Abstract
Hypoxia is known to reduce the expression of hepcidin, the master regulator of iron metabolism. However, it is not clear whether this response is primarily related to increased erythropoiesis driven by hypoxically stimulated erythropoietin or to a more direct effect of hypoxia on hepcidin expression. The germline loss-of-function VHLR200W mutation is common in Chuvashia, Russia, and also occurs elsewhere. VHLR200W homozygotes have elevated hypoxia-inducible factor 1α (HIF-1α) and HIF-2α levels, increased red cell mass, propensity to thrombosis, and early mortality. Ninety VHLR200W homozygotes and 52 controls with normal VHL alleles from Chuvashia, Russia, were studied under basal circumstances. In univariate analyses, serum hepcidin concentration was correlated positively with serum ferritin concentration and negatively with homozygosity for VHLR200W. After adjustment for serum erythropoietin and ferritin concentrations by multiple linear regression, the geometric mean (95% confidence interval of mean) hepcidin concentration was 8.1 (6.3-10.5) ng/mL in VHLR200W homozygotes versus 26.9 (18.6-38.0) ng/mL in controls (P < .001). In contrast, a significant independent relationship of serum erythropoietin, hemoglobin, or RBC count with hepcidin was not observed. In conclusion, up-regulation of the hypoxic response leads to decreased expression of hepcidin that may be independent of increased erythropoietin levels and increased RBC counts.
Introduction
Oxygen sensing is a fundamental physiologic function, and hypoxia-inducible factors (HIFs) are the principal transcriptional regulators of the response to hypoxia in mammalian cells.1 HIF is a heterodimer composed of an HIF-β subunit that is constitutively expressed and one of several HIF-α subunits that are regulated posttranslationally, principally by the oxygen tension. The von Hippel Lindau (VHL) protein and prolyl hydroxylase domain proteins (PHDs) are critical to the oxygen-related regulation of cellular HIF-α levels, which then determine the levels of HIF dimers. The VHL protein is the recognition component of an E3 ubiquitin-protein ligase complex that mediates proteasomal degradation of HIF-1α and HIF-2α under normoxic conditions.2 PHDs are enzymes that require oxygen as a substrate and that serve to hydroxylate HIF-1α and HIF-2α on specific proline residues; this proline hydroxylation is required for the interaction of HIF-1α and HIF-2α with VHL.1,3 Therefore, HIF-1 and HIF-2 levels increase in response to hypoxia, and this leads to increased expression of erythropoietin4 and either increased or decreased expression of other hypoxia-responsive genes.1,5-7
The R200W mutation of the VHL gene is present on the same haplotype in almost all persons of heterogeneous racial and ethnic background, indicating that the mutation may have originated in a founder before the divergence of the human races.8 Only one individual with this mutation present on a different haplotype has been reported.9 Homozygosity for VHLR200W is responsible for Chuvash polycythemia, the first recognized congenital disorder of augmented hypoxia sensing.10,11 Chuvash polycythemia is common in the Chuvash Republic of the Russian Federation,12 where approximately 200 cases are recognized among a population of approximately 1.5 million people, with an estimated heterozygosity frequency of 1.7% (V.G., unpublished observations, 2011) and in the Italian island of Ischia13 ; the condition also occurs sporadically in other parts of the world.9,14,15 Homozygosity for VHLR200W leads to up-regulation of HIF-1 and HIF-2 under normoxic conditions.11,16 Several of the target genes of HIF are up-regulated in Chuvash polycythemia, including those for endothelin-1, glucose transporter 1, plasminogen activator inhibitor-1, transferrin, the transferrin receptor, and VEGF.11,17,18 Matched cohort, case-control, and other analyses have shown that homozygosity is associated with lower systemic blood pressure, higher pulmonary artery pressure, and other changes in pulmonary vascular physiology. It is also associated with varicose veins, vertebral and hepatic hemangiomas, lower white blood cell and platelet counts, increased serum concentrations of inflammatory cytokines, changes in plasma thiol concentrations, arterial and venous thrombosis, major bleeding episodes, cerebral vascular events, and premature mortality. Malignant tumors typical of classic VHL tumor predisposition syndrome have not been found, and no increased risk of cancer has been demonstrated.17-22
Hepcidin is the master regulator of systemic iron metabolism, leading to decreased iron absorption and increased iron storage in macrophages through its interaction with ferroportin.23,24 Expression of hepcidin in the liver is up-regulated by a bone morphogenetic protein receptor complex in response to intracellular iron stores25,26 and is also stimulated by elevated circulating levels of transferrin bound to iron.25-28 Expression in the liver is also up-regulated by IL-6 in response to inflammation.29 In contrast, hepcidin responds to hypoxia with decreased expression,30,31 but it is not clear whether this is a direct response to up-regulation of HIFs31 or to an indirect response related to erythropoietin signaling32 or to increased erythropoiesis itself.33 The present study was conducted to determine whether the VHLR200W genotype leads directly to decreased serum levels of hepcidin or whether such an effect is mediated by an increase in erythropoietin expression and increased erythropoiesis.
Methods
Research protocol
The Howard University institutional review board approved the research and all participants provided written informed consent in accordance with the Declaration of Helsinki. The study was carried out in the Chuvash Autonomous Republic of the Russian Federation, which is located approximately 650 kilometers southeast of Moscow along the Volga River. Patients with the diagnosis of Chuvash polycythemia, relatives of patients, and community controls were studied. Participants were > 20 years of age, were recruited from the community, and were in their usual state of health. The study participants were characterized by medical history, physical examination including blood pressure and body weight, and laboratory tests of the peripheral blood. Serum samples were collected from 2004-2008 and stored at −70°C. Collection and storage of samples from Chuvash polycythemia patients and controls was identical. Samples were transported in dry shippers with liquid nitrogen or on dry ice. They were analyzed for hepcidin in 2010, and had undergone 2 or fewer freeze-thaw cycles. Approximately 1/2 of the patients with Chuvash polycythemia had been treated with phlebotomy within the year before the date of the study. The other half had either never undergone phlebotomy or had received phlebotomy > 1 year before the date of the study.
Laboratory procedures
The complete blood count was performed by an automated analyzer (Sysmex XT 2000i; Sysmex Corporation). Serum ferritin concentration was determined by enzyme immunoassay (Ramco Laboratories). Serum concentration of erythropoietin was determined by ELISA (R&D Systems). Serum hepcidin was measured by competitive ELISA, as described previously.34 Serum albumin, total protein, and iron concentrations and total iron binding capacity were determined by Quest Diagnostics using spectrophotometric methodology. The globulin fraction, the albumin/globulin ratio, and the transferrin saturation were calculated.
Statistics
The primary study comparison was between VHLR200W homozygotes and genotypically normal subjects with regard to serum hepcidin concentration using multiple linear regression. For hepcidin concentrations below the detection limit, we assigned a value of 2.6 ng/mL, which is halfway between the limit of detection of 5.2 ng/mL and 0. Skewed continuous variables were log-transformed to approximate a normal distribution. Generalized linear models were also applied to validate the linear regression. The clinical characteristics of the VHLR200W homozygotes and controls were assessed with the Student t test or Pearson χ2 test. Bivariate relationships of various measurements with hepcidin were performed with Spearman correlation. Analyses were performed with Stata 10.0 software (StataCorp).
Results
Clinical characteristics according to VHL genotype
The clinical characteristics of the study participants are summarized in Table 1. Mean ages were 43 years in 90 VHLR200W homozygotes compared with 49 years in 52 controls (P = .008). Females made up slightly more than half of both cohorts. Histories of alcohol consumption, substantial bleeding in the past year, thrombosis, and systemic hypertension were not different in VHLR200W homozygotes compared with controls. Body mass index was lower in VHLR200W homozygotes than controls (P = .004), and history of smoking was higher (P = .024).
Clinical characteristics of study participants according to VHLR200W status
| . | Controls (n = 52) . | VHLR200W homozygotes (n = 90) . | P . |
|---|---|---|---|
| Age, y | 49 (14) | 43 (13) | .008 |
| Female sex, no (%) | 34 (65%) | 52 (58%) | .4 |
| History of smoking, no (%) | 6 (12%) | 25 (28%) | .024 |
| History of alcohol consumption, no (%) | 9 (17%) | 16 (18%) | .9 |
| History of bleeding in the past year (principally menorrhagia or gastrointestinal), no (%) | 3 (6%) | 8 (9%) | .5 |
| History of thrombosis, no (%) | 5 (10%) | 3 (3%) | .12 |
| Phlebotomy in past year, no (%) | 0 | 46 (51%) | < .001* |
| Body mass index, kg/m2 | 25.5 (5.2) | 23.1 (3.4) | .004* |
| Systolic blood pressure, mmHg | 132 (23) | 122 (19) | .010 |
| Diastolic blood pressure, mmHg | 84 (11) | 81 (10) | .14 |
| Mean blood pressure, mmHg | 100 (14) | 95 (12) | .031 |
| . | Controls (n = 52) . | VHLR200W homozygotes (n = 90) . | P . |
|---|---|---|---|
| Age, y | 49 (14) | 43 (13) | .008 |
| Female sex, no (%) | 34 (65%) | 52 (58%) | .4 |
| History of smoking, no (%) | 6 (12%) | 25 (28%) | .024 |
| History of alcohol consumption, no (%) | 9 (17%) | 16 (18%) | .9 |
| History of bleeding in the past year (principally menorrhagia or gastrointestinal), no (%) | 3 (6%) | 8 (9%) | .5 |
| History of thrombosis, no (%) | 5 (10%) | 3 (3%) | .12 |
| Phlebotomy in past year, no (%) | 0 | 46 (51%) | < .001* |
| Body mass index, kg/m2 | 25.5 (5.2) | 23.1 (3.4) | .004* |
| Systolic blood pressure, mmHg | 132 (23) | 122 (19) | .010 |
| Diastolic blood pressure, mmHg | 84 (11) | 81 (10) | .14 |
| Mean blood pressure, mmHg | 100 (14) | 95 (12) | .031 |
Results are means (SD) unless otherwise indicated.
Significant after the Bonferroni correction for multiple comparisons.
Complete blood count, iron measures, erythropoietin, and hepcidin concentrations according to VHL genotype
After adjustment for sex by multiple linear regression, the geometric mean (95% confidence interval of the mean [95% CI]) hemoglobin concentration was 17.3 (16.8-17.8) g/L in the VHLR200W homozygotes and 12.8 (12.3-13.3) g/L in controls without mutated VHL alleles (P < .001). The mean value for mean corpuscular volume was lower among the VHLR200W homozygotes than controls (P < .001), which is consistent with their frequent history of phlebotomy. The white blood cell and platelet counts were lower in the VHLR200W homozygotes (P ≤ .002). The serum ferritin concentration, serum iron concentration, and transferrin saturation were lower in VHLR200W homozygotes compared with controls, and the total iron binding capacity was significantly higher (P < .001). The serum ferritin concentration was ≤ 25 μg/L in 60 of 90 VHLR200W homozygotes (67%) compared with 12 of 52 controls (23%), also consistent with the frequent history of phlebotomy in the VHLR200W homozygotes. Serum concentrations of erythropoietin were higher and of hepcidin were lower in the VHLR200W homozygotes (P < .001; Table 2).
Laboratory tests according to VHLR200W status
| . | N . | Controls . | N . | VHLR200W homozygotes . | P . |
|---|---|---|---|---|---|
| Hemoglobin, g/dL* | 52 | 12.8 (12.3-13.3) | 90 | 17.3 (16.8-17.8) | < .001† |
| RBCs, ×106/μL | 52 | 4.3 (4.2-4.5) | 90 | 6.5 (6.3-6.7) | < .001† |
| Mean corpuscular volume, fL | 52 | 89 (87-91) | 79 | 80 (78-82) | < .001† |
| Mean corpuscular hemoglobin concentration, g/dL | 52 | 32.9 (32.3-33.5) | 80 | 32.9 (32.4-33.5) | .8 |
| WBCs, ×1000/μL | 52 | 6.5 (6.2-6.9) | 90 | 5.6 (5.3-6.0) | .002† |
| Platelets, ×1000/μL | 52 | 248 (232-265) | 90 | 208 (193-224) | < .001† |
| Ferritin, μg/L* | 50 | 53 (39-73) | 77 | 11 (9-15) | < .001† |
| Iron, μg/dL | 52 | 68 (60-78) | 88 | 36 (29-45) | < .001† |
| Iron-binding capacity, μg/dL | 52 | 354 (339-370) | 77 | 483 (465-503) | < .001† |
| Transferrin saturation, % | 52 | 19 (17-22) | 77 | 10 (8-12) | < .001† |
| Protein, g/dL | 52 | 7.2 (7.0-7.4) | 88 | 7.4 (7.3-7.5) | 0.10 |
| Albumin, g/dL | 52 | 4.4 (4.3-4.5) | 88 | 4.4 (4.3-4.5) | 0.6 |
| Globulin, g/dL | 52 | 2.8 (2.7-2.9) | 88 | 3.0 (2.9-3.0) | 0.008 |
| Albumin/globulin ratio | 52 | 1.6 (1.5-1.7) | 88 | 1.5 (1.4-1.5) | 0.006 |
| Erythropoietin, IU/L | 50 | 9.7 (8.6-11.1) | 77 | 51.5 (42.3-62.8) | < .001† |
| Hepcidin, ng/mL | 52 | 40.8 (29.6-56.3) | 90 | 6.0 (5.0-7.6) | < .001† |
| . | N . | Controls . | N . | VHLR200W homozygotes . | P . |
|---|---|---|---|---|---|
| Hemoglobin, g/dL* | 52 | 12.8 (12.3-13.3) | 90 | 17.3 (16.8-17.8) | < .001† |
| RBCs, ×106/μL | 52 | 4.3 (4.2-4.5) | 90 | 6.5 (6.3-6.7) | < .001† |
| Mean corpuscular volume, fL | 52 | 89 (87-91) | 79 | 80 (78-82) | < .001† |
| Mean corpuscular hemoglobin concentration, g/dL | 52 | 32.9 (32.3-33.5) | 80 | 32.9 (32.4-33.5) | .8 |
| WBCs, ×1000/μL | 52 | 6.5 (6.2-6.9) | 90 | 5.6 (5.3-6.0) | .002† |
| Platelets, ×1000/μL | 52 | 248 (232-265) | 90 | 208 (193-224) | < .001† |
| Ferritin, μg/L* | 50 | 53 (39-73) | 77 | 11 (9-15) | < .001† |
| Iron, μg/dL | 52 | 68 (60-78) | 88 | 36 (29-45) | < .001† |
| Iron-binding capacity, μg/dL | 52 | 354 (339-370) | 77 | 483 (465-503) | < .001† |
| Transferrin saturation, % | 52 | 19 (17-22) | 77 | 10 (8-12) | < .001† |
| Protein, g/dL | 52 | 7.2 (7.0-7.4) | 88 | 7.4 (7.3-7.5) | 0.10 |
| Albumin, g/dL | 52 | 4.4 (4.3-4.5) | 88 | 4.4 (4.3-4.5) | 0.6 |
| Globulin, g/dL | 52 | 2.8 (2.7-2.9) | 88 | 3.0 (2.9-3.0) | 0.008 |
| Albumin/globulin ratio | 52 | 1.6 (1.5-1.7) | 88 | 1.5 (1.4-1.5) | 0.006 |
| Erythropoietin, IU/L | 50 | 9.7 (8.6-11.1) | 77 | 51.5 (42.3-62.8) | < .001† |
| Hepcidin, ng/mL | 52 | 40.8 (29.6-56.3) | 90 | 6.0 (5.0-7.6) | < .001† |
Results are geometric means (95% CI).
Adjusted for sex.
Significant after the Bonferroni correction for multiple comparisons.
Univariate correlates of hepcidin by VHL genotype
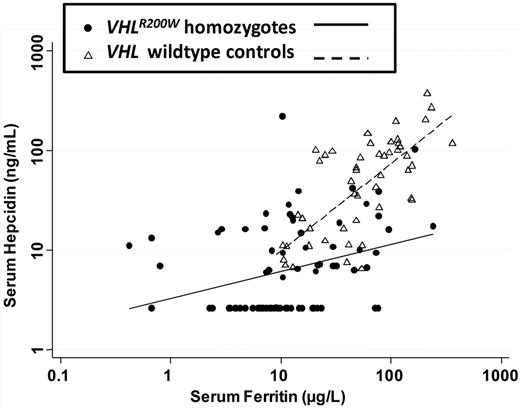
Table 3 and Figure 1 show that serum hepcidin concentration was correlated strongly with the serum ferritin concentration in both VHLR200W homozygotes and controls. The correlations of serum hepcidin with serum ferritin were stronger than the correlations of hepcidin with serum iron, total iron-binding capacity, and transferrin saturation. Figure 1 also demonstrates that hepcidin concentrations were lower in VHLR200W homozygotes compared with controls with similar ferritin concentrations. The slope of the regression line for hepcidin as a function of ferritin was significantly lower in VHLR200W homozygotes compared with controls (P = .002).
Spearman correlation of serum hepcidin concentration with clinical and laboratory variables
| . | Controls . | VHLR200W homozygotes . | ||
|---|---|---|---|---|
| N . | Rho (P) . | N . | Rho (P) . | |
| Age | 52 | 0.26 (.06) | 90 | 0.12 (.3) |
| Female sex | 52 | −0.19 (.19) | 90 | −0.02 (.8) |
| History of smoking | 52 | 0.10 (.5) | 90 | −0.05 (.7) |
| History of alcohol consumption | 52 | 0.01 (.9) | 90 | 0.16 (.12) |
| History of bleeding in the past year | 52 | −0.21 (.14) | 90 | −0.14 (.19) |
| History of thrombosis | 52 | −0.08 (.6) | 90 | 0.14 (.18) |
| Body mass index | 52 | 0.18 (.2) | 90 | 0.11 (.3) |
| Systolic blood pressure | 52 | 0.21 (.14) | 90 | −0.05 (.7) |
| Diastolic blood pressure | 52 | 0.05 (.7) | 90 | −0.07 (.5) |
| Hemoglobin | 52 | 0.29 (.038) | 90 | 0.08 (.5) |
| RBCs, ×106 | 52 | 0.20 (.16) | 90 | −0.03 (.8) |
| Mean corpuscular volume | 52 | −0.04 (.8) | 79 | 0.08 (.5) |
| Mean corpuscular hemoglobin concentration | 52 | 0.18 (.2) | 80 | −0.07 (.5) |
| WBCs | 52 | 0.30 (.031) | 90 | −0.03 (.8) |
| Platelets | 52 | 0.12 (.4) | 90 | 0.07 (.5) |
| Ferritin | 50 | 0.68 (< .0001)* | 77 | 0.36 (.001)* |
| Iron | 52 | 0.14 (.3) | 88 | 0.07 (.5) |
| Iron-binding capacity | 52 | −0.33 (.019) | 77 | −0.09 (.4) |
| Iron saturation | 52 | 0.23 (.11) | 77 | 0.12 (.3) |
| Protein | 52 | 0.32 (.021) | 88 | −0.05 (.7) |
| Albumin | 52 | 0.42 (.002)* | 88 | −0.14 (.19) |
| Globulin | 52 | 0.03 (.8) | 88 | 0.07 (.5) |
| Albumin/globulin ratio | 52 | 0.13 (.3) | 88 | −0.17 (.10) |
| Erythropoietin | 50 | −0.33 (.021) | 77 | −0.06 (.6) |
| . | Controls . | VHLR200W homozygotes . | ||
|---|---|---|---|---|
| N . | Rho (P) . | N . | Rho (P) . | |
| Age | 52 | 0.26 (.06) | 90 | 0.12 (.3) |
| Female sex | 52 | −0.19 (.19) | 90 | −0.02 (.8) |
| History of smoking | 52 | 0.10 (.5) | 90 | −0.05 (.7) |
| History of alcohol consumption | 52 | 0.01 (.9) | 90 | 0.16 (.12) |
| History of bleeding in the past year | 52 | −0.21 (.14) | 90 | −0.14 (.19) |
| History of thrombosis | 52 | −0.08 (.6) | 90 | 0.14 (.18) |
| Body mass index | 52 | 0.18 (.2) | 90 | 0.11 (.3) |
| Systolic blood pressure | 52 | 0.21 (.14) | 90 | −0.05 (.7) |
| Diastolic blood pressure | 52 | 0.05 (.7) | 90 | −0.07 (.5) |
| Hemoglobin | 52 | 0.29 (.038) | 90 | 0.08 (.5) |
| RBCs, ×106 | 52 | 0.20 (.16) | 90 | −0.03 (.8) |
| Mean corpuscular volume | 52 | −0.04 (.8) | 79 | 0.08 (.5) |
| Mean corpuscular hemoglobin concentration | 52 | 0.18 (.2) | 80 | −0.07 (.5) |
| WBCs | 52 | 0.30 (.031) | 90 | −0.03 (.8) |
| Platelets | 52 | 0.12 (.4) | 90 | 0.07 (.5) |
| Ferritin | 50 | 0.68 (< .0001)* | 77 | 0.36 (.001)* |
| Iron | 52 | 0.14 (.3) | 88 | 0.07 (.5) |
| Iron-binding capacity | 52 | −0.33 (.019) | 77 | −0.09 (.4) |
| Iron saturation | 52 | 0.23 (.11) | 77 | 0.12 (.3) |
| Protein | 52 | 0.32 (.021) | 88 | −0.05 (.7) |
| Albumin | 52 | 0.42 (.002)* | 88 | −0.14 (.19) |
| Globulin | 52 | 0.03 (.8) | 88 | 0.07 (.5) |
| Albumin/globulin ratio | 52 | 0.13 (.3) | 88 | −0.17 (.10) |
| Erythropoietin | 50 | −0.33 (.021) | 77 | −0.06 (.6) |
Significant after the Bonferroni correction for multiple comparisons.
The relationship of serum hepcidin concentration and serum ferritin concentration in VHLR200W homozygotes and controls. The Spearman correlation between hepcidin and ferritin was 0.68 (P < .0001) in controls and 0.36 (P = .001) in VHLR200W homozygotes. Forty-four of 90 VHLR200W homozygotes versus 1 of 50 controls with overlapping serum ferritin concentrations had hepcidin levels that were below the limit of detection, which is consistent with a relationship between VHLR200W homozygosity and a reduction in hepcidin expression.
The relationship of serum hepcidin concentration and serum ferritin concentration in VHLR200W homozygotes and controls. The Spearman correlation between hepcidin and ferritin was 0.68 (P < .0001) in controls and 0.36 (P = .001) in VHLR200W homozygotes. Forty-four of 90 VHLR200W homozygotes versus 1 of 50 controls with overlapping serum ferritin concentrations had hepcidin levels that were below the limit of detection, which is consistent with a relationship between VHLR200W homozygosity and a reduction in hepcidin expression.
Independent relationships of hepcidin to VHLR200W homozygosity, erythropoietin, and ferritin
By multiple linear regression, serum hepcidin concentration was significantly lower in VHLR200W homozygotes than controls without mutated VHL alleles after adjustment for serum ferritin and serum erythropoietin (Table 4). In ANOVA and after adjustment for ferritin and erythropoietin, the geometric mean (95% CI) hepcidin concentration was 8.1 (6.3-10.5) ng/mL in VHLR200W homozygotes versus 26.9 (18.6-30.0) ng/mL in controls (P < .0001). The independent inverse relationship of hepcidin with VHLR200W homozygosity persisted in subgroup analyses of subjects with low iron stores on the basis of serum ferritin concentration ≤ 25 μg/L or sufficient iron stores based on serum ferritin concentration > 25 μg/L. In contrast, none of these analyses showed a significant, independent relationship of erythropoietin with hepcidin. Although not shown in Table 4, hemoglobin concentration did not have a significant independent correlation with hepcidin concentration if it was substituted for erythropoietin in these models or if it was included along with erythropoietin.
Variables having an independent association with log hepcidin concentration by multiple linear regression
| . | Beta (95% CI) . | P . |
|---|---|---|
| All subjects (n=126)* | ||
| VHLR200W homozygosity | −0.52 (−0.74 to −0.30) | < .0001 |
| Serum ferritin (log) | 0.43 (0.27-0.58) | < .0001 |
| Serum erythropoietin (log) | −0.06 (−0.30-0.18) | .6 |
| Subjects with ferritin ≤ 25 μg/L (n = 71)† | ||
| VHLR200W homozygosity | −0.42 (−0.72 to −0.13) | .006 |
| Serum ferritin (log) | 0.05 (−0.22-0.33) | .7 |
| Serum erythropoietin (log) | −0.05 (−0.33-0.24) | .7 |
| Subjects with ferritin > 25 μg/L (n = 55)‡ | ||
| VHLR200W homozygosity | −0.38 (−0.72 to −0.05) | .026 |
| Serum ferritin (log) | 0.78 (0.41-1.16) | .006 |
| Serum erythropoietin (log) | −0.31 (−0.70-0.09) | .13 |
| . | Beta (95% CI) . | P . |
|---|---|---|
| All subjects (n=126)* | ||
| VHLR200W homozygosity | −0.52 (−0.74 to −0.30) | < .0001 |
| Serum ferritin (log) | 0.43 (0.27-0.58) | < .0001 |
| Serum erythropoietin (log) | −0.06 (−0.30-0.18) | .6 |
| Subjects with ferritin ≤ 25 μg/L (n = 71)† | ||
| VHLR200W homozygosity | −0.42 (−0.72 to −0.13) | .006 |
| Serum ferritin (log) | 0.05 (−0.22-0.33) | .7 |
| Serum erythropoietin (log) | −0.05 (−0.33-0.24) | .7 |
| Subjects with ferritin > 25 μg/L (n = 55)‡ | ||
| VHLR200W homozygosity | −0.38 (−0.72 to −0.05) | .026 |
| Serum ferritin (log) | 0.78 (0.41-1.16) | .006 |
| Serum erythropoietin (log) | −0.31 (−0.70-0.09) | .13 |
One observation was removed because of outlier (R2 = 0.58).
One observation was removed because of outlier (R2 = 0.19).
R2 = 0.53.
The serum hepcidin concentration decreases rapidly after phlebotomy (within 1-2 days), but the serum ferritin concentration takes longer to respond. Therefore, we repeated the multiple linear regression analysis with 44 controls with normal VHL alleles and 44 VHLR200W homozygotes whose last phlebotomy was more than 1 year before the time of the study or who had never had a phlebotomy. The VHLR200W homozygous state had an independent negative relationship with serum hepcidin concentration (β = −0.37, 95% CI −0.66 to −0.04, P = .014) and serum ferritin concentration had a significant positive relationship (β = 0.59, 95% CI 0.40-0.79, P < .0001), but erythropoietin did not have a significant independent relationship with hepcidin (P = .5). Hemoglobin concentration also did not have a significant relationship with hepcidin (data not shown).
Discussion
Hepcidin expression is reduced by both hypoxia and anemia,30 but the mechanisms for this effect are still under discussion. Under normoxic and iron-sufficient conditions, HIF-1α and HIF-2α undergo proteasomal degradation in a process that is mediated by the VHL protein, the recognition component of an E3 ubiquitin-protein ligase complex.2 HIF-1α and HIF-2α are subject to hydroxylation on specific proline residues by prolyl hydroxylase domain protein 2 (PHD2), an iron-dependent enzyme that requires oxygen as a substrate, and proline hydroxylation is required for the interaction of HIF-1α and HIF-2α with VHL.1,35 Therefore, HIF-1α and HIF-2α levels increase in response to hypoxia or iron deficiency, and this leads to increased expression of erythropoietin.4
Several lines of evidence indicate that the reduction in hepcidin expression associated with hypoxia and anemia might be mediated by erythropoietin or by the increased erythropoiesis associated with increased erythropoietin. Erythropoietin injection leads to decreased circulating hepcidin levels in humans36 and to reduced murine hepatic hepcidin gene expression in vivo.30,37,38 Stimulation of a hepatoma cell line with erythropoietin led to decreased hepcidin mRNA and protein,39 and studies in hepatocytes cultured in vitro suggested that erythropoietin may directly down-regulate hepcidin through erythropoietin receptor signaling and regulation of C/EBPα.32 However, in vivo, the effect of erythropoietin on reducing hepcidin expression depends on intact erythropoiesis,33,40 suggesting an indirect effect of erythropoietin on reducing hepcidin expression. Such an effect might be mediated by soluble factors derived from erythroblasts41,42 or by the effects of heightened erythropoiesis on iron levels.40
Evidence is also available for a more direct effect of HIFs on hepcidin expression. It has been reported that the promoter region of the murine hepcidin gene contains candidate HIF recognition elements, and that HIF-1 binds to and negatively transactivates the hepcidin promotor.31 Inactivation of hepatic HIF-1α leads to a reduced ability to down-regulate hepcidin in the setting of iron deficiency.31 Stabilization of HIF-1α and HIF-2α in the liver contributes to the induction of matriptase-243 and stabilization of HIF-1α to the induction of furin.44 Maptriptase-2 and furin cleave HJV to sHJV, which leads to a decrease in BMP-6 activation via interaction with HJV and to a reduction in hepcidin expression.44,45
In the present study, circulating hepcidin concentrations were significantly decreased among individuals with congenital up-regulation of HIF-1α and HIF-2α due to homozygosity for VHLR200W compared with the levels in control individuals with wild-type VHL. The significant association of lower serum hepcidin concentration with homozygosity for VHLR200W persisted after adjustment by multiple linear regression for serum ferritin concentration and for serum erythropoietin concentration or RBC count, which were also significantly altered in the VHLR200W homozygotes. Therefore, our findings are consistent with results showing that HIFs are engaged in coordinate regulation of iron metabolism and erythropoiesis through up-regulation of erythropoietin and down-regulation of hepcidin.31 In contrast, the present study did not observe independent relationships between erythropoietin, hemoglobin concentration, or RBC count and hepcidin. Therefore, our results are consistent with the idea that hypoxia signaling may suppress hepcidin in vivo, independently of the effects of erythropoietin, hemoglobin concentration, or RBC counts.
A limitation to this study is that we did not study hepcidin expression at the cellular level. The multiple linear regression model of serum hepcidin concentration in this study does not exclude the possibility that erythropoietin down-regulates hepcidin expression. Another limitation is that inflammatory cytokines tend to be elevated in Chuvash polycythemia,19 and cytokine levels were not measured in this study. However, increased inflammatory cytokines tend to increase hepcidin expression rather than lead to the decreased levels observed in this paper. Therefore, failure to account for cytokine levels would tend to bias the analysis against our finding of reduced hepcidin levels associated with VHLR200W homozygosity rather than in favor of this finding.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grant no. UH1-HL03679-05 from the National Heart, Lung, and Blood Institute (to V.R.G.) and the Office of Research on Minority Health; by Howard University General Clinical Research Center grant MO1-RR10284; and by National Institutes of Health grants R01HL079912-01 (to V.R.G.), and R01HL50077-14 (to J.T.P.).
National Institutes of Health
Authorship
Contribution: V.R.G. and J.T.P. designed and conducted the study, interpreted the data, and wrote the manuscript; G.Y.M., A.I.S., D.J.O., and L.A.P. designed and conducted the study; X.N., T.A., and S.N. conducted the study and wrote the manuscript; M.N. analyzed the data and wrote the manuscript; and T.G. conducted the study, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.T.P. has received research funding and served as a consultant for Amgen. V.R.G. has received research funding from Amgen and Merck and served as a consultant for Amgen, Merck, and Fibrogen. D.J.O. has received research funding from Amgen. The remaining authors declare no competing financial interests.
Correspondence: Victor R. Gordeuk, MD, Center for Sickle Cell Disease, Howard University, 2041 Georgia Ave NW, Washington, DC 20060; e-mail: vgordeuk@howard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal