Abstract
Relapsed or refractory (rel/ref) classical Hodgkin lymphoma (cHL) remains a clinical challenge, with limited effective treatment options available after stem cell transplantation. In a multicenter phase 2 study, the efficacy of lenalidomide in rel/ref cHL patients was evaluated at a dose of 25 mg/d on days 1-21 of a 28-day cycle. Patients remained on lenalidomide until disease progression or an unacceptable adverse event (AE) occurred. Thirty-eight cHL patients were enrolled with a median of 4 (range, 2-9) prior therapies; 87% had undergone prior stem cell transplantation and 55% of patients did not respond to their last prior therapy. Of 36 evaluable patients, responses were 1 complete remission (CR), 6 partial remissions (PRs), and 5 patients with stable disease (SD) for ≥ 6 months resulting in an International Working Committee (IWC) objective overall response rate (ORR) of 19% and a cytostatic ORR of 33%. Decreased chemokine (CCL17 and CCL22) plasma levels at 2 weeks were associated with a subsequent response. The treatment was well tolerated, and the most common grade 3/4 AEs were neutropenia (47%), anemia (29%), and thrombocytopenia (18%). Four patients discontinued lenalidomide because of rash, elevated transaminases/bilirubin, and cytopenias. We provide preliminary evidence of lenalidomide's activity in patients with rel/ref cHL, and therefore exploration of lenalidomide in combination with other active agents is warranted. This trial is registered at www.ClinicalTrials.gov as NCT00540007.
Introduction
Based primarily on clinical advances optimizing systemic cytotoxic chemotherapy over the last several decades, patients with newly diagnosed classical Hodgkin lymphoma (cHL) have an excellent prognosis after frontline therapy, which results in an ∼ 3- to 5-year progression-free survival (PFS) rate of 75%.1 The standard approach for relapsed cHL patients, salvage chemotherapy followed by autologous stem cell transplantation (ASCT), results in long-term disease-free survival for 50%-60% of patients.2 However, cHL patients who have refractory disease after salvage chemotherapy, who relapse after ASCT, or those who are not candidates for ASCT remain a clinical challenge, with limited effective treatments and a median overall survival (OS) of ∼ 2-3 years. Although there are several promising agents currently under investigation in cHL,3 novel therapies that are well tolerated with minimal long-term complications are needed for these patients.
Lenalidomide is a Food and Drug Administration (FDA)–approved antineoplastic drug for relapsed/refractory (rel/ref) multiple myeloma and myelodysplastic syndrome harboring a 5q− chromosomal abnormality. Extensive clinical investigations indicate both a manageable toxicity profile and promising clinical activity of lenalidomide in several B-cell malignancies.4,5 Initial activity in rel/ref multiple myeloma,6 chronic lymphocytic leukemia,7 and indolent8 or aggressive9 non-Hodgkin lymphoma was observed using a daily oral dose of 25 mg/d on days 1-21 of a 28-day cycle. This dose was established in an initial phase 1 study in multiple myeloma, and has been used in a large number of B-cell malignancy patients, with the most frequent adverse events (AEs) consisting of neutropenia and thrombocytopenia. Therefore, lenalidomide appears to be a well-tolerated novel agent with clinical activity in patients with a wide spectrum of B-cell malignancies.
Lenalidomide has a diverse set of potential mechanisms of action, and is proposed to directly induce the death of malignant B cells and to indirectly affect the tumor microenvironment, having immunomodulatory and anti-angiogenic properties.10 Recent advances have identified the microenvironment that surrounds the Hodgkin-Reed Sternberg (HRS) cell as being critical for cHL pathogenesis. Specific alterations that promote HRS survival and may have prognostic significance include cytokine skewing toward a Th2 cytokine profile, production of suppressive cytokines such as IL-10 and TGF-β, and production of chemokines including CCL17 and CCL22.11 These effects are accompanied by an altered immune cell infiltrate that includes regulatory T-cells but contains fewer CD8+ T and natural killer cytotoxic lymphocytes with concurrent functional deficiencies. Most recently, lymph node infiltration by CD68+ macrophages has been identified as a poor prognostic indicator in cHL.12 This pathogenic HRS-supporting network of cells, cytokines, and altered immunity provides novel pathways for therapeutic intervention. Because lenalidomide has been shown to modify many of the changes favorable to the HRS microenvironment and appears active in other B-cell malignancies, we postulated that lenalidomide may have clinical activity in cHL. This hypothesis was tested in an investigator-initiated, open-label, single-arm, multicenter, phase 2 study of single-agent lenalidomide in rel/ref cHL patients.
Methods
Patient eligibility
Patients ≥ 18 years of age with a World Health Organization (WHO) diagnosis of cHL13 that was rel/ref after at least 1 prior systemic therapy were eligible for the study. Patients must have relapsed after ASCT or have been deemed not suitable candidates for ASCT; prior allogeneic stem cell transplantation was allowed. Refractory disease was defined as a failure to achieve a complete remission (CR) or partial remission (PR) to the last prior treatment regimen. Additional inclusion criteria included: Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, measurable disease ≥ 1 cm, adequate renal (serum creatinine ≤ 1.5 times the upper limit of normal), hepatic (bilirubin ≤ 2.0 mg/dL and aspartate aminotransferase/alanine aminotransferase ≤ 3 times the upper limit of normal), and hematopoietic (absolute neutrophil count [ANC] ≥ 1000/μL and platelets ≥ 50 000/μL) function. Patients who were pregnant or nursing were not eligible. The study (NCT00540007) was conducted in accordance with the Declaration of Helsinki, approved by the institutional review boards of all participating institutions, and all patients provided written informed consent.
Study design and treatment
This was an open-label, prospective, single-arm, multicenter, phase 2 study of oral lenalidomide initiated at a dose of 25 mg/d on days 1-21 of a 28-day cycle. Lenalidomide was provided by the manufacturer (Celgene). Dose reductions were allowed for AEs in 5-mg increments to a lowest allowable dose of 5 mg daily. No dose reescalation was permitted, and dose modifications were defined prospectively in the protocol. The following were required before initiating a new cycle of lenalidomide: ANC ≥ 1000/μL; platelet count ≥ 50 000/μL; lenalidomide-related rash, allergic reaction, or cardiac arrhythmia resolved to ≤ grade 1; and other lenalidomide-related AEs resolved to ≤ grade 2 severity. If these criteria were not met, lenalidomide was delayed for 1 week and the patient reassessed; once these criteria were met, the patient was restarted on lenalidomide with a 1 dose level reduction. If these criteria occurred at the 5-mg daily dose, lenalidomide therapy was discontinued. Selected AEs also led to immediate discontinuation of lenalidomide therapy, including desquamating rash (any grade), grade 4 rash, ≥ grade 3 erythema multiforme, ≥ grade 3 cardiac toxicity, grade 4 lenalidomide-related anemia, grade 4 allergic reaction, or intrauterine pregnancy. Hematologic AE dose modifications also included holding lenalidomide for febrile neutropenia until ANC recovered to ≥ 1500/μL. Patients continued to receive lenalidomide therapy until an unacceptable AE occurred or the disease progressed. Growth factors were not administered routinely, but were allowed as clinically indicated for neutropenia or anemia. Patients used aspirin (81 or 325 mg) daily as prophylactic anticoagulation, whereas those deemed to be at high risk of deep venous thrombosis by the treating physician were prescribed warfarin or low-molecular-weight heparin. All AEs were evaluated and coded using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0.
Response criteria and statistical analysis
Responses were assessed using International Working Committee (IWC) criteria,14 and restaging computed tomography (CT) or positron emission tomography–CT were performed after cycles 2, 4, 6, and then every 3 cycles. At Washington University School of Medicine, all response assessments were performed by a blinded image response assessment team (led by M.J.S.). For all other sites, response assessments were performed by the site principal investigator. The primary end point of the study was overall response rate (ORR = CR + PR) defined by the IWC criteria. In addition, patients who obtained prolonged stable disease (SD) of ≥ 6 months were combined with CR and PR to calculate the cytostatic ORR (CR + PR + SD ≥ 6 months). Secondary end points included duration of remission, time-to-treatment failure, PFS (time from enrollment to progression or death), and OS. Simon's optimal 2-stage design was used with a sample size (n = 12 in first stage, n = 35 evaluable patients total) based on an α of 0.10 and a power of 0.9 to detect the target ORR of ≥ 30% over an unacceptable ORR of ≤ 10%. Three patients did not receive at least 2 cycles of lenalidomide (2 because of AEs, 1 because of rapidly progressive disease) and, therefore, additional patients were accrued to a total of 38. For efficacy analysis, results are reported for the entire cohort (n = 38) and for those patients evaluable per protocol definitions, including the patient with rapidly progressive disease during cycle 1 (n = 36). ORR was calculated along with 95% confidence interval (95% CI), and Kaplan-Meier analysis was used to estimate PFS and OS. The assessment of baseline clinical characteristics of disease response was assessed by comparison of proportions using the Fisher exact test (categorical values) or the Wilcoxon 2-sample test (continuous variables).
Plasma cytokine assessment
Plasma was obtained before and during lenalidomide therapy. Plasma was immediately processed, aliquoted, and frozen at −70°C for subsequent analysis of cytokines in batch. ELISA assays were performed in duplicate for CCL17/TARC and CCL22/MDC (R&D Systems) following the manufacturer's instructions. Graphical analysis using box-whisker (Tukey) plots was performed using Prism Version 5.00 software (GraphPad).
Results
Patient characteristics and treatment with lenalidomide
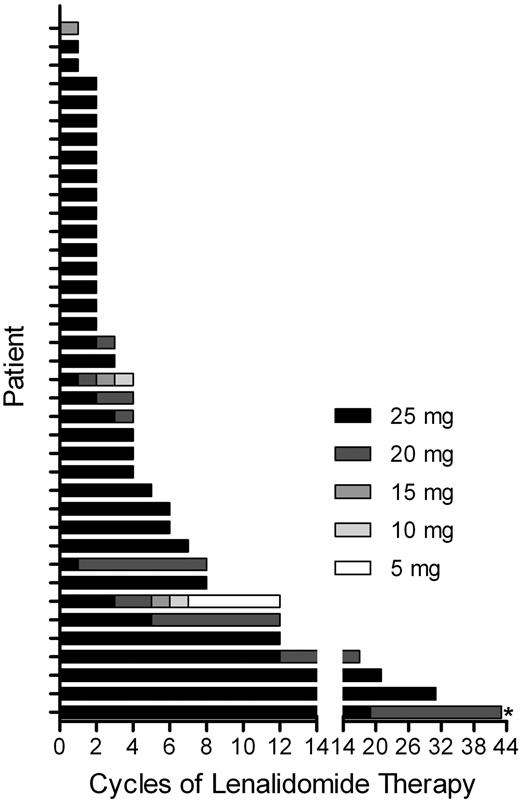
From October 2007 to May 2009, 38 patients were enrolled in this study, with a median age of 34 years (range, 25-63). Thirty-three patients had received prior stem cell transplantation (29 ASCT, 1 syngeneic, 3 ASCT and allogeneic), and 21 patients (55%) were refractory to their last prior therapy. The median number of prior therapies was 4 (range, 2-9) and the median time from last prior therapy to enrollment in this study was 3.5 months (range, 1-66). Additional patient characteristics are listed in Table 1. Of the 38 enrolled patients, 2 were removed from the study before receiving 2 cycles of lenalidomide because of AEs—grade 4 elevated bilirubin and alanine aminotransferase (n = 1) and grade 2 desquamating rash (n = 1)—and were considered not evaluable for efficacy analysis. A third patient was removed from the study because of rapid disease progression during cycle 1 and was considered evaluable for response. The median number of lenalidomide cycles administered per patient was 4 (range, 1 to ≥ 43). The lenalidomide dosages administered for each patient are shown in Figure 1. Median time of follow-up from enrollment in this study was 20 months (range, 1-43).
Patient characteristics (N = 38)
| Sex, n (%) | |
| Male | 15 (39) |
| Female | 23 (61) |
| Age, y | |
| Median | 34 |
| Range | 25-62 |
| Race, n (%) | |
| White | 34 (89) |
| Black | 2 (5) |
| Hispanic | 1 (3) |
| Asian | 1 (3) |
| Histology, n (%) | |
| Nodular sclerosis | 30 (79) |
| Mixed cellularity | 4 (10.5) |
| Classical, not-otherwise-specified | 4 (10.5) |
| Disease status, n (%) | |
| Relapsed | 17 (45) |
| Refractory | 21 (55) |
| B symptoms, n (%) | |
| Yes | 13 (34) |
| No | 25 (66%) |
| Performance status, n (%) | |
| 0 | 22 (58) |
| 1 | 13 (34) |
| 2 | 3 (8) |
| Prior chemotherapy regimens, n | |
| Median | 4 |
| Range | 2-9 |
| Prior radiation therapy, n (%) | |
| Yes | 24 (63) |
| No | 14 (37) |
| Prior stem cell transplantation, n (%) | |
| Yes | 33 (87) |
| No | 5 (13) |
| Prior stem cell transplantation type, n (%) | |
| Autologous | 29 (76) |
| Syngeneic | 1 (3) |
| Autologous and allogeneic | 3 (8) |
| Time from last therapy to enrollment, mo | |
| Median | 3.5 |
| Range | 1-66 |
| Sex, n (%) | |
| Male | 15 (39) |
| Female | 23 (61) |
| Age, y | |
| Median | 34 |
| Range | 25-62 |
| Race, n (%) | |
| White | 34 (89) |
| Black | 2 (5) |
| Hispanic | 1 (3) |
| Asian | 1 (3) |
| Histology, n (%) | |
| Nodular sclerosis | 30 (79) |
| Mixed cellularity | 4 (10.5) |
| Classical, not-otherwise-specified | 4 (10.5) |
| Disease status, n (%) | |
| Relapsed | 17 (45) |
| Refractory | 21 (55) |
| B symptoms, n (%) | |
| Yes | 13 (34) |
| No | 25 (66%) |
| Performance status, n (%) | |
| 0 | 22 (58) |
| 1 | 13 (34) |
| 2 | 3 (8) |
| Prior chemotherapy regimens, n | |
| Median | 4 |
| Range | 2-9 |
| Prior radiation therapy, n (%) | |
| Yes | 24 (63) |
| No | 14 (37) |
| Prior stem cell transplantation, n (%) | |
| Yes | 33 (87) |
| No | 5 (13) |
| Prior stem cell transplantation type, n (%) | |
| Autologous | 29 (76) |
| Syngeneic | 1 (3) |
| Autologous and allogeneic | 3 (8) |
| Time from last therapy to enrollment, mo | |
| Median | 3.5 |
| Range | 1-66 |
Summary of lenalidomide administration. Graphical summary of lenalidomide administration to 38 cHL patients, with individual patients on the y-axis and the number of cycles and dose of lenalidomide administered on the x-axis. If an intra-cycle dose reduction was performed, the lowest dose used for a given cycle is indicated. Lenalidomide was administered on days 1-21 of a 28-day cycle, initiated at 25 mg/d. *Ongoing lenalidomide therapy.
Summary of lenalidomide administration. Graphical summary of lenalidomide administration to 38 cHL patients, with individual patients on the y-axis and the number of cycles and dose of lenalidomide administered on the x-axis. If an intra-cycle dose reduction was performed, the lowest dose used for a given cycle is indicated. Lenalidomide was administered on days 1-21 of a 28-day cycle, initiated at 25 mg/d. *Ongoing lenalidomide therapy.
Response and survival
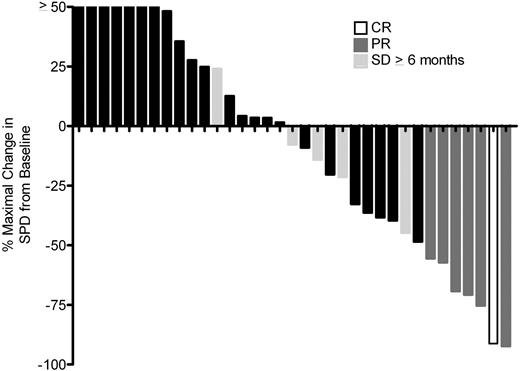
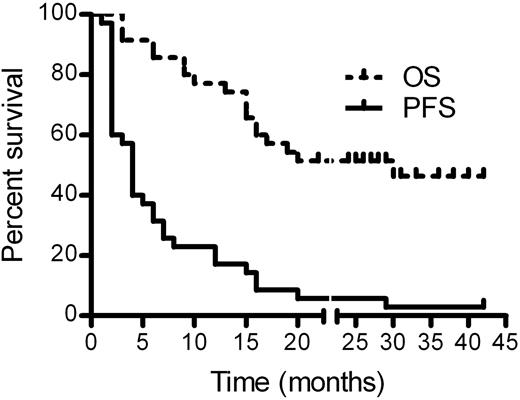
For the 36 response-evaluable patients, we observed 1 CR, 6 PRs, and 5 patients with SD ≥ 6 months for an IWC ORR of 19.4% (95% CI: 8%-36%) and a cytostatic ORR of 33.3% (95% CI: 19%-51%; Table 2). For the entire cohort of 38 patients, the IWC ORR was 18.4% (95% CI: 8%-34%) and the cytostatic ORR was 31.6% (95% CI: 18%-49%). Median duration of CR/PR was 6 months (range, 4 to ≥ 24), and median time-to-treatment failure in responders (CR/PR/SD ≥ 6 months) was 15 months (range, 4 to ≥ 43). Characteristics of responding patients are listed in Table 3. Lenalidomide treatment was continued for ≥ 43 cycles in 1 patient who had SD for 17 cycles and then achieved a PR at cycle 18. Of the 12 responding patients, 8 (67%) had failed to respond to their last prior therapy. Eleven evaluable patients had B symptoms present before therapy, and 6 (55%) had improvement or resolution based on assessment by their treating physician, with 1 patient not assessable. The percentage maximal decrease in the sum of the products of the greatest diameter from baseline is shown in Figure 2, with 18 of 36 (50%) evaluable patients demonstrating a decrease in tumor size. Serial CT images are shown for patient 001001, who remains on lenalidomide therapy after 43 cycles (40 months) in a continuing PR (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Baseline clinical and laboratory parameters (Table 1) were assessed for association with response, and the lack of B symptoms at relapse was the only factor associated with response (CR/PR/SD ≥ 6 months; P = .03). For the entire study population, the median PFS was 4 months (95% CI, 2-6) and the median OS was 20 months (95% CI: 15-not estimated [NE], Figure 3).
Response rates for entire cohort (N = 38) and per protocol response evaluable patients (n = 36)
| Type of response . | Patients, n . | Entire cohort, % . | Response evaluable, % . |
|---|---|---|---|
| CR | 1 | 2.6% | 2.8% |
| PR | 6 | 15.7% | 16.6% |
| SD > 6 months | 5 | 13.2% | 13.9% |
| ORR (CR + PR) | 7 | 18.4% | 19.4% |
| Cytostatic ORR (CR + PR + SD ≥ 6 mo) | 12 | 31.6% | 33.3% |
| Type of response . | Patients, n . | Entire cohort, % . | Response evaluable, % . |
|---|---|---|---|
| CR | 1 | 2.6% | 2.8% |
| PR | 6 | 15.7% | 16.6% |
| SD > 6 months | 5 | 13.2% | 13.9% |
| ORR (CR + PR) | 7 | 18.4% | 19.4% |
| Cytostatic ORR (CR + PR + SD ≥ 6 mo) | 12 | 31.6% | 33.3% |
Characteristics of patients with CR, PR, or SD > 6 months
| Age, y . | Sex . | Response to lenalidomide . | Cycles to best response, n . | Time to treatment failure, mo . | Cycles of lenalidomide, n . | Relapsed or refractory . | Prior therapies, n . | Prior XRT . | Prior transplantations . | Time to enrollment, mo* . | Histology . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 | Female | CR | 2 | 6 | 6 | Refractory | 2 | Yes | Auto | 5 | NS |
| 42 | Female | PR | 18 | NA | ≥ 43 | Refractory | 5 | Yes | Auto | 6 | NS |
| 31 | Male | PR | 6 | 12 | 12 | Refractory | 2 | Yes | Auto | 4 | NS |
| 48 | Male | PR | 2 | 13 | 12 | Refractory | 6 | Yes | Auto | 2 | NS |
| 37 | Male | PR | 2 | 6 | 6 | Refractory | 5 | Yes | Auto | 3 | NS |
| 32 | Male | PR | 4 | 4 | 4 | Relapsed | 4 | No | Auto+Allo | 12 | NOS |
| 25 | Female | PR | 4 | 8 | 8 | Relapsed | 5 | Yes | Auto | 9 | NS |
| 33 | Female | SD > 6 mo | 6 | 12 | 12 | Relapsed | 3 | Yes | Auto | 36 | MC |
| 25 | Female | SD > 6 mo | 6 | 30 | 30 | Refractory | 4 | Yes | Auto | 1 | NS |
| 50 | Male | SD > 6 mo | 6 | 17 | 17 | Refractory | 3 | No | Auto | 2 | MC |
| 33 | Female | SD > 6 mo | 6 | 8 | 8 | Relapsed | 4 | Yes | Auto | 3 | NS |
| 29 | Female | SD > 6 mo | 6 | 21 | 21 | Refractory | 3 | Yes | Auto | 10 | NS |
| Age, y . | Sex . | Response to lenalidomide . | Cycles to best response, n . | Time to treatment failure, mo . | Cycles of lenalidomide, n . | Relapsed or refractory . | Prior therapies, n . | Prior XRT . | Prior transplantations . | Time to enrollment, mo* . | Histology . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52 | Female | CR | 2 | 6 | 6 | Refractory | 2 | Yes | Auto | 5 | NS |
| 42 | Female | PR | 18 | NA | ≥ 43 | Refractory | 5 | Yes | Auto | 6 | NS |
| 31 | Male | PR | 6 | 12 | 12 | Refractory | 2 | Yes | Auto | 4 | NS |
| 48 | Male | PR | 2 | 13 | 12 | Refractory | 6 | Yes | Auto | 2 | NS |
| 37 | Male | PR | 2 | 6 | 6 | Refractory | 5 | Yes | Auto | 3 | NS |
| 32 | Male | PR | 4 | 4 | 4 | Relapsed | 4 | No | Auto+Allo | 12 | NOS |
| 25 | Female | PR | 4 | 8 | 8 | Relapsed | 5 | Yes | Auto | 9 | NS |
| 33 | Female | SD > 6 mo | 6 | 12 | 12 | Relapsed | 3 | Yes | Auto | 36 | MC |
| 25 | Female | SD > 6 mo | 6 | 30 | 30 | Refractory | 4 | Yes | Auto | 1 | NS |
| 50 | Male | SD > 6 mo | 6 | 17 | 17 | Refractory | 3 | No | Auto | 2 | MC |
| 33 | Female | SD > 6 mo | 6 | 8 | 8 | Relapsed | 4 | Yes | Auto | 3 | NS |
| 29 | Female | SD > 6 mo | 6 | 21 | 21 | Refractory | 3 | Yes | Auto | 10 | NS |
Auto indicates autologous SCT; Allo, allogeneic SCT; NS, nodular sclerosis; MC, mixed cellularity; NOS, not otherwise specified; and NA, not applicable
Time to enrollment indicates the length of time between the last prior therapy and enrollment in the current study.
Waterfall plot of maximal decrease in sum of the products of the greatest diameter from baseline (before therapy) for 35 cHL patients treated with ≥ 2 cycles of lenalidomide. Two patients who went off-study during cycle 1 or 2 of lenalidomide because of AEs are not included, and off-study data from 1 patient with rapidly progressive disease during cycle 1 were not available. Patients with CR, PR, or SD > 6 months are annotated.
Waterfall plot of maximal decrease in sum of the products of the greatest diameter from baseline (before therapy) for 35 cHL patients treated with ≥ 2 cycles of lenalidomide. Two patients who went off-study during cycle 1 or 2 of lenalidomide because of AEs are not included, and off-study data from 1 patient with rapidly progressive disease during cycle 1 were not available. Patients with CR, PR, or SD > 6 months are annotated.
Kaplan-Meier PFS and OS estimates. Survival analysis included 35 patients with evaluable disease completing at least 2 cycles of lenalidomide. Median PFS was 4 months (95% CI: 2-6) and the median OS was 20 months (95% CI: 15-NE).
Kaplan-Meier PFS and OS estimates. Survival analysis included 35 patients with evaluable disease completing at least 2 cycles of lenalidomide. Median PFS was 4 months (95% CI: 2-6) and the median OS was 20 months (95% CI: 15-NE).
Toxicity
In general, the treatment was well tolerated, and the most common grade 3 or 4 AEs were neutropenia (47%), leukopenia (29%), anemia (26%), lymphopenia (24%), and thrombocytopenia (18%) (Table 4). Grade 3 or 4 infections occurred in only 3 patients (n = 2 pneumonia with no organism identified, n = 1 parainfluenza virus). Lenalidomide dose reductions are depicted in Figure 1. The lenalidomide dose was reduced in 7 patients because of cytopenias (n = 3), fatigue (n = 2), elevated aspartate aminotransferase (n = 1), and neuropathy (n = 1) and discontinued in 4 patients for rash (n = 2), elevated bilirubin/transaminases (n = 1), and cytopenias (n = 1). One patient had a grade 2 tumor flare reaction during cycle 2 of lenalidomide that was treated with 3 days of steroids and resolved in ≤ 1 week. No venous thrombosis or tumor lysis syndrome events were observed. Two patients died during the study. The first was hospitalized with evidence of rapidly progressing disease when lenalidomide was initiated, received 19 doses, and had continued rapid progression resulting in death 3 days after lenalidomide was discontinued. The second patient received a total of 16 cycles of lenalidomide with SD, requiring dose reduction to 20 mg at cycle 13 for neutropenia. During cycle 17, lenalidomide was put on hold because of cytopenias for > 2 weeks and the patient was found to have died at home of an unknown cause.
Grade 3 or 4 AEs in 38 rel/ref cHL patients, n (%)
| Characteristic . | Grade 3 . | Grade 4 . | Total Grade 3 and 4 . |
|---|---|---|---|
| Hematologic | |||
| Neutropenia | 13 (34) | 5 (13) | 18 (47) |
| Leukopenia | 9 (24) | 2 (5) | 11 (29) |
| Anemia | 8 (21) | 2 (5) | 10 (26) |
| Lymphopenia | 7 (18) | 2 (5) | 9 (24) |
| Thrombocytopenia | 5 (13) | 2 (5) | 7 (18) |
| Febrile neutropenia | 1 (3) | 1 (3) | |
| Nonhematologic | |||
| Fatigue | 3 (8) | 3 (8) | |
| AST | 3 (8) | 3 (8) | |
| ALT | 1 (3) | 1 (3) | 2 (5) |
| Bilirubin | 1 (3) | 1 (3) | 2 (5) |
| Sensory neuropathy | 2 (5) | 2 (5) | |
| Dehydration | 2 (5) | 2 (5) | |
| Infection without neutropenia | 2 (5) | 2 (5) | |
| Infection with neutropenia | 1 (3) | 1 (3) | |
| Edema | 2 (5) | 1 (3) | |
| Dyspnea | 1 (3) | 1 (3) | |
| Pleural effusion | 1 (3) | 1 (3) | |
| Alkaline phosphatase | 1 (3) | 1 (3) | |
| Pain, abdominal | 1 (3) | 1 (3) | |
| Metabolic/laboratory | |||
| Low potassium | 2 (5) | 1 (3) | 3 (8) |
| Low sodium | 1 (3) | 1 (3) | 2 (5) |
| Low albumin | 1 (3) | 1 (3) | |
| Low calcium | 1 (3) | 1 (3) | |
| High calcium | 1 (3) | 1 (3) | |
| Low phosphorous | 1 (3) | 1 (3) |
| Characteristic . | Grade 3 . | Grade 4 . | Total Grade 3 and 4 . |
|---|---|---|---|
| Hematologic | |||
| Neutropenia | 13 (34) | 5 (13) | 18 (47) |
| Leukopenia | 9 (24) | 2 (5) | 11 (29) |
| Anemia | 8 (21) | 2 (5) | 10 (26) |
| Lymphopenia | 7 (18) | 2 (5) | 9 (24) |
| Thrombocytopenia | 5 (13) | 2 (5) | 7 (18) |
| Febrile neutropenia | 1 (3) | 1 (3) | |
| Nonhematologic | |||
| Fatigue | 3 (8) | 3 (8) | |
| AST | 3 (8) | 3 (8) | |
| ALT | 1 (3) | 1 (3) | 2 (5) |
| Bilirubin | 1 (3) | 1 (3) | 2 (5) |
| Sensory neuropathy | 2 (5) | 2 (5) | |
| Dehydration | 2 (5) | 2 (5) | |
| Infection without neutropenia | 2 (5) | 2 (5) | |
| Infection with neutropenia | 1 (3) | 1 (3) | |
| Edema | 2 (5) | 1 (3) | |
| Dyspnea | 1 (3) | 1 (3) | |
| Pleural effusion | 1 (3) | 1 (3) | |
| Alkaline phosphatase | 1 (3) | 1 (3) | |
| Pain, abdominal | 1 (3) | 1 (3) | |
| Metabolic/laboratory | |||
| Low potassium | 2 (5) | 1 (3) | 3 (8) |
| Low sodium | 1 (3) | 1 (3) | 2 (5) |
| Low albumin | 1 (3) | 1 (3) | |
| Low calcium | 1 (3) | 1 (3) | |
| High calcium | 1 (3) | 1 (3) | |
| Low phosphorous | 1 (3) | 1 (3) |
Plasma cytokine/chemokine assessment
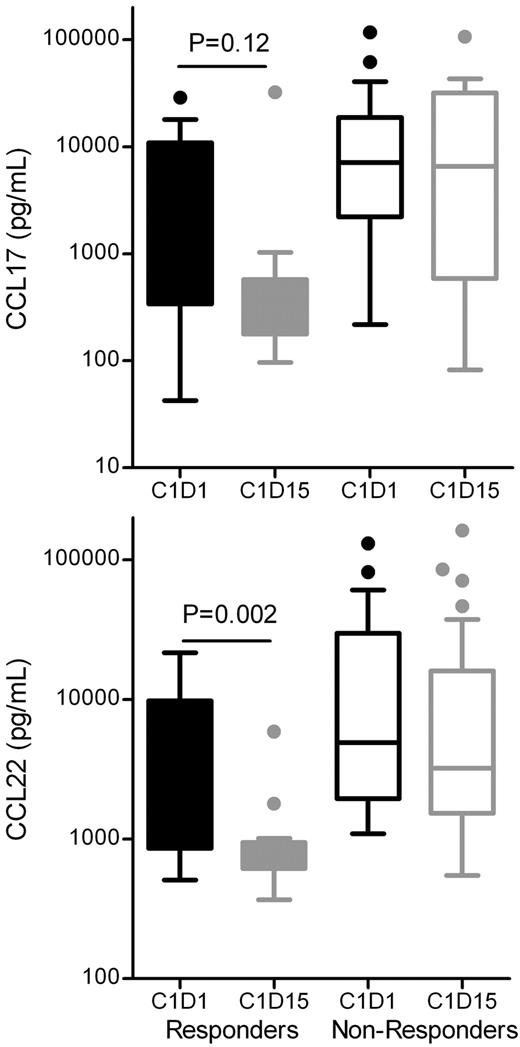
Serum CCL17 (TARC) and CCL22 (MDC) have been shown previously to be elevated in cHL patients and to decrease following clinical responses.15-17 CCL17 and CCL22 plasma levels were assessed before and after 2 weeks of lenalidomide therapy in all patients, and a significant association between CCL22 decrease and subsequent clinical responses was observed, with a nonsignificant trend for CCL17 (Figure 4). In contrast, baseline CCL17 or CCL22 levels did not predict for a subsequent clinical response to lenalidomide therapy in our rel/ref cHL patients (data not shown).
CCL17/TARC and CCL22/MDC changes. Shown are CCL17/TARC and CCL22/MDC changes from pretherapy cycle 1 day 1 (C1D1) and cycle 1 day 15 (C1D15) for responders (CR/PR/SD ≥ 6 months) and nonresponders (SD < 6 months, PD). Box-whisker plots depict pretherapy and C1D15 plasma CCL17 or CCL22 concentrations. P values were determined using the Wilcoxon signed rank test. There was no significant difference between C1D1 and C1D15 in nonresponders.
CCL17/TARC and CCL22/MDC changes. Shown are CCL17/TARC and CCL22/MDC changes from pretherapy cycle 1 day 1 (C1D1) and cycle 1 day 15 (C1D15) for responders (CR/PR/SD ≥ 6 months) and nonresponders (SD < 6 months, PD). Box-whisker plots depict pretherapy and C1D15 plasma CCL17 or CCL22 concentrations. P values were determined using the Wilcoxon signed rank test. There was no significant difference between C1D1 and C1D15 in nonresponders.
In vitro survival of cHL cell lines after lenalidomide exposure
Several indirect mechanisms may lead to lenalidomide responses in cHL patients; however, a direct assessment of the impact of lenalidomide on the growth or survival of cHL cell lines has not been reported. Therefore, the L428 and KMH2 cHL cell lines were treated with lenalidomide, and no effects on cell viability after 72 hours were observed (supplemental Figure 2). These findings indicate that lenalidomide does not directly impact cell growth or survival of these cHL cell lines in vitro.
Discussion
Lenalidomide has evidence of modest clinical activity when administered at a dose of 25 mg/d on days 1-21 of a 28-day cycle in patients with rel/ref cHL. In this heavily pretreated patient population, objective responses were observed in 19% of patients (median duration, 6 months), and 50% of evaluable patients had a reduction in tumor size on lenalidomide. An additional 14% of patients had prolonged (> 6 months) stabilization of disease, resulting in a cytostatic or clinically meaningful response rate of 33%, with a median time-to-treatment failure of 15 months. B symptoms improved on lenalidomide in 55% of affected patients. The most frequent AEs were neutropenia, anemia, and thrombocytopenia, but only 1 patient developed neutropenic fever, and 3 patients experienced grade 3 or 4 infections. The tolerability of 25 mg/d lenalidomide in rel/ref patients who have previously undergone ASCT is remarkable, and suggests that the degree of myelosuppression from lenalidomide may depend on the underlying hematologic disease. In our study, patients without B symptoms were significantly more likely to respond (CR/PR/SD ≥ 6 months) based on a univariate analysis, but there were no other demographic or clinical factors that predicted for lenalidomide response in rel/ref cHL patients within our relatively small sample set. Preliminary results from another study using this dose and schedule in patients with rel/ref cHL identified 2 PRs and 7 patients with SD of 14 evaluable patients.18 In addition, a German Hodgkin Study Group (GHSG) program consisting of n = 10 rel/ref cHL patients treated with lenalidomide at 25 mg/d on days 1-28 of a 28-day cycle reported 4 PRs and 1 CR in 10 selected rel/ref cHL patients (ORR = 50%).19 A subsequent presentation on this GHSG program (n = 31) reported 1 CR and 8 PR for an ORR of 29%.20 As a well-tolerated oral agent, these results suggest that further studies both to optimize the dose and schedule of lenalidomide in cHL and to investigate combinations with other active agents are warranted. Accordingly, we have recently completed accrual of a second cohort of rel/ref cHL patients to evaluate a continuous schedule (lenalidomide 25 mg/d on days 1-28 of a 28-day schedule), and the clinical data analysis and follow-up are ongoing.
Several new agents have recently been investigated in phase 1 or 2 studies in rel/ref cHL patients, with promising results. Brentuximab vedotin (BV; SGN-35) is an anti–CD30-monomethyl auristatin E Ab-drug conjugate that demonstrated impressive response rates in both phase 117 (54% at doses of ≥ 1.2 mg/kg) and phase 221 (75% at a dose of 1.8 mg/kg) studies when administered every 3 weeks. The median PFS as assessed by an independent review in the pivotal phase 2 study was 6 months.21 Several oral histone deacetylase (HDAC) inhibitors have recently been reported for patients with rel/ref cHL. Panobinostat (LBH589) is a pan-deacetylase inhibitor with response rates in rel/ref cHL patients of 38% in a phase 1/2 trial with short follow-up22 and 27% in an international phase 2 study with a median PFS of 5.7 months.23 MGCD0103 is a selective HDAC1/2 inhibitor that was administered in one study at 85 or 110 mg 3 times per week in 4-week cycles; this treatment resulted in an ORR of 20% (1 of 5 evaluable patients, 85 mg) or 38% (8 of 21 evaluable patients, 110 mg); the PFS was not reported.24 Vorinostat was also evaluated in 25 rel/ref cHL patients at 200 mg twice daily, which was associated with an ORR of 4%, suggesting that activity in cHL is not an HDAC inhibitor class effect, but may depend on the precise HDACs (or other pathways) targeted. Everolimus (RAD001) is an oral mTOR inhibitor that was investigated at 10 mg/d in 19 rel/ref cHL patients, yielding an ORR of 47%, with a median time-to-progression of 7 months.25 Therefore, lenalidomide has an IWC ORR comparable to panobinostat, both of which are modest compared with BV. However, the median PFS rates for BV, panobinostat, and lenalidomide is comparable in rel/ref cHL patients (6, 6, and 4 months, respectively), with each agent having subsets of patients who benefit from disease control for > 12 months. Combination studies in rel/ref cHL are currently in development for BV + lenalidomide (Cancer and Leukemia Group B), and panobinostat + lenalidomide and everolimus + lenalidomide26 are also being explored. In addition, the feasibility of using lenalidomide as maintenance therapy after ASCT is under evaluation.27 Lenalidomide in combination with Adriamycin, vinblastine, and dacarbazine (AVD) is being explored as frontline therapy in older (age 61-74 years) patients with cHL in Germany.28 These studies will provide important insights into the optimal combinations and settings to incorporate lenalidomide therapy for cHL patients.
How does lenalidomide achieve clinical responses in cHL? In the present study, we show that, at serum concentrations achieved in patients treated with 25 mg/d lenalidomide,6 lenalidomide failed to arrest growth or kill cHL cell lines in vitro. Whereas this provides evidence that lenalidomide may act through indirect mechanisms in cHL, in vitro effects on immortalized HRS cell lines may not recapitulate HRS pathophysiology in patients. Our understanding of the importance of the HRS microenvironment in cHL pathogenesis and progression has expanded over the last several decades.29 Unfortunately, there are no spontaneous mouse models of cHL available, hampering our ability to investigate the indirect mechanisms of lenalidomide in vivo. Because of its pleiotropic effects, there are several indirect mechanisms through which lenalidomide may act in this clinical context. Cytokine or chemokine modulation is one indirect mechanism whereby lenalidomide may alter the HRS microenvironment in vivo.29 CCL17 and CCL22 levels were detected in rel/ref cHL patients before therapy, and decreases in 2-week CCL17 and CCL22 plasma protein levels were correlated with response. Whereas the trend was not formally significant for CCL17, this was influenced by a high degree of baseline heterogeneity in our relatively small sample set. Alterations in these chemokines have been reported in responding cHL patients with other agents.15-17 Van den Berg et al first discovered that CCL17 was highly expressed by HRS cells in cHL pathology specimens.30 Weihrauch et al retrospectively reported CCL17 levels in the serum of 62 cHL patients selected from GHSG clinical studies, and found that CCL17 levels were correlated with freedom from treatment failure and survival, including posttherapy measurements.16 Niens et al evaluated chemokine serum levels in 133 untreated cHL patients and 334 controls, and found a significant increase of 82% (CCL17) and 57% (CCL22) in cHL patients compared with controls and a high correlation coefficient for concurrent increased levels of these 2 chemokines.15 Recently, Younes et al reported a phase 1 study of brentuximab vedotin (SGN-35) in rel/ref cHL, and also observed a decrease in CCL17 levels after therapy.17 Therefore, CCL17 and CCL22 may be useful biomarkers of response for multiple agents in cHL, and modulation by lenalidomide may contribute to clinical response. However, because multiple therapies resulted in decreased chemokine levels, this may also reflect a change in disease burden rather than a specific mechanism of action for lenalidomide. Other indirect immunomodulatory mechanisms such as Th cell modulation, augmentation of CD8 or natural killer cell responses, or tumor recognition remain to be explored in cHL.
In summary, lenalidomide administered at 25 mg/d on days 1-21 of 28-day cycles induced objective and cytostatic responses in a subset of rel/ref cHL patients and was very well tolerated in this patient population. Further study of lenalidomide in cHL with alternative doses and schedules and in combination with other agents is therefore warranted.
The online version of this article contains a data supplement.
*Presented in part at the American Society of Hematology 2009 Annual Meeting, December 5-8, 2009, New Orleans, LA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by Celgene Corporation, the ASCO Foundation (Young Investigator Award to T.A.F.), and the Foundation for Barnes-Jewish Hospital and its generous donors (to N.L.B.). T.A.F. is also supported by a National Institutes of Health grant (K08HL093299). The authors recognize the support of the Biostatistics Core, Clinical Trials Core, Imaging Response Assessment Team, and Tissue Procurement Core of the Siteman Cancer Center, which is funded by a National Cancer Institute Comprehensive Cancer Center support grant (P30 CA091842).
National Institutes of Health
Authorship
Contribution: T.A.F. and N.L.B. conceived and designed the study; T.A.F., A.F.C., K.A.B., T.S.F., D.D.H., A.G., N.D.W.-J., K.R.C., and N.L.B. provided study materials or patients; S.L., T.A.F., K.T., M.J.S., S.E.S., and C.R.K. collected and assembled the data; S.L., T.A.F., K.T., M.J.S., S.E.S., C.R.K., and N.L.B. analyzed and interpreted the data; T.A.F. and N.L.B. wrote the manuscript; and T.A.F., S.L., K.T., M.J.S., A.F.C., K.A.B., T.S.F., D.D.H., A.G., S.E.S., C.R.K., N.D.W.-J., K.R.C., and N.L.B. approved the final manuscript. All authors had access to the primary clinical trial data.
Conflict-of-interest disclosure: T.A.F. and N.L.B. received research funding from Celgene for this clinical study. K.R.C. performs consulting for Celgene (advisory board and education for peripheral T-cell lymphoma). A.G. received research funding from Celgene for this clinical study and has been on the advisory board for Celgene. The remaining authors declare no competing financial interests.
Correspondence: Todd A. Fehniger, Division of Oncology, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8056, St Louis, MO 63110; e-mail: tfehnige@wustl.edu.