Abstract
The endothelium plays a pivotal role in vascular inflammation. Here we study bone morphogenetic protein (BMP) signaling in endothelial inflammation and in particular the role of BMPER, an extracellular BMP modulator that is important in vascular development and angiogenesis. Using the BMP antagonist dorsomorphin or BMP2 as an agonist we show that BMP signaling is essential for the inflammatory response of vascular endothelial cells as demonstrated by intravital microscopy. We found that BMPER is decreased in inflammation similar to vascular protective genes like KLF2 and eNOS. Using in vitro and in vivo models we show that BMPER is down-regulated through the TNFα-NFκB-KLF2 signaling pathway. Functionally, lack of BMPER induced by siRNA or in BMPER+/− mice confers a proinflammatory endothelial phenotype with reduced eNOS levels and enhanced expression of adhesion molecules leading to increased leukocyte adhesion and extravasation in ex vivo and in vivo experiments. Vice versa, addition of BMPER exerts endothelium protective functions and antagonizes TNFα induced inflammation. Mechanistically, we demonstrate that these effects of BMPER are dependent on BMP signaling because of enhanced NFκB activity. In conclusion, the BMP modulator BMPER is a new protective regulator of vascular inflammation that modulates leukocyte adhesion and migration in vitro and in vivo.
Introduction
The endothelium plays a pivotal role in the response to vascular inflammation. Inflammatory stimuli such as TNFα induce endothelial dysfunction and confer a proadhesive endothelial phenotype.1 These events are important under physiologic conditions in wound healing as well as in inflammatory diseases like sepsis or atherosclerosis. Endothelial dysfunction initiates a cascade of events with leukocyte adhesion in a central position. It is of outstanding importance to understand the underlying molecular mechanisms of this process and to identify new mediators of a proadhesive endothelial phenotype.
In endothelial dysfunction hundreds of genes are induced but inhibition of genes is a relatively rare event.1 Among these few genes Kruepple like factor 2 (KLF2) and endothelial NO synthase (eNOS) are well characterized examples.2 Consistent with down-regulation in endothelial dysfunction KLF2 is up-regulated by laminar (physiologic) flow conditions or statin therapy.3,4 KLF2 then up-regulates the expression of protective endothelial factors, such as eNOS.5 ENOS and NO attenuated inflammatory response via inhibition of endothelial adhesion molecules VCAM-1 and ICAM-1 in vitro and in vivo.6,7 Vice versa reduction of eNOS and endothelial NO caused endothelial dysfunction at the vascular wall reflected by proinflammatory endothelial phenotype with increasing leukocyte endothelial interaction.7-9
Bone morphogenetic proteins (BMPs) are important regulators in blood vessel formation and vascular disease.10 BMPs are expressed in atheroprone regions of blood vessels and are down-regulated in the endothelium under protective conditions such as statin therapy.11-14 Correspondingly, BMP2 expression is increased by exposure of endothelial cells to proinflammatory stimuli such as TNFα. Once up-regulated, BMP2 and BMP4 induce a proinflammatory endothelial phenotype resulting in enhanced leukocyte adhesion to the endothelial surface in vitro.12,15-17 Accordingly, chronic infusion of BMP4 in mice leads to endothelial dysfunction and arterial hypertension.18 BMPs are regulated at the extracellular level by direct interaction with BMP modulators. BMP endothelial precursor cell–derived regulator (BMPER) is a secreted glycoprotein that binds directly to BMPs and modulates their function in a dose dependent manner. In gain of function assays BMPER behaves as a BMP-antagonist,19,20 whereas in loss of function models BMPER may exert pro-BMP functions.21-23 Mechanistically, BMPER regulates BMP signaling by interfering with the receptor binding site of BMPs.24-26 BMPER was originally identified in a screen for differentially expressed proteins in embryonic endothelial precursor cells.19 In mouse and zebrafish, it is expressed at the time and at sites of vasculogenesis, consistent with a regulatory role for BMPER in vascular events.19,21 Lack of BMPER in zebrafish embryos results in severely perturbed intersomitic angiogenesis.21 Consistent with this vascular phenotype BMPER may confer proangiogenic activity in a dose-dependent fashion and it is essential for BMP function in endothelial cells.14 Our previous work demonstrates that BMPER is up-regulated by statins involving Rho/Rock signaling.27 Here we characterize BMPER as a novel regulator of vascular inflammation that mediates anti-inflammatory activity on the endothelium.
Methods
Plasmids, antibodies, and reagents
Monoclonal anti–human BMPER antibody (MAB1956), monoclonal anti–human ICAM-1 antibody (BBA3), recombinant human BMPER (1956-CV), and human TNFα (210-TA) were purchased from R&D Systems. VCAM-1 antibody (sc-13160) from Santa Cruz Biotechnology, ICAM-1 antibody (#4915) from Cell Signaling, and eNOS antibody (610296) from BD Biosciences were used for Western blotting. The KLF2 plasmid was a generous gift of MK Jain, Cleveland and the plasmid coding for the NFκB suprerrepressor pcDNA3-IκBα-SR, lacking the amino-terminal 70 amino acids required for TNFα-induced degradation of IκBα, was a friendly gift from C. Scheidereit (Max-Delbrueck Zentrum, Berlin).
Cell culture
Human umbilical vein endothelial cells (HUVECs) are cultured in endothelial cell growth medium (Provitro) with 10% FBS. Cultures were kept at 37°C in a 5% CO2 humidified atmosphere.
Immunocytochemistry
HUVECs were seeded on glass coverslips and were stimulated for 24 hours with TNFα alone or in combination with human BMPER (500ng/mL). Cells were fixed in ice-cold methanol/ acetone at −20°C for 10 minutes and were blocked with 10% donkey serum for 30 minutes at room temperature. Subsequently HUVECs were incubated with a polyclonal ICAM-1 antibody, donkey anti–goat-FITC antibody, and DAPI for nuclear staining.
Promoter constructs
Transfection and luciferase assay
HUVECs were plated at a density of 1 × 105/well in a 6-well dish. Transient transfections were performed using Fugene 6 (Roche Molecular Biochemics) according to the instructions provided by the manufacturer. Cells were transfected using 2 μg of luciferase reporter vector and 0.5 μg of the pCMV β-galactosidase (pCMV β-Gal) as a transfection control. Twenty-four–hour posttransfection luciferase activity and β-galactosidase activity were measured. β-galactosidase activity was used to normalize for transfection efficiency. Data shown as mean ± SD of triplicates of at least 3 independent experiments.
Quantitative real-time PCR
Quantitative PCR analysis was performed using the real-time PCR detection system (Bio-Rad) and the MyiQ lightcycler software (Bio-Rad). Human RNA-polymerase II was used for normalization. Quantification was calculated using the ΔΔCT method.
siRNA transfection
SiRNA for human BMPER, human KLF2 and a nonspecific control siRNA were purchased from Invitrogen. HUVECs were transfected with siRNA using Lipofectamine RNAiMAX according to the manufacturer's protocol. Cells were harvested for protein isolation.
Western blot
HUVECs were stimulated with indicated concentrations of TNFα in endothelial cell growth medium (Provitro) with 1% FBS. Cell lysates were resolved on a reducing polyacrylamide gel, plotted onto a nitrocellulose membrane (Amersham Bioscience) and blocked with 3% nonfat dry milk powder in PBS/Tris with 0.1% Tween 20 for 2 hours at room temperature (20-22°C). The membrane was then incubated with primary antibody overnight at 4°C. After 1 hour of incubation with the secondary antibody, proteins were visualized using ECL reagent (Amersham). All western blots were repeated at least 3 times and quantified data are shown.
Dynamic adhesion assays
HUVECs were plated in a 35-mm dish and cultured to a confluence of 90%. PBMCs from healty donors were isolated by ficoll separation and stained with CFDA (10μM). PBMCs were activated with PMA (200 ng/mL) and were allowed to adhere for 10 minutes at the endothelial surface. The GlykoTech flow chamber was assembled with the dish at the bottom of the resulting parallel flow chamber. Chambers and tubes were filled with PBS without serum before the experiment. Subsequently shear stress was applied with a syringe pump (Harvard apparatus PHD 2000) with flow rates of 0.5 dyne/cm2 for a total of 10 minutes/well. Adherent cells were counted under a microscope by blinded investigators. All experiments were performed at least 3 times.
Mice
Heterozygous BMPER mice are available at The Jackson Laboratory (Stock Number: 007554; Strain Name: B6SJL-BMPERtm1Emdr/J) and were generated and described by Zakin et al.29 A targeting vector was used to place an in-frame lacZ reporter gene in the first exon of the BMPER gene and a neomycin resistance cassette downstream of lacZ. Homozygous BMPER mice die at birth because of respiratory failure. They display extensive skeletal abnormalities, microopthalmia, and exencephaly. Heterozygous mice have no obvious phenotype.23,29 All animal studies were approved by the local ethics committee (Regierungspräsidium Freiburg) and performed according to the respective guidelines.
Intravital microscopy of mesenteric venules
Mice were pretreated before surgery with an intraperitoneal injection of murine TNFα (10 ng/g body weight [bw]) alone or in combination with recombinant BMPER (0.33μg/g bw, retroorbitally), BMP2 (0.33μg/g bw IP), dorsomorphin (13μg/g bw IP). After 4 hours mice were anesthetized by IP injection of Ketamin and Xylazin. Additional sedation was administered as needed during the intravital microscopy observation time. Leukocytes were fluorescently labeled in vivo by retroorbital injection of 50 μL Rhodamine 6G (1mg/mL, Sigma-Aldrich). A loop of ileal mesentery was exteriorized through a midline incision in the abdominal wall and was placed in a temperature controlled plastic chamber for observation of the peri-intestinal microcirculation by intravital microscopy. Leukocyte interactions with the endothelial vessel wall was recorded for 1 minute at least in 3 veins/mouse. Leukocytes were grouped according to the duration of their interaction with the venular wall. Leukocytes that were stationary for > 30 seconds were defined as adherent and were counted as cells that adhere to 100 μm vessel length (adherent cells/100 μm). The average diameter of examined venules was normalized to 100 μm. Leukocyte adhesion was quantified by investigators blinded to the genotype of the mice.
Peritonitis model
Eight- to 12-week-old male mice were administered 2 mL of 4% thioglykollate (Sigma-Aldrich) intraperitoneally. After 4 hours mice were euthanisized by cervicooccipital dislocation. Cells were recovered by peritoneal lavage with 6 mL of RPMI medium and counted using a hemocytometer.
Statistical analysis and quantification
Statistical analyses were performed using GraphPad Prism 4.0. In vitro data are presented as mean ± SD and in vivo data as mean ± SEM. Comparisons were calculated by Student t test (2-sided, unpaired). Results were considered statistically significant when P < .05. Densitometric analysis of Western blots was performed using Quantity One 1-D Analysis Software (Version 4.4; Bio-Rad) and levels of significance were calculated by 1-sample t test.
Results
Dorsomorphin inhibits leukocyte adhesion to the vascular wall in vivo whereas BMP2 stimulates leukocyte adhesion and is up-regulated in vascular inflammation
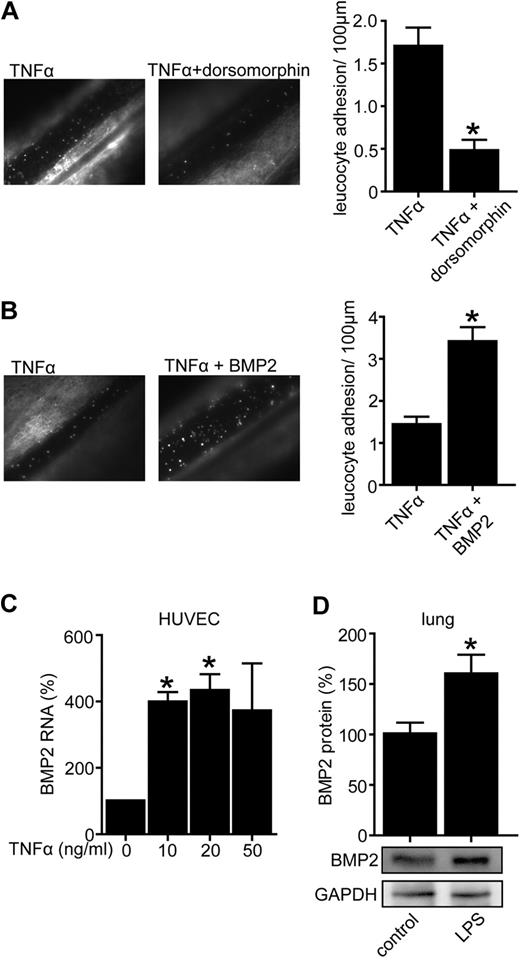
We set out to evaluate the role of BMPs in leukocyte adhesion to the vascular wall, which is a key feature of vascular inflammation. Therefore we antagonized BMP signaling in an in vivo model of leukocyte adhesion using the synthetic BMP-inhibitor dorsomorphin, a small molecule that inhibits BMP type I receptor Alk2, Alk3, and Alk6 and prevents activity of diverse BMPs such as BMP2, BMP4, BMP6, and BMP7.30-32 As shown in Figure 1A, dorsomorphin strongly decreased the number of adhering leukocytes suggesting an important role of BMPs in this process. Next, we aimed to assess the expression and function of BMPs in inflammation and chose BMP2 as a representative protagonist of the BMP family. To functionally test if BMP2 modulates leukocyte-endothelial interactions we injected C57BL/6 mice intraperitoneally with BMP2 or control buffer. To facilitate a baseline level of leukocyte adhesion TNFα (10 ng/g bw; IP) was also injected in both groups. Indeed BMP2 increased leukocyte adhesion to vascular endothelium as demonstrated by intravital microscopy consistent with a proinflammatory effect in vivo (Figure 1B). Given the proinflammatory role of BMP2 we next aimed to assess if BMP2 itself is regulated during vascular inflammation. Therefore inflammation was induced by TNFα in HUVECs and BMP2 expression was quantified by real time-PCR. Complementing previous data we found that BMP2 is up-regulated in endothelial cell inflammation (Figure 1C).33 These results were confirmed in vivo in mice injected intraperitoneally with lipopolysaccharide (LPS) to induce a systemic inflammation. Indeed BMP2 protein levels were higher in lungs of mice treated with LPS compared with controls (Figure 1D). Lungs were chosen for BMPER analysis because previous data demonstrates that lung is one of the organs in which BMPER is expressed in adulthood.19,34 Taken together, the synthetic BMP antagonist dorsomorphin has anti-inflammatory properties whereas BMP2, as a prototypic BMP agonist, is up-regulated in vascular inflammation and displays proinflammatory features in leukocyte-endothelium interaction.
Dorsomorphin inhibits leukocyte adhesion to the vascular wall in vivo, whereas BMP2 is up-regulated in vascular inflammation and stimulates leukocyte adhesion. (A) BMP-antagonist dorsomorphin prevents TNFα-mediated leukocyte adhesion in vivo. Four hours after injection of TNFα (10 ng/g bw IP, n = 5) alone or in combination with dorsomorphin (13 μg/g bw IP, n = 6) leukocyte adhesion was quantified by intravital microscopy of mesenterial venules. (B) BMP2 increased TNFα-induced leukocyte adhesion in vivo. C57BL/6 mice were injected with TNFα alone (10 ng/g bw IP, n = 5) or in combination with recombinant BMP2 (0.33 μg/g bw IP, n = 6). Four hours after injection leukocyte adhesion was quantified by intravital microscopy of mesenterial venules (*P < .05 versus control). (C) TNFα enhances BMP2 RNA expression. HUVECs were exposed to TNFα with indicated concentrations. Twenty-four hours after TNFα-stimulation cells were lysed and BMP2 RNA expression was assessed by quantitative real-time PCR. (D) LPS increased BMP2 protein in vivo as demonstrated by Western blotting. C57BL/6 mice were injected with LPS for 3 days (2.5 μg/g/d). Lungs were minced in RIPA buffer to obtain protein. Lysates were used for Western blotting. GAPDH was used as loading control.
Dorsomorphin inhibits leukocyte adhesion to the vascular wall in vivo, whereas BMP2 is up-regulated in vascular inflammation and stimulates leukocyte adhesion. (A) BMP-antagonist dorsomorphin prevents TNFα-mediated leukocyte adhesion in vivo. Four hours after injection of TNFα (10 ng/g bw IP, n = 5) alone or in combination with dorsomorphin (13 μg/g bw IP, n = 6) leukocyte adhesion was quantified by intravital microscopy of mesenterial venules. (B) BMP2 increased TNFα-induced leukocyte adhesion in vivo. C57BL/6 mice were injected with TNFα alone (10 ng/g bw IP, n = 5) or in combination with recombinant BMP2 (0.33 μg/g bw IP, n = 6). Four hours after injection leukocyte adhesion was quantified by intravital microscopy of mesenterial venules (*P < .05 versus control). (C) TNFα enhances BMP2 RNA expression. HUVECs were exposed to TNFα with indicated concentrations. Twenty-four hours after TNFα-stimulation cells were lysed and BMP2 RNA expression was assessed by quantitative real-time PCR. (D) LPS increased BMP2 protein in vivo as demonstrated by Western blotting. C57BL/6 mice were injected with LPS for 3 days (2.5 μg/g/d). Lungs were minced in RIPA buffer to obtain protein. Lysates were used for Western blotting. GAPDH was used as loading control.
BMPER is down-regulated in vascular inflammation
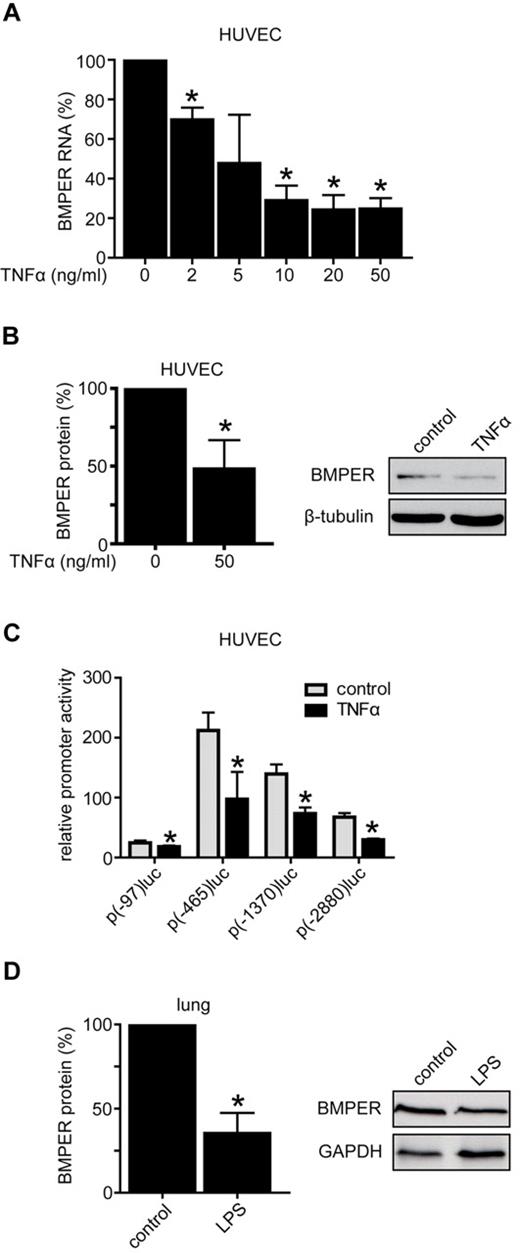
To understand the role of the BMP modulator BMPER in vascular inflammation we investigated the effect of endothelial inflammation on BMPER expression. In the first set of experiments we treated HUVECs with TNFα and found that BMPER expression is down-regulated at the RNA and protein level in a dose-dependent manner using quantitative real-time PCR and Western blotting (Figure 2A-B). To confirm these data in vivo we injected LPS IP into mice and quantified BMPER expression in comparison to control injected animals. Consistent with inflammation-triggered down-regulation of BMPER, protein expression was reduced in LPS treated mice (Figure 2D). To determine whether reduced BMPER levels are a consequence of inhibition of BMPER promoter activity, we performed luciferase reporter assays using different fragments of the BMPER promoter to drive the luciferase reporter.28 HUVECs were incubated with TNFα or control buffer for 24 hours after transfection of the BMPER promoter reporter constructs. Indeed, TNFα significantly reduced the activities of all 4 BMPER promoter fragments, demonstrating a regulation of BMPER expression at the transcriptional level (Figure 2C). Thus, these results unequivocally show that BMPER expression is down-regulated in endothelial inflammation in vitro and in vivo.
BMPER is down-regulated in vascular inflammation. (A) Stimulation of HUVECs with indicated TNFα concentrations for 24 hours down-regulated BMPER RNA expression in a concentration dependent manner as shown by quantitative real-time PCR of at least 3 independent experiments. RNA expression was analyzed using specific primers for human BMPER and human RNA polymerase II (hRPII). (B) BMPER protein expression in HUVECs as shown by Western blotting was also decreased after incubation with TNFα (50ng/mL) for 72 hours. β-tubulin was used as loading control. Expression was quantified by densitometric analysis of 3 independent experiments (right panel: representative Western blot). (C) TNFα inhibited BMPER promoter activity in HUVECs. Cells were transfected with the respective BMPER promoter constructs and luciferase activity was quantified after 24 hours TNFα (50 ng/mL) treatment. Values represent the mean ±SD of 3 independent experiments normalized to β-galactosidase (*P < .05 versus control). (D) LPS decreased BMPER protein levels in vivo as demonstrated by Western blotting. C57BL/6 mice were injected with LPS for 3 days (2.5 μg/g/d). Lungs were minced in RIPA buffer to obtain protein. Lysates were used for Western blotting. GAPDH was used as loading control (right panel: representative Western blot).
BMPER is down-regulated in vascular inflammation. (A) Stimulation of HUVECs with indicated TNFα concentrations for 24 hours down-regulated BMPER RNA expression in a concentration dependent manner as shown by quantitative real-time PCR of at least 3 independent experiments. RNA expression was analyzed using specific primers for human BMPER and human RNA polymerase II (hRPII). (B) BMPER protein expression in HUVECs as shown by Western blotting was also decreased after incubation with TNFα (50ng/mL) for 72 hours. β-tubulin was used as loading control. Expression was quantified by densitometric analysis of 3 independent experiments (right panel: representative Western blot). (C) TNFα inhibited BMPER promoter activity in HUVECs. Cells were transfected with the respective BMPER promoter constructs and luciferase activity was quantified after 24 hours TNFα (50 ng/mL) treatment. Values represent the mean ±SD of 3 independent experiments normalized to β-galactosidase (*P < .05 versus control). (D) LPS decreased BMPER protein levels in vivo as demonstrated by Western blotting. C57BL/6 mice were injected with LPS for 3 days (2.5 μg/g/d). Lungs were minced in RIPA buffer to obtain protein. Lysates were used for Western blotting. GAPDH was used as loading control (right panel: representative Western blot).
Inflammation induced down-regulation of BMPER is mediated by NFkB and KLF2
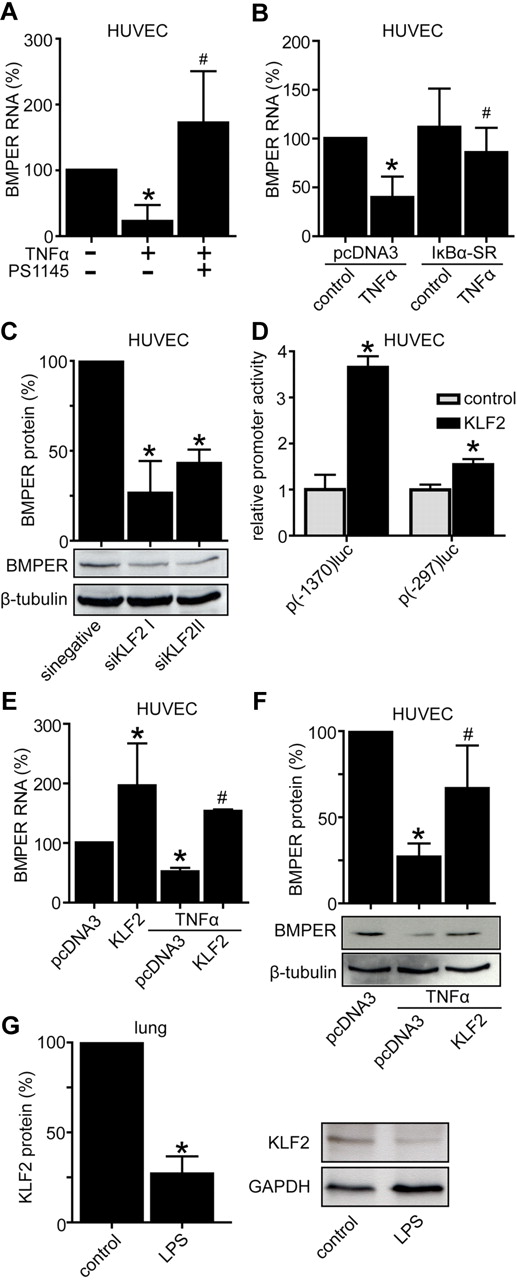
To test if inflammation-induced BMPER down-regulation is NFκB-dependent, we used 2 separate approaches. First, we exposed HUVECs to TNFα in the presence of the NFκB inhibitor PS1145 or control buffer. Indeed, PS1145 prevented down-regulation of BMPER expression induced by TNFα (Figure 3A). Second, to increase the specificity of NFκB inhibition in response to TNFα in endothelial cells we transfected a plasmid coding for IκBα-SR, a superrepressor of NFκB activity. Four hours after transfection endothelial cells were exposed to TNFα for additional 16 hours. As expected from our previous experiment overexpressed IκBα-SR abolished down-regulation of BMPER by TNFα, indicating that regulation of BMPER in inflammation is NFκB dependent (Figure 3B).
Inflammation induced down-regulation of BMPER is mediated by NFκB and KLF2. (A) HUVECs were treated with TNFα (50 ng/mL) alone or in combination with NFκB inhibitor PS1145 (10μM). After 24 hours cells were lysed and RNA was reverse transcribed. BMPER levels were quantified by real-time PCR. (B) NFκB inhibition by overexpression of superrepressor IκBα-SR in endothelial cells abolished down-regulation of BMPER RNA by TNFα. (C) KLF2 knockdown by transfection of specific KLF2 siRNA reduced BMPER protein levels shown by Western blotting with anti–human BMPER antibody. β-tubulin was used as loading control. (D) KLF2 increased BMPER promoter activity in endothelial cells. HUVECs were transiently cotransfected by a KLF2 coding plasmid and indicated BMPER promoter constructs p(-1370)luc or p(-297)luc. After 24 hours cells were lysed and BMPER promoter activity was quantified by reporter assays. Values represent the mean ± SD of 3 independent experiments normalized to β-galactosidase. (E) Overexpression of KLF2 increases BMPER RNA level and attenuates down-regulation of BMPER RNA (E) and protein (F) by TNFα. At day 1 HUVECs were transfected with a KLF2 coding plasmid and after 24 hours stimulated with TNFα (50 ng/μL). Afterward, HUVECs were lysed and RNA was isolated and reverse transcribed. KLF2, BMPER, and hrpII were quantified by real-time PCR. Each experiment was performed at least 3 times with similar results. (G) LPS reduced KLF2 protein expression in vivo. KLF2 protein levels were analyzed in LPS treated lungs and compared with control lungs using Western blotting (*P < .05 versus control; #P < .05 versus pcDNA3 + TNFα).
Inflammation induced down-regulation of BMPER is mediated by NFκB and KLF2. (A) HUVECs were treated with TNFα (50 ng/mL) alone or in combination with NFκB inhibitor PS1145 (10μM). After 24 hours cells were lysed and RNA was reverse transcribed. BMPER levels were quantified by real-time PCR. (B) NFκB inhibition by overexpression of superrepressor IκBα-SR in endothelial cells abolished down-regulation of BMPER RNA by TNFα. (C) KLF2 knockdown by transfection of specific KLF2 siRNA reduced BMPER protein levels shown by Western blotting with anti–human BMPER antibody. β-tubulin was used as loading control. (D) KLF2 increased BMPER promoter activity in endothelial cells. HUVECs were transiently cotransfected by a KLF2 coding plasmid and indicated BMPER promoter constructs p(-1370)luc or p(-297)luc. After 24 hours cells were lysed and BMPER promoter activity was quantified by reporter assays. Values represent the mean ± SD of 3 independent experiments normalized to β-galactosidase. (E) Overexpression of KLF2 increases BMPER RNA level and attenuates down-regulation of BMPER RNA (E) and protein (F) by TNFα. At day 1 HUVECs were transfected with a KLF2 coding plasmid and after 24 hours stimulated with TNFα (50 ng/μL). Afterward, HUVECs were lysed and RNA was isolated and reverse transcribed. KLF2, BMPER, and hrpII were quantified by real-time PCR. Each experiment was performed at least 3 times with similar results. (G) LPS reduced KLF2 protein expression in vivo. KLF2 protein levels were analyzed in LPS treated lungs and compared with control lungs using Western blotting (*P < .05 versus control; #P < .05 versus pcDNA3 + TNFα).
Based on published studies demonstrating down-regulation of KLF2 expression by TNFα in an NFκB-dependent manner and on our earlier BMPER promoter analyses showing that KLFs up-regulate BMPER expression, we aimed to investigate if KLF2 is involved in TNFα-mediated down-regulation of BMPER.2,28 For this purpose we pursued 4 different approaches:
First, we transfected HUVECs with siRNA specific for KLF2 (siKLF2) or nonspecific control siRNA (sinegative) and quantified BMPER protein levels. Knockdown of KLF2 to ∼ 45% of baseline level significantly reduced BMPER expression (Figure 3C, supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Second, we over-expressed KLF2 along with 2 different BMPER promoter constructs in endothelial cells. As expected from our previous findings that KLF15 transactivates the BMPER promoter, KLF2 over-expression also significantly increased BMPER promoter activity in endothelial cells (Figure 3D). Third, we over-expressed KLF2 in HUVECs and quantified endogenous BMPER RNA levels by quantitative real-time PCR. Indeed, KLF2 enhanced BMPER RNA expression, indicating that KLF2 is a positive-acting trans factor of BMPER expression (Figure 3E). Finally, over-expressed KLF2 was able to prevent TNFα-induced down-regulation of BMPER expression as quantified by real-time PCR and Western blotting (Figure 3E-F). Taken together these data clearly demonstrate that reduction of BMPER expression follows the TNFα-NFκB-KLF2-signaling pathway.
Additional support for this finding comes from an in vivo experiment that we analyzed KLF2 protein levels in murine lungs after induction of systemic inflammation by LPS (Figure 3G). LPS significantly reduced KLF2 protein expression in vivo in parallel to BMPER expression supporting the notion that down-regulation of KLF2 in vascular inflammation leads to reduced BMPER expression in vitro and in vivo (Figure 3). Together, these data identify BMPER as a downstream target of KLF2 in response to inflammatory stimuli.
Loss of BMPER decreases eNOS levels and increases the expression of endothelial adhesion molecules ICAM-1 and VCAM-1
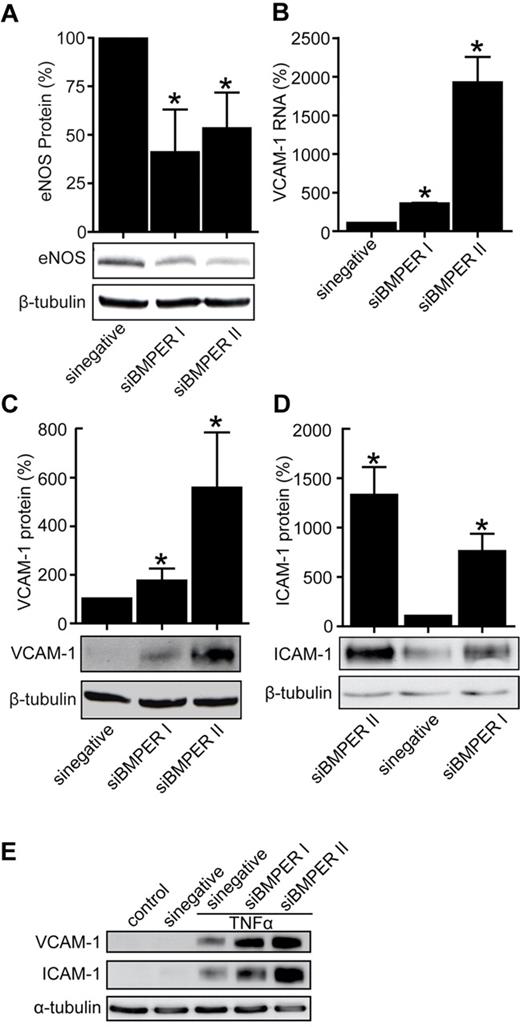
Based on our data that BMPER is down-regulated in endothelial cells in the setting of vascular inflammation, we hypothesized that loss of BMPER may reflect a novel mechanism in endothelial cells to induce inflammation. We postulated that BMPER is involved in the control of regulators of inflammation such as eNOS or the adhesion molecules ICAM-1 and VCAM-1. Supporting this hypothesis, siRNA-based knockdown of BMPER in HUVECs resulted in reduced eNOS and increased ICAM-1 and VCAM-1 expression (Figure 4A-D). These data demonstrate that loss of BMPER has similar proinflammatory consequences in endothelial cells as exposure to TNFα and that BMPER and TNFα may therefore act along the same pathway.
Loss of BMPER decreases eNOS levels and increases the expression of endothelial adhesion molecules ICAM-1 and VCAM-1. (A) SiRNA-based BMPER knockdown reduces eNOS protein levels. HUVECs were transfected with either of 2 specific BMPER siRNAs. After 48 hours, cell lysates were used for Western blotting with an anti–human eNOS (A) and an anti-BMPER antibody (supplemental Figure 2). β-tubulin was used as loading control. BMPER knockdown enhanced VCAM-1 RNA (B) shown real-time PCR, VCAM-1 protein (C), and ICAM-1 (D) expression levels as demonstrated by immunoblotting (*P < .05 versus sinegative). (E) ICAM-1 and VCAM-1 expression is enhanced in HUVECs with TNFα treatment after BMPER knockdown. HUVECs were transfected with scrambled or BMPER specific siRNA and after 36 hours cells were exposed to TNFα for additional 6 hours. Cells were lysed and lysates were used for Western blotting.
Loss of BMPER decreases eNOS levels and increases the expression of endothelial adhesion molecules ICAM-1 and VCAM-1. (A) SiRNA-based BMPER knockdown reduces eNOS protein levels. HUVECs were transfected with either of 2 specific BMPER siRNAs. After 48 hours, cell lysates were used for Western blotting with an anti–human eNOS (A) and an anti-BMPER antibody (supplemental Figure 2). β-tubulin was used as loading control. BMPER knockdown enhanced VCAM-1 RNA (B) shown real-time PCR, VCAM-1 protein (C), and ICAM-1 (D) expression levels as demonstrated by immunoblotting (*P < .05 versus sinegative). (E) ICAM-1 and VCAM-1 expression is enhanced in HUVECs with TNFα treatment after BMPER knockdown. HUVECs were transfected with scrambled or BMPER specific siRNA and after 36 hours cells were exposed to TNFα for additional 6 hours. Cells were lysed and lysates were used for Western blotting.
To assess if BMPER knockdown promotes expression of adhesion molecules in the presence of proinflammatory cytokines, endothelial cells were transfected with scrambled siRNA or 2 different BMPER siRNAs. Twenty-four hours post transfection cells were exposed to TNFα for 10 hours. TNFα resulted in enhanced induction of VCAM-1 and ICAM-1 in cells with BMPER knocked down demonstrating that loss of BMPER enhances inflammatory effects of TNFα (Figure 4E).
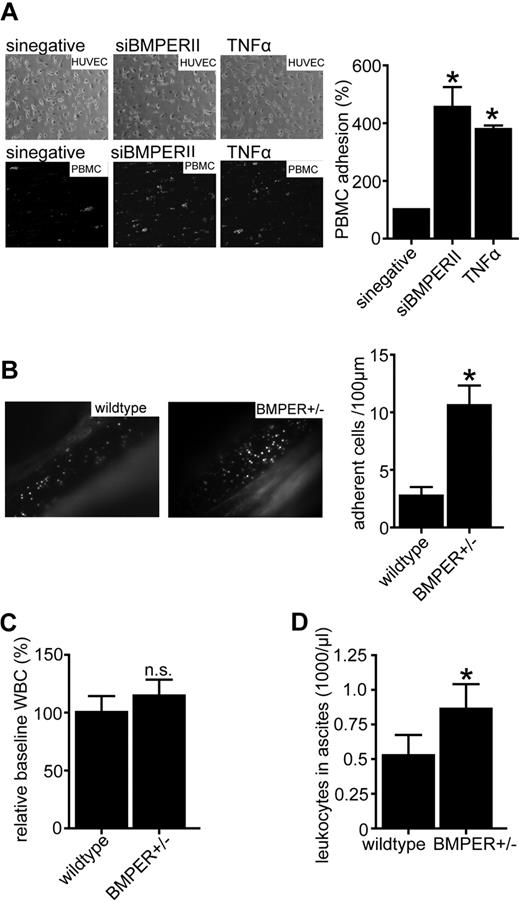
Loss of BMPER induces leukocyte adhesion and extravasation
Based on these findings we tested if loss of BMPER has similar functional consequences in endothelial cell inflammation as addition of TNFα. To address this question we investigated endothelial cell-leukocyte interactions in the flow chamber. Mononuclear cells (PBMCs) were allowed to attach to endothelial cells in which BMPER had been silenced by specific siRNA (siBMPERII) compared with control siRNA (sinegative). As expected from the previous set of experiments, depletion of BMPER led to a significant increase in leukocyte adhesion suggesting proinflammatory consequences of reduced BMPER levels (Figure 5A). These ex vivo flow chamber results were confirmed in vivo using intravital microscopy. At baseline there were no differences in leukocyte adhesion in BMPER± deficient mice compared with control (data not shown). Four hours after injection of TNFα leukocyte adhesion was increased in heterozygous BMPER mice compared with wild-type littermates (Figure 5B). Having found that BMPER deficiency increases leukocyte adhesion to the vascular wall, we next asked whether loss of BMPER would also result in increased neutrophil extravasation from the vascular bed. To address this question we used the mouse model of thioglycollate-induced peritonitis. At baseline there was no significant difference in peripheral white blood cell counts between BMPER± and wild-type control mice although a trend toward increased white blood cell counts was detectable when BMPER was reduced (Figure 5C). But, after a 4-hour challenge with thioglycollate significantly more white blood cells had extravaded into the peritoneum of BMPER± mice compared with wild-type controls (Figure 5D). In conclusion, these findings demonstrate that loss of BMPER has proinflammatory consequences in terms of endothelial adhesion molecule expression as well as leukocyte-endothelium interaction and leukocyte extravasation in vitro and in vivo.
Loss of BMPER induces leukocyte adhesion and extravasation. (A) Loss of BMPER increases dynamic adhesion of PBMCs on the endothelial surface in flow chamber experiments. Endothelial cells were transfected with siBMPERII or scrambled siRNA. TNFα-stimulated endothelial cells served as a positive control. PMA (phorbol-12 myristate-13acetate) activated PBMCs attached on the endothelial surface (left panel: representative images; top: phase contrast, bottom fluorescence). (B) Intravital microscopy of mesenterial veins revealed more leukocyte adhesion in BMPER± mice (n = 6) compared with control (n = 5). Mice were treated with TNFα (10ng/g bw IP) 4 hours before intravital microscopy. Leukocytes and thrombocytes were labeled with Rhodamin 6G (*P < .05). (C) At baseline levels, there was no difference in peripheral white blood cell (WBC) counts between BMPER± and wild-type control mice. (D) Leukocyte extravasation is increased in thioglycollate-induced peritonitis in BMPER ±mice. At 4 hours after challenge with 2 mL of 4% thioglycollate IP BMPER ±mice (n = 5) showed more extravasation of leukocytes to inflamed peritoneum compared with control mice (n = 5). Leukocytes were collected by peritoneal lavage and total leukocyte count was analyzed by hemocytometer (*P < .05 versus WT control).
Loss of BMPER induces leukocyte adhesion and extravasation. (A) Loss of BMPER increases dynamic adhesion of PBMCs on the endothelial surface in flow chamber experiments. Endothelial cells were transfected with siBMPERII or scrambled siRNA. TNFα-stimulated endothelial cells served as a positive control. PMA (phorbol-12 myristate-13acetate) activated PBMCs attached on the endothelial surface (left panel: representative images; top: phase contrast, bottom fluorescence). (B) Intravital microscopy of mesenterial veins revealed more leukocyte adhesion in BMPER± mice (n = 6) compared with control (n = 5). Mice were treated with TNFα (10ng/g bw IP) 4 hours before intravital microscopy. Leukocytes and thrombocytes were labeled with Rhodamin 6G (*P < .05). (C) At baseline levels, there was no difference in peripheral white blood cell (WBC) counts between BMPER± and wild-type control mice. (D) Leukocyte extravasation is increased in thioglycollate-induced peritonitis in BMPER ±mice. At 4 hours after challenge with 2 mL of 4% thioglycollate IP BMPER ±mice (n = 5) showed more extravasation of leukocytes to inflamed peritoneum compared with control mice (n = 5). Leukocytes were collected by peritoneal lavage and total leukocyte count was analyzed by hemocytometer (*P < .05 versus WT control).
BMPER attenuates leukocyte adhesion in vitro and in vivo as a consequence of increased eNOS levels and decreased ICAM-1 and VCAM-1 expression
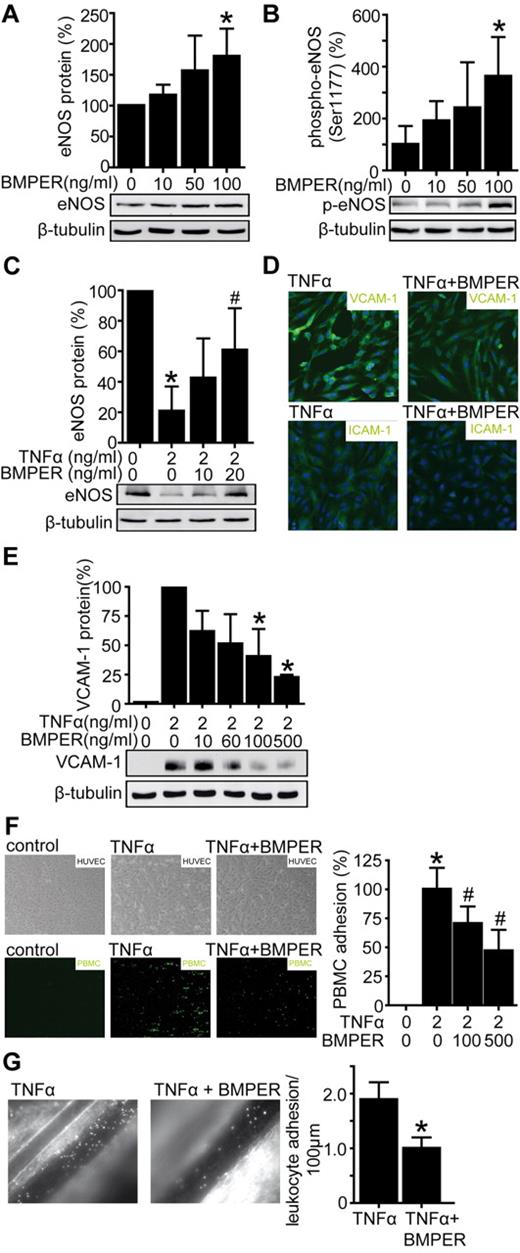
If loss of BMPER has proinflammatory consequences, we next asked if BMPER may exert protective and anti-inflammatory functions in endothelium when present in excessive amounts. To answer this question we exposed HUVECs to BMPER or control buffer and assessed eNOS protein levels by Western blotting (Figure 6A). Indeed BMPER increased eNOS protein levels in a concentration-dependent manner in endothelial cells after 24 hours.
BMPER attenuates leukocyte adhesion in vitro and in vivo as a consequence of increased eNOS levels and decreased ICAM-1 and VCAM-1 expression. (A) HUVECs were exposed to recombinant BMPER and cell lysates were used for Western blotting with a human anti-eNOS antibody. β-tubulin served as a loading control. (B) BMPER enhances eNOS activity by phosphorylation at the serin 1177. HUVECs were exposed to indicated BMPER concentrations for 30 minutes and cell lysates were used for Western blotting. (C) BMPER accelerates the recovery of eNOS levels in cells exposed to TNFα. HUVECs were treated with TNFα (2 ng/mL) for 12 hours and afterward cells were exposed to cell culture medium in the presence of different BMPER concentrations. (D) BMPER inhibits TNFα induced ICAM-1 and VCAM-1 expression in HUVECs demonstrated by immuncytochemistry (green fluorescence). HUVECs were stimulated for 12 hours with TNFα (2 ng/mL) alone or in combination with human recombinant BMPER (500 ng/mL). DAPI (blue) was used for nuclear staining. (D) BMPER inhibits TNFα induced VCAM-1 protein quantified by Western blotting with anti–human VCAM-1 antibody (*P < .05 versus control). HUVECs were stimulated with TNFα (2 ng/mL) alone or in combination with human recombinant BMPER (doses as indicated). Cell lysates were used for Western blotting and β-tubulin served as loading control. (E) BMPER inhibits leukocyte adhesion to endothelial cells under dynamic flow. HUVECs were incubated with TNFα (2 ng/mL) alone or in combination with BMPER for 12 hours (doses as indicated). PMA-activated PBMCs attached on the endothelial surface (*P < .05 versus control; #P < .05 versus TNFα). (F) BMPER prevents TNFα-mediated leukocyte adhesion in C57BL/6 mice. Four hours before intravital microscopy TNFα (10 ng/g bw IP, n = 5) was injected alone or in combination with recombinant BMPER (0.33 μg/g bw, retro-orbital, n = 6). BMPER attenuates TNFα-induced leukocyte adhesion, confirming the potent anti-inflammatory effects of BMPER (*P < .05 versus TNFα).
BMPER attenuates leukocyte adhesion in vitro and in vivo as a consequence of increased eNOS levels and decreased ICAM-1 and VCAM-1 expression. (A) HUVECs were exposed to recombinant BMPER and cell lysates were used for Western blotting with a human anti-eNOS antibody. β-tubulin served as a loading control. (B) BMPER enhances eNOS activity by phosphorylation at the serin 1177. HUVECs were exposed to indicated BMPER concentrations for 30 minutes and cell lysates were used for Western blotting. (C) BMPER accelerates the recovery of eNOS levels in cells exposed to TNFα. HUVECs were treated with TNFα (2 ng/mL) for 12 hours and afterward cells were exposed to cell culture medium in the presence of different BMPER concentrations. (D) BMPER inhibits TNFα induced ICAM-1 and VCAM-1 expression in HUVECs demonstrated by immuncytochemistry (green fluorescence). HUVECs were stimulated for 12 hours with TNFα (2 ng/mL) alone or in combination with human recombinant BMPER (500 ng/mL). DAPI (blue) was used for nuclear staining. (D) BMPER inhibits TNFα induced VCAM-1 protein quantified by Western blotting with anti–human VCAM-1 antibody (*P < .05 versus control). HUVECs were stimulated with TNFα (2 ng/mL) alone or in combination with human recombinant BMPER (doses as indicated). Cell lysates were used for Western blotting and β-tubulin served as loading control. (E) BMPER inhibits leukocyte adhesion to endothelial cells under dynamic flow. HUVECs were incubated with TNFα (2 ng/mL) alone or in combination with BMPER for 12 hours (doses as indicated). PMA-activated PBMCs attached on the endothelial surface (*P < .05 versus control; #P < .05 versus TNFα). (F) BMPER prevents TNFα-mediated leukocyte adhesion in C57BL/6 mice. Four hours before intravital microscopy TNFα (10 ng/g bw IP, n = 5) was injected alone or in combination with recombinant BMPER (0.33 μg/g bw, retro-orbital, n = 6). BMPER attenuates TNFα-induced leukocyte adhesion, confirming the potent anti-inflammatory effects of BMPER (*P < .05 versus TNFα).
Next, we focused on the short-term effects of BMPER and assessed effects of BMPER on eNOS phosphorylation, which has been shown to regulate eNOS activity.35,36 HUVECs were exposed to BMPER for 30 minutes with indicated concentrations and eNOS phosphorylation (Ser1177) was significantly higher compared with control, indicating that BMPER increases eNOS activity (Figure 6B). Next, we aimed to investigate if BMPER exerts protective effects in endothelial cells under inflammatory conditions. To induce inflammation in vitro, HUVECs were treated with TNFα for 12 hours. Cell culture medium was changed and cells were allowed to recover with or without additional BMPER (Figure 6C). As expected eNOS levels were reduced after TNFα exposure. In line with a beneficial role of BMPER in endothelial cell regeneration, eNOS levels were higher when cells were subsequently incubated with BMPER instead of control. To further characterize anti-inflammatory effects of BMPER, HUVECs were exposed to TNFα alone or in combination with BMPER. Indeed, BMPER protected endothelial cells in a dose-dependent manner from TNFα-induced expression of proinflammatory ICAM-1 and VCAM-1 as shown by immunocytochemistry and Western blotting (Figure 6D-E). Thus, BMPER exerts protective anti-inflammatory effects in endothelial cells. To investigate the functional consequences of decreased adhesion molecule expression by BMPER, we induced endothelial cell dysfunction in HUVECs using TNFα with or without the addition of BMPER. Indeed, increasing concentrations of BMPER protected endothelial cells from adhesion of leukocytes in these flow chamber experiments (Figure 6F). Based on this conclusive set of in vitro and ex vivo experiments, we aimed to confirm the endothelium protective characteristics of BMPER in vivo. Endothelial inflammation was induced in C57BL/6 mice by injection of TNFα IP. Animals were then subjected to IV injections with BMPER protein or control, and leukocyte adhesion to the endothelium was quantified by intravital microscopy. As shown in Figure 6G, treatment with recombinant BMPER attenuated leukocyte adhesion significantly in vivo. Altogether, our data unequivocally demonstrates that BMPER exerts anti-inflammatory functions on the endothelium in vitro and in vivo.
Loss of BMPER causes endothelial inflammation involving a BMP-dependent mechanism
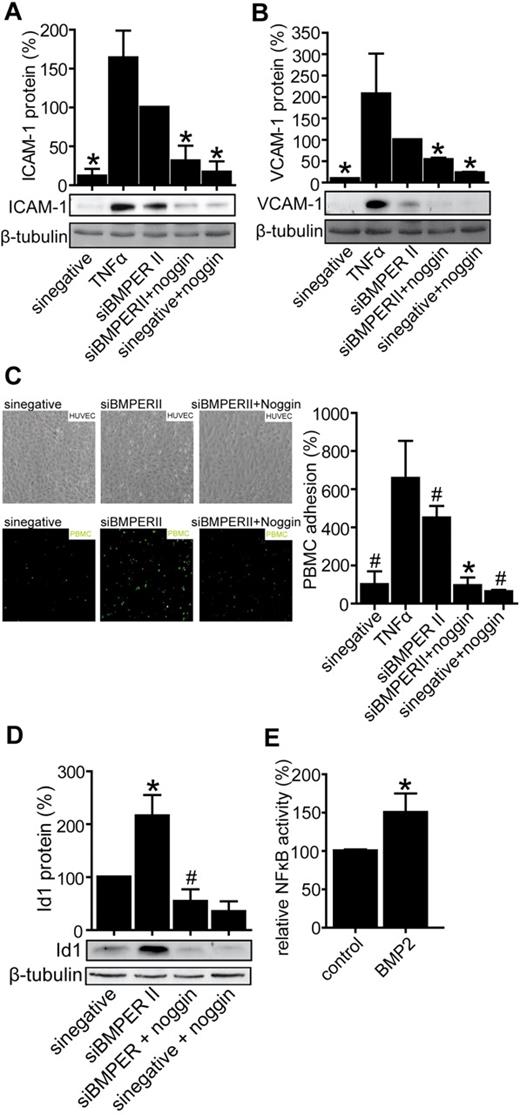
Previous studies have elegantly shown that BMPER inhibits BMPs most probably by direct interaction with BMPs and inhibition of BMP binding to their receptor.24,26 Based on this data, here we hypothesized that BMPER exerts its anti-inflammatory effects by inhibiting the binding of proinflammatory BMP2 to its receptor. Vice versa, loss of BMPER may cause inflammation by allowing enhanced BMP activity. Thus, we aimed to test if BMPER effects are dependent on the BMP pathway. To inhibit BMP pathway activity, we used for further experiments BMP antagonist noggin, a homodimer, which is highly conserved and antagonizes BMP activity by binding BMPs and blocking BMP epitopes that are needed for interaction with BMP receptors.37 Indeed, under these conditions the proinflammatory effects in BMPER-depleted cells were prevented as reflected by decreased ICAM-1 and VCAM-1 expression in vitro as well as reduced leukocyte-endothelial interaction in flow chamber experiments (Figure 7). As shown in Figure 7A and B, the induction of adhesion protein expression by loss of BMPER was rescued in the presence of noggin. Accordingly leukocyte-endothelial cell interaction, which was enhanced in the absence of BMPER, was reduced when BMP activity was blunted by noggin (Figure 7C).
Loss of BMPER causes endothelial inflammation involving a BMP-dependent mechanism. The BMP antagonist noggin attenuates expression of adhesion molecules in vitro reflected by ICAM-1 protein (A) and VCAM-1 protein (B) after BMPER knockdown. HUVECs were transfected with control siRNA or siBMPERII. Endothelial cell medium was changed and cells were treated with or without noggin (500 ng/mL). HUVECs transfected with control siRNA were exposed to TNFα and served as a positive control. Cells were lysed and used for Western blotting with anti–human ICAM-1 antibody (A), anti–human VCAM-1 antibody (B) or anti–human Id1 antibody (D). β-tubulin was used as loading control. (C) Noggin prevents leukocyte adhesion on endothelial cells transfected with BMPER siRNA in flow chamber experiments. Endothelial cells were transfected with siBMPERII or control siRNA and were exposed to noggin as indicated. TNFα stimulated endothelial cells served as a positive control. PMA activated PBMCs attached on the endothelial surface. (D) Noggin diminishes up-regulation of Id1 protein expression after BMPER knockdown. (E) BMP2 increased NFκB activity in NIH3T3 fibroblasts. NIH3T3 cells were transfected with the NFκB-responsive luciferase construct and exposed to recombinant BMP2 (100 ng/mL) for 24 hours. Afterward cells were lysed and NFκB activity was quantified in reporter assay and normalized to β-galactosidase activity (*P < .05 versus siBMPER; #P < .05 versus sinegative).
Loss of BMPER causes endothelial inflammation involving a BMP-dependent mechanism. The BMP antagonist noggin attenuates expression of adhesion molecules in vitro reflected by ICAM-1 protein (A) and VCAM-1 protein (B) after BMPER knockdown. HUVECs were transfected with control siRNA or siBMPERII. Endothelial cell medium was changed and cells were treated with or without noggin (500 ng/mL). HUVECs transfected with control siRNA were exposed to TNFα and served as a positive control. Cells were lysed and used for Western blotting with anti–human ICAM-1 antibody (A), anti–human VCAM-1 antibody (B) or anti–human Id1 antibody (D). β-tubulin was used as loading control. (C) Noggin prevents leukocyte adhesion on endothelial cells transfected with BMPER siRNA in flow chamber experiments. Endothelial cells were transfected with siBMPERII or control siRNA and were exposed to noggin as indicated. TNFα stimulated endothelial cells served as a positive control. PMA activated PBMCs attached on the endothelial surface. (D) Noggin diminishes up-regulation of Id1 protein expression after BMPER knockdown. (E) BMP2 increased NFκB activity in NIH3T3 fibroblasts. NIH3T3 cells were transfected with the NFκB-responsive luciferase construct and exposed to recombinant BMP2 (100 ng/mL) for 24 hours. Afterward cells were lysed and NFκB activity was quantified in reporter assay and normalized to β-galactosidase activity (*P < .05 versus siBMPER; #P < .05 versus sinegative).
To follow this hypothesis in more detail we analyzed expression of inhibitor of differentiation (Id1) protein, an established marker of BMP activity under same conditions.38-40 Id1 is a negative regulator of basic-helix-loop-helix (bHLH) transcription factors and is induced by BMPs in endothelial cells. Along the same line of argument we found that Id1 was increased after BMPER depletion in HUVECs and its expression was normalized by the addition of noggin (Figure 7D). These results indicate that inflammation caused by loss of BMPER is a consequence of increased BMP activity. To understand the molecular mechanism, how increased BMP activity leads to endothelial inflammation, we set out to examine the effect of BMPs on NFκB activity, which plays a pivotal role in inflammatory response. BMP2-enhanced NFκB activity in reporter assays (Figure 7E) reveal that loss of BMPER leads to inflammation because of enhanced BMP-induced NFκB activity.
Discussion
In the present work, we identify the BMP modulator BMPER as a new protective regulator of endothelial inflammation. Endothelial inflammation is a key pathophysiologic feature of cardiovascular diseases such as sepsis, arterial hypertension, and atherosclerosis.3,41-43 Moreover, the endothelium is critically involved in the biologic response to inflammation.1
BMP2 and BMP4 have been reported to induce proinflammatory vascular responses. BMP2 leads to endothelial dysfunction reflected by increased monocyte adhesion on the endothelial surface and to reduced relaxation of arteries in response to acetylcholine. Similarly, BMP4 promotes the expression of ICAM-1 and induces leukocyte adhesion in a reactive oxidative stress (ROS)–dependent manner.12,15,16 Chronic infusion of BMP4 leads to endothelial dysfunction and finally arterial hypertension in mice.18,44 Taking this into consideration, we asked if antagonizing BMP activity may inhibit vascular inflammation. In fact, when we treated mice with the BMP antagonist dorsomorphin we observed reduced leukocyte adhesion to the vascular wall reflecting reduced endothelial inflammation (Figure 1A). Thus, a tight control of BMP activity appears crucial for endothelial cell homeostasis. BMP activity is regulated at multiple levels, but predominantly by extracellular BMP modulators that have been shown to be differentially expressed in endothelial dysfunction.45
Our group has identified the novel BMP modulator BMPER, which is necessary for fine-tuning BMP activity in angiogenesis and vascular development.14,19,21 BMPER is a member of the BMP family and interacts directly with BMP2, BMP4, and BMP6.14 It has been shown by others that BMPER inhibits the receptor binding site of BMP2.25 In genetic models, BMPER behaves as a context-dependent BMP modulator during organogenesis.23,34 For example, in lung development BMPER functions as a BMP antagonist.34 The fact that BMPs are important in endothelial inflammation guided us to characterize the expression and function of BMPER in this context. We found that, in contrast to BMP2, BMPER is down-regulated on inflammatory stimuli and may thus belong to the group of endothelial protective factors such as KLF2 (Figure 2-3). KLF2 is considered to be a key transcriptional regulator of vascular homeostasis because its complete absence results in abnormal blood vessel formation and embryonic death and its hemicygous deficiency results in increased atherosclerosis in apolipoprotein E-deficient mice.46-48 KLF2 overexpression up-regulates eNOS, inhibits NFκB activity, and strongly suppresses the expression of proinflammatory cytokines, chemokines, and adhesion molecules such as IL-6, IL-8, IL-15, MCP-1, E-selectin, and VCAM-1. Therefore KLF2 mediates an “anti-adhesive” endothelial phenotype.3-5 Notably, KLF2 and BMPER follow the same regulatory pattern involving NFκB and up-regulation by statins.2,3,27 Our observation that BMPER is positively regulated by KLF2 is concordant with microarray data reported by others.3 Together, these findings suggest that KLF2 and BMPER act along the same pathway, and that BMPER may confer at least some of the protective functions of KLF2.
Supporting this notion we found that loss of BMPER induces endothelial inflammation in vitro and in vivo as reflected by decreased eNOS levels. Reduced eNOS levels are known to cause endothelial dysfunction at the vascular wall and the dysfunctional endothelium expresses adhesion molecules such as VCAM-1 and ICAM-1 leading to a proinflammatory endothelial phenotype reflected by increased leukocyte endothelial interaction.8,9 Concordantly to these studies, loss of BMPER increased expression of adhesion molecules and leukocyte adhesion to vascular endothelium as well as facilitates monocyte emigration from the vascular bed (Figures 4–5). Vice versa, eNOS/NO attenuates inflammatory response via inhibition of endothelial adhesion molecules.6 Consistently BMPER increases eNOS activity and expression levels and consequently reduces the expression of adhesion molecules and inhibits leukocyte adhesion in several ex vivo and in vivo assays (Figure 6).
Together these results led us to the conclusion that BMPER has anti-inflammatory properties.
Our findings concerning the BMP modulator BMPER are in agreement with reports that BMP antagonists modulate vascular inflammation.45,49 For example, for Matrix Gla protein (MGP) it was shown that both loss or gain of function results in inhibition of vascular inflammation.49 Interestingly, in MGP−/−; Apoe−/− mice BMPER is strongly up-regulated as reported by Yao et al. Our results reported here, help to explain the surprising finding that loss of MGP has anti-inflammatory consequences. Finally, we have shown that anti-inflammatory and protective effects of BMPER are indeed dependent on BMP signaling, which controls inflammatory cell response by NFκB (Figure 7A-E).
Taken together, we identify BMPER for the first time as a modulator of vascular inflammation and postulate a chain of events with BMPER in a central position. Furthermore, our findings support the notion that fine-tuned BMP signaling is essential to maintain vascular homeostasis and that BMP antagonism may be a future therapeutic strategy in vascular inflammation.
To transfer our results in human disease, we aim to determine the role of BMP activity in sepsis in humans. Therefore prospective studies will analyze expression levels of BMPs and BMP modulators such as BMPER as potential biomarkers in septic patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bianca Engert and Stefanie Wintrich for their outstanding technical assistance; Katharina Zeschky for excellent support with the flow chamber set up and the peritonitis model; and Marco Idzko (University of Freiburg, Department of Pneumology, Freiburg) provided essential support with the in vivo model of LPS induced inflammation.
This work was supported by Deutsche Forschungsgemeinschaft SFB-TR23 (A1) and Mo973/6-1 to M.M. and by intramural funds to T.H. and M.M.
Authorship
Contribution: T.H. designed and performed research, analyzed data, and wrote the paper; R.R. performed research and analyzed data; E.K. and L.G. performed research; J.H. and S.G. analyzed data; D.D. contributed analytical tools; C.P. wrote the paper; and M.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Moser, MD, University of Freiburg, Dept Medicine III, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: martin.moser@uniklinik-freiburg.de.