Abstract
Inflammation is increasingly recognized as a critical mediator of angiogenesis, and unregulated angiogenic response is involved in human diseases, including cancer. Proinflammatory prostaglandin E2 (PGE2) is secreted by many cell types and plays important roles in the process of angiogenesis via activation of cognate EP1-4 receptors. Here, we provide evidence that PGE2 promotes the in vitro tube formation of human microvascular endothelial cells, ex vivo vessel outgrowth of aortic rings, and actual in vivo angiogenesis. Use of EP subtype-selective agonists and antagonists suggested EP4 mediates the prostaglandin-induced tube formation, and this conclusion was substantiated with small interfering RNA to specifically knockdown the EP4 expression. EP4 couples to Gαs, leading to activation of protein kinase A (PKA). Inhibition of PKA activity or knockdown of PKA catalytic subunit γ with RNAi attenuates the PGE2-induced tube formation. Further, knocking down the expression of Rap1A, HSPB6, or endothelial NO synthase, which serve as PKA-activatable substrates, inhibits the tube formation, whereas knockdown of RhoA or glycogen synthase kinase 3β that are inactivated after phosphorylation by PKA increases the tube formation. These results support the existence of EP4-to-PKA angiogenic signal and provide rationale for use of selective EP4 signal inhibitors as a probable strategy to control pathologic angiogenesis.
Introduction
Blood vessel formation (angiogenesis) is involved in various human physiologic and pathologic conditions, including wound healing, cancer, and neovascular diseases of the eye. Our basic knowledge of regulatory mechanisms involved in angiogenesis is derived primarily from observations with vascular endothelial growth factor (VEGF) as the angiogenic inducer. However, recent clinical and basic science discoveries indicate that the angiogenic response is more complex and may involve (many) other factors.1 Emerging evidence supports a role for inflammation in angiogenesis and suggests mutual dependency of the 2 processes. During inflammatory reactions, immune cells synthesize and secrete proangiogenic factors that promote neovascularization. The newly formed vasculature, in turn, contributes to sustain the inflammation by facilitating recruitment of inflammatory cells to the affected sites.2 However, molecular messengers involved in the relay of information between the 2 compartments remain to be fully identified.
Prostaglandins are naturally occurring lipids that are produced from cyclooxygenase (COX)–mediated metabolism of arachidonic acid. The prostaglandins are abundantly expressed in inflammatory sites and act locally to regulate pathologic responses. Notably, COX2 expression is up-regulated in a variety of cell types present in diseased organs, including tumors where its expression correlates with poor prognosis. Prostaglandin E2 (PGE2) is the predominant prostaglandin in solid tumors and is synthesized in the cancer epithelial, blood vessel endothelial, and immune cells.3 The PGE2 exerts its effects in an autocrine and paracrine fashion and its effect on cancer cell growth, survival, and migration as well as immune cell responses are well established.4 Less characterized, however, are the mechanisms and effects of PGE2 on endothelial cells and angiogenesis.
PGE2 affects target cells by activating 4 cognate receptors named EP1, EP2, EP3, and EP4 that belong to the large family of G protein–coupled receptors.5 PGE2-mediated activation of individual EPs leads to the exchange of GDP for GTP on the Gα subunits and subsequent dissociation of the Gα-GTP from the Gβγ subunits. In most cells, PGE2-bound EP1 couples to Gq and induces activation of protein kinase C through mobilization of intracellular Ca+2 ions. EP3 couples to Gi and inhibits synthesis of cAMP, thereby attenuating activity of the cAMP-dependent protein kinase (PKA). Both EP2 and EP4 couple to Gs, leading to increased synthesis of the cAMP and consequent activation of PKA. The Gβγ subunits also transduce signals in response to EP stimulation, including transactivation of epidermal growth factor receptor and its multiple downstream effectors. Hence, PGE2 transduces the receptor subtype-specific signaling events.5
Available evidence suggests involvement of PGE2 in angiogenesis. In the tumor milieu, for example, PGE2 may act on cancer cells, thereby prompting them to produce proangiogenic factors such as VEGF, basic fibroblast growth factor (bFGF), and the chemokine CXCL1 that, in turn, activate endothelial cells to instantiate angiogenesis.6-8 PGE2 may also act directly on the endothelial cells to promote their angiogenic response: the stimulation with PGE2 was reported to increase expression of CXCR4, thereby promoting the endothelial cell migration.9 Nonetheless, it remains largely unclear just how PGE2 promotes angiogenesis. Here, we investigated the possible involvement of EPs and their selective downstream effectors in angiogenesis with the use of in vitro endothelial cell tube formation, ex vivo aortic ring outgrowth, and in vivo angiogenesis assays. The results show that PGE2 promotes angiogenesis through activation of endothelial cell–expressed EP4 and PKA catalytic γ subunit. Moreover, the results identified Rap1A, HSPB6, and endothelial NO synthase (eNOS), which serve as PKA substrates to become activated, as mediators of angiogenesis, and RhoA and glycogen synthase kinase-3β (GSK3β), which are inactivated after phosphorylation by PKA, as inhibitors of angiogenesis, suggesting the multiple PKA substrates may act independently but coordinately to balance the angiogenic response. These results provide rationale for the targeting of EP4 and PKA to limit pathologic angiogenesis.
Methods
Reagents
Antibodies were obtained as follows: anti-VASP from Chemicon; anti-PKA Cα, anti-GSK3β, anti–phospho-Ser9-GSK3β, and anti-actin from Cell Signaling; anti-PKA Cβ, anti-PKA Cγ from Abcam; anti-eNOS from BD Biosciences, anti-CD31 from Santa Cruz Biotechnology; and anti-RhoA from Cytoskeleton. Secondary Abs were from Jackson ImmunoResearch Laboratories. Chemicals were obtained as follows: PGE2, PGE1-OH, sulprostone, butaprost, AH23848, and AH6809 from Cayman Chemical; isoproterenol, db-cAMP, Rp-cAMPS, H-89, and LiCl from Sigma-Aldrich; purified recombinant GST-fusion full-length PKA Cα, Cβ, and Cγ proteins from Echelon; PKI from Calbiochem; and VEGF from Cell Signaling. Control and targeted small interfering RNAs (siRNAs) were from Dharmacon.
Cell culture
Neonatal human dermal microvascular endothelial cells (HMVECs; Lonza) were grown in endothelial growth medium-2 at 37°C in a humidified 95% air and 5% CO2 atmosphere. Where indicated, cells were starved for 16 hours in endothelial basal medium-2 (EBM-2; Lonza) supplemented with 0.1% (wt/vol) BSA. Cells were used for ≤ 8 passages. Knockdown of indicated endogenous genes was achieved with ON-TARGETplus SMARTpool siRNA (Dharmacon). Cells grown on 6-well plates to 40% confluence were transfected with 50-100nM siRNA and 3-4 μL DharmaFECT 2 reagent. For all assays, pooled transfected cells were equally divided to ensure the identical cell populations, and cell starvation commenced 56 hours after transfection. All experiments were repeated a minimum of 3 times.
In vitro tube formation
The 96-well plates were coated with 40 μL/well growth factor-reduced Matrigel (BD Biosciences) and incubated for 30 minutes at 37°C to allow for gel polymerization. After starvation, HMVECs were trypsinized, counted, and seeded at 1.5-2.0 × 104 cells/well in the presence or absence of indicated reagents. Images of forming tubes were captured 6 hours later with the use of a Leica Microsystems 4000B microscope equipped with a RTKE Spot camera (model 7.2 color mosaic) and Spot Version 4.1.3 software (Diagnostic Instruments). The number of branching points of the tubular network in the entire fields (at 50× magnification 5×/0.5 NA objective at 25°C) of each well was quantified with ImageJ software (National Institutes of Health). Experiments were performed in triplicate and repeated 3 times.
CAM assay
Fertilized chicken eggs (Chicken Houses Plus) were maintained in an egg incubator (Chicken Houses Plus) for 10 days. A small window (10 × 10 mm) was made at the blunt end of the egg to expose the chorioallantoic membrane (CAM). PGE2 or recombinant VEGF was applied every 12 hours onto the entire exposed surface of the CAMs. Windows were sealed with transparent tape, and the eggs were incubated for 3 days. Eggs were then placed on ice for 30 minutes, and the CAMs were harvested and washed with PBS. After peeling off the upper protective nonvessel layer of the CAM, the remaining intact basal layer was spread on a glass coverslip and analyzed under light microscopy as indicated for the in vitro tube formation assay.10 Vessel quantification was performed by counting the branching points in 4-6 randomly chosen fields (50× magnification) for each CAM. Experiments were initiated with 12 eggs for each group and repeated 3 times.
Aortic ring outgrowth assay
Thoracic aorta harvested from 8-week-old C57BL/6 mice were rinsed with EBM-2 medium, cleaned of periadventitial fat and connective tissues, sectioned into 1-mm rings, washed with RPMI 1640 culture medium supplemented with penicillin and streptomycin, and placed into 96-well plates precoated with Matrigel. Consecutive pieces of aortic rings were divided into 3 groups and incubated in EBM-2 medium supplemented with 2% FBS. Ring tissues were treated daily with PGE2 or VEGF, and cell sprouting was photographed on day 7 using the same equipment described for the in vitro tube formation assay. The area of the vessel outgrowth was quantified with ImageJ software (National Institutes of Health).11 Two rings from each of a total of 4 aortas were used for each assay that was repeated 3 times.
cAMP measurement
HMVECs were treated with agonist for 5 minutes, and the cAMP levels were measured with a direct cAMP enzyme immunoassay kit (Assay Designs) according to the manufacturer's instructions. All assays were performed in triplicate and repeated 3 times.
Real-time PCR
Total RNA was extracted with Trizol Reagent (Invitrogen), and a total of 2 μg was reverse-transcribed (in a final volume of 20 μL) with the use of SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer's instructions. Real-time PCR containing 0.5 μL of cDNA and 10 μL of iQ SYBR Green Supermix (Bio-Rad) in a volume of 20 μL was performed in triplicate with the use of iCycler detection system (Bio-Rad). Target gene expression was normalized to GAPDH expression. The primers were as follows: EP4 sense, 5′-TGC TCT TCT TCA GCC TGT CC-3′, and EP4 antisense, 5′-AGA CTG CAA AGA GCG TGA GG-3′; PKACA sense, 5′-GAT GCT GGT GAA ACA CAA GG-3′, and PKACA antisense, 5′-TTT CAT TCA GGG TGT GTT CG-3′; PKACB sense, 5′-GGG CTA CAA TAA GGC AGT GG-3′, and PKACB antisense, 5′-TGG TGG GTC TGC AAA GAA TG-3′; PKACG sense, 5′-CTG AGC AAA GGC TAC AAC AAG G-3′, and PKACG antisense, 5′-GAT CTT CTC GTA GAT CTG GAT GG-3′; and GAPDH sense, 5′-GGT CAT GAG TCC TTC CAC GAT-3′, and GAPDH antisense, 5′-CAT GGG TGT GAA CCA TGA GAA-3′.
Western blotting
Appropriately treated HMVECs were lysed with RIPA buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 2mM EDTA, 1% NP-40, and 0.1% SDS) containing fresh phenylmethylsulfonyl fluoride (1mM), protease inhibitor cocktail (Sigma-Aldrich), and phosphatase inhibitor cocktail (Sigma-Aldrich). Total cell lysate (5-50 μg/lane) or recombinant proteins (10-160 ng/lane) were separated on 8%-12% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked in 4% BSA/PBST (0.1% Tween-20 in PBS) at ambient temperature for 1 hour, incubated with primary rabbit anti-VASP (1:1000), rabbit anti-GSK3β (1:1000), rabbit anti–pSer9-GSK3β (1:750), mouse anti-RhoA (1:1000), mouse anti-βactin (1:10 000), mouse anti-eNOS (1:1000), rabbit anti-PKA Cα (1:1000), rabbit anti-PKA Cβ (1:1000), or rabbit anti-PKA Cγ (1:1000) Abs at 4°C for 16 hours, followed by washing and incubation with HRP-coupled anti–mouse or anti–rabbit Abs (1:5000) for 1 hour. Signals were visualized with ECL plus Western blotting detection reagent (GE Healthcare) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). Blots were imaged with HP Scanjet 4370.
Animal studies
All experiments involving mice were done according to a protocol reviewed and approved by the Institutional Animal Care and Use Committee at the University of Florida. Sterile polyvinyl acetal CF-50 round sponges (diameter, 8 mm; thickness, 3 mm; kind gift from Dr J. M. Davidson, Vanderbilt University) were implanted under the dorsal skin of 9-week-old female C57BL/6 mice (17-20 g of body weight; n = 4-5 animals/treatment).12 One week after implantation, sponges were directly injected every other day for 14 days with vehicle (DMSO), PGE2 (8.8 ng/g body weight), AH23848 (25 or 125 ng/g body weight), or H89 (11 or 55 ng/g body weight). The sponges were harvested and immediately photographed. Each sponge was cut into 2 parts, with one part weighed, immersed into 0.5 mL of distilled water, chopped into small pieces, and stored at 4°C overnight (for hemoglobin analysis). The second part was fixed in buffered formalin phosphate (Fisher Scientific) at ambient temperature for 16 hours (for blood vessel density determination). The amount of hemoglobin in the sponges was determined with the Drabkin reagent (Sigma-Aldrich) as recommended by the manufacturer.
Immunohistochemistry
Paraffin-embedded sponges were sectioned (6 μm), deparaffinized in xylene, and rehydrated in graded alcohol. The sections were subjected to heat-induced Ag retrieval with Target Retrieval Solution (Dako), and nonspecific proteins were blocked with Protein Block (Dako). Tissues were probed with a rabbit anti-CD31 (1:50) Ab at 4°C overnight, washed, and incubated with a HRP-conjugated anti–rabbit secondary Ab (1:100). Sections were developed with AEC+Substrate (Dako), counterstained with hematoxylin, and mounted with Faramount aqueous mounting medium (Dako). Four microscopic images of the fields (200× magnification using 20×/0.40 NA objective at 25°C) in each section that contain the greatest microvessel density (hotspots) were taken. Any red staining of cell or cell cluster that was separate from adjacent microvessels was considered a single, countable vessel, and the 3 highest vessel counts for each section were used for statistical analysis.13
Statistics
Data are expressed as means ± SEMs. Statistical analysis was performed with 1-way ANOVA with Bonferroni posttest, and a P < .05 was considered statistically significant. All analyses were performed and all graphs were generated with the use of Microsoft Excel. Figure axis labels were created with Adobe Illustrator CS5 (Adobe Systems).
Results
PGE2 promotes angiogenesis
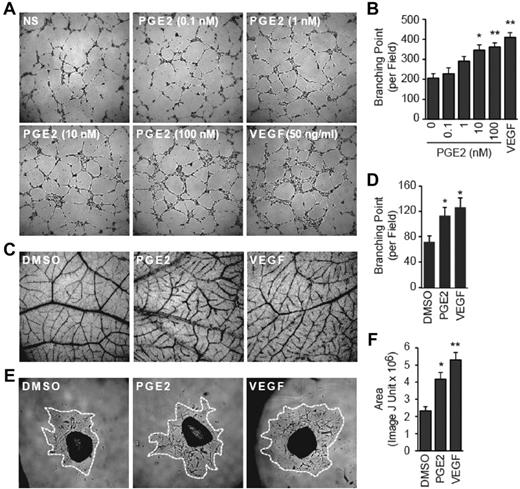
We used 3 distinct experimental approaches, namely in vitro tube formation of HMVECs, in vivo chicken CAM blood vessel formation, and ex vivo mouse thoracic aorta ring outgrowth to probe angiogenic effects of PGE2. The PGE2 exerted a dose-dependent increase in the tube formation of HMVECs (Figure 1A) as quantitated by the number of branching points (Figure 1B). Similarly, the treatment with PGE2 increased blood vessel density (Figure 1C) and branching point number (Figure 1D) in the CAM assay. Finally, treatment with PGE2 promoted sprouting from aortic rings as measured by the increased area of vessel outgrowth (Figure 1E-F). Remarkably, effects of PGE2 on the HMVEC tube formation, CAM angiogenesis, and aorta outgrowth were comparable to those achieved with VEGF, supporting the conclusion that PGE2 is a potent proangiogenic factor.
PGE2 promotes angiogenesis. (A-B) PGE2 promotes the in vitro tube formation. HMVECs were seeded on growth factor–reduced Matrigel-coated wells in the absence or presence of PGE2 or VEGF. The tube formation images at 50× magnification in the central field of the well were taken 6 hours later (A), and the branching points were counted with ImageJ software (National Institutes of Health). (B) Each point represents the mean ± SEM of 3 experiments performed in triplicate. (C-D) PGE2 promotes angiogenesis. Fertilized chicken eggs were incubated for 10 days, and CAMs were treated with PGE2 (1μM) or VEGF (100 ng/mL) twice per day for a total of 3 days. Blood vessel images at a 50× magnification were photographed (C), and branching points quantified with the ImageJ software (National Institutes of Health; D). The experiments were initiated with 12 eggs for each group and were culminated with 4-6 evaluable CAMs. Each point represents the mean ± SEM of 3 independent experiments. (E-F) PGE2 promotes aortic ring vessel outgrowth. Mouse thoracic aorta were sectioned into 1-mm rings and placed into growth factor–reduced Matrigel-coated wells. The rings were treated daily with PGE2 (100nM) or VEGF (50 ng/mL) for a total of 6 days, and 50× magnification images were recorded. The area of the cell outgrowth was quantified with the ImageJ (National Institutes of Health). (F) Two rings per aorta from a total of 4 aortas were used for each group, and the assay was repeated 3 times. Each point represents the mean ± SEM of 5 evaluable outgrowths. (B,D,F) *P < .05 and **P < .01 versus vehicle groups.
PGE2 promotes angiogenesis. (A-B) PGE2 promotes the in vitro tube formation. HMVECs were seeded on growth factor–reduced Matrigel-coated wells in the absence or presence of PGE2 or VEGF. The tube formation images at 50× magnification in the central field of the well were taken 6 hours later (A), and the branching points were counted with ImageJ software (National Institutes of Health). (B) Each point represents the mean ± SEM of 3 experiments performed in triplicate. (C-D) PGE2 promotes angiogenesis. Fertilized chicken eggs were incubated for 10 days, and CAMs were treated with PGE2 (1μM) or VEGF (100 ng/mL) twice per day for a total of 3 days. Blood vessel images at a 50× magnification were photographed (C), and branching points quantified with the ImageJ software (National Institutes of Health; D). The experiments were initiated with 12 eggs for each group and were culminated with 4-6 evaluable CAMs. Each point represents the mean ± SEM of 3 independent experiments. (E-F) PGE2 promotes aortic ring vessel outgrowth. Mouse thoracic aorta were sectioned into 1-mm rings and placed into growth factor–reduced Matrigel-coated wells. The rings were treated daily with PGE2 (100nM) or VEGF (50 ng/mL) for a total of 6 days, and 50× magnification images were recorded. The area of the cell outgrowth was quantified with the ImageJ (National Institutes of Health). (F) Two rings per aorta from a total of 4 aortas were used for each group, and the assay was repeated 3 times. Each point represents the mean ± SEM of 5 evaluable outgrowths. (B,D,F) *P < .05 and **P < .01 versus vehicle groups.
PGE2 exerts proangiogenic effects by activating EP4
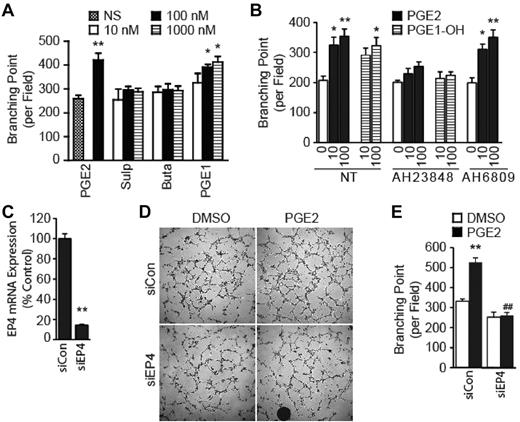
PGE2 binds to and activates 4 cognate receptors named EP1, EP2, EP3, and EP4.5 To determine which EP subtype(s) transduce the PGE2-mediated angiogenic response, we first determined expression level of the EP genes with the use of quantitative real-time PCR. Results evidenced expression of all EP genes in the HMVECs (data not shown). We used selective EP subtype agonists and show that stimulation of HMVECs with the EP1/EP3-selective sulprostone or EP2-selective butaprost failed to recapitulate the PGE2 effects on the tube formation (Figure 2A). Distinctly, the selective activation of EP4 with PGE1-OH significantly enhanced ability of the HMVECs to form tubes (Figure 2A).
PGE2 promotes the tube formation by activating EP4. (A) Effect of EP agonists on the tube formation. HMVECs were stimulated with PGE2, sulprostone (Sulp), butaprost (Buta), or PGE1-OH (PGE1). Tubular networks were quantified with ImageJ software (National Institutes of Health). Each point represents the mean ± SEM of values obtained from 3 independent experiments performed in triplicate. (B) Effect of EP antagonists on the tube formation. HMVECs were treated with AH23848 or AH6809 in the presence or absence of PGE2 or PGE1-OH. Tubular networks were quantified with ImageJ software (National Institutes of Health). Each point represents the mean ± SEM of values obtained from 3 independent experiments performed in triplicate. (C) Knockdown of endogenous EP4 expression. HMVECs were transfected with control scrambled siRNA (siCon) or siRNA targeting EP4 (siEP4) and allowed to grow for 60 hours. Total RNA was extracted and subjected to quantitative real-time PCR to determine expression of the EP4 gene. Expression of EP4 gene in the siEP4 sample is presented as a fraction of that in the siCon sample. Each point represents the mean ± SEM of 3 independent experiments. (D) Knockdown of EP4 expression inhibits the PGE2-induced tube formation. Representative images were obtained after 78 hours of transfection with siRNA. (E) Quantification of the tube formation after EP4 knockdown. Each point represents the mean ± SEM of triplicate samples, and experiments were repeated 3 times. ##P < .01 versus corresponding treatment in the siCon group. (A-C,E) *P < .05 and **P < .01, in comparison to corresponding control groups.
PGE2 promotes the tube formation by activating EP4. (A) Effect of EP agonists on the tube formation. HMVECs were stimulated with PGE2, sulprostone (Sulp), butaprost (Buta), or PGE1-OH (PGE1). Tubular networks were quantified with ImageJ software (National Institutes of Health). Each point represents the mean ± SEM of values obtained from 3 independent experiments performed in triplicate. (B) Effect of EP antagonists on the tube formation. HMVECs were treated with AH23848 or AH6809 in the presence or absence of PGE2 or PGE1-OH. Tubular networks were quantified with ImageJ software (National Institutes of Health). Each point represents the mean ± SEM of values obtained from 3 independent experiments performed in triplicate. (C) Knockdown of endogenous EP4 expression. HMVECs were transfected with control scrambled siRNA (siCon) or siRNA targeting EP4 (siEP4) and allowed to grow for 60 hours. Total RNA was extracted and subjected to quantitative real-time PCR to determine expression of the EP4 gene. Expression of EP4 gene in the siEP4 sample is presented as a fraction of that in the siCon sample. Each point represents the mean ± SEM of 3 independent experiments. (D) Knockdown of EP4 expression inhibits the PGE2-induced tube formation. Representative images were obtained after 78 hours of transfection with siRNA. (E) Quantification of the tube formation after EP4 knockdown. Each point represents the mean ± SEM of triplicate samples, and experiments were repeated 3 times. ##P < .01 versus corresponding treatment in the siCon group. (A-C,E) *P < .05 and **P < .01, in comparison to corresponding control groups.
To gain confidence in the conclusion that EP4 mediates the PGE2-induced tube formation, we used 2 additional approaches: EP subtype-specific ligand antagonist and gene knockdown with siRNA. Treatment with selective EP4 antagonist AH23848 inhibited both PGE2- and PGE1-OH–induced tube formation (Figure 2B). However, the treatment with EP1/EP2/EP3 antagonist AH6809 evidenced little effect on either the PGE2- or PGE1-OH–regulated tube formation (Figure 2B), further supporting the conclusion that EP4 transduces the angiogenic response. Finally, we modulated endogenous EP4 expression with the use of siRNA to directly implicate it in the tube formation. Quantitative real-time PCR analysis evidenced a > 80% reduction in the EP4 gene levels by siEP4, in comparison to scrambled siCon (Figure 2C). The knockdown of EP4 markedly reduced the PGE2-induced tube formation (Figure 2D-E). Together, these results provide direct evidence that EP4 mediates the PGE2-induced HMVECs tube formation.
PGE2 promotes the cAMP-dependent tube formation
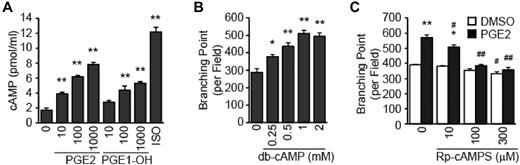
EP4 couples to heterotrimeric Gs proteins, and the most studied effector of activated Gαs-GTP is adenylyl cyclase that synthesizes soluble second messenger cAMP.5 We are able to show that stimulation of HMVECs with PGE2 or PGE1-OH increases accumulation of intracellular cAMP in a dose-dependent manner (Figure 3A), demonstrating the coupling of EP4 to Gαs. Significantly, treatment with the cAMP mimetic db-cAMP promotes the dose-dependent increase in the tube formation (Figure 3B), suggesting the EP4-mediated tube formation involves cAMP. In support of this idea, we find that treatment with the cAMP antagonist Rp-cAMPS attenuates the PGE2-mediated tube formation (Figure 3C). These results support the existence of a PGE2→EP4 →cAMP→HMVEC tube formation signaling cascade.
PGE2 induces angiogenesis via cAMP. (A) PGE2 and PGE1-OH treatment increases cAMP accumulation. HMVECs were treated with PGE2 (in nM), PGE1-OH (in nM), or isoproterenol (ISO; 1μM), and cAMP levels were determined with a cAMP enzyme immunoassay. Each point represents the mean ± SEM of triplicate samples, and experiments were repeated 3 times. (B) cAMP mimetic db-cAMP induces the tube formation. HMVECs were treated with db-cAMP for 6 hours followed by quantitation of branching points, as described. (C) cAMP competitive analog Rp-cAMPS inhibits the PGE2-induced tube formation. HMVECs were treated with Rp-cAMPS in the presence or absence of PGE2 (100nM) for 6 hours followed by quantitation of branching points. Each point represents the mean ± SEM of triplicate samples, and experiments were repeated 3 times. #P < .05 and ##P < .01 versus corresponding treatment in the control group. For all panels, *P < .05, **P < .01, compared with appropriate control group.
PGE2 induces angiogenesis via cAMP. (A) PGE2 and PGE1-OH treatment increases cAMP accumulation. HMVECs were treated with PGE2 (in nM), PGE1-OH (in nM), or isoproterenol (ISO; 1μM), and cAMP levels were determined with a cAMP enzyme immunoassay. Each point represents the mean ± SEM of triplicate samples, and experiments were repeated 3 times. (B) cAMP mimetic db-cAMP induces the tube formation. HMVECs were treated with db-cAMP for 6 hours followed by quantitation of branching points, as described. (C) cAMP competitive analog Rp-cAMPS inhibits the PGE2-induced tube formation. HMVECs were treated with Rp-cAMPS in the presence or absence of PGE2 (100nM) for 6 hours followed by quantitation of branching points. Each point represents the mean ± SEM of triplicate samples, and experiments were repeated 3 times. #P < .05 and ##P < .01 versus corresponding treatment in the control group. For all panels, *P < .05, **P < .01, compared with appropriate control group.
PKA Cγ mediates the tube formation
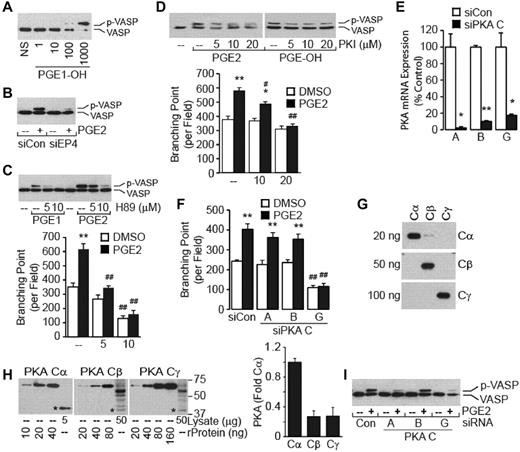
cAMP modulates the cellular response primarily by activating PKA, Epac (a guanine nucleotide exchange factor for small GTPase Rap), and/or ion channels.14 PKA activity may be determined with vasodilator-stimulated protein (VASP) phosphorylation as a surrogate indicator.15 Treatment with the selective EP4 agonist PGE1-OH induced a dose-dependent increase in the VASP phosphorylation (Figure 4A). Knockdown of endogenous EP4 expression with siRNA evidenced a dramatic reduction in the PGE2-mediated VASP phosphorylation (Figure 4B), supporting the notion that EP4 transduces the PKA activation signal. To strengthen the conclusion that a cAMP→PKA signal mediates the VASP phosphorylation, we examined the time- and dose-dependent effects of db-cAMP and Rp-cAMPS. Treatment of HMVECs with db-cAMP exhibited dose- and time-dependent increases in the VASP phosphorylation with maximal signal achieved at a dose of 1mM after 5 minutes of stimulation (data not shown). In accord with these results, treatment with the cAMP antagonist Rp-cAMPS inhibited the PGE2- and PGE1-OH–mediated VASP phosphorylation (data not shown).
PGE2 induces angiogenesis through PKA. (A) PGE1-OH induces the VASP phosphorylation. HMVECs were stimulated with PGE1-OH (in nM) for 5 minutes. Cell monolayers were lysed and subjected to Western blot analysis with the use of anti-VASP Abs. Note that the phosphorylation of VASP retards its migration on SDS-PAGE. (B) Knockdown of EP4 attenuates the PGE2-induced VASP phosphorylation. Cells transiently transfected with siCon or siEP4 were treated with PGE2 (100nM) for 5 minutes and analyzed for VASP phosphorylation content as in panel A. Inhibition of PKA activity with H89 (C) or PKI (D) attenuates the prostaglandin-mediated VASP phosphorylation and tube formation. HMVECs were pretreated, or not, with PKA inhibitor H89 for 30 minutes (C) or PKI for 1 hour (D) followed by stimulation with PGE2 (100nM for H89 and 10nM for PKI) or PGE1-OH (100nM) for 5 minutes for the VASP phosphorylation (top) or 6 hours for the tube formation (bottom). Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. *P < .05, **P < .01, compared with corresponding vehicle sample; #P < .05, ##P < .01, compared with corresponding treatment sample. (E) Knockdown of PKA catalytic subunits. HMVECs were transiently transfected with siCon or siRNA targeting genes encoding the individual PKA catalytic subunits. Quantitative PCR analysis was performed 60 hours after transfection. *P < .05, **P < .01, compared with corresponding siCon sample. (F) Knockdown of PKA Cγ attenuates the PGE2-induced tube formation. Cells transfected with siCon or siRNA targeting gene encoding the individual PKA catalytic subunit for 72 hours were used for the tube formation assay. Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. **P < .01, compared with vehicle-treated samples; ##P < .01, compared with corresponding treatment samples. (G) Detection of PKA catalytic subunit proteins. Recombinant PKA Cα, Cβ, and Cγ GST-fusion proteins were subjected to Western blot analysis with the use of anti-Cα, anti-Cβ, or anti-Cγ Abs. Numbers on the left indicate loaded amount of each protein. (H) Expression of PKA catalytic subunit proteins in HMVECs. Recombinant PKA Cα, Cβ, and Cγ GST-fusion proteins were used as standards. Intensity of bands was determined with ImageJ (National Institutes of Health). Data are presented as fold change in expression of PKA catalytic subunit, where the value of Cα was arbitrarily assigned 1.0. Numbers on the right indicate molecular mass in kDa. The * denotes endogenous catalytic subunit protein. (I) Effect of knocking down expression of PKA catalytic subunits on PGE2-induced VASP phosphorylation as determined by Western blotting with the use of anti-VASP Abs.
PGE2 induces angiogenesis through PKA. (A) PGE1-OH induces the VASP phosphorylation. HMVECs were stimulated with PGE1-OH (in nM) for 5 minutes. Cell monolayers were lysed and subjected to Western blot analysis with the use of anti-VASP Abs. Note that the phosphorylation of VASP retards its migration on SDS-PAGE. (B) Knockdown of EP4 attenuates the PGE2-induced VASP phosphorylation. Cells transiently transfected with siCon or siEP4 were treated with PGE2 (100nM) for 5 minutes and analyzed for VASP phosphorylation content as in panel A. Inhibition of PKA activity with H89 (C) or PKI (D) attenuates the prostaglandin-mediated VASP phosphorylation and tube formation. HMVECs were pretreated, or not, with PKA inhibitor H89 for 30 minutes (C) or PKI for 1 hour (D) followed by stimulation with PGE2 (100nM for H89 and 10nM for PKI) or PGE1-OH (100nM) for 5 minutes for the VASP phosphorylation (top) or 6 hours for the tube formation (bottom). Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. *P < .05, **P < .01, compared with corresponding vehicle sample; #P < .05, ##P < .01, compared with corresponding treatment sample. (E) Knockdown of PKA catalytic subunits. HMVECs were transiently transfected with siCon or siRNA targeting genes encoding the individual PKA catalytic subunits. Quantitative PCR analysis was performed 60 hours after transfection. *P < .05, **P < .01, compared with corresponding siCon sample. (F) Knockdown of PKA Cγ attenuates the PGE2-induced tube formation. Cells transfected with siCon or siRNA targeting gene encoding the individual PKA catalytic subunit for 72 hours were used for the tube formation assay. Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. **P < .01, compared with vehicle-treated samples; ##P < .01, compared with corresponding treatment samples. (G) Detection of PKA catalytic subunit proteins. Recombinant PKA Cα, Cβ, and Cγ GST-fusion proteins were subjected to Western blot analysis with the use of anti-Cα, anti-Cβ, or anti-Cγ Abs. Numbers on the left indicate loaded amount of each protein. (H) Expression of PKA catalytic subunit proteins in HMVECs. Recombinant PKA Cα, Cβ, and Cγ GST-fusion proteins were used as standards. Intensity of bands was determined with ImageJ (National Institutes of Health). Data are presented as fold change in expression of PKA catalytic subunit, where the value of Cα was arbitrarily assigned 1.0. Numbers on the right indicate molecular mass in kDa. The * denotes endogenous catalytic subunit protein. (I) Effect of knocking down expression of PKA catalytic subunits on PGE2-induced VASP phosphorylation as determined by Western blotting with the use of anti-VASP Abs.
We used H89 and PKI as diagnostic tools to directly implicate PKA in angiogenesis.16 Both H89 (Figure 4C) and PKI (Figure 4D) inhibited the PGE2- and PGE1-OH–mediated VASP phosphorylation in a dose-dependent manner. Significantly, the treatment with H89 (Figure 4C) or PKI (Figure 4D) also inhibited the PGE2-induced tube formation, providing direct support for an EP4→ cAMP → PKA signal to promote the tube formation.
The mammalian PKA family consists of 4 regulatory subunits RIα, RIβ, RIIα, and RIIβ and 3 catalytic subunits Cα, Cβ, and Cγ, and each of these subunits is encoded by a unique gene. In the inactive state, PKA exists as a tetrameric holoenzyme composed of 2 regulatory subunits bound to 2 catalytic subunits. Binding of cAMP to the regulatory subunits results in the release and activation of monomeric catalytic subunits.17 To identify the PKA subunit(s) involved in the tube formation, we knocked down expression of the individual catalytic subunits in the HMVECs (Figure 4E). Whereas the knockdown of the genes encoding PKA Cα or Cβ showed little effect on the PGE2-induced tube formation, the knockdown of PKA Cγ dramatically decreased the tube formation (Figure 4F). To exclude the possibility that PKA Cγ protein is disproportionately (over)expressed in the endothelial cells, we determined expression of individual PKA catalytic subunit proteins (Figure 4G-H). Results show selectivity of used Abs (Figure 4G) and show the high expression of PKA Cα in comparison to PKA Cβ or Cγ subunits (Figure 4H). Functionally, the knockdown of the genes encoding PKA Cα or Cγ, but not Cβ, attenuated the PGE2-induced VASP phosphorylation (Figure 4I).Together, these results introduce the selective participation of the PKA Cγ subunit in the HMVEC tube formation and propose its selective effectors (vide infra) in the process of angiogenesis.
Select PKA substrates mediate the PGE2-controlled tube formation
The knockdown of genes encoding individual PKA catalytic subunits suggested involvement of PKA substrate(s) in the tube formation process. To begin to identify the PKA effector(s) involved in the HMVEC tube formation, we screen a representative siRNA library to target select PKA substrate genes.18 The PKA substrates mediate multiple cellular responses, including cell migration and invasion (RhoA, Rap1A, Rap1B, Rap1GAP), mitogenic signaling (Csk, Ras-GRF1, Raf1), transcriptional regulation (CREB), apoptosis and cell survival (GSK3α, GSK3β), trafficking (HSPB6, synapsin1, VASP), and endothelial function (eNOS). Knockdown of representative protein expression (Figure 5A) illustrated the high efficiency. Remarkably, the knockdown of endogenous HSPB6, eNOS, or Rap1A resulted in a substantial reduction in the PGE2-induced tube formation (Figure 5B). In contrast, the knockdown of endogenous RhoA or GSK3β significantly increased both basal and PGE2-induced tube formation (Figure 5B-C). It is interesting to note that PKA phosphorylation inhibits activity of GSK3β and RhoA but increases activity of HSPB6, eNOS, and Rap1A. These results support the idea that select PKA substrates mediate the PGE2-induced HMVEC tube formation.
PKA substrates mediate the PGE2 proangiogenic signal. (A) Knockdown efficiency of representative targets. HMVECs were transiently transfected with scrambled siRNA (CN) or siRNA targeting indicated genes (KD) and allowed to recover for 72 hours. Cell lysates were collected and analyzed by immunoblotting with the use of the corresponding Abs. (B) Quantitation of HMVEC tube formation. HMVECs transfected with siRNA corresponding to the indicated gene or scramble siRNA for 72 hours were seeded into growth factor–reduced Matrigel in the presence of vehicle DMSO or PGE2 (100nM) for 6 hours, followed by counting the branching points, as described. The branching points of the tubular network of cells transfected with scramble siRNA and treated with DMSO were set to 100. All other branching point counts are presented as the fraction of this control value. Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. *P < .05, compared with vehicle in the control group; ##P < .01, compared with corresponding treatment in the control group. (C) Representative images depicting tube formation of HMVECs transfected with siRNA targeting GSK3β (siGSK3β) or scrambled siRNA (siCon) after treatment, or not, with PGE2 (100nM) for 6 hours. (D) PGE2 promotes the dose-dependent GSK3β phosphorylation. HMVECs were treated with PGE2 (in nM) for 5 minutes (left) or LiCl (in mM) for 5 minutes (right). Cell lysates were analyzed by Western blotting with the use of phospho-Ser9 or total GSK3β Abs. (E) LiCl promotes the HMVEC tube formation. HMVECs were subjected to tube formation assay in the presence of LiCl or NaCl (both in mM) for 6 hours, followed by measurement of branching points of the tubular network, as described. Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. *P < .05, **P < .01, compared with NaCl group. (F) Knockdown of PKA Cγ attenuates PGE2-induced GSK3β phosphorylation. HMVECs transfected with siCon or siPKA that targeted individual catalytic subunits were stimulated, or not, with PGE2 (100nM) for 5 minutes, and cell lysates were subjected to Western blot analysis with the use of anti–phospho-Ser9 (top) or total (bottom) GSK3β Abs.
PKA substrates mediate the PGE2 proangiogenic signal. (A) Knockdown efficiency of representative targets. HMVECs were transiently transfected with scrambled siRNA (CN) or siRNA targeting indicated genes (KD) and allowed to recover for 72 hours. Cell lysates were collected and analyzed by immunoblotting with the use of the corresponding Abs. (B) Quantitation of HMVEC tube formation. HMVECs transfected with siRNA corresponding to the indicated gene or scramble siRNA for 72 hours were seeded into growth factor–reduced Matrigel in the presence of vehicle DMSO or PGE2 (100nM) for 6 hours, followed by counting the branching points, as described. The branching points of the tubular network of cells transfected with scramble siRNA and treated with DMSO were set to 100. All other branching point counts are presented as the fraction of this control value. Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. *P < .05, compared with vehicle in the control group; ##P < .01, compared with corresponding treatment in the control group. (C) Representative images depicting tube formation of HMVECs transfected with siRNA targeting GSK3β (siGSK3β) or scrambled siRNA (siCon) after treatment, or not, with PGE2 (100nM) for 6 hours. (D) PGE2 promotes the dose-dependent GSK3β phosphorylation. HMVECs were treated with PGE2 (in nM) for 5 minutes (left) or LiCl (in mM) for 5 minutes (right). Cell lysates were analyzed by Western blotting with the use of phospho-Ser9 or total GSK3β Abs. (E) LiCl promotes the HMVEC tube formation. HMVECs were subjected to tube formation assay in the presence of LiCl or NaCl (both in mM) for 6 hours, followed by measurement of branching points of the tubular network, as described. Each point represents the mean ± SEM of values obtained from 3 experiments performed in triplicate. *P < .05, **P < .01, compared with NaCl group. (F) Knockdown of PKA Cγ attenuates PGE2-induced GSK3β phosphorylation. HMVECs transfected with siCon or siPKA that targeted individual catalytic subunits were stimulated, or not, with PGE2 (100nM) for 5 minutes, and cell lysates were subjected to Western blot analysis with the use of anti–phospho-Ser9 (top) or total (bottom) GSK3β Abs.
We used GSK3β to further establish the PGE2-regulated signal pathway involved in the tube formation. Results show that PGE2 induces the dose-dependent increase in GSK3β phosphorylation (Figure 5D left) that was inhibited, in a dose-dependent manner, by the EP4 antagonist AH23848 and PKA inhibitor H89 (data not shown). For this set of experiments, we used the GSK3β activity modulator lithium chloride as a control and could confirm its dose-dependent effect to increase the GSK3β phosphorylation (Figure 5D right). Expectedly, the treatment with lithium chloride, but not control sodium chloride, also increased the tube formation (Figure 5E). To provide a direct link between PKA and PGE2-controlled GSK3β phosphorylation, genes encoding the individual PKA catalytic subunits were knocked down with siRNA. The knockdown of PKA Cα or Cβ exhibited no significant effects on the PGE2-induced GSK3β phosphorylation (Figure 5F). However, the knockdown of PKA Cγ obliterated the PGE2-induced GSK3β phosphorylation (Figure 5F), mirroring the effects seen on tube formation (Figure 4F). Collectively, these results reiterate a role for PKA Cγ and GSK3β in the HMVECs tube formation.
PGE2 promotes the EP4- and PKA-dependent angiogenesis
To corroborate the results of in vitro tube formation with actual angiogenesis, we implanted acetal sponges12 subcutaneously in the flanks of mice. Appropriately treated sponge scaffolds were inspected visually, and they exhibited an intensified reddish color after the PGE2 treatment (8.8 ng/g body weight), in comparison to vehicle (Figure 6A). Importantly, the similar treatment with AH23848 (25 or 125 ng/g body weight) or H89 (11 or 55 ng/g body weight) evidenced a decrease in the reddish color, compared with vehicle-treated sponges (Figure 6A). Sponge reddish color reflects hemoglobin content, and the direct measurement of hemoglobin concentration showed the PGE2-mediated increase (182.3% vs DMSO control) but (the dose-dependent) AH23848- and H89-induced decrease of hemoglobin content in the sponges (Figure 6B), providing quantitative changes in the function of the blood vessels.
EP4 and PKA mediate the in vivo angiogenesis. (A) Images of polyvinyl acetal sponges harvested from mice. The sponges were implanted in the flank of C57BL/6 mice and injected with PGE2 (8.8 ng/g body weight), AH23848 (25 or 125 ng/g body weight), or H89 (11 or 55 ng/g body weight) every 2 days for 2 weeks. (B) Hemoglobin content in the sponges as determined with the Drabkin reagent. Each point represents the mean ± SEM of values obtained from 4 to 5 sponges. *P < .05, **P < .01, compared with corresponding vehicle group. Low, AH23848 (25 ng/g body weight) and H89 (11 ng/g body weight) and high, AH23848 (125 ng/g body weight) and H89 (55 ng/g body weight), groups. (C) Expression of CD31+ cells by immunohistochemistry. Harvested sponges were sectioned and stained with the endothelial cell marker CD31 Ab. Sections were counterstained with hematoxylin to visualize nuclei. (D) Blood vessel density in harvested sponges as quantitated by counting red-staining–positive cells or cell clusters. Three 200× magnification fields containing the most prominent hotspots were counted for each section. Each point represents the mean ± SEM of values obtained from 4 to 5 sponges. *P < .05, **P < .01, compared with corresponding vehicle group. Low, AH23848 (25 ng/g body weight) and H89 (11 ng/g body weight) and high, AH23848 (125 ng/g body weight) and H89 (55 ng/g body weight), groups. (E) Schematic presentation of PGE2-induced angiogenic signal in HMVECs (see text for details).
EP4 and PKA mediate the in vivo angiogenesis. (A) Images of polyvinyl acetal sponges harvested from mice. The sponges were implanted in the flank of C57BL/6 mice and injected with PGE2 (8.8 ng/g body weight), AH23848 (25 or 125 ng/g body weight), or H89 (11 or 55 ng/g body weight) every 2 days for 2 weeks. (B) Hemoglobin content in the sponges as determined with the Drabkin reagent. Each point represents the mean ± SEM of values obtained from 4 to 5 sponges. *P < .05, **P < .01, compared with corresponding vehicle group. Low, AH23848 (25 ng/g body weight) and H89 (11 ng/g body weight) and high, AH23848 (125 ng/g body weight) and H89 (55 ng/g body weight), groups. (C) Expression of CD31+ cells by immunohistochemistry. Harvested sponges were sectioned and stained with the endothelial cell marker CD31 Ab. Sections were counterstained with hematoxylin to visualize nuclei. (D) Blood vessel density in harvested sponges as quantitated by counting red-staining–positive cells or cell clusters. Three 200× magnification fields containing the most prominent hotspots were counted for each section. Each point represents the mean ± SEM of values obtained from 4 to 5 sponges. *P < .05, **P < .01, compared with corresponding vehicle group. Low, AH23848 (25 ng/g body weight) and H89 (11 ng/g body weight) and high, AH23848 (125 ng/g body weight) and H89 (55 ng/g body weight), groups. (E) Schematic presentation of PGE2-induced angiogenic signal in HMVECs (see text for details).
We performed immunohistochemical staining of the sponge tissues to show the blood vessel densities with the use of the endothelial cell marker CD31 Ab. Results show a significant increase in immunoreactive cells and vessel-like structures in the PGE2-treated group (Figure 6C). In contrast, a significant decrease in detectable CD31+ cells was observed in the AH23848- or H89-treated groups (Figure 6C). Quantitation of the stained cells or cell clusters that were separated from adjacent microvessels indicated a dose-dependent decrease in the vessel density after treatment with AH23848; there was a 8.8% and 29.6% decrease in vessel density on treatment with 25 ng/g of body weight and 125 ng/g of body weight, respectively, of H89 (Figure 6D). Concordantly, the treatment with 11 ng/g of body weight and 55 ng/g of body weight of AH23848 prompted a 37.6% and 42.5%, respectively, decrease in the number of stained cells per vessel (Figure 6D). Collectively, these data show a direct role for EP4 and PKA in angiogenesis and provide rationale for their targeting as a way to limit uncontrolled angiogenic responses.
Discussion
Inflammatory mediators are now recognized for their role in not only mounting a proper immune reaction after exogenous pathogenic attack but also in the body's response to innate diseases. Prostaglandins, specifically PGE2, exert immunomodulatory roles under both physiologic and pathologic conditions. The PGE2 affects target cells by activating cognate receptors EP1-4 that belong to the G protein–coupled receptor superfamily of cell surface–expressed receptors. The central finding of this work is that PGE2 acts directly on endothelial cells, thereby promoting assembly of new blood vessels through selective activation of the EP4 and PKA Cγ signal pathway. As depicted schematically in Figure 6E, the EP4-mediated angiogenic signaling cascade may encompass heterotrimeric Gαs protein, cAMP, and PKA Cγ and its selective substrates.
PGE2 is a product of COX2, and there is ample evidence to implicate COX2 as an angiogenesis mediator. For instance, IL-1β and bone morphogenic proteins (BMP) induce angiogenesis in a manner that depends on COX2 activity; treatment with COX2 inhibitors attenuates both IL-1–19 and BMP620-mediated angiogenesis. Moreover, the IL-1β- and BMP6-mediated corneal angiogenesis and microvessel outgrowth, respectively, are markedly attenuated in COX2−/−, compared with wild-type animals.19,20 Proangiogenic effects of COX2 can be inhibited by nonsteroidal anti-inflammatory drugs, and the inhibitory effects of nonsteroidal anti-inflammatory drugs can be reversed by the addition of exogenous PGE2,21 implying COX2 promotes angiogenesis, at least in part, via PGE2 signaling systems. Remarkably, protein kinase CK2 regulates the expression of COX2 and drives retinal neovascularization.22
The PGE2 may induce angiogenesis by acting indirectly on a variety of cell types to produce proangiogenic factors. Indeed, PGE2 stimulation of airway smooth muscle,23 endometrium,7 or colon8 cancer cells leads to increased production of VEGF, bFGF, or CXCL1 that, in turn, act on target endothelial cells to promote the angiogenesis. Interestingly, the PGE2-induced increase in VEGF expression was reported to be mediated by EP1 in colon cancer cells,24 EP2 and EP4 in ovarian cancer and airway smooth muscle cells,23,25 and EP3 in fibroblasts.26 Hence, multiple signaling networks instigated by the various EPs can mediate the PGE2-induced angiogenic response, and the choice of involved pathway(s) may depend on the target (cancer) cell type.
In addition, PGE2 may elicit angiogenic responses by acting directly on endothelial cells via a reported multiplicity of mechanisms that are dictated by cell type and context. In postcapillary venular endothelial cells, stimulation with PGE2 induced the EP3-mediated activation of the nonreceptor tyrosine kinase c-Src that, in turn, activated matrix metalloproteases, leading to the transactivation of FGF receptors and the consequent angiogenic response.27 The PGE2-mediated activation of matrix metalloproteases also leads to increased expression of TGFβ that, in turn, activates the Smad3 pathway, thereby prompting angiogenesis.28 In HMVECs, the stimulation with PGE2 was reported to act through EP2 and EP4 to increase expression of CXCR4, which facilitates angiogenesis.9 Under these scenarios, PGE2 promotes the angiogenesis via activation of distinct ligand-receptor pairs that themselves transduce the angiogenic response. In the HMVECs that we used in this study, we observed no measurable effect of PGE2 on the expression of VEGF (data not shown). Rather, our results with EP4-selective agonists, complemented with EP4-selective antagonists and EP4 knockdown with siRNA, evidence the EP4-directed angiogenic response. Similar use of complementary biologic and pharmacologic activators and inhibitors showed a role for cAMP and its target PKA in the EP4-mediated angiogenesis.
The EP4 signals through heterotrimeric Gs proteins, leading to enhanced synthesis of cAMP and activation of PKA. Our results with the use of mimetic and competitor pharmacologics place cAMP as a mediator of the PGE2-transduced angiogenesis. Consistent with this conclusion, several lines of evidence support a role for cAMP in angiogenesis. First, stimulation with forskolin, a potent inducer of cAMP accumulation, promotes the human umbilical cord endothelial cell proliferation, migration, and tube formation.29 Second, blockade of cAMP degradation with the phosphodiesterase 3 inhibitor cilostazol enhances the ability of human aortic endothelial cells to form tubes.30 Third, activation of β-adrenoceptors, which couple to Gs and increase cAMP accumulation, promotes the tumor angiogenesis.31
There are 3 catalytic subunits of PKA, and our results emphasize the important role of the Cγ subunit in the EP4-induced angiogenesis: knockdown of the individual genes encoding Cα and Cβ subunits showed little effect on the PGE2-induced tube formation, whereas the knockdown of Cγ abolished it. Nonetheless, results also show that the combined knockdown of Cα and Cβ subunits attenuated the PGE2-induced tube formation (data not shown), suggesting substrate(s) overlap of the PKA catalytic subunits that are critical for angiogenesis. Notably, the PKA catalytic subunits display selective tissue distribution and substrate specificity.32,33 All subunits are detected in HUVECs, although their expression is differentially regulated by IL-1β and IFN.34,35 Despite the high degree of sequence homology among the 3 PKA catalytic subunits, emerging evidence suggests the substrate specificity. Exemplar HSV thymidine kinase is regulated by the Cγ, but not Cα, subunit.33 Our results imply that VASP is phosphorylated by PKA Cα or Cγ, but not Cβ, whereas GSK3β is phosphorylated by Cγ but not Cα or Cβ, suggesting the individual PKA catalytic subunits use specific substrates.
Our results suggest a role for select PKA substrates in the PGE2-mediated angiogenic response. Results from the knockdown of individual genes that encode for PKA substrates justify the conclusion that eNOS, HSPB6, and Rap1A exert proangiogenic signals, whereas RhoA and GSK3β exhibit antiangiogenic effects. The eNOS is a well-established regulator of vascular tone, and there is evidence to show that it stimulates angiogenesis by increasing levels of NO, leading to the activation of cGMP-dependent protein kinase signaling.34 PKA activates eNOS by phosphorylating it on Ser635 or Ser1177. HSPB6, a member of the small heat shock protein family, can be activated by PKA after phosphorylation of Ser16 and is implicated in multiple processes, including myocardial infarction.36 Enhanced activation of Akt and increased secretion of VEGF and bFGF were observed in HSPB6-engineered mesenchymal stem cells, resulting in improved function of infarcted myocardium and elevated angiogenesis in myocardial tissue.37 Rap1A is a member of the Ras family of small GTP-binding proteins, and it regulates multiple cellular functions, including adhesion, polarity, and growth. The Rap1A activity can be regulated by PKA, which phosphorylates it on Ser180.38 It was shown that Rap1A is essential for in vitro endothelial cell angiogenic sprouting, migration, adhesion, and affinity to integrin.39 Rap1A was also suggested to mediate bFGF- and VEGF-induced angiogenesis.40,41 Our results show that knockdown of PKA-activatable eNOS, HSPB6, or Rap1A impairs angiogenesis, but their direct involvement in the PKA-controlled angiogenesis remains to be established.
The activity of small GTPase RhoA, a member of the Ras superfamily, can be negatively affected by PKA-dependent phosphorylation of Ser188.42 RhoA and its downstream effectors, the RhoA kinases ROCK1 and ROCK2, regulate a number of cellular processes, including cell motility, proliferation, and cytoskeletal architecture. Evidence suggests that PKA-phosphorylated RhoA permits the proangiogenic signals. Activated β-adrenoceptors induce the PKA-dependent RhoA phosphorylation, which is deficient to activate ROCK,43 and inhibition of ROCK induces angiogenesis.44 Moreover, a group of microtubule inhibitors was observed to elicit potent antiangiogenic and vascular-disrupting effects by elevating RhoA activity.45,46 GSK3β, an isoform of the highly conserved and ubiquitously expressed GSK3, phosphorylates not only the originally identified glycogen synthase but also a broad range of other substrates implicated in metabolism, microtubule dynamics, embryonic development, and tumorigenesis. PKA phosphorylates GSK3β on Ser9, thereby inhibiting its activity,47 and it was reported that activated GSK3β promotes the endothelial cell apoptosis and disruption of cell matrix attachment.47 In addition, inhibition of GSK3β abolishes the antiangiogenic effects because of blockade of growth factor signals,48 and transplantation of endothelial progenitor cells expressing catalytically inactive GSK3β into ischemic hind limbs of athymic mice significantly improves the blood flow, limb salvage, and tissue capillary density.49 Accordingly, our results show that the knockdown of RhoA or GSK3β that become inactivated after phosphorylation by PKA increases the angiogeneic response. Nonetheless, the roles of RhoA and GSK3β in PKA-regulated angiogenesis remain to be determined and will require further studies.
In summary, our findings show a new PGE2-regulated proangiogenic pathway in endothelial cells that involves EP4, PKA, and select PKA substrates. Interference of the expression or activation of these key molecules may provide benefit in the management of inflammation- and angiogenesis-related diseases, including cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J. M. Davidson for providing sponges and Dr Z. Hu, Dr E. Eruslanov, Ms L. Lynch, and Ms E. Grigson for technical assistance. They also thank Dr P. Chakraborty for helpful comments on the manuscript and members of the Daaka laboratory for helpful suggestions and discussion.
This work was supported, in part, by the National Cancer Institute (Public Health Service grant CA129155, Y.D.).
National Institutes of Health
Authorship
Contribution: Y.Z. and Y.D. designed the experiments; Y.Z. performed all experiments in this work; and Y.Z. and Y.D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yehia Daaka, University of Florida College of Medicine, 2033 Mowry Rd, CGRC Rm 462, Gainesville, FL 32610; e-mail: ydaaka@ufl.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal