Abstract
VWF is a plasma protein that binds platelets to an injured vascular wall during thrombosis. When exposed to the shear forces found in flowing blood, VWF molecules undergo lateral self-association that results in a meshwork of VWF fibers. Fiber formation has been shown to involve thiol/disulfide exchange between VWF molecules. A C-terminal fragment of VWF was expressed in mammalian cells and examined for unpaired cysteine thiols using tandem mass spectrometry (MS). The VWF C2 domain Cys2431-Cys2453 disulfide bond was shown to be reduced in approximately 75% of the molecules. Fragments containing all 3 C domains or just the C2 domain formed monomers, dimers, and higher-order oligomers when expressed in mammalian cells. Mutagenesis studies showed that both the Cys2431-Cys2453 and nearby Cys2451-Cys2468 disulfide bonds were involved in oligomer formation. Our present findings imply that lateral VWF dimers form when a Cys2431 thiolate anion attacks the Cys2431 sulfur atom of the Cys2431-Cys2453 disulfide bond of another VWF molecule, whereas the Cys2451-Cys2468 disulfide/dithiol mediates formation of trimers and higher-order oligomers. These observations provide the basis for exploring defects in lateral VWF association in patients with unexplained hemorrhage or thrombosis.
Introduction
VWF is a large, multimeric glycoprotein responsible for chaperoning the blood coagulation cofactor factor VIII and tethering platelets to the site of vascular endothelial injury.1 It is synthesized by vascular endothelial cells and megakaryocytes and circulates as a series of multimers containing variable numbers of 500-kDa dimeric units. Circulating multimers range between 500 and 20 000 kDa in size, and although any size multimer is able to chaperone factor VIII,2 it is the only the largest sizes that are able to effectively tether platelets. Each subunit contains binding sites for collagen and for platelet glycoproteins Ib and αIIbβ3.1
Multimer size is regulated in the circulation. An excess of high-molecular-weight multimers can cause unwanted thrombosis and is associated with thrombotic thrombocytopenic purpura,3 whereas a paucity of large multimers is associated with the bleeding disorder VWD.1 Under normal conditions, the size of VWF multimers is controlled by ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type-1 motifs) proteolysis of the Tyr1605-Met1606 peptide bond in the A2 domain.4
The tertiary and quaternary structure of VWF is influenced by the shear forces typically found in flowing blood. VWF undergoes a shear-dependent conformational change,5 and individual molecules can self-associate under both static6 and shear conditions.7-9 VWF reversibly transitions from a loosely coiled ball to an elongated structure in response to shear.5,10,11 Self-association of VWF has been reported in different systems. Soluble VWF perfused over a VWF-coated surface undergoes “homotypic” self-association that facilitates platelet adhesion.9 In addition, VWF self-associates into a flexible clump at a critical shear rate and, when perfused over a collagen-coated surface, forms a web of fibers that binds platelets.7,10 VWF fibers retain the shape acquired under shear when the shear is removed.
Endothelial cell–derived VWF also forms fibers when exposed to shear. VWF secreted by sheared endothelial cells forms “strings” that are several cell diameters in length and also bind platelets.8,12,13 The mechanism of formation of the VWF strings involves cysteine thiol/disulfide exchange, because the formation of the strings is inhibited by thiol alkylation12 and the string structure is disrupted by the small thiol N-acetylcysteine.14 Mature VWF, both purified from plasma and made recombinantly, contains unpaired cysteine thiols12,15-17 that have been localized to the N-terminal D3 and C-terminal C domains.12,13,17 Presumably, one or more of these cysteine thiols is involved in VWF self-association.
In the present study, we explored the thiol/disulfide mechanism of self-association of VWF. The location and abundance of the unpaired cysteines in the C-terminal part of VWF was elucidated using mass spectrometry (MS), and their involvement in lateral association was examined by mutating the cysteine residues. The Cys2431-Cys2453 and possibly Cys2451-Cys2468 thiols/disulfides in the C2 domain were found to mediate lateral association.
Methods
VWF expression constructs
C-terminal fragments of human VWF cDNA (OriGene) were cloned into the pCS2+ expression plasmid, which contained a chordin signal peptide to direct secretion followed by a c-Myc tag. C1-CK (residues 2255-2813), C1-C3 (residues 2255-2648), and C2 (residues 2429-2495) constructs were made. The Cys2431, Cys2453, Cys2451, and Cys2468 residues in the C2 expression construct were mutated to alanine, individually or in pairs, using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). All primers were from Sigma-Aldrich.
Expression and analysis of recombinant VWF fragments
The human embryonic kidney cell line HEK 293 was cultured in DMEM (Invitrogen) supplemented with 10% FCS, 2mM l-glutamine, 10 units/mL of penicillin, and 10 μg/mL of streptomycin. T75 flasks of cells at ∼ 70% confluence were transfected with 25 ng of vector DNA using poly(ethyleneimine) (Sigma-Aldrich)18 and incubated for 5 days at 37°C and 5% CO2. The conditioned medium was collected and centrifuged to remove cellular debris. Samples were resolved on NuPAGE Novex 4%-12% Bis-Tris Gel (Invitrogen) with MOPS running buffer under nonreducing and reducing conditions, and transferred to polyvinylidene difluoride membrane (Millipore). Proteins were detected by Western blot using 1:1500 dilutions of either peroxidase-conjugated anti–c-Myc mAbs (Roche) or anti-VWF rabbit polyclonal Abs (Dako). Chemiluminescence films were analyzed using a GS-700 Imaging Densitometer and Multi-Analyst software (Bio-Rad).
Purification and analysis of the C1-CK fragment
Conditioned medium (10 mL) was incubated with Red c-Myc Agarose (Sigma-Aldrich; 0.5 mL of packed beads) on a rotating wheel overnight at 4°C. The beads were collected, washed 3 times with TBS, and incubated with 50μM 3-(N-maleimidylpropionyl)biocytin (MPB; Invitrogen) for 60 minutes at 25°C. The beads were washed 3 times with TBS and the protein eluted from the beads with 0.05% SDS for 30 minutes at 70°C. The C1-CK fragment (10-20 μg) was resolved on NuPAGE Novex 4%-12% Bis-Tris Gel with MOPS running buffer under nonreducing conditions, stained with colloidal Coomassie, or transferred to polyvinylidene difluoride membrane and blotted with 1:1500 dilutions of either anti-VWF rabbit polyclonal Abs or streptavidin peroxidase (Dako) to detect the MPB label.
MS
Conditioned medium was incubated with Red c-Myc Agarose on a rotating wheel overnight at 4°C. The beads were collected, washed 3 times with TBS, and incubated with 50mM iodoacetamide for 30 minutes at 25°C. The beads were washed 3 times with TBS and the fragment eluted from the beads with 0.05% SDS for 30 minutes at 70°C. The C1-CK fragment was resolved on NuPAGE Novex 4%-12% Bis-Tris Gel under nonreducing conditions and stained with colloidal Coomassie. The protein band was excised from the gel, destained, dried, and incubated with 100mM DTT in 25mM NH4CO2 for 1 hour at 25°C. The gel slice was washed with NH4CO2, dried, and incubated with 50mM methyl methanethiolsulfonate (Sigma-Aldrich) in 25mM NH4CO2. The slices were washed and dried before digestion of the C1-CK fragment with 20 ng/μL of trypsin (Promega) and 50 ng/μL of Glu-C endopeptidase (Roche) in 25mM NH4CO2 overnight at 25°C.
Peptides were eluted from the slices with 5% formic acid, 50% acetonitrile for 30 minutes at 25°C and separated by nano-LC on a Ultimate 3000 HPLC (Dionex) using a fritless nano column (75 μ × ∼ 10 cm) containing C18 medium (5 μ, 200 Å Magic; Michrom). Peptides were resolved using a linear gradient of H2O:CH3CN (98:2, 0.1% formic acid) to H2O:CH3CN (64:36, 0.1% formic acid) at 250 nL/min for 60 minutes. Positive ions were generated by electrospray and analyzed in a LTQ FT Ultra (Thermo Electron) mass spectrometer operated in data-dependent MS/MS acquisition mode. MS data were searched using Mascot (V2.2, Matrix Science) against the nonredundant database from the National Center for Biotechnology Information. Search parameters were: precursor tolerance 10 ppm and product ion tolerances ± 0.4 Da. Cys-carboxyamidomethyl and Cys-methyldisulfide were selected as variable modifications with full tryptic cleavage of up to 5 missed cleavages. To determine the extent of alkylated cysteine residues in the C1-CK fragment, the relative ion abundance of peptides containing Cys-carboxyamidomethyl and Cys-methyldisulfide was used. To calculate ion abundance of peptides, extracted ion chromatograms were generated using the XCalibur Qual Browser software (Version 2.0.7, Thermo). The area was calculated using the automated peak detection function built into the software.
Structure prediction
Results
The domain structure of mature VWF and the recombinant C-domain fragments produced and analyzed in this study are described in Figure 1A.
UniProt domain structure of mature VWF and the C-domain fragments analyzed in this study. The mature N-terminus begins with a protease inhibitor I8 domain (I8, residues 772-828), followed by a D domain (residues 856-1074), a cysteine-rich domain (CR, residues 1053-1127), another I8 domain (residues 1140-1196), 3 type A domains (residues 1275-1458, 1496-1669 and 1689-1871), another D (residues 1938-2153) and I8 (residues 2199-2255) domain, 3 type C domains (residues 2255-2328, 2429-2495 and 2580-2645), and finishes with the C-terminal knot (CK, residues 2724-2812). Fragments encompassing the C1-CK (residues 2255-2813), C1-C3 (residues 2255-2648), and C2 (residues 2429-2495) domains were expressed in mammalian HEK cells and purified from the conditioned medium. The residue numbering is that for the preproprotein.
UniProt domain structure of mature VWF and the C-domain fragments analyzed in this study. The mature N-terminus begins with a protease inhibitor I8 domain (I8, residues 772-828), followed by a D domain (residues 856-1074), a cysteine-rich domain (CR, residues 1053-1127), another I8 domain (residues 1140-1196), 3 type A domains (residues 1275-1458, 1496-1669 and 1689-1871), another D (residues 1938-2153) and I8 (residues 2199-2255) domain, 3 type C domains (residues 2255-2328, 2429-2495 and 2580-2645), and finishes with the C-terminal knot (CK, residues 2724-2812). Fragments encompassing the C1-CK (residues 2255-2813), C1-C3 (residues 2255-2648), and C2 (residues 2429-2495) domains were expressed in mammalian HEK cells and purified from the conditioned medium. The residue numbering is that for the preproprotein.
Identification of the unpaired cysteine residues in the VWF C1-CK fragment
To elucidate the mechanism of lateral association of VWF, we first chose to analyze the redox status of the cysteine residues in the VWF C1-CK fragment. Myc-tagged C1-CK fragment was expressed in mammalian HEK cells and secretion was directed by a chordin signal sequence. The fragment was purified from the conditioned medium by c-Myc affinity chromatography. The protein was homogeneous by staining with colloidal Coomassie and was recognized by anti-VWF polyclonal Abs (Figure 2A). The C1-CK fragment has a molecular weight of ∼ 120 kDa, which is consistent with a dimer linked by disulfide bonds through the C-terminal knot.20 The fragment also labeled with a biotin-linked maleimide, indicating that it contains unpaired cysteine thiols (Figure 2A). This is in accordance with several reports of labeling of native plasma and recombinant VWF with thiol alkylators.12,15-17
Identification of the unpaired cysteine residues in the VWF C1-CK fragment. (A) The C1-CK fragment contains unpaired cysteine thiols. The fragment was expressed in mammalian HEK cells, purified from the conditioned medium, labeled with a biotin-linked maleimide (MPB), and resolved on SDS-PAGE. The fragment was visualized by staining with colloidal Coomassie or blotted with either anti-VWF Abs or with streptavidin-peroxidase to detect the MPB label. (B) Amino acid sequence and peptide coverage (underlined) of the VWF C1-CK fragment by tandem MS analysis. Seventy percent of the residues and 68% of the cysteines were covered in the analysis. (C) Tandem mass spectrum of the GCDVCTCTDME peptide showing Cys2453 (underlined) labeled with carboxyamidomethyl (free cysteine) and Cys2448 and Cys2451 labeled with methyldisulfide (paired cysteines). The accurate mass spectrum of the peptide is also shown in the inset (observed [M + 2H]2+ = 663.1872 m/z; expected [M + 2H]2+ = 663.1859 m/z).
Identification of the unpaired cysteine residues in the VWF C1-CK fragment. (A) The C1-CK fragment contains unpaired cysteine thiols. The fragment was expressed in mammalian HEK cells, purified from the conditioned medium, labeled with a biotin-linked maleimide (MPB), and resolved on SDS-PAGE. The fragment was visualized by staining with colloidal Coomassie or blotted with either anti-VWF Abs or with streptavidin-peroxidase to detect the MPB label. (B) Amino acid sequence and peptide coverage (underlined) of the VWF C1-CK fragment by tandem MS analysis. Seventy percent of the residues and 68% of the cysteines were covered in the analysis. (C) Tandem mass spectrum of the GCDVCTCTDME peptide showing Cys2453 (underlined) labeled with carboxyamidomethyl (free cysteine) and Cys2448 and Cys2451 labeled with methyldisulfide (paired cysteines). The accurate mass spectrum of the peptide is also shown in the inset (observed [M + 2H]2+ = 663.1872 m/z; expected [M + 2H]2+ = 663.1859 m/z).
The C1-CK fragment was digested with trypsin and Glu-C endopeptidase and the peptides were analyzed using tandem MS. Seventy percent of the amino acids and 68% of the cysteine residues of the fragment were covered in the analysis (Figure 2B). Both the unpaired cysteines and the disulfide-bonded cysteines were identified in the analysis. The unpaired cysteines were alkylated with iodoacetamide and the disulfide-bonded cysteine thiols with methyl methanethiolsulfonate after reduction with DTT. A tandem mass spectrum of the GCDVCTCTDME peptide (residues 2447-2457) is shown in Figure 2C. Based on the peptide fragmentation pattern, it is evident that Cys2453 in this peptide is labeled with carboxyamidomethyl (the iodoacetamide adduct). The ratio of carboxyamidomethyl to methyldisulfide (the methyl methanethiolsulfonate adduct) labeling represents the fraction of the cysteine in the population that is in the reduced state. Peptides with ratios > 0.1 are listed in Table 1. The remaining cysteine-containing peptides with ratios < 0.1 are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cys2453 in the C2 domain is clearly the most abundant unpaired cysteine of those analyzed in the C1-CK protein. The ratio of labeling for this cysteine was 3.00, compared with the next highest of 0.25 for Cys2574. Further results and discussion of this analysis is found in the supplemental materials.
Identification and quantification of the unpaired cysteine residues in the C1-CK fragment
| Peptide . | Free cysteine residue . | Abundance in sample . | Ratio of carboxyamidomethyl to methyldisulfide . | |
|---|---|---|---|---|
| Carboxyamidomethyl . | Methyldisulfide . | |||
| GCDVCTCTDME | 2453 | 521 147 | 173 452 | 3.00 |
| VAQCSQKPCEDSCR | 2468/2473 | 36 701 | 284 780 | 0.13 |
| TSACCPSCR | 2574 | 161 978 | 656 725 | 0.25 |
| TVMIDVCTTCR | 2600 | 43 408 | 180 645 | 0.24 |
| EENNTGECCGR | 2640 | 95 707 | 561 872 | 0.17 |
| IPGTCCDTCEEPE | 2715/2716 | 194 631 | 914 022 | 0.21 |
| Peptide . | Free cysteine residue . | Abundance in sample . | Ratio of carboxyamidomethyl to methyldisulfide . | |
|---|---|---|---|---|
| Carboxyamidomethyl . | Methyldisulfide . | |||
| GCDVCTCTDME | 2453 | 521 147 | 173 452 | 3.00 |
| VAQCSQKPCEDSCR | 2468/2473 | 36 701 | 284 780 | 0.13 |
| TSACCPSCR | 2574 | 161 978 | 656 725 | 0.25 |
| TVMIDVCTTCR | 2600 | 43 408 | 180 645 | 0.24 |
| EENNTGECCGR | 2640 | 95 707 | 561 872 | 0.17 |
| IPGTCCDTCEEPE | 2715/2716 | 194 631 | 914 022 | 0.21 |
The ratio of carboxyamidomethyl (free cysteines) to methyldisulfide (paired cysteines) labeling represents the fraction of the cysteine (underlined) in the population that is in the reduced state. Peptides with ratios > 0.1 are listed. The remaining cysteine containing peptides detected by mass spectrometry have ratios < 0.1 and are listed in supplemental Table 1.
Although this analysis covered only 68% of the cysteine residues in the C1-CK fragment, the relevant cysteines with respect to lateral association of VWF are likely all accounted for based on the findings of Choi et al.12 These investigators used a thiol-reactive matrix (thiopropyl-Sepharose) to capture the VWF C-terminal fragment that contains unpaired cysteine(s). As shown by MS analysis, the fragment consisted of residues 2435-2464, 2479-2493, and 2516-2535 and contained 7 cysteines, all of which were covered in our analysis (Figure 2B). Choi et al concluded that 1, 2, or possibly all 7 cysteine residues in the fragment were unpaired.12 Our analysis indicated that Cys2453 was the unpaired thiol that reacted with thiopropyl-Sepharose in their study.
Homology of the VWF C domains and joiner regions
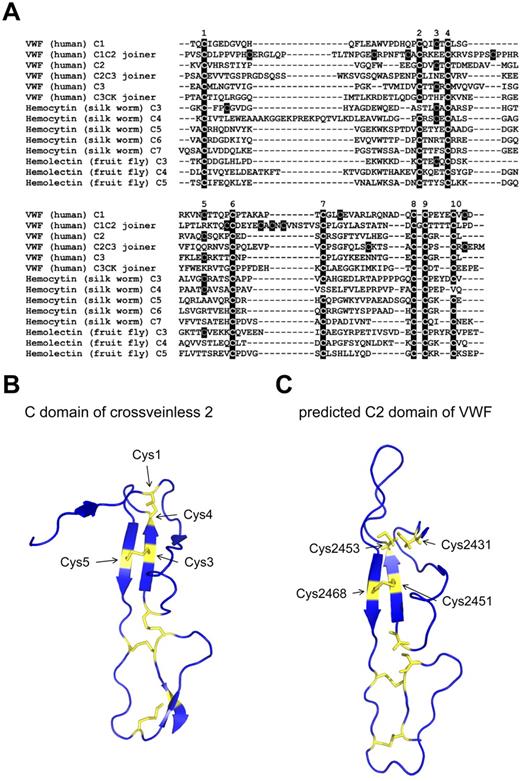
The C domain is 60-80 amino acids in length and is defined by a conserved spacing of 10 cysteine residues.21 In many cases, the domains have low sequence homology. Interestingly, we noticed that the C-domain joiner regions shared 8 of the 10 conserved C-domain cysteine residues (Figure 3A). All 3 C-joining regions in human VWF are missing Cys3 and Cys5, a pattern shared with several hemolectin (fruit fly) and hemocytin (silk worm) C domains (Figure 3A). Hemolectin and hemocytin are insect hemocyte-specific proteins that are believed to be important in the maintenance of invertebrate hemostasis and the immune system.22 A family of single C-domain proteins (SVC) that is also missing Cys3 and Cys5 has been described in the fruit fly.23 Based on X-ray crystal and predicted structures of the C domain, Cys3 and Cys5 form a disulfide pair (see next section). Whereas it is not possible to determine whether the joining regions and other hemolectin/hemocytin domains missing Cys3-Cys5 truly form the C fold without structural evidence, it is a reasonable assumption that they adopt a C fold. By analyzing the conservation of disulfide bonds in eukaryotes, we have recently shown that disulfide-bonded cysteines are highly conserved and that, once these cysteines are gained, they are rarely lost in protein evolution.24 It may be that the C domains missing the Cys3-Cys5 disulfide bond represent a less-evolved domain.
Analysis of the VWF C domains and joiner regions and predicted structure of the C2 domain of VWF. (A) Alignment of human VWF C domains and joining regions with C domains from hemocytin (silk worm) and hemolectin (fruit fly) using the Clustal 2.1 multiple sequence alignment tool. Insect C domains are derived and numbered as described previously.41 The 10 disulfide-forming cysteines in C domains are labeled above the alignment. (B) Ribbon structure of the C domain of crossveinless 2 (PDB ID 3BK3) showing the 5 disulfide bonds in yellow. The cysteine residues that form the Cys1-Cys4 and Cys3-Cys5 disulfide bonds are indicated. The X-ray structure has a resolution of 2.7 Å.25 (C) Predicted structure of the C2 domain of VWF. The structure was derived using the crossveinless 2 and procollagen 2A C-domain structures and the I-TASSER protein structure prediction tool.19 The C-score is −1.05. The cysteine residues are indicated in yellow. Cys2451 and Cys2468 form a disulfide bond in this structure, whereas Cys2431 and Cys2453 are unpaired.
Analysis of the VWF C domains and joiner regions and predicted structure of the C2 domain of VWF. (A) Alignment of human VWF C domains and joining regions with C domains from hemocytin (silk worm) and hemolectin (fruit fly) using the Clustal 2.1 multiple sequence alignment tool. Insect C domains are derived and numbered as described previously.41 The 10 disulfide-forming cysteines in C domains are labeled above the alignment. (B) Ribbon structure of the C domain of crossveinless 2 (PDB ID 3BK3) showing the 5 disulfide bonds in yellow. The cysteine residues that form the Cys1-Cys4 and Cys3-Cys5 disulfide bonds are indicated. The X-ray structure has a resolution of 2.7 Å.25 (C) Predicted structure of the C2 domain of VWF. The structure was derived using the crossveinless 2 and procollagen 2A C-domain structures and the I-TASSER protein structure prediction tool.19 The C-score is −1.05. The cysteine residues are indicated in yellow. Cys2451 and Cys2468 form a disulfide bond in this structure, whereas Cys2431 and Cys2453 are unpaired.
Predicted structure of the C2 domain of VWF
The structures of 2 C domains have been determined: the C domain of crossveinless 2 (X-ray structure, PDB ID 3BK3)25 and procollagen 2A (NMR structure, PDB ID 1U5M).26 The structure of the crossveinless 2 C domain is shown in Figure 3B. The 10 cysteine residues of the domain form 5 disulfide bonds. The disulfide connectivity is Cys1-Cys4, Cys2-Cys8, Cys3-Cys5, Cys6-Cys9, and Cys7-Cys10. An analysis of the disulfide bonds in this structure is shown in Table 2.
| Chain . | Cys1 residue† . | Cys1 solvent‡ . | Cys2 residue . | Cys2 solvent‡ . | Cα-Cα, ŧ . | Configuration . |
|---|---|---|---|---|---|---|
| 1 | 9 (1) | 17 | 31 (4) | 0 | 6.04 | +/−RHSpiral |
| 26 (2) | 26 | 60 (8) | 4 | 4.89 | +/−RHHook | |
| 29 (3) | 4 | 38 (5) | 16 | 3.98 | −RHStaple | |
| 43 (6) | 46 | 61 (9) | 35 | 5.39 | −LHSpiral | |
| 50 (7) | 37 | 64 (10) | 48 | 6.03 | −LHHook | |
| 2 | 9 (1) | 21 | 31 (4) | 0 | 5.96 | +/−RHSpiral |
| 26 (2) | 31 | 60 (8) | 12 | 5.08 | +/−RHHook | |
| 29 (3) | 9 | 38 (5) | 12 | 4.04 | −RHStaple | |
| 43 (6) | 54 | 61 (9) | 34 | 5.51 | −LHSpiral | |
| 50 (7) | 36 | 64 (10) | 57 | 6.12 | −RHSpiral |
| Chain . | Cys1 residue† . | Cys1 solvent‡ . | Cys2 residue . | Cys2 solvent‡ . | Cα-Cα, ŧ . | Configuration . |
|---|---|---|---|---|---|---|
| 1 | 9 (1) | 17 | 31 (4) | 0 | 6.04 | +/−RHSpiral |
| 26 (2) | 26 | 60 (8) | 4 | 4.89 | +/−RHHook | |
| 29 (3) | 4 | 38 (5) | 16 | 3.98 | −RHStaple | |
| 43 (6) | 46 | 61 (9) | 35 | 5.39 | −LHSpiral | |
| 50 (7) | 37 | 64 (10) | 48 | 6.03 | −LHHook | |
| 2 | 9 (1) | 21 | 31 (4) | 0 | 5.96 | +/−RHSpiral |
| 26 (2) | 31 | 60 (8) | 12 | 5.08 | +/−RHHook | |
| 29 (3) | 9 | 38 (5) | 12 | 4.04 | −RHStaple | |
| 43 (6) | 54 | 61 (9) | 34 | 5.51 | −LHSpiral | |
| 50 (7) | 36 | 64 (10) | 57 | 6.12 | −RHSpiral |
Calculated using the disulfide bond analysis tool.40 The protein crystallized as a dimer and the analysis of both chains (1 and 2) is shown.
The number in the bracket is the order of the cysteine in the primary sequence from 1-10.
Solvent accessibility is the area of the cysteine residue exposed to solvent in approximately Å2.
Distance between the α carbon atoms of the 2 cysteine residues.
The configuration of a disulfide bond can be described by the 5 bond angles of the cystine.27 There are 20 possible disulfide bond configurations using this criterion. The Cys1-Cys4 bond links a β-strand (Cys4) to a loop structure (Cys1) and has a +/−RHSpiral configuration. Cys1 is exposed to solvent. The Cys3-Cys5 disulfide is nearby (∼ 10 Å) the Cys1-Cys4 bond and has an interesting structure. It has a −RHStaple configuration and the cysteines link adjacent β-strands of a small antiparallel β-sheet. Both Cys3 and Cys5 are exposed to solvent. This type of disulfide bond has been associated with controlling mature protein function, the so-called allosteric disulfides.27 For example, the allosteric disulfides in the immune coreceptor CD428 and the HIV envelope protein gp12029 are −RHStaple bonds. A feature of −RHStaple bonds is the close proximity of the α-carbon atoms of the 2 cysteine residues that can impart strain on the bond.27,30 The distance between the α-carbons of the Cys3-Cys5 bond is 4.01 Å (Table 2, average of the 2 structures), compared with a mean of 5.62 Å for all disulfides in a nonredundant set of X-ray structures.31
The crossveinless 2 and procollagen 2A C domain structures were used to predict the structure of the VWF C2 domain using the I-TASSER protein structure prediction tool.19 It is apparent that the structures have a similar architecture and the pairing of the cysteine residues is the same (Figure 3C). Cys2431 and Cys2453 are predicted to be unpaired in this structure, but would form the Cys1-Cys4 disulfide bond. Cys2451 and Cys2468 form the Cys3-Cys5 disulfide bond. The structure prediction was not compatible with any other cysteine pairings. The finding that Cys2453 is unpaired in the C1-CK fragment (Figure 2) implies that the Cys2431-Cys2453 disulfide bond is redox active. Peptides containing Cys2431 were not resolved in the MS analysis, but this residue is predicted to be unpaired based on this structural analysis.
Oligomerization of the VWF C1-C3 and C2 fragments
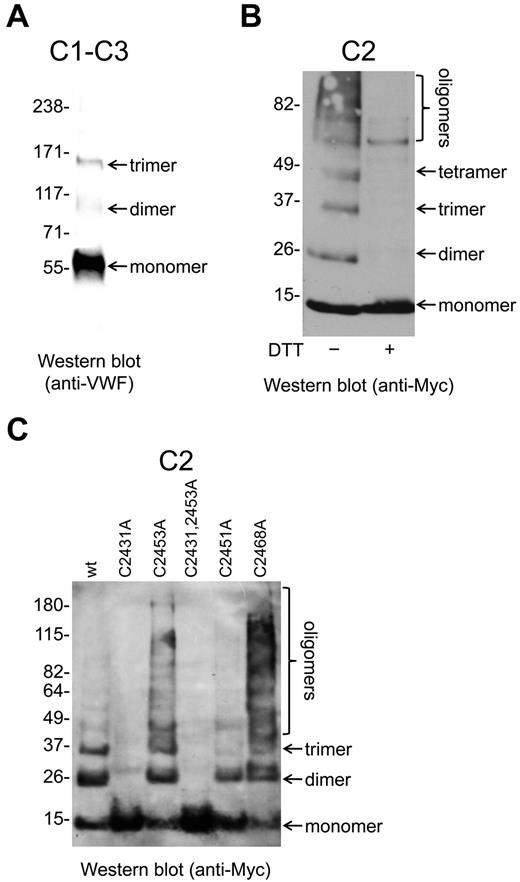
Myc-tagged VWF C1-C3 and C2 fragments (Figure 1) were expressed in mammalian HEK cells, purified from the conditioned medium by c-Myc affinity chromatography, resolved on SDS-PAGE, and examined by Western blot (Figure 4). The C1-C3 fragment was recognized by anti-VWF polyclonal Abs under nonreducing conditions but not when reduced with DTT The C2 fragment was not recognized by anti-VWF Abs, but was detected using Abs against the c-Myc tag.
Oligomerization of the VWF C2 domain is mediated by the Cys2431-Cys2453 and Cys2451-Cys2468 disulfides/dithiols. (A) The VWF C1-C3 fragment was expressed in mammalian HEK cells and purified from the conditioned medium. The fragment was resolved on SDS-PAGE and blotted with anti-VWF polyclonal Abs. The fragment was present as monomers, dimers, and trimers. (B) The Myc-tagged VWF C2 fragment was expressed in mammalian HEK cells and purified from the conditioned medium. The fragments were resolved on SDS-PAGE and blotted with anti-Myc Abs. The C2 domain was present as monomers, dimers, trimers, tetramers, and higher-order oligomers. The C2 domain multimers were disulfide-linked, because they resolved to monomers on incubation with DTT. (C) Wild-type or mutants of the Cys2431-Cys2453 and Cys2451-Cys2468 disulfide bonds of the Myc-tagged C2 fragment were expressed in mammalian HEK cells, purified from the conditioned medium, resolved on SDS-PAGE, and blotted with anti-Myc Abs. The wild-type C2 fragment was present as monomers, dimers, trimers, and higher-order oligomers. The C2431A and C2431A,C2453A C2 mutants were only present as monomers, the Cys2451A mutant only as monomers and dimers, whereas the C2453A and C2468A mutants demonstrated enhanced oligomerization.
Oligomerization of the VWF C2 domain is mediated by the Cys2431-Cys2453 and Cys2451-Cys2468 disulfides/dithiols. (A) The VWF C1-C3 fragment was expressed in mammalian HEK cells and purified from the conditioned medium. The fragment was resolved on SDS-PAGE and blotted with anti-VWF polyclonal Abs. The fragment was present as monomers, dimers, and trimers. (B) The Myc-tagged VWF C2 fragment was expressed in mammalian HEK cells and purified from the conditioned medium. The fragments were resolved on SDS-PAGE and blotted with anti-Myc Abs. The C2 domain was present as monomers, dimers, trimers, tetramers, and higher-order oligomers. The C2 domain multimers were disulfide-linked, because they resolved to monomers on incubation with DTT. (C) Wild-type or mutants of the Cys2431-Cys2453 and Cys2451-Cys2468 disulfide bonds of the Myc-tagged C2 fragment were expressed in mammalian HEK cells, purified from the conditioned medium, resolved on SDS-PAGE, and blotted with anti-Myc Abs. The wild-type C2 fragment was present as monomers, dimers, trimers, and higher-order oligomers. The C2431A and C2431A,C2453A C2 mutants were only present as monomers, the Cys2451A mutant only as monomers and dimers, whereas the C2453A and C2468A mutants demonstrated enhanced oligomerization.
The C1-C3 fragment was present as molecular weight species of ∼ 55 kDa, ∼ 110 kDa, and ∼ 160 kDa (Figure 4A). These sizes are consistent with monomers, homodimers, and homotrimers of the fragment, respectively. The C2 domain was present as molecular weight species of ∼ 10 kDa, ∼ 20 kDa, ∼ 30 kDa, ∼ 40 kDa, and larger oligomers (Figure 4B). These sizes are consistent with monomers, dimers, trimers, tetramers, and higher-order oligomers, respectively. All of the C2 species were disulfide linked, because they resolved to monomers on reduction with DTT (Figure 4B).
Oligomerization of the VWF C2 domain fragment is mediated by the Cys2431-Cys2453 and Cys2451-Cys2468 disulfides/dithiols
Mutants of the Cys2431-Cys2453 and Cys2451-Cys2468 disulfide bonds of the C2 fragment were analyzed for differential states of oligomerization. The Cys2431-Cys2453 bond was targeted because it was found to be reduced in the majority of the C1-CK molecules (Figure 2). We also targeted the Cys2451-Cys2468 disulfide bond for the following reasons: (1) this bond is uniquely missing in the C joiner regions and in some C domains in other organisms, which suggests a functional relevance; (2) the bond has all of the features of other functional (or allosteric) disulfides; and (3) the bond is very close to the Cys2431-Cys2453 bond in the tertiary structure, which suggests a relationship between the 2.
The C2431A and C2431A,C2453A C2 mutants were monomers only, the Cys2451A mutant monomers and dimers, and the C2453A and C2468A mutants demonstrated enhanced oligomerization compared with wild-type protein (Figure 4C). The C2451A,C2468A double mutant did not appear in the conditioned medium of the HEK cells, suggesting that the protein was recognized as misfolded and targeted for degradation.
Discussion
There is emerging evidence that lateral self-association of VWF is important for the hemostatic function of the protein. This interaction can result in large fibers of VWF (also called strings or ropes) that form a meshwork. The formation of collagen-tethered VWF meshes at sites of vascular injury is expected to be better able to capture platelets in flowing blood than individual molecules. The mechanism by which VWF molecules self-associate was explored in this study. Self-association has been reported to be covalent and involve unpaired cysteine residues.12,14 Considering the cluster of potential free thiols in and around the C2 domain,12,13 we focused on identifying the particular cysteines involved in this region and understanding how they exchange between VWF molecules during lateral self-association.
The following observations can be made from our results with the C-terminal VWF fragments and modeling of the C2 domain structure. The redox state of the Cys2431-Cys2453 disulfide bond in the C2 domain is ∼ 75% reduced and ∼ 25% oxidized. This suggests that the bond is reduced during or after secretion by a reductase. Ablating Cys2431 and both Cys2431 and Cys2453 in the C2 fragment by mutagenesis resulted in production of monomers only. This implies that the C2 dimers are at least held together by interchain disulfide bonds between Cys2431 residues. Ablating Cys2451 resulted in production of monomers and dimers only. This observation implies that trimers and higher-order oligomers of the C2 domain are held together by interchain disulfide bonds between Cys2451 and possibly Cys2431 residues. Ablating Cys2453 and Cys2468 resulted in enhanced multimerization of the C2 fragment compared with the wild-type protein. This suggests that the rate-limiting step in multimerization is the reduction of the Cys2431-Cys2453 and Cys2451-Cys2468 disulfide bonds; that is, cleaving the bonds by mutating the cysteine residues not involved in interchain disulfides removes the energy burden of reducing the bonds. These conclusions are brought together in the proposed mechanism of lateral association of VWF shown in Figure 5.
Proposed mechanism of lateral association of VWF. The Cys2431-Cys2453 disulfide bond in the C2 domain (shown in red) is reduced by a reductase in blood or in the injured vascular wall. The Cys2431 thiolate anion (present as a certain percentage of all thiols by action of the buffer) attacks the Cys2431 sulfur atom of the Cys2431-Cys2453 disulfide bond of another VWF molecule resulting in a disulfide-linked VWF lateral dimer. A VWF trimer may be formed when the Cys2451 thiolate anion (shown in green) of one of the VWF molecules in the dimer attacks the Cys2431 sulfur atom of the Cys2431-Cys2453 disulfide bond of a third VWF molecule. Higher-order oligomers of VWF may be formed by the addition of other molecules by the same reaction.
Proposed mechanism of lateral association of VWF. The Cys2431-Cys2453 disulfide bond in the C2 domain (shown in red) is reduced by a reductase in blood or in the injured vascular wall. The Cys2431 thiolate anion (present as a certain percentage of all thiols by action of the buffer) attacks the Cys2431 sulfur atom of the Cys2431-Cys2453 disulfide bond of another VWF molecule resulting in a disulfide-linked VWF lateral dimer. A VWF trimer may be formed when the Cys2451 thiolate anion (shown in green) of one of the VWF molecules in the dimer attacks the Cys2431 sulfur atom of the Cys2431-Cys2453 disulfide bond of a third VWF molecule. Higher-order oligomers of VWF may be formed by the addition of other molecules by the same reaction.
The Cys2431-Cys2453 disulfide bond in one molecule of VWF is reduced by a reductase, and the resulting Cys2431 thiolate attacks the same disulfide bond in another VWF molecule, forming the dimer. A third VWF molecule can be added to the dimer when the Cys2451 thiolate of one of the molecules in the dimer attacks the Cys2431-Cys2453 disulfide bond of the third molecule. Other molecules can then added by the same reaction. This is the simplest mechanism that can explain the data and is consistent with the findings that have been published to date. Considering that native VWF consists of variable numbers of homodimers linked through the N-terminal CR domain (Figure 1), the Cys2451-Cys2468 disulfide bond does not have to be involved to form trimers and higher-order oligomers of this molecule. The Cys2431-Cys2453 disulfide/dithiol of different monomeric units of the multimer can simply exchange with other monomeric units of another multimer. Studies of lateralization of cysteine mutants of the full-length protein secreted by cells under shear will answer this question.
Our future studies will focus on the identity of the reductase that cleaves the Cys2431-Cys2453 and Cys2451-Cys2468 disulfide bonds. Two candidates are thioredoxin and protein disulfide isomerase (PDI). Thioredoxin has been shown to cleave the Cys288-Cys326 disulfide bond in β2-glycoprotein I in plasma,32-34 as well as in 2 other disulfide bonds with the same −RHStaple configuration as the VWF Cys2451-Cys2468 bond.28,29 Conversely, PDI35-37 is on the surface of platelets38 and platelet microparticles39 and so will be in the vicinity of VWF during thrombus formation. Moreover, PDI ligands inhibit thrombus formation in vivo.36,37 Identifying the reductase is important because its action appears to be the rate-limiting step in the lateral association of VWF.
The redox chemistry of VWF lateral association is predicted to be influenced by compounds that react with the C2 domain disulfides/dithiols. Two plasma proteins that may play a role in this chemistry are β2-glycoprotein I and ADAMTS13. β2-Glycoprotein I reduced with thioredoxin forms disulfide-linked complexes with VWF.33 This suggests that the Cys288 and/or Cys326 thiols of reduced β2-glycoprotein I can attack the Cys2431-Cys2453 (or Cys2451-Cys2468) disulfide of VWF. Similarly, ADAMTS13 contains unpaired cysteine thiols in the C-terminal region that react with the C2 domain of VWF.17 Another layer of control of VWF lateral association may be redox active small molecules generated during inflammation. Activation of inflammatory cells (neutrophils and macrophages) results in the production of large amounts of myeloperoxidase-generated HOCl, which rapidly reacts with cysteine thiols. By influencing thiol/disulfide–exchange events in VWF, HOCl may promote or inhibit VWF fiber formation.
The importance of lateral association of VWF for its hemostatic role in humans has not been measured. Defects of lateralization would not be reflected in abnormal results in standard tests of VWF antigen, activity, or multimer patterns. It is possible, therefore, that impaired VWF lateralization is responsible for hemostatic defects in some patients with apparently normal VWF and platelet function. From the results presented herein, point mutations in cysteine residues 2431 and 2451 result in impaired lateral association that may present as bleeding, whereas mutations in cysteine residues 2453 and 2468 result in enhanced lateral association that may present as thrombosis. An assay for VWF lateralization that could be performed in whole blood or plasma would be required to identify these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bruno Reversade for the pCS2+ expression plasmid containing the chordin signal peptide to direct secretion and Vivien Chen for helpful discussions.
This study was supported by grants from the National Health and Medical Research Council of Australia, the Cancer Council New South Wales, and the Cancer Institute New South Wales.
Authorship
Contribution: T.G. performed the experiments and contributed to the experimental design; J.W.H.W. performed the homology analysis and contributed to the analysis of the data; C.S. contributed to the analysis of the data; and P.J.H. conceived the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Philip Hogg, Director, Lowy Cancer Research Centre, Level 2, Corner High and Botany Streets, University of New South Wales, Sydney, NSW 2052, Australia; e-mail: p.hogg@unsw.edu.au.

![Figure 2. Identification of the unpaired cysteine residues in the VWF C1-CK fragment. (A) The C1-CK fragment contains unpaired cysteine thiols. The fragment was expressed in mammalian HEK cells, purified from the conditioned medium, labeled with a biotin-linked maleimide (MPB), and resolved on SDS-PAGE. The fragment was visualized by staining with colloidal Coomassie or blotted with either anti-VWF Abs or with streptavidin-peroxidase to detect the MPB label. (B) Amino acid sequence and peptide coverage (underlined) of the VWF C1-CK fragment by tandem MS analysis. Seventy percent of the residues and 68% of the cysteines were covered in the analysis. (C) Tandem mass spectrum of the GCDVCTCTDME peptide showing Cys2453 (underlined) labeled with carboxyamidomethyl (free cysteine) and Cys2448 and Cys2451 labeled with methyldisulfide (paired cysteines). The accurate mass spectrum of the peptide is also shown in the inset (observed [M + 2H]2+ = 663.1872 m/z; expected [M + 2H]2+ = 663.1859 m/z).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/19/10.1182_blood-2011-06-360297/4/m_zh89991180970002.jpeg?Expires=1765901901&Signature=HT6-MJfxDu2ymbFKq-Pm1AMhUw1Dc8SuWgSm0ZK2mDf5B1ewPX8WCl3fzE83nvIR-QdOOGK-LpXN8bXoUPNYObjyYjhLNvNoCmUi~jr2AfqqUy96I5-MlKjZ3WIUd2Bd4BuutpMs4KroWVuAfa6QDrggyG7jkSNECV3GdGk6gToGOxYvIxDv9EViHcJna7N6wLZ4nvzuW7W2qJB64ManUS9f2WrvsmUT-hNDfSaOvj33bZZogDJ2zPVBB~kS7PRIHeANWnLnnQKpIRblaCgKJxuHvsiaJkUClEouYU5mSauWiEiemBvcXmObzz3HOmv64hDyyiyq6w7JidxHDukfNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal