
It has been reported that PMNs may phagocytose late-stage parasitized RBCs.36 Such cells were stained with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM), which enabled intracellular parasites to be visualized (P.D., S. Heber, S. Baumeister, K.L., K.R., S.C.B., and S. Bhakdi, unpublished data, September 2011), and incubated with PE-labeled PMNs in the presence of active serum for 30 minutes. However, erythrophagocytosis could never be observed (Figure 4A).
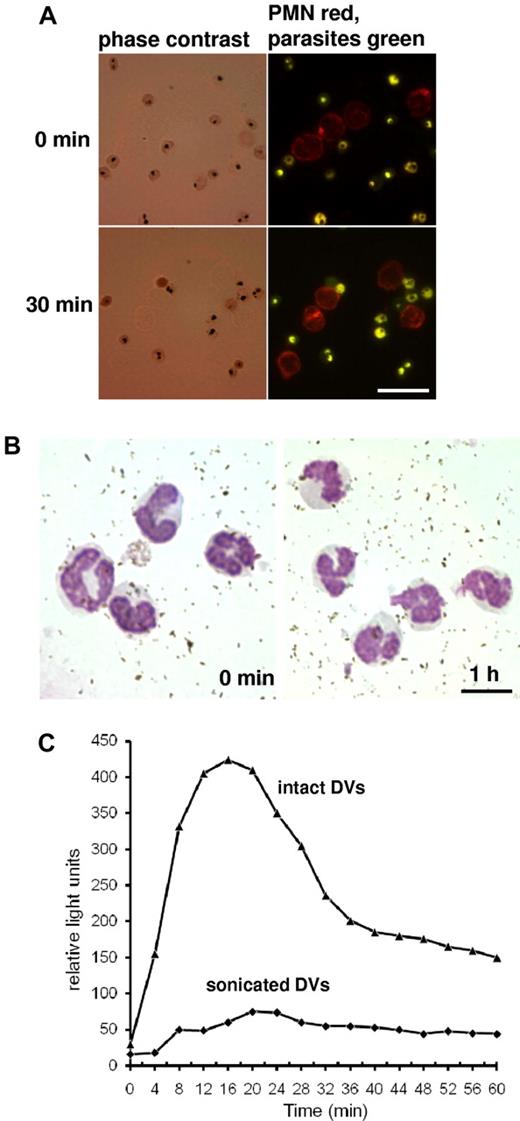
PMNs do not phagocytose late-stage pRBCs or hemozoin in nonimmune serum. (A) pRBCs were stained with BCECF-AM, a nonfluorescent acetoxymethylester that is enzymatically hydrolyzed to fluorescent BCECF, to visualize parasites and intracellular DVs, and incubated with PE-labeled PMNs in active human serum. No erythrophagocytosis could be discerned after 30 minutes. Scale bar indicates 20 μm. (B) DVs were disrupted by sonication and hemozoin was isolated from a Percoll gradient and incubated with PMNs in active serum. No evidence for phagocytic uptake and no appearance of cells with characteristic malaria pigment could be discerned in Giemsa-stained smears. Scale bar indicates 10 μm. (C) Luminol-based chemiluminescence assay were performed in PMNs and active serum upon incubation with intact DVs or with sonicated DVs (hemozoin). Representative results are shown from 1 of 3 similar experiments.
PMNs do not phagocytose late-stage pRBCs or hemozoin in nonimmune serum. (A) pRBCs were stained with BCECF-AM, a nonfluorescent acetoxymethylester that is enzymatically hydrolyzed to fluorescent BCECF, to visualize parasites and intracellular DVs, and incubated with PE-labeled PMNs in active human serum. No erythrophagocytosis could be discerned after 30 minutes. Scale bar indicates 20 μm. (B) DVs were disrupted by sonication and hemozoin was isolated from a Percoll gradient and incubated with PMNs in active serum. No evidence for phagocytic uptake and no appearance of cells with characteristic malaria pigment could be discerned in Giemsa-stained smears. Scale bar indicates 10 μm. (C) Luminol-based chemiluminescence assay were performed in PMNs and active serum upon incubation with intact DVs or with sonicated DVs (hemozoin). Representative results are shown from 1 of 3 similar experiments.
Isolated hemozoin lacking the encasing DV-membrane is not phagocytosed
Malaria pigment is microscopically detectable in circulating PMNs of patients with malaria, and the relative number of pigment-containing PMNs provide a prognostic criterion for disease outcome.26,27 The question of how the pigment enters the cell has never been addressed. A tacit assumption is that DVs are labile structures from which hemozoin is released and then phagocytosed. However, we have found that the DV membrane is remarkably stable, requiring extreme measures to effect its disruption in vitro (P.D., S. Heber, S. Baumeister, K.L., K.R., S.C.B., and S. Bhakdi, unpublished data, September 2011). Once freed of the encasing membrane, the pigment disperses into small, crystalline unit structures devoid of complement-activating properties and morphologically distinct from the compact deposits visible within intact DVs (P.D., S. Heber, S. Baumeister, K.L., K.R., S.C.B., and S. Bhakdi, unpublished data, September 2011). This agrees with published data showing that the intravesicular malaria pigment consists of multiple aggregates of hemozoin crystals.17 It is these aggregates and not the dispersed crystals that present as malaria pigment in circulating leukocytes.26,27 In the next experiment, DVs were disrupted by sonication or by detergent lysis, and hemozoin was purified by centrifugation through Percoll. The isolated hemozoin was incubated in 10% NHS with PMNs for 1 hour. No microscopic evidence for attraction of PMNs to and uptake of isolated hemozoin could be obtained, and cells presenting with the characteristic malaria pigment were not observed (Figure 4B). In agreement with these observations, chemiluminescence assays revealed that sonication of DVs immediately destroyed their ROS-inducing property (Figure 4C). The same was found when isolated hemozoin was applied to the cells (not shown).
DV-induced ROS-production in PMNs does not suppress merozoite reinvasion
Merozoites have been reported to be susceptible to the cytotoxic action of ROS.37 This conclusion was based on experiments with murine malaria merozoites and may not be extrapolatable to P falciparum. Nevertheless, it was of interest to determine whether ROS generation might indirectly serve a protective function because of bystander killing of the parasites. In the next experiment, a 10-fold excess of noninfected RBCs was added to enriched, late-stage pRBCs and cultures were maintained for 24 hours in the presence or absence of PMNs, which were added in supraphysiological numbers (∼ 1:100 RBCs) so that no effects would be missed. Figure 5Ai depicts the control without PMNs, showing the presence of freshly infected cells with ring-stage parasites. When PMNs were present in the culture, these were seen to have phagocytosed the DVs (Figure 5Aii). However, ring forms could still be discerned and quantification of infected cells by flow cytometric analyses of DNA-stained samples generated superimposable curves (Figure 5Aiii left peaks, uninfected nonfluorescing cells; right peaks, infected cells). Therefore, ROS generation accompanying phagocytic uptake of DVs by PMNs led to no reduction in the infective capacity of merozoites. This experiment was performed 3 times with PMNs from different donors with the same result.
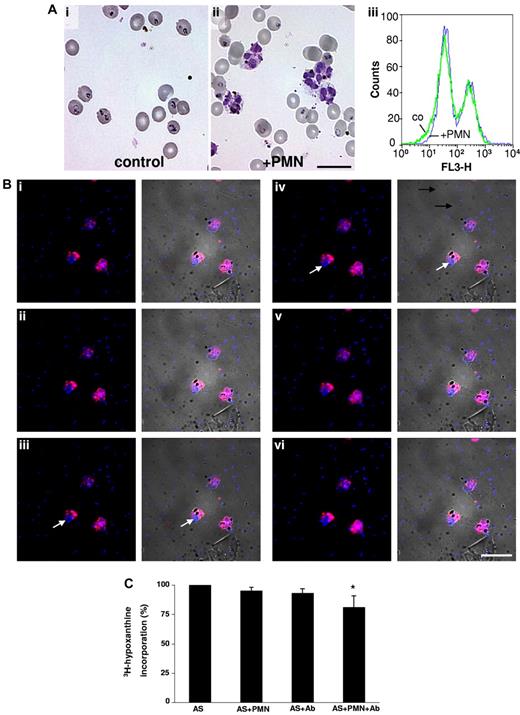
Lack of protective effect of PMNs on merozoite reinvasion. (A) Late-stage pRBCs were allowed to rupture and new infection was allowed to proceed in active human serum in the absence (i) or presence (ii) of PMNs. Twenty-four hours later, ring forms could be seen microscopically in both cases. Infected erythrocytes were detected by fluorescent staining of parasite DNA with hydroethidium-bromide (iii). Flow cytometric analyses revealed identical infection rates in the presence and absence of PMNs (right peaks: fluorescing infected cells; left peaks: nonfluorescing, noninfected cells). (B) Detection of merozoite phagocytosis mediated by Abs against P falciparum. The experiment shown in Figure 3B was repeated in the presence of 1 mg/mL of IgG isolated from a pool of 5 sera containing high-titered Abs against P falciparum. Stacked images (i-vi, top to bottom) now revealed that merozoites (blue) had been phagocytosed alongside with the DVs. Left rows: fluorescence; right rows: merged fluorescence and phase-contrast images. Arrows: phagocytosed merozoites colocalizing with DVs in the central plane (iii-iv) of a cell. Similar results were obtained with IgG from 5 individual sera with high-titered Abs against P falciparum. Scale bar indicates 20 μm. (C) Paucity of protective effects of PMNs and specific Abs upon parasite reinvasion. The 3H-hypoxanthine DNA-incorporation assay was used and values obtained in active serum alone were defined as 100%. Results obtained with 5 high-titered Abs are shown. 3H-hypoxanthine incorporation was assessed in active serum (AS) in the presence of either PMNs or IgG (Ab) or in the presence of PMNs and IgG. A small but significant reduction in 3H-hypoxanthine incorporation was observed in the presence of Abs plus PMNs compared with the control (n = 5; *P < .001).
Lack of protective effect of PMNs on merozoite reinvasion. (A) Late-stage pRBCs were allowed to rupture and new infection was allowed to proceed in active human serum in the absence (i) or presence (ii) of PMNs. Twenty-four hours later, ring forms could be seen microscopically in both cases. Infected erythrocytes were detected by fluorescent staining of parasite DNA with hydroethidium-bromide (iii). Flow cytometric analyses revealed identical infection rates in the presence and absence of PMNs (right peaks: fluorescing infected cells; left peaks: nonfluorescing, noninfected cells). (B) Detection of merozoite phagocytosis mediated by Abs against P falciparum. The experiment shown in Figure 3B was repeated in the presence of 1 mg/mL of IgG isolated from a pool of 5 sera containing high-titered Abs against P falciparum. Stacked images (i-vi, top to bottom) now revealed that merozoites (blue) had been phagocytosed alongside with the DVs. Left rows: fluorescence; right rows: merged fluorescence and phase-contrast images. Arrows: phagocytosed merozoites colocalizing with DVs in the central plane (iii-iv) of a cell. Similar results were obtained with IgG from 5 individual sera with high-titered Abs against P falciparum. Scale bar indicates 20 μm. (C) Paucity of protective effects of PMNs and specific Abs upon parasite reinvasion. The 3H-hypoxanthine DNA-incorporation assay was used and values obtained in active serum alone were defined as 100%. Results obtained with 5 high-titered Abs are shown. 3H-hypoxanthine incorporation was assessed in active serum (AS) in the presence of either PMNs or IgG (Ab) or in the presence of PMNs and IgG. A small but significant reduction in 3H-hypoxanthine incorporation was observed in the presence of Abs plus PMNs compared with the control (n = 5; *P < .001).
At this juncture, it was of interest to determine whether the failure of PMNs to prevent parasite reinvasion might be rectified in the presence of specific Abs. Experiments were undertaken with IgG from 5 patients with high Ab titers, from a pool of high-titered sera from 10 patients, and from 5 patients with low Ab titers.
Phagocytosis experiments were performed as in the experiment shown in Figure 3, and reinvasion was quantified by measuring DNA incorporation of 3H-hypoxanthine. No effects of low-titered Abs could be discerned in any experiments. However, in the presence of high-titered Abs, merozoites were observed to be phagocytosed along with comparable numbers of DVs (Figure 5B). Inspection of 100 PMNs revealed that, although the merozoites outnumbered DVs by an order of magnitude in the incubation mixture, a phagocyte was never seen harboring merozoites alone. Therefore, it appeared that preferential uptake of DVs persisted even in the presence of the Abs. This might have reduced each cell's capacity to phagocytose merozoites, allowing the majority to escape phagocytosis. Indeed, only small Ab-mediated reductions of parasite reinvasion could be detected in the 3H-hypoxanthine incorporation assays, even though the PMNs were present in 10-fold higher numbers in these experiments (Figure 5C).
The capacity to produce ROS is blunted in DV-laden PMNs
ROS generation is subject to multiple pathways of feedback regulation,38,39 so the possibility that ingestion of DVs might lead to impaired respiratory burst on subsequent bacterial challenge emerged. PMNs in 10% serum were incubated for 60 minutes in the presence or absence of 2-4 DVs per cell and subsequently challenged with S aureus. Figure 6A shows the chemiluminescence recordings observed. In control PMNs, S aureus challenge provoked a sustained generation of ROS. DVs induced the initial bell-shaped chemiluminescence response, but thereafter, the second burst of ROS provoked by S aureus was blunted. Identical results were found with PMNs from 4 different donors.
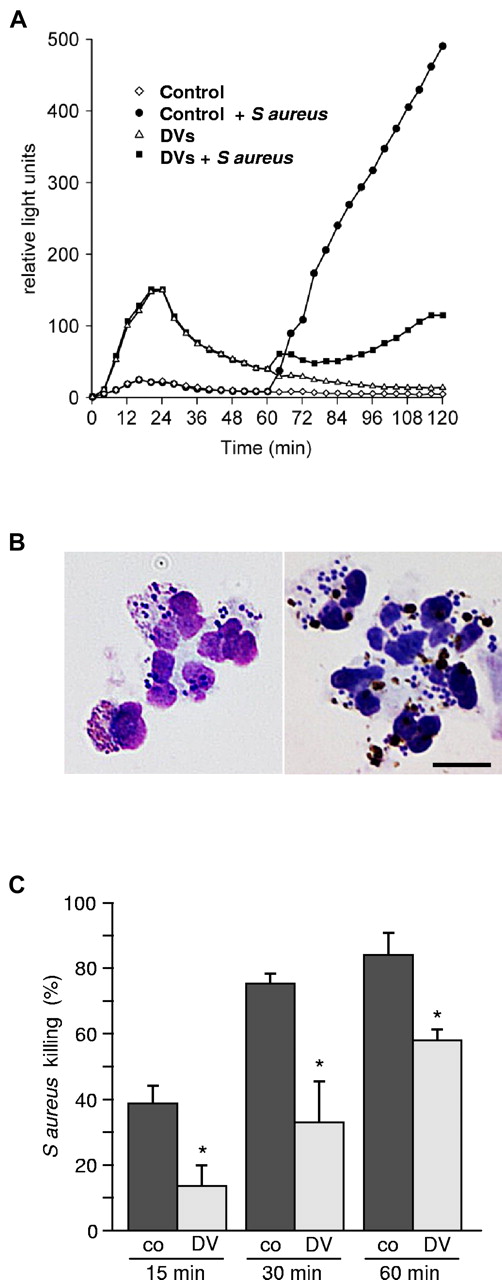
Phagocytosis of DVs leads to impairment of PMNs-function. (A) ROS generation was detected using the luminol-based chemiluminescence assay. Control PMNs incubated for 60 minutes in medium and subsequently challenged with S aureus mounted a vigorous response. Incubation of cells with DVs induced an initial bell-shaped response, but ROS generation upon subsequent bacterial challenge was conspicuously blunted. (B) Microscopic examination undertaken 20 minutes after S aureus challenge revealed effective phagocytic uptake of the bacteria in both cases (left: control PMNs; right: PMNs preloaded with DVs). Scale bar indicates 10 μm. (C) Assessment of bacterial killing revealed marked impairment of bactericidal activity in DV-laden cells. One representative experiment of 4 independent experiments is shown. Results are expressed as means of triplicates ± SD; *P < .001 compared with control.
Phagocytosis of DVs leads to impairment of PMNs-function. (A) ROS generation was detected using the luminol-based chemiluminescence assay. Control PMNs incubated for 60 minutes in medium and subsequently challenged with S aureus mounted a vigorous response. Incubation of cells with DVs induced an initial bell-shaped response, but ROS generation upon subsequent bacterial challenge was conspicuously blunted. (B) Microscopic examination undertaken 20 minutes after S aureus challenge revealed effective phagocytic uptake of the bacteria in both cases (left: control PMNs; right: PMNs preloaded with DVs). Scale bar indicates 10 μm. (C) Assessment of bacterial killing revealed marked impairment of bactericidal activity in DV-laden cells. One representative experiment of 4 independent experiments is shown. Results are expressed as means of triplicates ± SD; *P < .001 compared with control.
DV uptake reduces microbicidal activity of PMNs
These findings prompted phagocytosis and killing assays. Cell-rich plasma obtained after dextran sedimentation of erythrocytes from heparinized blood was spiked with 2-5 DV/PMN and incubated for 60 minutes at 37°C. The PMNs were then challenged with 10-20 S aureus per cell. Microscopy revealed that the capacity of DV-loaded PMNs to subsequently ingest the bacteria remained unimpaired, and most of the bacteria were phagocytosed as in the controls after 15-20 minutes (Figure 6B). However, colony counting undertaken after detergent solubilization of the PMNs led to the striking finding that the capacity of DV-laden cells to kill ingested bacteria was significantly compromised (Figure 6C). ROS reduction did not directly impact oxygen-independent microbicidal mechanisms, so the killing function was reduced but not entirely abrogated.
Five to 10% of African children with severe malaria suffer from sepsis episodes, half of which are caused by enteric nontyphoid Salmonellae.12-14 Phagocytic killing assays were therefore also performed with a clinical isolate of S typhimurium. Enteric Salmonellae display widely varying degrees of serum complement resistance. This unpredictable potential confounder was eliminated by conducting assays with isolated PMNs in the presence of 50% C8-deficient human serum. Killing could then be attributed solely to the microbicidal function of the neutrophils. As found with S aureus, a significant reduction of bactericidal capacity was observed for S typhimurium in PMNs that had been laden with DVs (20-minute kill in controls: 48% ± 5% and in DV-laden cells: 20% ± 2%; n = 4, *P < .001).
Discussion
Lysis of each parasitized erythrocyte in P falciparum malaria liberates one DV along with infectious merozoites into the bloodstream.16,40 High parasitemia is therefore inseparably associated with high loads of DVs at the sites of RBC rupture. It is all the more remarkable that, whereas seminal work on the biogenesis and biochemical events occurring within the DV is ongoing,34,35,41-43 only one group of investigators has been conducting studies with naturally released DVs, and these are devoted to their effects on monocyte functions.44-48 The first study was conducted with unpurified malarial pigment obtained by hypotonic lysis of infected erythrocytes. In that study, evidence was presented that the pigment was phagocytosed by human macrophages, and that this provoked a respiratory burst that was blunted upon subsequent provocation. Bactericidal assays were not performed, but it was surmised that this immune suppression might bear general clinical relevance when malarial pigment reached the macrophages in the spleen and other organs.44 The fact that most DVs will probably be ingested not by tissue macrophages but rather by the surrounding PMNs has never been considered before the present work, an oversight that likely derives from several reasons. There is a tendency to assume that the malaria pigment itself is endowed with biologic properties, and that synthetic hematin, which is considered equivalent to natural malaria pigment,17,18 is readily available. The popularity of hematin as a research tool is understandable, but its use could not have led to present discoveries because free pigment lacks all of properties described herein. In this context, it is not common knowledge that the DV is released as an intact, enveloped organelle and it has not been recognized that the DV membrane is remarkably stable. Therefore, a widespread assumption is that malaria pigment itself rapidly contacts the host environment. Consequently, the possibility has been missed that the DV membrane may fulfill biologic functions that are entirely distinct from those of isolated hematin or hemozoin.
The starting point of the present investigation was our recent discovery that the DV membrane is endowed with the capacity to dually activate the alternative complement and intrinsic clotting pathways (P.D., S. Heber, S. Baumeister, K.L., K.R., S.C.B., and S. Bhakdi, unpublished data, September 2011). Binding of complement marks a particle for phagocytosis, and experiments described herein naturally followed. Isolated DVs were indeed found to be rapidly phagocytosed in a complement-dependent fashion, which raised the possibility that engulfment of DVs represents the major pathway leading to the presence of malaria pigment in PMNs of patients. The latter is a widespread finding, attesting to the generality of the phenomenon. Previous concepts have envisaged phagocytosis of hemozoin crystals or schizonts to be responsible, but no evidence for either could be obtained in this study. Sonicated DVs or isolated hemozoin did not induce a respiratory burst and were not detectably taken up by the PMNs. We also could not observe any phagocytosis of late-stage parasitized RBCs in nonimmune serum. The possibility that specific Abs might alter the latter situation, as suggested in an early study,36 is not excluded. However, PMNs containing ingested parasitized RBCs are seldom seen in clinical samples, so we propose at this stage that malaria pigment in the PMNs of patients derives mainly from phagocytosis of intact DVs after their extrusion into the blood. Electron micrographs of both PMNs and macrophages bearing malarial pigment have been published.49 The findings are highly suggestive for 2 reasons. First, although the cells had not phagocytosed parasitized erythrocytes, aggregates of hemozoin crystals could be seen in the cell cytoplasm. Such aggregates do not persist when the pigment is artificially liberated from the organelle in vitro. Second, the aggregates can be seen to be surrounded by membrane structures in the electron micrographs. These findings are fully in agreement with our hypothesis that phagocytosis of DVs underlies the appearance of malaria pigment in the cells.
Whether DVs might be preferentially phagocytosed was investigated in the presence and absence of high-titered Abs against P falciparum. Synchronized late-stage pRBCs were used, enabling the time point of schizont rupture to be closely monitored so that the notoriously short-lived merozoites could be retrieved together with the DVs. Culture was performed in active instead of heat-inactivated serum so that complement activation would immediately occur upon erythrocyte rupture. Staining of merozoite DNA was undertaken before the addition of surface-labeled PMNs, rendering rapid fluorescent microscopic analyses feasible. These experiments revealed the striking fact that DVs but not merozoites were selectively opsonized and phagocytosed by PMNs in the presence of active, nonimmune serum. In the presence of high-titered Abs, phagocytosis of merozoites was also observed. However, preferential uptake of DVs still appeared to persist. Therefore, although merozoites outnumbered DVs by an order of magnitude, as also occurs in vivo, PMNs were never observed to contain merozoites only. If preferential uptake of DVs really takes place, this might impinge on each cell's capacity to ingest merozoites.
ROS generation, the central element in the microbicidal machinery of mammalian phagocytes, is subject to feedback regulation via multiple pathways.38,39 Induction of the respiratory burst by phagocytosed DVs might therefore be followed by a state of hyporesponsiveness. Indeed, the capacity to mount a respiratory burst on subsequent bacterial challenge was severely compromised after DV uptake. PMNs preloaded with DVs were still fully capable of phagocytosing bacteria. However, their microbicidal capacity was reduced. This was shown using the classic target S aureus and a clinical isolate of S typhimurium. The latter was used because noninvasive Salmonella are the leading cause of septicemia in African children with severe malaria, and these experiments now offer a possible explanation for this finding.
Having served its physiologic purpose, the liberated DV appears to function as a decoy, and is used by the parasite to divert and derange central elements of the innate immune system. The intrinsic clotting and alternative pathway simultaneously become activated on its surface. Both pathways generate potent mediator molecules that may trigger and sustain microcirculatory disturbances and vascular leakage, which contribute to the clinical syndromes of malaria. DVs from other Plasmodium species are probably endowed with similar properties. Complement activation has been shown in monkeys infected with P coatneyi50 and in mice infected with P berghei.51 High levels of parasitemia exceeding 10% are found almost exclusively in P falciparum infections, and capillaric sequestration of pRBCs will further heighten the local load of DVs. Other than the living parasite, the organelle is then preferentially taken up by blood phagocytes. This may initially provide benefit to the host by restricting activation of complement and coagulation. Differences in PMN-dependent clearing capacity may explain why high parasitemia is sometimes tolerated and vice versa. However, the price for this indirectly beneficial PMN function may ultimately be high. As parasitemia increases, so will the detraction of the cells away from their true targets. We found that high-titer-specific Abs invoked some phagocytosis of merozoites. This finding would appear to confirm earlier studies in which Abs from patients reportedly promoted phagocytosis of merozoites by neutrophils.52,53 However, merozoite preparations described in those studies were retrieved as a dark band from Percoll gradients and thus may have contained appreciable numbers of DVs. We are not aware of any previous study in which phagocytosis of merozoites and DVs has been cleanly differentiated, such as is illustrated herein in Figures 3 and 5.
Possibly because efficient ingestion of DVs still prevailed, PMNs contained relatively small numbers of merozoites and the majority of parasites remained outside of the cells. In agreement with this observation, substantive reductions in the rates of reinvasion could also not be discerned. These findings are preliminary because they were performed with only a small number of antisera and one laboratory strain of P falciparum. Homologous Abs might more efficiently redirect PMN phagocytosis toward merozoites to afford some protection. However, our findings agree with earlier studies in which little32 or no54,55 protective effects of specific Abs plus PMNs could be observed in vitro. Monocytes apparently synergized more efficiently with the Abs,55 but the significance of this finding remains unclear because the cells were used at unphysiologically high numbers. Most recently, the presence of Abs provoking strong ROS responses in PMNs upon incubation with a mixture of merozoites and DVs was reported to be correlated with better in vivo protection compared with Abs that induced little ROS production.56 Whether ROS generation is correlated with uptake of merozoites requires further investigation. These open questions notwithstanding, it appears quite clear that in vivo protection mediated by anti-merozoite Abs can generally not be very efficient, because patients in endemic regions often have circulating Abs that can neither inhibit infection nor totally suppress disease progression.
DV uptake and cellular activation could cause PMNs to remain mainly sequestered in the microcirculation. If that is the case, then the actual number of DV-laden phagocytes would probably be considerably higher than suggested by the mere numbers of circulating, hemozoin-containing cells. Sequestered, activated PMNs possibly augment pathologic processes in the microcirculation. At the same time, their systemic overloading may gradually set the stage for septicemic complications to develop whenever bacterial pathogens chance to gain entry into the circulation (Figure 7). The leading roles played by Salmonellae and Enterobacteriaceae in African children may derive simply from the high endemic prevalence of these agents. Should the principle tenets of this investigation turn out to be correct, the DV would emerge as a major, multifaceted determinant of parasitic pathogenicity. Clinically oriented studies are called for to test this hypothesis.
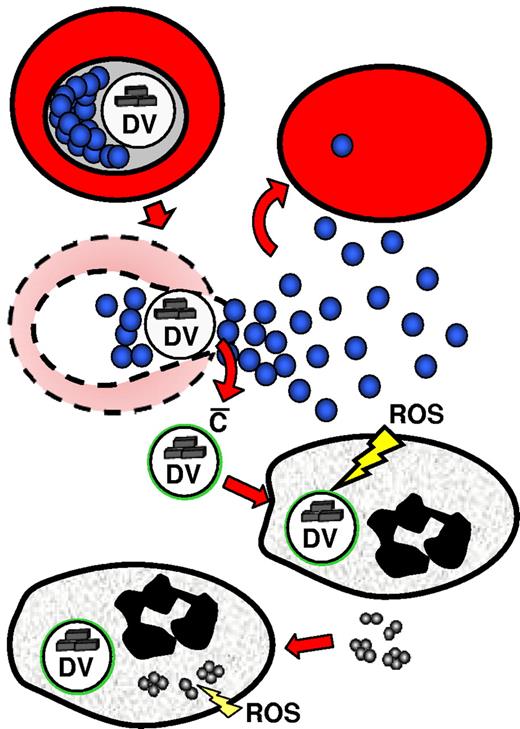
Schematic presentation of the concept of immune decoy by the DV. Rupture of a parasitized cell liberates one DV along with 32 merozoites. In nonimmune serum, complement is activated only on the DV, so exclusive uptake of the vesicle follows, leaving merozoites free to invade new cells. ROS generation in response to DV uptake is unable to harm the merozoites, but instead drives the cells into a state of functional exhaustion so that efficient killing of subsequently engulfed bacteria is no longer ensured.
Schematic presentation of the concept of immune decoy by the DV. Rupture of a parasitized cell liberates one DV along with 32 merozoites. In nonimmune serum, complement is activated only on the DV, so exclusive uptake of the vesicle follows, leaving merozoites free to invade new cells. ROS generation in response to DV uptake is unable to harm the merozoites, but instead drives the cells into a state of functional exhaustion so that efficient killing of subsequently engulfed bacteria is no longer ensured.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Walter Hitzler and Roland Conradi for continued supply of erythrocytes, banked human blood, and human sera, and Markus Radsak for the gift of anti-CD16 Abs.
This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 490 (to S. Bhakdi), Sonderforschungsbereich 593 (to K.L. and S. Baumeister), Sonderforschungsbereich 877 (to K.R.), the Cluster of Excellence “Inflammation at Interfaces” (to K.R.), and the Thai Infectious Disease Network (to P.D., R.U., S.C.B., and S. Bhakdi).
Authorship
Contribution: S. Bhakdi and S.C.B. conceived the project; S. Bhakdi, K.R., and P.D. designed the research; P.D. and S. Bhakdi performed the experiments; R.L. performed the electron microscopy; S. Bhakdi, P.D., S. Baumeister, K.L., R.U., and K.R. analyzed the data; and S. Bhakdi wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sucharit Bhakdi, Department of Medical Microbiology and Hygiene, University Medical Center, Johannes Gutenberg University, Hochhaus Augustusplatz, 55202 Mainz, Germany; e-mail: sbhakdi@uni-mainz.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal